Abstract
Piperlongumine (PL), isolated from the fruit of Long pepper, Piper longum, is a cancer-inhibiting compound that selectively kills tumor cells while sparing their normal counterparts. Here we evaluated the efficacy with which PL suppresses malignant B cells derived from a newly developed, double-transgenic mouse model of human endemic Burkitt lymphoma (BL), designated mCD40-LMP1/iMycEμ. PL inhibited tumor cell proliferation in a concentration-dependent manner and induced apoptosis of neoplastic but not normal B cells. Treatment with PL resulted in downregulation of EBV-encoded LMP1, cellular Myc, constitutive NF-κB activity, and a host of LMP1-Myc-NF-κB-regulated target genes including Aurka, Bcat1, Bub1b, Ccnb1, Chek1, Fancd2, Tfrc and Xrcc6. Of note, p21Cip1-encoding Cdkn1a was suppressed independent of changes in Trp53 mRNA levels and p53 DNA-binding activity. Considering the central role of the LMP1-NF-κB-Myc axis in B-lineage neoplasia, these findings further our understanding of the mechanisms by which PL inhibits B-lymphoma and provide a preclinical rationale for the inclusion of PL in new interventions in blood cancers.
Keywords: Cancer therapy and prevention, transgenic mouse model of human endemic Burkitt lymphoma, Epstein Barr virus, NF-κB, p21-encoding Cdkn1a
1. Introduction
Piperlongumine (PL), a constituent of Long pepper, Piper longum, demonstrates tumor-inhibiting, immunomodulatory, and antioxidant properties [1]. We were first to report that PL kills the high-grade B-cell tumor, Burkitt lymphoma (BL), but leaves normal B lymphocytes alive [2]. This finding added BL to a long list of cancer cell lines – including melanoma as well as bladder, breast and lung tumors – in which low micromolar concentrations of PL exhibit high levels of cytotoxicity [3]. To translate the promise of PL into actionable approaches to cancer therapy and prevention, it is necessary to elucidate the biological mechanism by which the compound inhibits malignant growth.
Hallmarks of human endemic BL include both infection with Epstein Bar virus (EBV) followed by expression of EBV-encoded LMP1 [4] and deregulation of the cellular oncoprotein MYC [5]. Both LMP1 and MYC promote B-cell proliferation and survival, using distinct but interconnected pathways [6,7]. LMP1 functionally mimics signaling of CD40 [4], a key downstream event of which is activation of the nuclear factor kappa-B (NF-κB) family of transcription factors, which regulate numerous target genes governing B-cell activation, proliferation and protection from apoptosis [8]. Constitutive NF-κB signaling is key to the development and therapy resistance of many cancers, including B-cell lymphoma [9,10,11]. Cooperative functions of LMP1, NF-κB and MYC have been well documented in B-cell lymphoma; e.g., LMP1 activates NF-κB [12]; NF-κB is a positive regulator of MYC expression [13,14]; and MYC and NF-κB are required for EBV/LMP1-driven tumors [15]. Developing targeted drugs for inhibiting the LMP1-NF-κB-MYC pathway is a priority for research on EBV-associated lymphomas and other cancers.
We recently developed a double-transgenic mouse model of human BL, designated mCD40-LMP1/iMycE, which lends itself to the testing of pharmacological inhibitors of LMP1-NF-κB-Mycdriven B-cell tumors. Mice harboring the iMycEμ [16] and mCD40-LMP1 [17] transgenes are prone to BL-like tumors from which continuous cell lines can be readily established. Here, we took advantage of one of these cell lines, Hal2G1, to evaluate the possibility that PL kills BL-like tumors by inhibiting the LMP1-NF-κB-Myc pathway. Treatment with PL resulted in strong pathway suppression, leading to the downregulation of critical LMP1-NF-κB-Myc target genes including Aurka, Bcat1, Bub1b, Ccnb1, Chek1, Tfrc, Fancd2, Xrcc6 and Cdkn1a. Interestingly, PL-dependent inhibition of Cdkn1a, which encodes the p53 target and cell cycle inhibitor, p21CIP1, was not associated with changes in TP53 mRNA or DNA-binding activity of p53. These results further our understanding of the mechanism by which PL kills malignant B cells and underline the utility of strain mCD40-LMP1/iMycEμ mice for PL’s envisioned preclinical assessment in vivo.
2. Materials and Methods
2.1. Mice and tumor cell lines
C57BL/6 (B6) mice containing the mCD40-LMP1 [17] or iMycEμ [16] transgene were intercrossed to generate double-transgenic mCD40-LMP1/iMycEμ mice. Tumor cell lines, designated Hal, were generated from BL-like tumors harvested from these mice. Hal2G1 was derived from a tumor that had been propagated in vivo (first transplant generation or G1), whereas Hal1G0 was derived from a primary tumor (G0) from a different mouse. WEHI231 mouse B lymphoma cells were purchased from ATCC (ATCC, Manassas, VA). iMycEμ-1 lymphoblastic B-cell lymphoma (LBL) cells have been described previously [18]. All cell lines were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, at 37 °C in a humidified 5% CO2 incubator. Normal splenic B cells were isolated from B6 mice using CD45R (B220) MACS beads (Miltenyi Biotec, Auburn, CA). Human B-lymphocytes were isolated from blood donor PBMCs (peripheral blood mononuclear cells), using centrifugation in a Ficoll-Paque density gradient (30 min, 400×g) followed by fractionation on CD45R (B220) MACS columns.
2.2. Cellular and molecular assays
PL was purchased from Indofine (Hillsborough, NJ) and dissolved in DMSO prior to use. The final concentration of DMSO never exceeded 0.1%. MTS, trypan blue exclusion (TBE) and propidium iodide (PI) staining assays were employed to evaluate proliferation and survival of B cells. Expression levels of genes of interest were measured with the help of reverse transcription (RT) polymerase chain reaction (PCR) and quantitative PCR (qPCR). DNA binding activity of Myc, NF-κB and p53 was determined by electrophoretic mobility shift assay (EMSA). Statistical analysis used Student’s t test; p < 0.05 was considered significant. Additional details are provided in the Supplemental Methods section.
3. Results
3.1. PL inhibits growth and proliferation of mouse B lymphoma cells
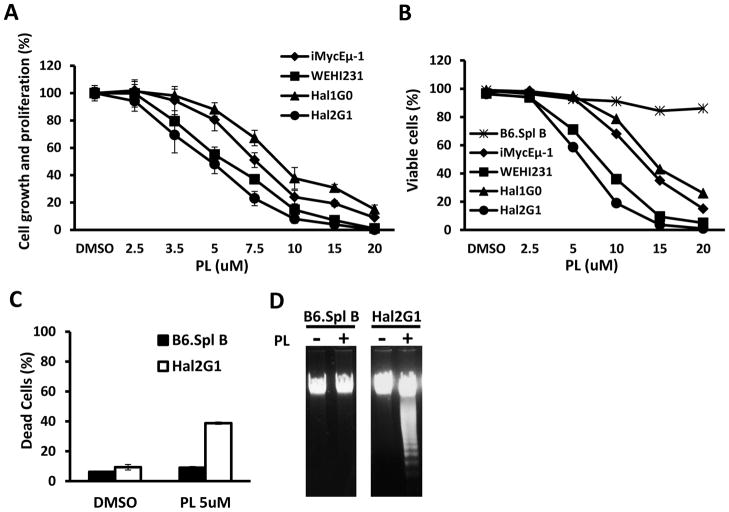
To evaluate the inhibitory effect of PL on mouse B-cell lymphoma, MTS assays were performed using Hal2G1, Hal1G0, iMycEμ-1 and WEHI231 cells treated with increasing concentrations of PL (2.5 μM – 20μM) for 24 hrs. Fig. 1A shows that PL inhibited 4 of 4 cell lines in a concentration-dependent manner. There were small differences in the susceptibility to the drug, reflected by different IC50 values: Hal2G1 cells were most sensitive to PL (IC50 = 5.1 μM), followed by Hal1G0 (7.0 μM), iMycEμ- 1 (7.6 μM) and the least sensitive line, WEHI231 (9.0 μM). Because of Hal2G1’s exquisite sensitivity to PL, the cell line was chosen as principal model system for the studies presented below.
Figure 1. PL-dependent growth inhibition and apoptosis.
(A) MTS assay, a measure of metabolic activity, was used as surrogate for cell proliferation. Cells (106/ml) were treated with the indicated concentrations of PL for 24 hrs. Results were normalized to cells treated with DMSO only (solvent control). Error bars indicate standard deviations of the mean determined in a representative experiment performed in triplicate. IC50 of PL was determined for each cell line and used for all following experiments unless otherwise stated.
(B) Cells were treated with PL as described in panel A and the fraction of dead cells was determined with the assistance of the TBE assay.
(C) Normal B splenocytes (black bars) or Hal2G1 cells (white bars) were treated for 24 hrs with 5 μM PL or left untreated (DMSO only). Cells were stained with PI (propodium iodide) and subjected to flow cytometry to determine the fraction of dead cells containing sub-G1 DNA content. Means and standard deviations (error bars) were determined in three independent experiments and plotted. Asterisk indicates statistical significance at p < 0.05 using the t test.
(D) DNA laddering of genomic DNA was readily apparent in PL-treated (+) Hal2G1 cells but not in Hal2G1 cells left untreated (−) or normal (treated or untreated) splenic B cells.
3.2. PL selectively induces apoptosis in mouse B lymphoma cells
To compare mouse B lymphoma with normal splenic B cells, we repeated the study depicted in Fig. 1A after inclusion of B220+ splenocytes from inbred B6 mice, using trypan blue exclusion to distinguish viable and dead cells. Fig. 1B shows that treatment with PL caused significant death in all lymphomas but not normal B cells. In agreement with that, flow cytometric analysis of DNA content of PI-stained Hal2G1 and normal B cells showed a greater than four-fold increase in the apoptotic sub-G1 fraction of Hal2G1 cells treated with 5 μM PL, yet only a negligible increase in normal B cells (Fig. 1C). Apoptotic death was confirmed by the detection of fragmented DNA in PL-treated Hal2G1 cells, which was not seen in normal B cells (Fig. 1D). These results demonstrated that PL selectively induced apoptosis in malignant but not normal B cells.
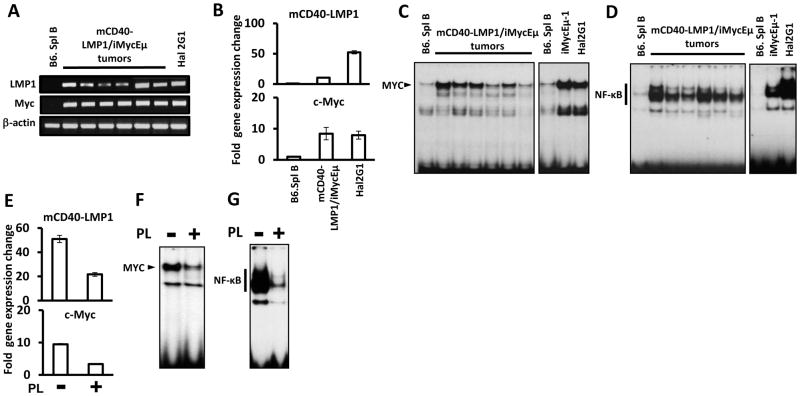
3.3. PL inhibits Myc and NF-κB activity
RT-PCR (Fig. 2A) and qPCR (Fig. 2B) were used to determine the expression of LMP1 and Myc in Hal2G1 cells and B-cell tumors obtained from 6 different mCD40-LMP1/iMycEμ-transgenic mice. Normal B cells were used as control. The levels of Myc message were comparable in Hal2G1 cells and B-lymphomas by qPCR (Fig. 2B bottom), but LMP1 was significantly higher in the cell line (Fig. 2B top). The latter was due, at least in part, to heterogeneities in LMP1 expression in the B-lymphomas (Fig. 2A). Next, EMSA was used to demonstrate the DNA-binding activity of Myc and NF-κB to their specific target sequences (Fig. 2C–D). Hal2G1 cells exhibit high levels of that activity, rendering the cell line a good model for inhibition studies using PL. Indeed, PL attenuated the expression of Myc (Fig. 2E bottom) and LMP1 (Fig. 2E top) in Hal2G1 cells, suggesting that PL either reduces activity at the MHC II Eκ promoter driving mCD40-LMP1 expression [17] or somehow negatively regulates stability of the transgenic transcript. This was not further investigated. More importantly, PL reduced the DNA-binding activity of Myc and NF-κB (Fig. 2F, G) in Hal2G1 cells, suggesting that PL-dependent apoptosis is mediated by inhibition of the LMP1-NF-κB-Myc axis.
Figure 2. PL inhibits the LMP1- NF-κB-Myc pathway.
(A) RT-PCR analysis of LMP1 and Myc expression in normal splenic B cells (lane 1), B-cell tumors obtained from 6 different mCD40-LMP1/iMycEμ-transgenic mice (lanes 2–7) and Hal2G1 cells (lane 8). The housekeeping gene, Actb, was used as control.
(B) qPCR analysis of the samples from panel A. PCR results were normalized to Hprt message levels and converted to fold gene expression change by dividing the normalized value from malignant B cells to that of normal B cells.
(C) EMSA of nuclear extracts prepared from normal B cells, lymphoma tissues and cell lines as indicated. Myc DNA-binding activity is indicated by labeled arrowhead.
(D) EMSA of the samples from panel C. NF-κB DNA-binding activity is indicated by thick vertical line.
(E) PL-dependent reduction of LMP1 and Myc mRNA levels in Hal2G1 cells, as determined by qPCR. Cells were cultured in presence (+) or absence (−) of PL for 24 hrs.
(F) NF-κB DNA-binding activity in Hal2G1 cells treated with PL as shown in panel E (+) or left untreated (−).
(G) Myc DNA-binding activity in Hal2G1 cells treated with PL as shown in panel E (+) or left untreated (−).
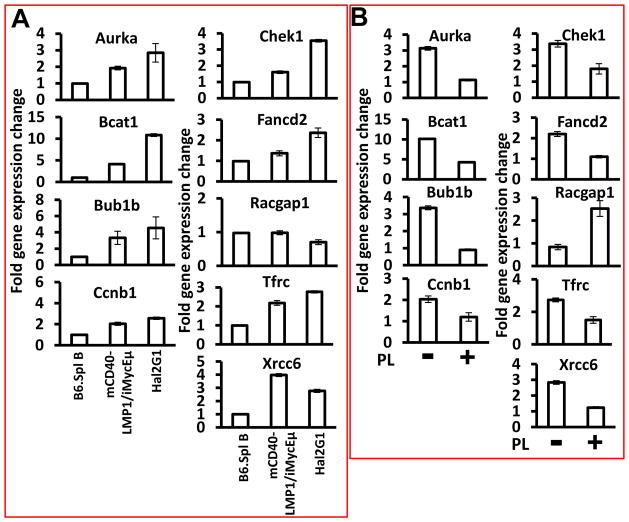
3.4. Treatment with PL results in downregulation of LMP1-NF-κB-Myc-dependent target genes
We next evaluated the expression of 40 putative LMP1-NF-κB-Myc targets to identify genes involved in PL-dependent inhibition of growth and survival of lymphoma cells. mRNA levels of Aurka, Bcat1, Bub1b, Ccnb1, Chek1, Fancd2, Tfrc and Xrcc6 were significantly elevated in mCD40-LMP1/iMycEμ tumors and Hal2G1 cells compared to normal B splenocytes (Fig. 3A), but this was not the case for Racgap1 (right column, center) and genes encoding Pax5, Blimp1, Xbp1, Bcl2, Bcl-xL and two cytokines, IL-6 and IL-10 (Suppl. Fig. 2). Treatment of Hal2G1 cells with PL resulted in downregulation of all overexpressed genes mentioned above, yet upregulation of Racgap1 (Fig. 3B). The latter was also seen in 4 of 4 human BL lines and mouse iMycEμ-1 cells (Suppl. Fig. 1). These results indicated that treatment with PL suppresses a set of LMP1-NF-κB-Myc-regulated target genes known to be important for B cell activation and survival.
Figure 3. PL-dependent expression changes of LMP1-NF-κB-Myc target genes.
(A) qPCR results indicating elevated expression of all indicated genes except Racgap1 in lymphomas from mCD40-LMP1/iMycEμ mice (n = 6) or Hal2G1 cells relative to normal B220+ splenocytes. Target gene expression was normalized to Hprt and converted to fold gene expression change by dividing the results from lymphomas or Hal2G1 cells to the value of normal B cells. Error bars represent the standard deviation of the mean from triplicate measurements.
(B) qPCR results showing that compared to Hal2G1 cells left untreated (−), all indicated genes except Racgap1 were down regulated upon treatment with PL for 24 hrs (+). Racgap1 was up regulated.
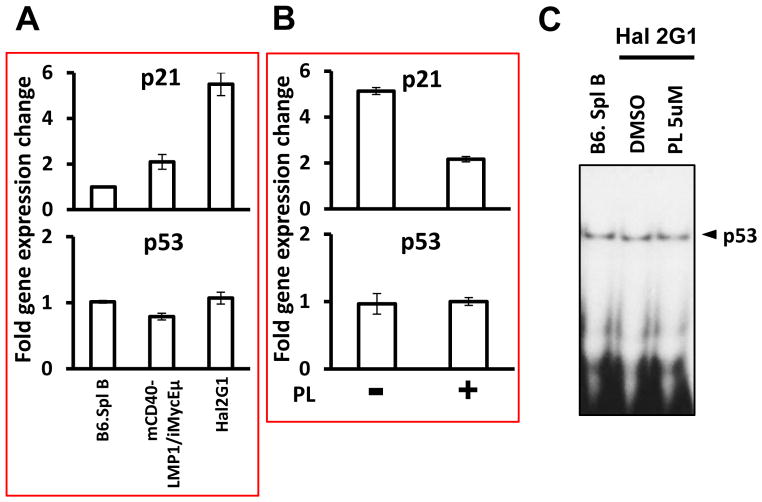
3.5. PL-dependent downregulation of p21 is not dependent on p53
A gene of special interest that was also elevated in mCD40-LMP1/iMycEμ-driven lymphoma and Hal2G1 cells relative to normal B220+ splenocytes was Cdkn1a (Fig. 4A top). This gene encodes p21Cip1, widely known as a tumor suppressor and direct target of the Trp53-encoded tumor suppressor, p53. Unlike Cdkn1a, Trp53 was not elevated in malignant B cells (Fig. 4A bottom). Just as for proliferation-associated genes, such as Aurka, Bub1b and Ccnb1, treatment of Hal2G1 with PL reduced p21 expression (Fig. 4B top) but had no effect on the level of Trp53 message (Fig. 4B bottom) or p53 DNA-binding activity (Fig. 4C). This phenomenon also held true in several other human BL cell lines and transgenic mouse models of B-lineage tumors (data not shown); these findings will be the subject of a separate report. In iMycEμ-1 cells, however, p21 and p53 were co-repressed (Suppl. Fig. 3), suggesting that discordant regulation of the two genes in Hal2G1 may be caused by LMP1. These results, understood with the knowledge that the p21 promoter has NF-κB binding sites and can be regulated by p50/RelB [19], suggest that p21 could function to promote tumor growth without a change in p53 activation.
Figure 4. Significant PL-dependent downregulation of p21-encoding Cdkn1a message in the face of unchanged p53 DNA-binding activity.
(A) qPCR results indicating elevated expression of Cdkn1a in mCD40-LMP1/iMycEμ-induced lymphomas (n = 6) or Hal2G1 cells compared to normal B220+ splenocytes. Cdkn1a expression was normalized to Hprt and converted to fold gene expression change by dividing the values from lymphomas or Hal2G1 cells to the value of normal B cells. Error bars represent the standard deviation of the mean from triplicate measurements.
(B) qPCR results showing that compared to Hal2G1 cells left untreated (−), Cdkn1a was down regulated in cells treated with PL for 24 hrs (+).
(C) EMSA showing no difference in DNA binding activity of p53 in normal B cells (lane 1) and Hal2G1 cells cultured in absence (lane 2) or presence of 5 μM PL for 24 hrs.
4. Discussion
Due to its demonstrable selectivity in cancer cells, PL has recently garnered much interest as an anti-cancer agent [2,3]. The study presented here extends previous reports on PL in cancer research to a newly developed mouse model of human EBV-associated endemic BL, mCD40-LMP1/iMycEμ, in which BL-like tumors develop predictably in presence of a normal immune system. Treatment with PL resulted in growth inhibition and selective killing of mouse BL-like cells including Hal2G1, but left normal splenic B cells alive. PL-induced cytotoxicity likely relied on a mechanism that includes suppression of LMP1-NF-κB-Myc target genes known to sustain cell proliferation and survival. We also speculate that the p53-independent inhibition of the survival-enhancing p21-Fancd2-Xrcc6 pathway is part of this mechanism. Although preliminary at this juncture, our findings provide a strong rationale for the continuing preclinical evaluation of PL, including determination of efficacy with which the compound inhibits mCD40-LMP1/iMycEμ-driven B-cell tumors in mice.
LMP1-NF-κB-Myc signaling governs the transcription of hundreds of target genes, of which we examined a subset of 40 genes using qPCR. Aurka, Bcat1, Bub1b, Ccnb1, Chek1, Fancd2, Tfrc and Xrcc6 all exhibited elevated expression in BL-like tumors, as well as remarkable downregulation upon exposure to PL. Human homologues of these genes are all involved in MYC-dependent cancer development and/or acquisition of drug resistance in patients with cancer. For example, in human and mouse B-cell lymphomas, AURKA is positively regulated by MYC at both the transcriptional and protein levels [20]. BCAT1, also a direct target of MYC, is expressed at significantly higher levels in tumor tissues than in controls [21]. AURKA, BUB1B and CCNB1, three regulators of cell cycle progression, have been implicated as key upregulated genes during EBV-induced transformation of B-lymphocytes [22]. Regulation of CHK1 and CHK2 by MYC promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma [23]. TFRC-encoded transferrin receptor is a critical downstream target of MYC in B-cell lymphoma [24]. Our finding that all these genes were overexpressed in BL-like tumors but coordinately downregulated upon treatment with PL implicates them as effector genes of the LMP1-NF-κB-Myc axis of great potential relevance for the treatment and prevention of B-cell neoplasia.
In contrast to the genes discussed above, Racgap1 was expressed at low levels in BL-like cells but significantly induced upon treatment with PL. Little is known about the biological function of Racgap1 in B-lymphoma, but its value as biomarker for early recurrence and heightened aggressiveness of solid cancers is increasingly being recognized [25,26,27]. Racgap1, a positive regulator of Rac activity, has been reported to play an important role in cytokinesis [28], differentiation of myeloid cells [29] and, interestingly, increased production of reactive oxygen species (ROS) [30]. The latter provides an intriguing parallel to the main mechanism by which PL is thought to kill solid cancers; i.e., drug-induced surge in ROS levels [3]. It is therefore possible that in BL-like tumors, PL-dependent killing relies in part on Racgap1-mediated ROS production. This warrants additional study.
Of special interest in our subset of 40 LMP1-NF-κB-Myc target genes were Cdkn1a, Fancd2 and Xrcc6. These genes were jointly over-expressed in BL-like tumors and jointly suppressed following treatment with PL. We postulate that these genes contribute to the maintenance of BL-like cells in two ways: evasion of apoptosis and enhanced repair of DNA damage. In support of this theory, Cdkn1a-encoded p21 promotes leukemia by a complex mechanism that includes enhanced DNA repair and prevention of p53-induced apoptosis [31]. Consistent with that, lymphomas arising in p21-deficient mice exhibit unusually high rates of apoptosis [32]. Fancd2 and the Ku70-encoding Xrcc6 gene are key players in the Fanconi anemia/early-onset breast cancer (FA/BRCA) DNA repair pathway and the canonical DNA double-strand break repair pathway, respectively. Interestingly, Cdkn1a, Fancd2 and Xrcc6 are all subject to regulation by NF-κB: p21 is transcriptionally regulated by RelB [19], which is important, for example, for TNFα–treated cells protected from UV induced- apoptosis by NF-κB-dependent activation of p21 [33]; NF-κB-mediated expression of Xrcc6 governs, in part, proliferation of some cell types [34]; p50/RelB regulates transcription of Fancd2, along with that of other genes, in the FA/BRCA pathway [35]. These findings support the hypothesis that PL-dependent killing of BL-like tumors is effected, at least in part, by downregulation of Cdkn1a, Fancd2 and Xrcc6.
Given that two hallmarks of cancer are sustained proliferation and evasion of apoptosis [36], our data suggest that LMP1-NF-κB-Myc-driven expression of Aurka, Bub1b and Ccnb1 is important for maintaining the former, whereas expression of Cdkn1a, Fancd2, Xrcc6 and perhaps Chek1 enables the latter in BL-like tumors. Much more work is necessary to better define the contributions of each of these genes to tumor development and treatment response. However, PL appears to target both hallmarks of cancer, lending credence to the proposition that the compound may afford new approaches to the chemoprevention and treatment of blood cancers.
Supplementary Material
Highlights.
Mouse model of human Burkitt lymphoma revealed cancer inhibition by PL.
Treatment with PL led to apoptosis of malignant but not normal B cells.
PL inhibited LMP1-NF-κB-Myc-dependent target genes including p21-encoding Cdkn1a.
PL holds promise for new interventions approaches to hematologic malignancies.
Acknowledgments
We thank Dr. Thomas Raife, University of Iowa DeGowin Blood Center, for the kind provision of leukoreduction chambers for preparation of peripheral blood B-lymphocytes. This work was funded by research grants from the NCI (R01CA151354; SJ) and NNSFC (81250110552; VT), as well as additional support by a Hyundai Hope on Wheels Research Scholar Grant (NLK), Roy J. Carver Charitable Trust Collaborative Pilot Grant (GAB and SJ) and the Iowa City Veterans Affairs Medical Center (GAB).
Abbreviations
- BL
Burkitt lymphoma
- PL
piperlongumine
Footnotes
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its nal citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh N, Kumar S, Singh P, Raj HG, Prasad AK, Parmar VS, Ghosh B. Piper longum Linn. Extract inhibits TNF-α-induced expression of cell adhesion molecules by inhibiting NF-κB activation and microsomal lipid peroxidation. Phytomedicine. 2008;15:284–291. doi: 10.1016/j.phymed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Han SS, Son DJ, Yun H, Kamberos NL, Janz S. Piperlongumine inhibits proliferation and survival of Burkitt lymphoma in vitro. Leukemia Research. 2013;37:146–154. doi: 10.1016/j.leukres.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 5.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proceedings of the National Academy of Sciences. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha A, Robertson ES. Epstein-Barr Virus–Associated B-cell Lymphomas: Pathogenesis and Clinical Outcomes. Clinical Cancer Research. 2011;17:3056–3063. doi: 10.1158/1078-0432.CCR-10-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levens DL. Reconstructing Myc. Genes & development. 2003;17:1071–1077. doi: 10.1101/gad.1095203. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Verma IM. NF- [kappa]B regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 9.Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Gutkind JS. Molecular cross-talk between the NF kappa B and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa I, Briones J, Bordes R, Brunet S, Martino R, Sureda A, Sierra J, Prat J. Activation of the NF-κB signalling pathway in diffuse large B-cell lymphoma: clinical implications. Histopathology. 2008;53:441–449. doi: 10.1111/j.1365-2559.2008.03139.x. [DOI] [PubMed] [Google Scholar]
- 11.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 12.Shair KHY, Bendt KM, Edwards RH, Bedford EC, Nielsen JN, Raab-Traub N. EBV Latent Membrane Protein 1 Activates Akt, NFκB, and Stat3 in B Cell Lymphomas. PLoS Pathog. 2007;3:e166. doi: 10.1371/journal.ppat.0030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Arsura M, Wu M, Duyao M, Buckler AJ, Sonenshein GE. Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. The Journal of Experimental Medicine. 1995;181:1169–1177. doi: 10.1084/jem.181.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M, Arsura M, Bellas RE, FitzGerald MJ, Lee H, Schauer SL, Sherr DH, Sonenshein GE. Inhibition of c-myc expression induces apoptosis of WEHI 231 murine B cells. Molecular and Cellular Biology. 1996;16:5015–5025. doi: 10.1128/mcb.16.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faumont N, Durand-Panteix S, Schlee M, Grömminger S, Schuhmacher M, Hölzel M, Laux G, Mailhammer R, Rosenwald A, Staudt LM, Bornkamm GW, Feuillard J. c-Myc and Rel/NF-κB Are the Two Master Transcriptional Systems Activated in the Latency III Program of Epstein-Barr Virus-Immortalized B Cells. Journal of Virology. 2009;83:5014–5027. doi: 10.1128/JVI.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SS, Kim JS, Tessarollo L, Owens JD, Peng L, Han SS, Tae Chung S, Torrey TA, Cheung WC, Polakiewicz RD, McNeil N, Ried T, Mushinski JF, Morse HC, Janz S. Insertion of c-Myc into Igh Induces B-Cell and Plasma-Cell Neoplasms in Mice. Cancer Research. 2005;65:1306–1315. doi: 10.1158/0008-5472.CAN-04-0268. [DOI] [PubMed] [Google Scholar]
- 17.Stunz LL, Busch LK, Munroe ME, Sigmund CD, Tygrett LT, Waldschmidt TJ, Bishop GA. Expression of the Cytoplasmic Tail of LMP1 in Mice Induces Hyperactivation of B Lymphocytes and Disordered Lymphoid Architecture. Immunity. 2004;21:255–266. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Su Han S, Shaffer A, Peng L, Chung S, Lim J, Maeng S, Su Kim J, McNeil N, Ried T, Staudt L, Janz S. Molecular and cytological features of the mouse B-cell lymphoma line iMycEmu-1. Molecular Cancer. 2005;4:40. doi: 10.1186/1476-4598-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bren GD, Solan NJ, Miyoshi H, Pennington KN, Pobst LJ, Paya CV. Transcription of the RelB gene is regulated by NF-kappaB. Oncogene. 2001;20:7722. doi: 10.1038/sj.onc.1204868. [DOI] [PubMed] [Google Scholar]
- 20.den Hollander J, Rimpi S, Doherty JR, Rudelius M, Buck A, Hoellein A, Kremer M, Graf N, Scheerer M, Hall MA. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116:1498–1505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa R, Yanagi H, Shen CS, Fujiwara Y, Noda M, Yagyu T, Gega M, Oshima T, Yamamura T, Okamura H. ECA39 is a novel distant metastasis-related biomarker in colorectal cancer. World Journal of Gastroenterology. 2006;12:5884. doi: 10.3748/wjg.v12.i36.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai Y, Tang Y, He F, Zhang Y, Cheng A, Gan R, Wu Y. Screening and functional analysis of differentially expressed genes in EBV-transformed lymphoblasts. Virology Journal. 2012;9:1–9. doi: 10.1186/1743-422X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Research. 2013;73:1219–1231. doi: 10.1158/0008-5472.CAN-12-1408. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell KA, Yu D, Zeller KI, Kim J-w, Racke F, Thomas-Tikhonenko A, Dang CV. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Molecular and Cellular Biology. 2006;26:2373–2386. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y, Liu M, Wang P, Ding X, Cao Y. Analysis of 20 genes at chromosome band 12q13: RACGAP1 and MCRS1 overexpression in nonsmall cell lung cancer, Genes. Chromosomes and Cancer. 2013;52:305–315. doi: 10.1002/gcc.22030. [DOI] [PubMed] [Google Scholar]
- 26.Wang SM, Ooi LLP, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clinical Cancer Research. 2011;17:6040–6051. doi: 10.1158/1078-0432.CCR-11-0557. [DOI] [PubMed] [Google Scholar]
- 27.Pliarchopoulou K, Kalogeras K, Kronenwett R, Wirtz R, Eleftheraki A, Batistatou A, Bobos M, Soupos N, Polychronidou G, Gogas H. Prognostic significance of RACGAP1 mRNA expression in high-risk early breast cancer: a study in primary tumors of breast cancer patients participating in a randomized Hellenic Cooperative Oncology Group trial. Cancer chemotherapy and pharmacology. 2013;71:245–255. doi: 10.1007/s00280-012-2002-z. [DOI] [PubMed] [Google Scholar]
- 28.Mishima M, Kaitna S, Glotzer M. Central Spindle Assembly and Cytokinesis Require a Kinesin-like Protein/RhoGAP Complex with Microtubule Bundling Activity. Developmental Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 29.Tonozuka Y, Minoshima Y, Bao YC, Moon Y, Tsubono Y, Hatori T, Nakajima H, Nosaka T, Kawashima T, Kitamura T. A GTPase-activating protein binds STAT3 and is required for IL-6–induced STAT3 activation and for differentiation of a leukemic cell line. Blood. 2004;104:3550–3557. doi: 10.1182/blood-2004-03-1066. [DOI] [PubMed] [Google Scholar]
- 30.Hordijk PL. Regulation of NADPH Oxidases: The Role of Rac Proteins. Circulation Research. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 31.Viale A, De Franco F, Orleth A, Cambiaghi V, Giuliani V, Bossi D, Ronchini C, Ronzoni S, Muradore I, Monestiroli S. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature. 2009;457:51–56. doi: 10.1038/nature07618. [DOI] [PubMed] [Google Scholar]
- 32.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nature Reviews Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basile JR, Eichten A, Zacny V, Münger K. NF-κB-Mediated Induction of p21Cip1/Waf1 by Tumor Necrosis Factor α Induces Growth Arrest and Cytoprotection in Normal Human Keratinocytes1 1 R01 CA81135 (K.M.), K16DE00275 (J.R.B.), DAAD Doktorandenstipendium im Rahmen des gemeinsamen HSP III von Bund und Ländern (A.E.) and a Senior Postdoctoral Fellowship from the New England Division of the American Cancer Society (V.Z.) Molecular Cancer Research. 2003;1:262–270. [PubMed] [Google Scholar]
- 34.Lim JW, Kim H, Kim KH. Expression of Ku70 and Ku80 Mediated by NF-κB and Cyclooxygenase-2 Is Related to Proliferation of Human Gastric Cancer Cells. Journal of Biological Chemistry. 2002;277:46093–46100. doi: 10.1074/jbc.M206603200. [DOI] [PubMed] [Google Scholar]
- 35.Yarde DN, Oliveira V, Mathews L, Wang X, Villagra A, Boulware D, Shain KH, Hazlehurst LA, Alsina M, Chen DT. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Research. 2009;69:9367–9375. doi: 10.1158/0008-5472.CAN-09-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.