Abstract
Pathologic processes intrinsic and extrinsic to the tendons have been proposed as the underlying cause of rotator cuff disease, but the precise etiology is not known. Tear formation is, in part, attributable to the accumulation of subrupture tendon fatigue damage. We review the molecular, mechanical, and structural changes induced in tendons subjected to controlled amounts of fatigue loading in an animal model of early tendinopathy. The distinct tendon responses to low and moderate levels of loading, as opposed to high levels, provide insight into the potential mechanisms for the therapeutic benefits of exercise in the treatment of rotator cuff tendinopathy. The progression of damage accumulation leading to fiber rupture and eventual tendon tearing seen with higher loading illustrates the progression from tendinopathy to full-thickness tearing. We hope that this more realistic animal model of tendon fatigue damage will allow better assessment of biologic, mechanical, tissue-engineering, and rehabilitation strategies to improve repair success.
Keywords: Rotator cuff tear, tendon fatigue damage, biologic, healing
Rotator cuff disease is the most common cause of shoulder pain, and more than 75,000 rotator cuff repairs are performed in the United States annually.23 Biopsy specimens taken from ruptured tendons suggest that full-thickness tears typically result from a chronic degenerative process rather than acute avulsion.5,11 Pathologic processes intrinsic and extrinsic to the cuff tendons have been proposed as the underlying cause, but the precise etiology is not known. Differentiating between primary and secondary causes is difficult because clinical data are limited to specimens taken from tendons in the end stages of disease, and little data exist describing early tendinopathy.
Clinical outcome studies increasingly suggest that the level of degeneration within repaired tendons is the most important factor influencing tendon-to-bone healing.8,20 An understanding of the mechanisms by which tendinopathy progresses to tendon rupture will be integral to the development of future treatments. Several clinical findings suggest that tear formation is partly attributable to the accumulation of subrupture tendon damage. These include a greater incidence of rotator cuff tears in the dominant extremity, an increase in incidence with increasing age, and the higher incidence of rotator cuff tears in overhead throwing athletes.24
Tendinopathy is also thought to be a precursor of Achilles, quadriceps, and patella tendon rupture. However, the pathologic progression from healthy tendon to tendinopathy and, eventually, tendon rupture is not fully defined. Animal models of tendinopathy have been developed to study tendon response to several potential etiologic factors. We review the molecular, mechanical, and structural changes induced in tendons subjected to controlled amounts of fatigue loading in an animal model of early tendinopathy.
Animal models
Several investigators have developed animal models of overuse or repetitive injury. Soslowsky et al17 used treadmill running to induce overuse injuries in the rotator cuff of rats. They demonstrated significant changes in the supraspinatus tendon compared with healthy controls, including increased cellularity and a decrease in collagen organization. Barbe et al4 trained rats to perform repetitive forelimb reaching and grabbing. They found tendon fibril fraying and an increased number of macrophages in the reaching limbs compared with the contralateral controls after 6 and 8 weeks.
These models have provided insight into the cumulative effects of repetitive loading but are limited in their ability to directly control the loads applied to the tendon. To address this limitation, we developed an in vivo rat patellar tendon model of fatigue damage accumulation that allows direct, precise control of the magnitude and frequency of the load applied to the tendon.6 Control of the fatigue-loading parameters allows for investigation of various clinically relevant scenarios by varying the loading parameters.
The patella tendon is uniquely suited for this type of study because it is easily accessible and allows for clamping of the patella and tibia without damage to the tendon (Fig. 1). In addition, patellar tendinopathy is commonly encountered in the clinic, particularly in jumping athletes.21 A rat model was chosen because it is a small animal used previously in tendon biomechanical studies but is large enough to allow region-specific examination of the loaded tendons. Although this model is effective for modeling the early development of tendinopathy, which is pertinent to the management of rotator cuff tears, it does not account for the complex planes of motion that are commonly encountered within the shoulder and therefore does not precisely replicate the effect of these on the development of rotator cuff tendinopathy.
Figure 1.
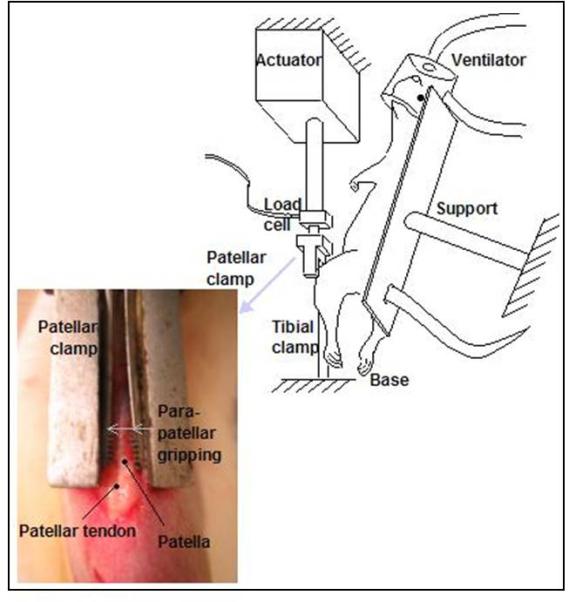
Schematic of experimental setup from Fung et al.6 The patella and the tibia of anesthetized rats are surgically exposed. Clamps are used to fix the tibia and connect the patella to a load cell allowing loading of the tendon without direct contact with the grips.
Experimental protocol
Left patellar tendons of anesthetized female retired-breeder Sprague Dawley rats are exposed through a midline incision.6 The tibia is clamped, maintaining the knee in approximately 30° of flexion. A custom clamp is used to grip the patella and is attached to a 50-lb load cell and actuator of a servohydrolic loading system (Fig. 1). The tendon itself is not clamped or damaged by this set up.
The fatigue-loading protocol has been previously described6 and consists of the following steps: A first diagnostic test is performed before fatigue loading to assess the initial state of the tendon. Fatigue loading is then applied for a number of cycles, ranging from 1 N to 40% of tendon ultimate load (defined by preliminary monotonic load experiments). The number of cycles is dictated by the desired severity of tendon damage. A second diagnostic test is then applied immediately after fatigue loading. The tendon is then allowed to recover for 45 minutes, after which a third diagnostic test is finally applied (Fig. 2). Mechanical parameters that are reflective of damage (damage parameters) have been identified.1 By comparing these damage indices between diagnostics 2 and 3, the recoverable effect of fatigue loading is determined. Similarly, by comparing these parameters between diagnostics 1 and 3, the nonrecoverable effect of fatigue loading is determined.1
Figure 2.
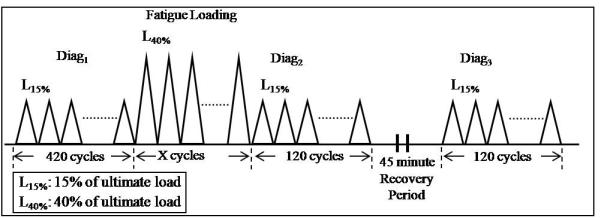
Representation of fatigue loading protocol.2
This model of in vivo fatigue loading has been used to evaluate the effects of low, moderate, and high levels of fatigue loading on the molecular, structural, and mechanical properties of the tendon. We have also used this model to identify parameters that are reflective of the damage induced in the tendon.
Molecular changes
Healthy tendon is a complex, highly organized material made up of collagen fibrils embedded in a matrix of proteoglycans. Tendons exhibit a close relationship between structural changes and molecular responses via mechanotransduction. Although inflammation is typically associated with the healing response of the tendon to laceration, cellular inflammation has not consistently been found in the biopsy specimens of degenerative rotator cuff tears, and the role of inflammation, especially its molecular mediators, in early tendinopathy remains a matter of debate.1,9,12 The biologic response in fatigue-loaded tendons is distinctly different from laceration and has been shown to include a hypertrophic molecular response to low levels of cyclic loading, similar to the physiologic stimulus of exercise, and also a catabolic response to high levels of fatigue damage, which could produce tendon rupture.
Sun et al19 used the rat fatigue-loading model to examine the effect of low (0.6% strain) and moderate (1.7% strain) levels of fatigue loading on expression of matrix metalloproteinase (MMP) -13 and interleukin-1β (IL-1β). The relationship between fatigue loading and MMP-13 was evaluated because MMP-13 contributes to the inflammation found in osteoarthritis, rheumatoid arthritis,22 and periodontal disease,10 and is found at increased levels in full-thickness rotator cuff tears.14 Similarly, IL-1β is the main inflammatory mediator in humans and rats and induces several MMPs in human tendons, including MMP-13.
Their results showed that loading rat patellar tendons in vivo to 0.6% strain (low) and 1.7% strain (moderate) produced distinctly different microstructural damage patterns and changes in expression of MMP-13 and IL-1β. Low strain loading resulted in kinked collagen deformation in isolated areas, with a mean damage area fracture of 4.1%. MMP-13 and IL-1β expression were suppressed nearly 70% compared with controls. Cyclic loading to moderate levels of fatigue produced some lateral fiber separation (tearing) and kinked fiber deformation, reflected in a higher mean damage area fraction (10.3%) than lower-level fatigue loading. MMP-13 and IL-1β messenger RNA (mRNA) levels, protein, and enzyme activity, all increased fivefold to sixfold compared with controls under these loading parameters, suggesting that MMP-13 and Il-1β activity are altered by fatigue loading in a dose-dependent fashion. Moderate fatigue loading, resulting in matrix damage, led to increased levels of these inflammatory mediators, whereas low strain loading, producing only matrix deformation without tearing, had a suppressive effect.
Fung et al6 used real-time polymerase chain reaction to analyze early changes in collagen subtype expression in response to varying amounts of fatigue loading and compared these with changes occurring after tendon laceration. In contrast to lacerated tendons, which showed only an increase in collagen (Col)-I expression, fatigue-loaded tendons demonstrated a more diverse response for all 3 Col subtypes, which varied between loading protocols:
Col-I was downregulated in tendons subjected to low-level fatigue at 1 and 3 days after loading. There was no change in Col-I expression after moderate fatigue loading, but high-level loading induced significant increases in Col-I expression at both times.
Col-III was increased 2.8-fold to 4-fold 1 day after low- and moderate-level fatigue loading but decreased to control levels at 3 days after low and moderate loading. After high-level loading, Col-III levels were similar to controls on day 1 but had increased 9.5-fold by day 3.
Col-V levels showed a similar pattern. They were increased 3.5-fold at day 1 and 3.2-fold to 5.8-fold at 3 days after fatigue at low and moderate damage. After high fatigue loading, they increased 16-fold after 1 day and 21-fold after 3 days.
Sun et al18 quantified mRNA expression for select collagens, MMPs and their inhibitor (TIMPs), 1 and 7 days after 100 cycles (low) and 7200 cycles (moderate) fatigue loading. Col-I, Col-III, and Col-V expression at 7 days after 7200 cycles were similar to those observed for moderate-level fatigue loading, as described by Fung et al.6 After 100 cycles of fatigue loading, MMP-3, MMP-13, and MMP-14, as well as Col-I and Col-XII, were upregulated. These upregulated MMPs are important in tendon remodeling and degradation of damaged matrix, and when considered in combination with increasing Col-I expression, these findings suggest that low levels of loading produce an adaptive, hypertrophic tendon response.
In contrast, a catabolic response was observed at 7200 cycles, marked by upregulation of Col-III and Col-V and downregulation of Col-I. The ratio of mRNA expression of Col-III to Col-I increased from 3:10 to 9:10 for 100 to 7200 cycles of fatigue loading. An increase in this ratio is seen in conditions of tendinopathy and may reflect poor matrix organization.16 Altered homeostasis is also reflected in the upregulation of TIMP-1 and TIMP-2, and downregulation of TIMP-4 for 7200 cycle fatigue. No significant change in TIMP activity was observed for 100-cycle fatigue loading, however.
Mechanical changes
Using real-time measures determined from the fatigue loading curves, Fung et al6 showed that peak cyclic tendon strain has 3 discrete phases during fatigue loading to failure. The primary phase was characterized by an increase in peak cyclic strain and tendon stiffness. This was followed by a transition to a secondary phase, which occurred between 300 and 500 cycles and was defined by a plateau in peak cyclic strain and no change in stiffness. The final, tertiary phase showed a steep increase in peak cyclic strain and a decrease in stiffness.
To assess the mechanical effects of fatigue loading, baseline hysteresis (defined as the area between the loading and unloading curves) and tangent stiffness from fatigue loading curves were compared at an initial cycle (cycle 15) and an endpoint cycle at low, moderate, and high levels of fatigue loading.6,7 Significant changes in stiffness and hysteresis were observed from cycle 15 to the endpoint at low, moderate, and high levels of fatigue loading. A significant increase in stiffness (~18%) was observed for low-level and moderate-level fatigue loading, whereas a significant decrease occurred after high-level fatigue loading (~18%). Similarly, a significant decrease in hysteresis occurred at low and moderate loading (~30%), but high-level fatigue loading produced an increase in hysteresis (~25%). The changes in stiffness and hysteresis between high and both low and moderate fatigue levels were significant. Increased stiffness and decreased hysteresis after lower levels of fatigue loading are likely due to fiber recruitment and redistribution of load from damaged to undamaged fibers. High-level loading produced more severe damage that may overwhelm this compensatory mechanism, leading to frank fiber rupture, reduced stiffness, and a steep increase in tendon strain that would ultimately lead to tendon failure with continued loading.
The recoverable and nonrecoverable changes in tendon fatigue response are currently being investigated by calculating the averages of certain mechanical parameters from the last 10 cycles of each diagnostic test and comparing these average values between diagnostics to identify parameters that can serve as indices of the damage induced.1 Differences in these parameters between Diag1 (prefatigue) and Diag3 (postrecovery) demonstrate non-recoverable changes, and differences between Diag2 (postfatigue) and Diag3 (postrecovery) define the recoverable effect of loading in the tendon.2
Several initial parameters were evaluated, including tendon elongation, hysteresis, stiffness of the loading and unloading portions of the load-displacement curves, and measures characterizing the end of the toe region. Non-recoverable changes were found in hysteresis, elongation, loading, and unloading stiffness.1 Changes in initial hysteresis and in loading and unloading stiffness were greater for a greater number of fatigue-loading cycles; however, elongation was not sensitive to the number of fatigue-loading cycles.
A relationship was identified between initial hysteresis loss and the stiffness of the tendon 7 days after fatigue loading. The relationship between the molecular response of the tendon 7 days after fatigue loading is currently being evaluated in the context of these initial damages indices, in addition to the number of fatigue-loading cycles, to gain greater insight into the effect of damage on the ability of the tendon to repair and heal.
Structural changes
The primary function of tendons is to bear tensile loads, and their tensile properties are largely derived from the structure and organization of Col-I. A hallmark feature of tendinopathy is disruption of tendon microarchitecture3,15 making inclusion of Col structure analysis critical to understanding the response to variable amounts of cyclic loading. Pathologic specimens taken from torn tendons show thinning and disorientation of Col fibers, myxoid degeneration, chondroid metaplasia, calcification, and vascular infiltration.9 Conventional histologic methods of tissue analysis are limited to 2 dimensional sections and may not fully demonstrate regional variation in tendon damage.
Second, harmonic imaging invokes the second-order optical property of Col using a near-infrared frequency laser and allows for imaging that characterizes tendon microstructure in 3-dimensions (3D).6 This imaging modality allows evaluation of tendon structure through thick plastic sections, thus minimizing sectioning artifact.13 Results of variable fatigue loading analyzed by this method showed 3D microstructure with progressively increasing density and variety of tendon damage with successive increases in loading.6
Tendons subjected to low-level loading were characterized by kinked deformations, whereas moderate loading produced fiber dissociation. Fiber thinning and out-of-plane discontinuity were found only in tendons subjected to high-level fatigue. Increasing damage levels resulted in increased anisotropy (Fig. 3). Qualitative assessment of the tendon’s midsubstance second harmonic generation microscopy images showed distinct differences that correlated to different levels of damage. Although control tendons exhibited aligned collagen fibers without matrix disruption, fatigue-loaded tendons displayed progressive structural damage and disorganization. Tendons loaded to low-level fatigue exhibited isolated fiber kinks. Tendons loaded to moderate-level fatigue showed similar changes but in a larger distribution, and widening of interfiber space was also present. Severe matrix disruption was found in high-level fatigue-loaded tendons.
Figure 3.
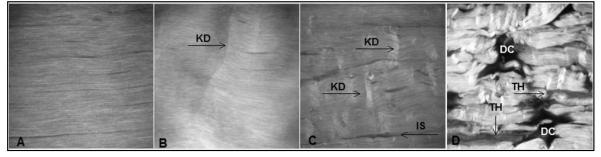
Increasing levels of fatigue loading resulted in progressive changes in tendon structure. (A) Non–fatigue-loaded tendons exhibited aligned collagen fibrils. (B) Kinked fiber deformations (KD, arrows) were characteristic of low level fatigue loaded tendons. (C) Kinked fiber deformations (KD, arrows) with widening of the inter fiber space (IS) were characteristic of moderate-level fatigue-loaded tendons. (D) Severe matrix disruption with fiber thinning (TH, arrows) and matrix discontinuities (DC) were characteristic of high-level fatigue-loaded tendons (field of view, 400 mm) Adapted from Fung et al.6
Conclusion
Despite its high prevalence, the inciting element for rotator cuff tendinopathy and tearing is not known. Rather than being a process that is purely intrinsic or extrinsic to the tendons itself, the origin of rotator cuff disease is likely multifactorial. Damage accumulation from repetitive use and aging likely contribute to the process.
This review describes the response of the tendon to fatigue loading using various parameters to improve our understanding of the early stages of tendinopathy. The distinct tendon responses to low and moderate levels of loading, as opposed to high levels, provide insight into the potential mechanisms for the therapeutic benefits of exercise in the treatment of rotator cuff tendinopathy and in the progression of damage accumulation to fiber rupture and eventual tendon tearing. This understanding of the pathologic progression of tendon disease will likely be the basis for future advances in the treatment of rotator cuff problems that could be aimed at altering the progression from tendinopathy to full-thickness tears.
Furthermore, optimizing the mechanical strength of rotator cuff repair constructs has improved clinical outcomes only marginally. Many biologic strategies to improve this have been tested in laceration models of healthy animal tendons that poorly simulate diseased and degenerated rotator cuff tendons. It is hoped that more realistic animal models of tendons subjected to repetitive fatigue damage will allow better assessment of biologic, mechanical, tissue-engineering, and rehabilitation strategies to improve repair success.
Footnotes
Disclaimer The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
This study was approved by the Mount Sinai Institutional Review Board.
References
- 1.Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. doi:10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andarawis-Puri N, Flatow EL. Tendon fatigue in response to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11:106–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670–5. doi: 10.1152/japplphysiol.00259.2009. doi:10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- 4.Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–76. doi: 10.1016/S0736-0266(02)00086-4. doi:10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codman EA. Complete rupture of the supraspinatus tendon. Operative treatment with report of two successful cases. 1911. J Shoulder Elbow Surg. 2011;20:347–9. doi: 10.1016/j.jse.2010.10.031. doi:10.1016/j.jse.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Fung DT, Wang VM, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier DM, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–9. doi: 10.1016/j.jbiomech.2009.08.039. doi:10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung DT, Wang VM, Laudier DM, Shine JH, Basta-Pljakic J, Jepsen KJ, et al. Subrupture tendon fatigue damage. J Orthop Res. 2009;27:264–73. doi: 10.1002/jor.20722. doi:10.1002/jor.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–28. doi: 10.1177/0363546506297539. doi:10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–20. doi: 10.1097/01.blo.0000092974.12414.22. doi:10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez M, Valenzuela MA, Lopez-Otin C, Alvarez J, Lopez JM, Vernal R, et al. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J Periodontol. 2006;77:1863–70. doi: 10.1902/jop.2006.050461. doi:10.1902/jop.2006.050461. [DOI] [PubMed] [Google Scholar]
- 11.Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–8. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 12.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–25. [PubMed] [Google Scholar]
- 13.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–5. doi: 10.1002/jor.20304. doi:10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 14.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–9. doi: 10.1177/0363546503262200. doi:10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 15.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36:533–8. doi: 10.1177/0363546507308549. doi:10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 16.Lui PP, Chan LS, Lee YW, Fu SC, Chan KM. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford) 2010;49:231–9. doi: 10.1093/rheumatology/kep384. doi:10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 17.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 18.Sun HB, Andarawis-Puri N, Li Y, Fung DT, Lee JY, Wang VM, et al. Cycle-dependent matrix remodeling gene expression response in fatigue-loaded rat patellar tendons. J Orthop Res. 2010;28:1380–6. doi: 10.1002/jor.21132. doi:10.1002/jor.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–61. doi: 10.1007/s11999-008-0278-4. doi:10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38:2435–42. doi: 10.1177/0363546510382835. doi:10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 21.Tiemessen IJ, Kuijer PP, Hulshof CT, Frings-Dresen MH. Risk factors for developing jumper’s knee in sport and occupation: a review. BMC Res Notes. 2009;2:127. doi: 10.1186/1756-0500-2-127. doi:10.1186/1756-0500-2-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collage-nase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–64. doi: 10.1186/ar401. doi:10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181–7. doi: 10.1016/j.jse.2006.06.013. doi:10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699–704. doi: 10.2106/JBJS.E.00835. doi:10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]


