Abstract
Osteomyelitis is a bacterial disease that can become chronic, and treatment often includes a surgical operation to remove infected bone. The aim of this study was to develop and investigate in vitro bone filling composite materials that release ciprofloxacin to kill any remaining bacteria and contain bioceramic to help the bone to heal. Three composites of poly(L-lactide-co-ε-caprolactone), β-tricalcium phosphate and ciprofloxacin were compounded using twin-screw extrusion and sterilized by gamma irradiation. Drug release and degradation of the composites were investigated in vitro for 52 weeks. The composite with 50 wt% of β-TCP had the most promising ciprofloxacin release profile. The ceramic component accelerated the drug release that occurred in three phases obeying first-order kinetics. Inhibition zone testing using bioluminescence showed that the released ciprofloxacin had effect in eradicating a common osteomyelitis causing bacteria Pseudomonas aeruginosa. During the in vitro degradation test series, molar weight of the polymer matrix of the composites decreased rapidly. Additionally, 1H-NMR analysis showed that the polymer had blocky structure and the comonomer ratio changed during hydrolysis. The tested composites showed great potential to be developed into bone filler materials for the treatment of osteomyelitis or other bone related infections.
Keywords: controlled drug delivery, ciprofloxacin, antibiotic, biodegradable, composite, poly(L-lactide-co-caprolactone)
Introduction
The objective of the work was to develop ciprofloxacin releasing and bioabsorbable bone defect filler material that also contains a ceramic component to aid bone healing. In this study, the in vitro drug release and degradation behavior of biodegradable ciprofloxacin releasing bone filling material, which has high β-TCP content of up to 60 wt%, for enhanced osteoconductivity, was investigated. Additionally, the effect of the released antibiotic against a common osteomyelitis causing bacteria Pseudomonas aeruginosa was tested using the bioluminescence method. The required length of the controlled antibiotic delivery is from three to six months. This length of time is considered adequate in the treatment of osteomyelitis.1 Furthermore, the degradation of the fillers should occur within a similar time frame.
Osteomyelitis is a severe complication that is challenging to treat. It is caused by bacteria, commonly Staphylococcus aureus, Pseudomonas aeruginosa or Staphylococcus epidermidis and leads to bone destruction.2 Traditionally, osteomyelitis is treated by surgical debridement of the infected tissues followed by a long course of intravenous or parenteral antibiotics.3-6 Fractures, especially open ones, implant surfaces, and external fracture fixations are examples of situations that are known to enhance bacterial adhesion. These conditions, if left untreated, may lead to a biofilm formation and osteomyelitis. These problems have been addressed in numerous reviews.7-9
The surgical debridement of the infected bone in the treating of osteomyelitis creates a defect in the bone called a dead space. Because bacteria may remain in the surrounding tissues, antibiotics are also needed in the treatment. Adequate concentrations of the antibiotic on the site of the dead space are difficult to achieve due to the poor circulation of blood in the infected bone tissue. Local delivery of the antibiotics provides an efficient way of delivering the drug in situ and achieving therapeutic levels of the drug. One of the greatest advantages in local drug therapy is that systemic adverse effects are avoided.10,11 The challenge is to keep the drug concentration at the therapeutic level and not to exceed toxic levels. Previous studies have shown that with local treatment, the systemic drug concentrations in the blood or other tissues are significantly lower than in the surrounding local tissues.11-17
Local biodegradable and antibiotic releasing systems have been studied both in vitro and in vivo11,12,15,16,18-22 and reviewed by many research groups.10 Koort et al. have studied ciprofloxacin releasing bone defect fillers with osteoconductive ceramic component in a localized osteomyelitis rabbit model and the results have been promising. Ciprofloxacin was found to penetrate bone well and higher local concentrations of ciprofloxacin could be achieved than by using systemic administration.10,13,14,23
Mäkinen24 has proposed a new clinical treatment algorithm in the treatment of osteomyelitis based on osteoconductive materials that release antibiotics locally. In this algorithm, the surgical debridement and the antibiotic treatment of the resulting dead space in the bone are performed in one operation. After treatment, no surgical removal of the antibiotic releasing implants or bone grafting is required due to the bioabsorbable and osteoconductive nature of the implants. The fillers developed in the current study may provide the osteoconductive and antibiotic releasing materials that Mäkinen has proposed. However, there is need to test the most promising composites further in vivo to prove their efficacy in living tissues.
Results and Discussion
The effect of processing and sterilization on the materials
The composite materials were manufactured using twin-screw extrusion and the resulting composites had ceramic particles and ciprofloxacin antibiotic evenly distributed in the polymer matrix due to the efficient mixing in the extrusion process. The composites are denoted PLCL + C [Poly(L-lactide-co-ε-caprolactone) (PLCL) with 8 wt% of ciprofloxacin in feed], PLCL + TCP50 + C [PLCL with 50 wt% of β-tricalcium phosphate (β-TCP) and 8 wt% of ciprofloxacin in feed] and PLCL + TCP60 + C (PLCL with 50 wt% β-TCP and 8 wt% of ciprofloxacin in feed). Processing caused only minor degradation in the composites. The weight average molecular weight (Mw) of the raw material was measured as 245,000 g/mol and the number average molecular weight (Mn) 150,000 g/mol. The processing of the composites caused a slight decrease both in the Mw and Mn. The decrease in the Mw was 8% for PLCL + C and 4% and 3% for the PLCL + TCP50 + C and the PLCL + TCP60 + C respectively and the decrease in the Mn was 12% for the PLCL + C and negligible (below 1%) for both PLCL + TCP50 + C and the PLCL + TCP60 + C. Polydispersity (PD) was slightly increased for PLCL + C (from 1.6 to 1.7) but no change was observed for PLCL + TCP50 + C and PLCL + TCP60 + C. As was expected, the sterilization using gamma irradiation with the measured dose of 29–35 kGy caused considerable degradation to all the composites. Gamma irradiation is known to affect polymers and cause degradation.25,26 The decrease was 20%, 45% and 50% for the PLCL + C, the PLCL + TCP50 + C, and the PLCL + TCP60 + C respectively, when compared with the initial Mw of the raw material.
The residual monomer content of the raw material was 0.08 wt% for the L-lactide monomer and below detection limit (< 0.02 wt%) for the ε-caprolactone monomer. The L-lactide and ε-caprolactone monomer contents of the processed samples were analyzed from two parallel samples of each manufactured composite and the amounts of both monomers were very low. The average L-lactide monomer content after processing was 0.05-mol% and caprolactone monomer content below 0.02-mol%. This indicates that no monomers were generated during processing in any of the studied composites. Because there were no differences in the monomer contents of the manufactured materials, it can be assumed that the monomers did not cause differences in the hydrolytic degradation behavior of the studied composites.25
Drug release from the materials
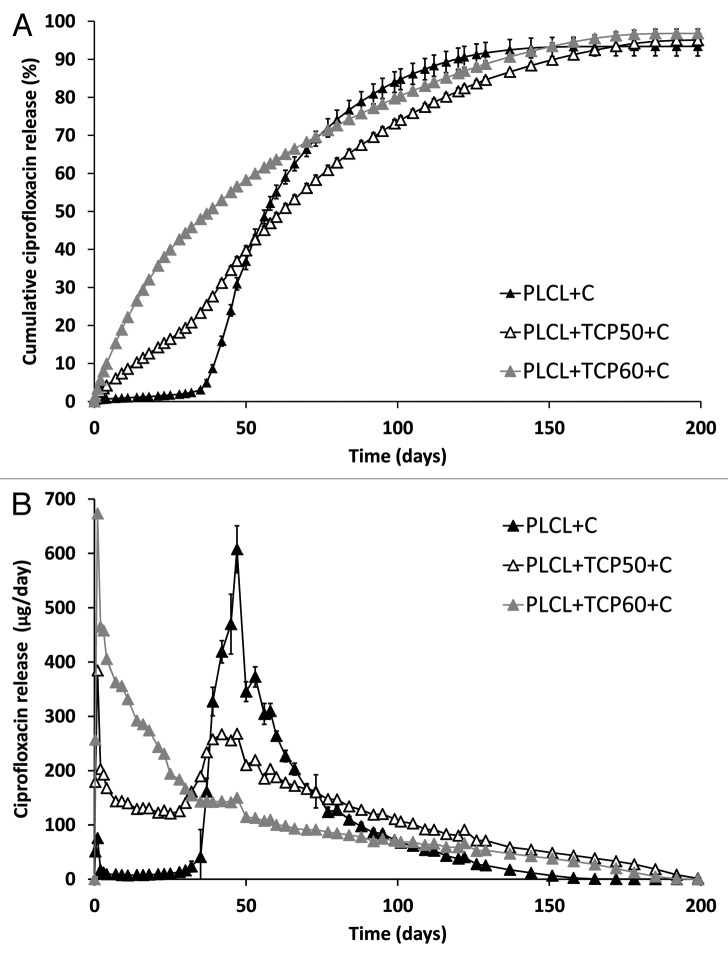
The initial ciprofloxacin contents of the composites were measured as 7.0 ± 0.4 wt% for PLCL + C, 8.1 ± 0.1 wt% for PLCL + TCP50 + C, and 7.5 ± 0.2 wt% for PLCL + TCP60 + C. The cumulative ciprofloxacin release from the studied materials is presented as Figure 1A, where the release occurring in three phases can be noticed. The first phase was the burst in the beginning of the drug release that lasted for one day. This is the phase when the drug molecules at the surface or near the surface are released.27,31 Although initial burst, which is typical for drug releasing polymeric materials, has been claimed as unwanted phenomenon,28,29 it may be beneficial when the target is to destroy remaining bacteria after the surgical debridement of infected tissue. If the burst is moderate, it may help to achieve the required antibiotic concentration in the tissue and eradicate bacteria or to prevent the attachment of bacteria to the operated bone or implant.10,30
Figure 1. The cumulative (A) and daily (B) release of ciprofloxacin from composites of poly(L-lactide-co-ε-caprolactone) (PLCL) and β-tricalcium phosphate (TCP) with initial TCP contents of 0 wt%, 50 wt% and 60 wt% and initial ciprofloxacin (C) content of 8 wt%. Results shown as averages with standard deviations (n = 5).
The second phase was from the day 1 until day 32, when the ciprofloxacin release from PLCL + C was very slow and the release from the composites with 50 and 60 wt% of β-TCP occurred steadily. The second phase was diffusion controlled31 and obeyed the first-order kinetics (R2 values 0.99 for both of the composites containing 50 wt% and 60 wt% of β-TCP). In first order kinetics, the release rate of the drug is dependent on the concentration and obeys Equation 1:
| (1) |
where A is the drug load at the time t, A0 is the initial drug load, and k is the rate constant. This equation results in a straight line when the logarithm of the remaining drug in the sample is plotted against time.
The third phase of the ciprofloxacin release started around day 32 of the release tests, where the release from PLCL + C and PLCL + TCP50 + C accelerated. This can be seen as a change in the release profile at 32 d in Figure 1A. There was, however, no notable change in the release profile at this point for the composite with 60-wt% of β-TCP. This was probably due to the porous structure of the composite caused by the high β-TCP content that enabled a high diffusion rate of ciprofloxacin out of the polymer matrix. In this case, the polymer degradation did not seem to have much of an effect on the ciprofloxacin release. After the phase change at 32 d, all the materials obeyed first-order kinetics (R2 values 0.99 for all the tested materials) but with different slopes than seen in the second phase.
It was noticed that the β-TCP content of the composites had a considerable effect on the ciprofloxacin release (Fig. 1A). β-TCP introduces porosity to the test samples and therefore enables accelerated diffusion of the drug out of the polymer matrix. The processing of the PLCL copolymer with β-TCP particles produces voids at the interfaces of the materials. In addition to the increased diffusion, β-TCP also increases the roughness of the surface; thus, it increases the surface area of the pellets and provides more area for the diffusion to happen. Other research groups have had rather similar results in studies concerning composites of bioceramics and biodegradable polymers containing antibiotics.16,18,22
Plain poly-ε-caprolactone is known to have good diffusion properties for low molecular weight substances (MW below 400 g/mol).32 However, the permeability falls when the ε-caprolactone content decreases in copolymers, e.g., in copolymer of DL-lactide and ε-caprolactone.33 The polymer studied here contains only 30% of the ε-caprolactone monomer and, therefore, the ciprofloxacin molecules that have a molar mass of 331 g/mol were not able to diffuse very well through the plain copolymer during the first 32 d.
At the time point of 32 d, the polymer degradation had apparently reached a level that enables accelerated diffusion for the plain copolymer as well as the composite with 50 wt% of β-TCP. At this point, the Mw of the polymer had decreased to the level of 30,000–34,000 g/mol and the Mn to the level of 17,000–20,000 g/mol. The mass loss of the test samples accelerated in a later stage of the test series, so in conclusion, the change in the diffusion properties due to the degradation of the polymer was most probably the reason for the acceleration of the drug release around 32 d.
The acceleration of the drug release can be easily seen in Figure 1B where the daily release of ciprofloxacin is presented. The release rate reached a peak at the time point of 50 d for all the tested materials, but the peak was most notable for the PLCL + C. A very small acceleration peak could also be observed at the time point of 120 d. At this point, most of the drug had diffused out from the tested materials and, therefore, the degradation degree of PLCL did not cause a larger peak to the daily release of ciprofloxacin.
As the steady zero order release is the ideal release profile in this case, the composite with 50% of β-TCP shows the most promising drug release properties for the treatment of osteomyelitis. The drug release of this composite continued up to 160 d with daily released concentrations above 2 μg/ml, which has been reported to be above the minimum inhibitory concentration (MIC) of the most common osteomyelitis pathogen Staphylococcus aureus.34,35 The slow release period after 160 d when the daily release of ciprofloxacin was decreased below 2 μg/ml is of concern because it may allow the development of resistant bacteria.10 However, the drug release rate and behavior in vivo may be different from what has been observed in vitro.
At the time point of 180 d, the drug release had decreased to a negligible level and 93–97% of the total ciprofloxacin loaded in the composites had been released. The test series was continued up to 392 d, but no further release of ciprofloxacin was detected.
The effect of the released ciprofloxacin against Pseudomonas aeruginosa
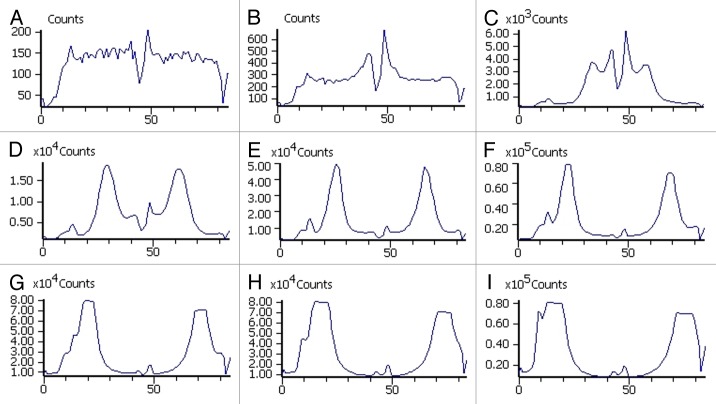
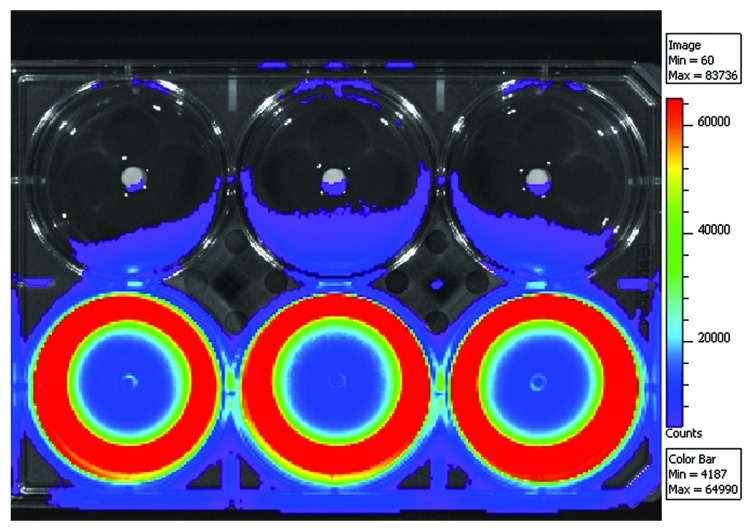
We studied the effect of the released ciprofloxacin against a common osteomyelitis causing bacteria Pseudomonas aeruginosa. The composite for this experiment contained 50 wt% of β-TCP and was chosen based on the most promising ciprofloxacin release results. A bacterial strain of P. aeruginosa engineered to emit light was used in the bacterial culture. The results of our bioluminescence measurements are shown in Figure 2 as light intensity levels (photon counts). The measurements were taken in one well at different time points (0, 2, 4, 6, 8, 10, 12, 14 and 16 h) of incubation. To illustrate the results, a 6-well plate cultured with P. aeruginosa and ciprofloxacin-releasing pellets on the lower row and with control pellets without antibiotic on the top row after 16 h of incubation is shown in Figure 3, as a false color picture. The light intensity levels of the control wells are not shown in Figure 2 because there were no significant changes observed in light intensity levels when compared with the results of antibiotic releasing pellets. All the light production in the control wells shut down at the time point of 6 h. This may have been due to quorum sensing mechanism36 where the bacteria react to possible stress by turning off the light production. The light seen in the top row wells of Figure 3 is not actual light production from the bacteria, but a reflection from the wells in the lower row. The first signs of the antibacterial activity were already seen after two hours of incubation both visually (false color picture) and in the light intensity levels (photon counting). The formation of the inhibition zone can be seen in Figure 2 as a developing valley in the middle of the graphs with lower light intensity that gradually spreads toward the edge of the well. A region with very high light intensity can be seen surrounding the developing valley. The inhibition zone can also be seen in Figure 3 as the dark blue zone in the middle of the wells that indicates dead bacteria. The effectiveness of ciprofloxacin released from the pellets in eradicating the bacteria and forming clear inhibition zones around the pellets in the wells was clearly seen. Bright yellow and red in the areas around the inhibition zones indicated increased light production. This increased light production preceded total decay in the light production. The reason for the decay in the light production was probably that when the bacteria came in contact with ciprofloxacin, the bacteria tried to survive by shutting down the non-vital systems consuming Adenosine triphosphate (ATP). As a result, more ATP was available for the bioluminescence pathway, and the light production increased.
Figure 2. Light intensity level as photon counts of Pseudomonas aeruginosa exposed to one ciprofloxacin releasing pellet in the middle of a well. The results are presented at different time points of incubation. A is at the time point of 0 h, B at 2 h, C at 4 h, D at 6 h, E at 8 h, F at 10 h, G at 12 h, H at 14 h and I at 16 h. Note the different scales of the y-axes.
Figure 3. Bioluminescence results of the ciprofloxacin containing (8 wt%) composites of poly(L-lactide-co-ε-caprolactone) and 50 wt% of β-tricalcium phosphate on a bacterial culture of light emitting Pseudomonas aeruginosa. Pellets containing ciprofloxacin are on the lower row and corresponding composites without ciprofloxacin are on the top row and act as negative controls.
In vitro degradation of the materials
Molecular structure
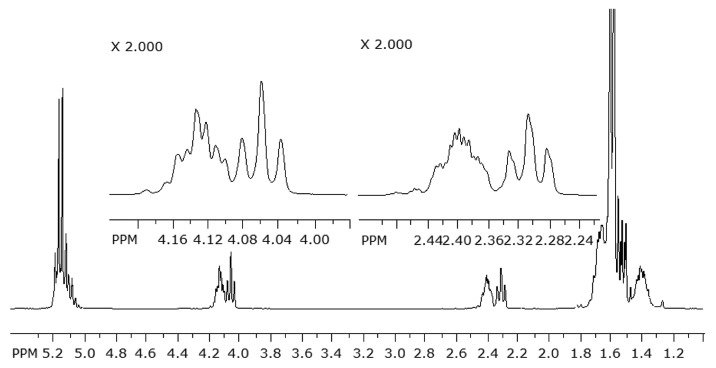
The 1H NMR spectra were measured from seven samples (raw material PLCL, PLCL + C at 0, 26 and 52 weeks in vitro and PLCL + TCP50 + C at 0, 26 and 52 weeks in vitro). Some signals were overlapping and could not be used for the analysis. The most informative signals of the polymer are shown in Figure 4 where a part of the PLCL raw material 1H NMR spectrum is seen. The polymer showed signals at δ 5.16 for the –CH group proton of the lactide comonomer, at δ 4.05–4.13 for the α-oxy methylene protons of the ε-caprolactone comonomer and at δ 2.3–2.4 for the protons of the methylene group of ε-caprolactone that is bonded to the carbonyl group. The signals of the caprolactone protons at δ 4.05–4.13 and at δ 2.3–2.4 were clearly split into two signals according to the position in the polymer chain. The triplet at δ 4.13 indicated the CH2 group in the ε-caprolactone fragment bonded to an L-lactide unit and the broader multiplet at δ 4.05 indicated the α-oxy methylene group bonded to another ε-caprolactone unit.37,38 The signal at δ 2.3–2.4 was split the same way. The triplet at δ 2.4 indicated a group bonded to a L-lactide group and the broader multiplet at δ 2.3 corresponded to a group that is bonded to another ε-caprolactone group.37,38 The comonomer ratios of the copolymer were calculated as the ratio of the integral of the signal at δ 5.16 to the average integrals of the caprolactone signals at δ 4.05–4.13 and at δ 2.3–2.4.38 The 1H NMR analysis showed that the L-lactide to ε-caprolactone ratio was increased from 68/32 of the raw material and the samples of 0 weeks to 76/24 of the PLCL + TCP50 + C at 52 weeks and 71/29 of the PLCL + C at 52 weeks. The results showed a similar effect of the changing of the comonomer ratio of the copolymer as the hydrolysis proceeds, as was seen in our earlier study with similar composites without ciprofloxacin (Ahola et al. Accepted to Journal of Biomaterials Applications). Jeong et al. have also reported this effect.37
Figure 4. Part of the 1H NMR spectrum of the poly(L-lactide-co-ε-caprolactone) raw material.
Because the copolymer properties depend not only on the comonomer composition but also on the distribution of the comonomers in the polymer chains, analysis of the microstructure of the polymer was also needed.38,39 The number average sequence lengths of L-lactide and ε-caprolactone were calculated according to Herbert39 and Fernández38 using Equations 2 and 3:
| (2) |
| (3) |
where (LA) and (CL) are the molar fractions of the L-lactide and ε-caprolactone comonomers in the copolymer and (LA - CL) is the average dyad relative fraction, which can be calculated from the 1H NMR data of the copolymer. The calculation is well explained in an article by Fernández.38 Additionally, the randomness factor, R, was calculated using Equation 4.
| (4) |
The randomness factor is 1 for a random copolymer and 0 for a block copolymer.38 The results of the average sequence length calculations showed that the polymer is rather blocky, R having values of 0.25 for the raw material and the samples prior to in vitro testing. The R factor decreased during degradation of the polymer to 0.18 for PLCL + TCP50 + C to 0.23 for the PLCL + C at 52 weeks indicating that the more random parts of the copolymer degrade first. The average sequence lengths of L-lactide were 13 in the beginning of the test series and increased to 22 and 15 for PLCL + TCP50 + C and PLCL + C respectively at 52 weeks. This also supports the fact that random parts of the copolymer having short blocks of the comonomers degraded first and thus the average sequence length was increased. The average sequence length of ε-caprolactone was not changed during hydrolysis and had values of 6–7 for all the analyzed samples.
Lemmouchi et al.40 have reported degradation results of ε-caprolactone and L-lactide having a comonomer ratio of 74/26 (caprolactone to lactide) before hydrolysis, changed so that the amount of ε-caprolactone comonomer in the polymer increased and L-lactide decreased. This supports the interpretation that polymer degradation depends largely on the microstructure of the polymer and the amorphous parts degrade first despite the comonomer ratio.40
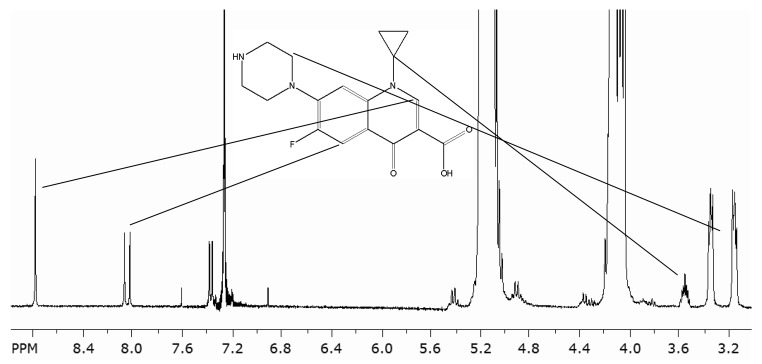
Signals of ciprofloxacin were visible only in the samples of 0 weeks. At 26 or 52 weeks the ciprofloxacin content in the samples was low and under the limit that could be detected in NMR. The integrals of protons of the quinoline ring at 8.80 and 8.06 ppm (Fig. 5) were in good agreement with the stoichiometric proportion 1:1 in both ciprofloxacin containing samples. Additionally, the signals of cyclopropane proton at 3.55 ppm, and the 8 protons in the piperazine ring, which appeared as a multiplet at 3.25 ppm showed good agreement with the stoichiometric values 1:8. Based on these results, it can be concluded that the ciprofloxacin molecule has maintained its original structure throughout the processing and sterilization steps. This result supports the findings of Koort et al. who concluded that processing and sterilization do not affect the bactericidal effect of ciprofloxacin.12-14,23
Figure 5. Part of the 1H NMR spectrum of poly(L-lactide-co-ε-caprolactone) and ciprofloxacin after processing and positions of the peaks assigned to the protons in the ciprofloxacin molecule.
Molecular weights, mass loss and water absorption
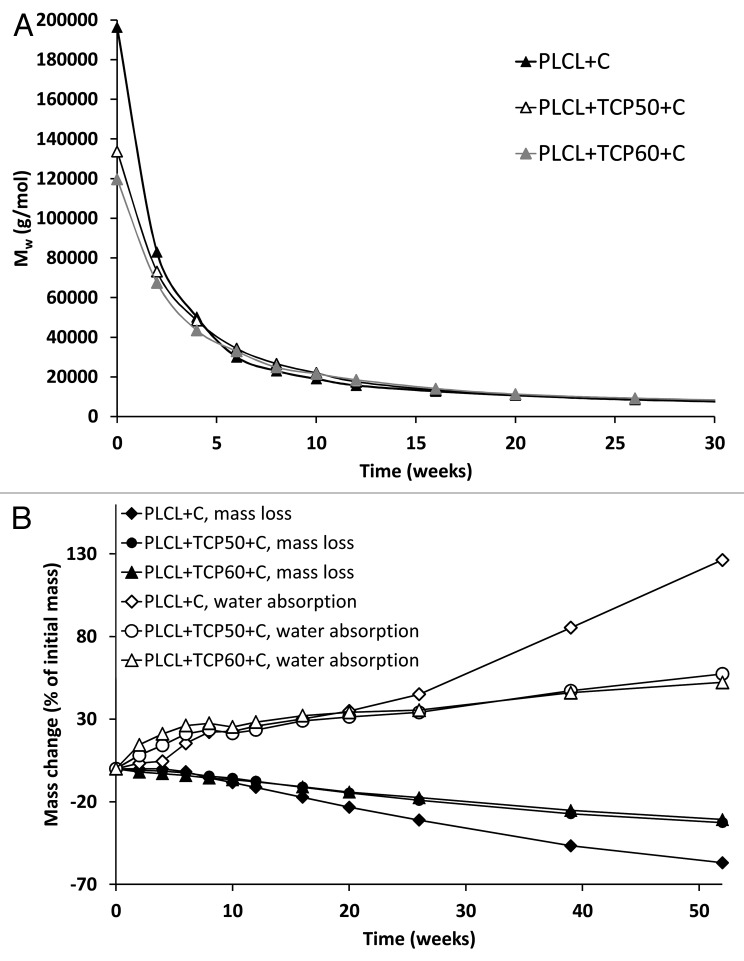
At the start of the hydrolysis test series, the molecular weights of PLCL + TCP50 + C (Mw = 134,000 g/mol and Mn = 69,000 g/mol), and PLCL + TCP60 + C (Mw = 120,000 g/mol and Mn = 70,000 g/mol) were considerably lower than of PLCL + C (Mw = 196,000 g/mol and Mn = 90,000 g/mol). Despite the differences, the degradation proceeded rapidly in all three composites and the differences were leveled out during the hydrolysis test series (Fig. 6A). There were no differences observed in the degradation behavior between the studied materials. We noticed the same kind of effect in our earlier study with composites without antibiotics (Ahola et al. Accepted to the Journal of Biomaterials Applications), as did Daculsi et al.26 At the time point of 4 weeks, the Mw values of the samples were already between 43,000 g/mol and 50,000 g/mol. The decrease of the molecular weight from this time point on was very similar for all the tested composites. After 52 weeks of hydrolysis at 37°C and pH 7.4, the molecular weights (both Mw and Mn) of all the composites had decreased by 97–98% to 2–3% of the initial value of the raw material. Polydispersity of the polymer decreased slightly and steadily throughout the test series, from 1.7–2.2 at 0 weeks to 1.2–1.3 at 52 weeks. The decrease indicated that Mw decreased more rapidly than Mn and that the molecular mass distribution was narrowed. The degradation of the aliphatic polyesters is known to occur first in the amorphous sections of the polymer and via random chain scission.41 The random chain scission caused the molecular weight of the polymer to decrease rapidly following first order kinetics with k having values of 2.0 × 10−3 1/h for the plain copolymer and 1.3 × 10−3 1/h for PLCL + TCP50 + C and 1.2 × 10−3 1/h for PLCL + TCP60 + C.
Figure 6. The weight average molar weight (Mw) (A), and mass loss and water absorption (B) of the studied composites as a function of time in vitro. The composites comprised of poly(L-lactide-co-ε-caprolactone) (PLCL), β-tricalcium phosphate (TCP) and ciprofloxacin (C) with initial TCP contents of 0 wt%, 50 wt%, and 60 wt% and ciprofloxacin content of 8 wt%. Error bars in part B are not visible due to the small values of standard deviations (n = 5).
The SEC distribution plots showed emerging bimodality at 16 weeks for all the tested materials. In our earlier studies, the same kind of bimodality was present in composites without ciprofloxacin antibiotic (Ahola et al. Accepted to the Journal of Biomaterials Applications) beginning from the 20th week of the test series. Bimodality in the SEC distribution curve can be explained with the blocky structure of the copolymer, which was shown by the 1H NMR analysis. The random parts of the copolymer degrade first, which might cause an increase in a certain part of the SEC distribution curve as the blocky parts consisting mainly of L-lactide monomers remain in the copolymer.
The mass loss of the test samples was steady and started from the very beginning of the test series (Fig. 6B). The first part of the mass loss was due to the drug release as ciprofloxacin was released from the composites. When the mass loss caused by the ciprofloxacin release was taken into account, it could be concluded that the mass loss caused by the polymer erosion started after 6 weeks for PLCL + TCP50 + C and PLCL + TCP60 + C and after 4 weeks for PLCL + C. The mass loss in the very beginning of the test series was greater for PLCL + TCP50 + C and PLCL + TCP60 + C than for PLCL + C, which correlated well with the drug release data. The dissolution of β-TCP was very slow as was also noted in our earlier study (Ahola et al. Accepted to the Journal of Biomaterials Applications) and it was not significant in the time scale of this study. The mass loss of the composites was almost linear during the whole 52-week test period (R2 values 0.98).
Water absorption in PLCL + TCP50 + C and PLCL + TCP60 + C was greater than in PLCL + C during the first 10 weeks (Fig. 6B). After 20 weeks, this behavior changed as the water absorption of PLCL + C accelerated and it absorbed more water than the other tested composites. The polymer content in this composition was greater than in PLCL + TCP50 + C and PLCL + TCP60 + C, and this naturally affected the total water absorption of the samples. As the degradation proceeded, there were more hydrophilic end groups in the polymer to absorb water. There was no notable difference in the water absorption between the composites with 50 wt% and 60 wt% of β-TCP. This might be due to the fact that the difference in the ceramic content in these composites was not enough to cause notable differences in the water absorption behavior.
β-TCP content of the materials
The relative amount of β-TCP, determined by thermogravimetric analysis, in the composites increased throughout the test series (Fig. 7A and B). This is because β-TCP dissolves very slowly compared with the polymer degradation (Ahola et al. Accepted to the Journal of Biomaterials Applications). This kind of behavior might be beneficial regarding bone healing, as the polymer disappears from the site and the osteoconductive β-TCP particles remain to help bone ingrowth. Additionally, the voids at the interfaces between the ceramic particles and polymer matrix may also enable bone ingrowth.
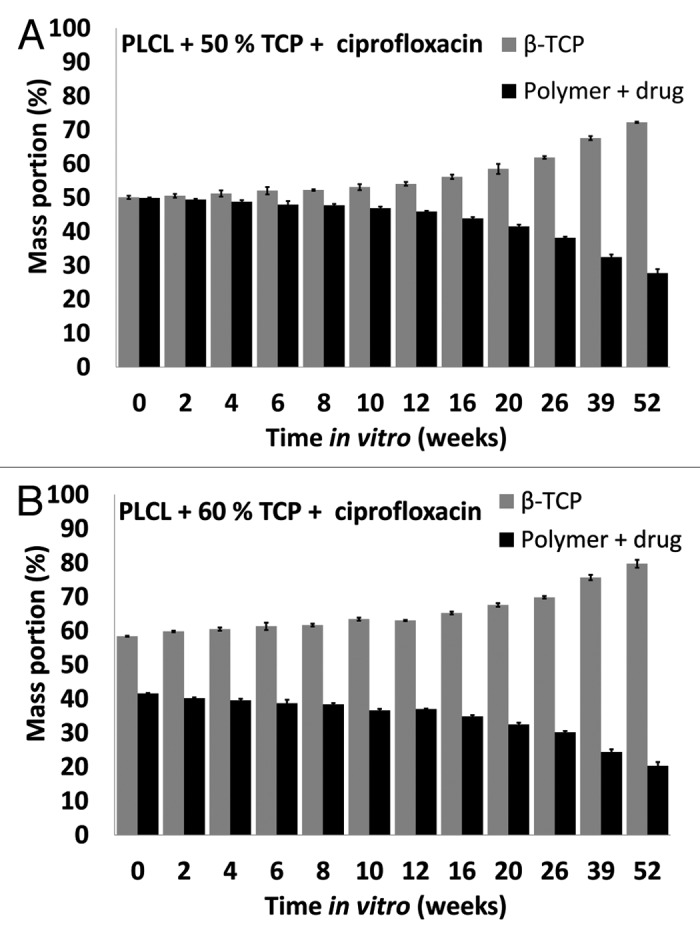
Figure 7. β-tricalcium phosphate (TCP) contents of the studied composites as a function of time in vitro. The composites comprised of poly(L-lactide-co-ε-caprolactone) (PLCL) and β-tricalcium phosphate (TCP) with initial TCP contents of 50 wt% and 60 wt% and ciprofloxacin (C) content of 8 wt%. Results shown as averages with standard deviations (n = 5).
Thermal properties
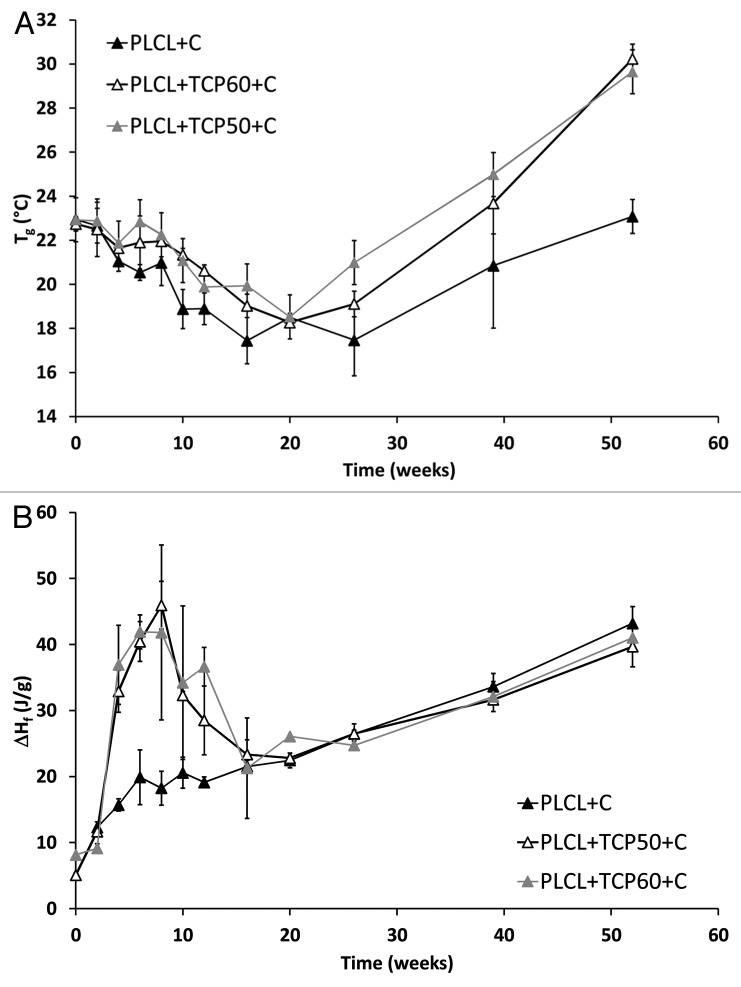
Compared with the similar composites without the ciprofloxacin antibiotic (Ahola et al. Accepted to the Journal of Biomaterials Applications), slightly higher Tgs were measured after processing and sterilization. Tgs were about 20°C for the composites without ciprofloxacin and 23°C for the composites containing ciprofloxacin. The changes in the Tgs of the materials during the in vitro test series are shown in Figure 8A. During the in vitro test series, there was a slow decrease in the Tg:s of the composites with ciprofloxacin until week 20 when the Tgs gradually decreased to 18°C. At the same time, the Mws of the polymers decreased dramatically affecting also the Tg. This behavior is well known with biodegradable polymers.42,43 After the time point of 20 weeks, the Tg:s began to increase ending up at 30°C for PLCL + TCP50 + C and PLCL + TCP60 + C and 23°C for PLCL + C at 52 weeks. At this time period, the loss of the short polymer chains has an increasing effect on the Tg42 as well as the fact that the ratio of the comonomers in the copolymer changed as the degradation proceeded. This change was seen in the 1H NMR analysis as an increase in the L-lactide content and decrease in ε-caprolactone content. The same kind of trend in Tg:s was observed with composites without ciprofloxacin, but it was more noticeable and the Tg:s decreased to 12–15°C during the first 12 weeks of the test series and started to increase after that (Ahola et al. Accepted to the Journal of Biomaterials Applications). Tgs of copolymers of L-lactide and ε-caprolactone are known to be strongly dependent on the comonomer composition. The Tg increases dramatically even with small increments of the L-lactide content.32 The melting temperatures and melting enthalpies were analyzed from the first heating of the samples because during the second heating, the melting peaks did not appear anymore due to the fast cooling in the DSC analysis. Most of the samples showed clear bimodality in the melting peaks indicating two kinds of crystals in the polymer. The reason for the bimodality may be imperfect crystals in the polymer which may be annealed during DSC scan. As a result, they melt, recrystallize and melt again in higher temperature, which shows a bimodal melting peak in the DSC scan.44 There were no clear trends in the melting temperatures of the composites. The bimodal melting peaks appeared mainly between 80°C and 120°C. There was a small decrease in the values between weeks 4 and 20 and a tendency to cold-crystallization at the 26th week.
Figure 8. Glass transition temperatures (Tg) (A) and melting enthalpies (ΔHf) (B) of the studied composites as a function of time in vitro. The composites comprised of poly(L-lactide-co-ε-caprolactone) (PLCL) and β-tricalcium phosphate (TCP) with initial TCP contents of 0 wt%, 50 wt% and 60 wt% and ciprofloxacin (C) content of 8 wt%. Results are shown as averages with standard deviations (n = 2–5).
Melting enthalpies, which describe the crystallinity of the polymer, were analyzed and corrected to correspond to the combined polymer and ciprofloxacin part of the composites. There was a dramatic increase in the crystallinity of the composites from the beginning of the test series until the time point of 6 weeks. After this, the crystallinity started to decrease until the 20 weeks time point and then started to increase again (Fig. 8B). With the composite without β-TCP, the same behavior was observed, but it was considerably weaker. When compared with similar composites without ciprofloxacin, there was a considerable difference in the changes in melting enthalpies. The melting enthalpy of the composites without ciprofloxacin increased steadily until the time point of 26 weeks (Ahola et al. Accepted to the Journal of Biomaterials Applications). In conclusion, the dramatic increase and decrease in melting enthalpies during weeks 0–20 is attributed to the presence of ciprofloxacin in the composites. The overall increase in the crystallinity of the polymer as hydrolysis proceeds is probably due to the increased proportion of L-lactide blocks in the copolymer as the amorphous and more random parts of the copolymer degrade first, which was shown by the 1H-NMR analysis. The L-lactide blocks are able to rearrange and form crystals due to the increased mobility of the polymer chains.
Microstructure
SEM micrographs showed pores of different sizes in the samples containing β-TCP. No notable differences were seen in the structures of composites with 50 wt% and 60 wt% of β-TCP. In the samples without β-TCP, no pores were seen after processing of the material, but some very small pores were formed during the in vitro study. In the Figure 9, SEM micrographs of composites with 60 wt% of β-TCP are shown. In the inner parts of the samples, porosity was seen throughout the hydrolysis test series (Fig. 9A-C). Pores reaching the size of 100–200 μm were observed as well as smaller pores. Additionally, the surfaces of the pellet-shaped samples were porous (Fig. 9D–F). The pores were created around the ceramic particles in the process when the composite in melt phase was drawn from the die of the extruder because there was practically no adhesion between the ceramic particles and the polymer.
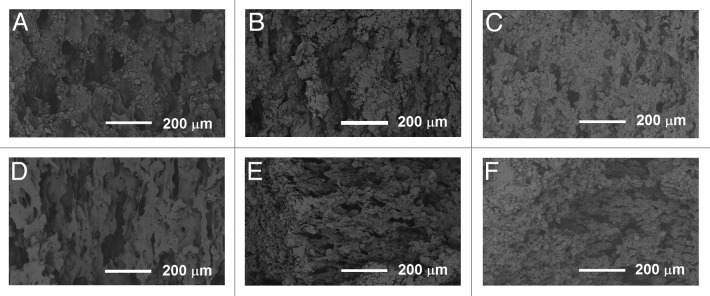
Figure 9. SEM micrographs of the composites of poly(L-lactide-co-ε-caprolactone), 60 wt% β-tricalcium phosphate and ciprofloxacin. (A–C) fractured surfaces after 0 weeks, 26 weeks, and 52 weeks in vitro, respectively. (D–F) specimen surfaces after 0 weeks, 26 weeks and 52 weeks in vitro, respectively.
Pores of a size over 100 μm have been reported to enhance bone ingrowth45 and would, therefore, be beneficial in the healing phase of the bone after the infection causing bacteria have been eradicated. Smaller pores are critical because they enable liquid, nutrition, and waste to flow in the material to and from the cells.
Conclusions
The tested composites with high β-TCP contents show desirable ciprofloxacin releasing properties as ciprofloxacin was steadily released during 160 d in concentrations above the MIC of Staphylococcus aureus. This is within the desired range when the objective is the treatment of osteomyelitis. The release obeyed first-order kinetics having a phase change at 32 d of the release test period. The increased porosity, introduced by β-TCP particles, enhanced the release of ciprofloxacin and removed the long lag phase in the ciprofloxacin release of the plain copolymer. 1H-NMR analysis showed that processing and sterilization did not affect the ciprofloxacin molecular structure. Additionally, the effect against a common osteomyelitis causing bacteria Pseudomonas aeruginosa was shown in cell culture study utilizing bioluminescence.
The polymer matrix of the composites, poly(L-lactide-co-ε-caprolactone) copolymer, was found to have very blocky structure where the L-lactide comonomers formed long blocks in the polymer structure. This also influenced the increase in crystallinity as the polymer degradation proceeded. The decrease in the molecular weight of the copolymer matrix followed the first-order kinetics. The composites with high β-TCP contents, especially the composite with 50 wt% of β-TCP, show great potential to be developed into a product for the treatment of osteomyelitis and other bone related infections. However, in vivo studies as well as clinical studies are needed to establish the effect of these materials when they are implanted in living tissue.
Materials and Methods
Materials
The copolymer that was used as the matrix in the composites was medical grade poly(L-lactide-co-ε-caprolactone) (PLCL, Purac Biomaterials) with the comonomer ratio of 70/30 and Mw of 246,000 g/mol. β-Tricalcium phosphate (β-TCP) (granule size < 38 μm) was used as the ceramic component and it was purchased from Plasma Biotal Ltd. Ciprofloxacin antibiotic (M = 331.4 g/mol) was purchased from Uquifa. Sörensen phosphate buffer solution was prepared according to the standard ISO 1581446 and the chemicals used for the buffer solution (Na2HPO4 and KH2PO4) were purchased from J.T. Baker. The chemicals used for the bioluminescence bacterial cultures were: Gentamicin sulfate (Sigma-Aldrich), Isopropyl-β-D-thiogalaktopyranoside (IPTG) (Fermentas, Lithuania), Trypton (Lab M Limited), Yeast extract (Lab M Limited), Sodium Chloride (Merck), Agar (Merck).
Processing
The components of the composite materials, that are PLCL polymer, β-TCP, and ciprofloxacin antibiotic, were dried for 72 h in vacuum at room temperature before processing. The materials were processed into rod-shaped billets with a diameter of approximately 2.5 mm with a co-rotating, custom-built intermeshing twin-screw extruder (L/D ratio 22.5) in a nitrogen atmosphere. PLCL copolymer, β-TCP and rifampicin antibiotic were delivered to the process with separate gravimetric screw feeders. The mixing of the components took place in the extruder. A haul-off unit was used to guide the extrudate from the die and the diameter of the billets was fine-tuned adjusting the speed of the haul-off unit. Three different composites were processed. Each composite had 8 wt% ciprofloxacin antibiotic in feed and different β-TCP contents (0, 50 and 60 wt%). These composites are denoted PLCL + C, PLCL + TCP50 + C and PLCL + TCP60 + C respectively. The billets were cut into approximately 2.5 mm long pellet-shaped samples. Before degradation and drug release tests were performed, the samples were packed and sterilized using gamma irradiation (minimum dose 25 kGy).
Drug release study
The drug release tests were conducted at 37°C in vitro. Weighed test samples (each test sample consisted of 15 pellets) were placed in brown glass bottles along with 20 ml Sörensen buffer solution. Five parallel test samples were tested for each composite material. The bottles were placed in an incubator shaker at 37°C. At predetermined time intervals, the buffer solution was withdrawn from each of the bottles and replaced with fresh solution. The amount of buffer solution and the periodical change to fresh buffer solution enabled sink conditions to be valid throughout the test series. The amount of released ciprofloxacin was determined from the buffer solution using a Unicam UV 500 spectrometer (ThermoSpectronic, Cambridge, United Kingdom) at maximum absorption wavelength of 271 nm. A wavelength area from 190 to 400 nm was scanned in order to detect possible changes in the ciprofloxacin molecular structure that cause deviation in the UV-spectrum. Ciprofloxacin concentrations were calculated using Beer-Lambert law and with the help of a standard curve prepared with known concentrations of ciprofloxacin.
Inhibition zone testing using bioluminescence imaging
The effect of a ciprofloxacin releasing composite material against a common osteomyelitis causing bacteria Pseudomonas aeruginosa was tested using bioluminescence imaging based on the ability of genetically engineered bacteria to emit light.47,48 In the bioluminescence method, the luminescence and the changes in it emitted by living microorganisms are measured. The changes in the luminescence are caused by lux gene-coded luciferase, which respond to the target analyte in a dose-dependent manner.47,48
The antibiotic containing composite material chosen for this study was the composite containing 50 wt% of β-TCP (PLCL + TCP50 + C) based on the most promising drug release results. Composite of PLCL and 50 wt% β-TCP without antibiotics was used as control.
An engineered bacteria strain of Pseudomonas aeruginosa PAO-LAC carrying plasmid pUCP24GW49 was used as the biosensor. The P. aeruginosa here had been engineered to act as a SOS-response sensor, thereby providing information of the agents that influence chromosomal DNA homeostasis, among others. The bacteria were cultured on antibiotic plates overnight at 30°C and 300 rpm (1 mM IPTG, 10 µg/ml gentamycin), and suitable colonies were moved into liquid culturing in LB (5 g/l yeast extract, 10 g/l tryptone and 5 g/l NaCl). If the bacteria did not produce luminescence as wished in the morning, 1/50 dilution was made in a culture tube and it was incubated at 37°C and 300 rpm for three hours. The level of luminescence of the cultures was measured by using Plate ChameleonTM multilabel counter 1.001 (Hidex Ltd.), and at volume of 200 µl. Counts of 1.1–2.3 × 106 were found satisfying.
Layers of LB-agar (agar 15 g/l, 2 ml) were cast into 6-well plate, and the controls and antibiotic containing composite pellets were placed on top of them (one pellet per well). Bacteria were mixed with soft LB-agar (7.5 g/l) solution and cast on top of the first layers. The amount of bacterial culture was dependent on the luminescence level. After solidification, the plate was taken to the imaging station of Xenogen VivoVision IVIS® Lumina luminescence camera (Caliper LifeSciences). Pictures were taken every 20 min for 16 h with exposure time of 30 sec. The pictures were analyzed using Living Image® 3.1 program (Caliper LifeSciences).
In vitro degradation study
Degradation tests were conducted according to the standard ISO 1581446 at 37°C in vitro. Five parallel test samples, each consisting of 15 pellets, of each composite material were tested at each time point. First, weighed test samples were placed in brown glass bottles with 20 ml Sörensen buffer solution. Then, the bottles were placed in an incubator shaker at 37°C. The pH of the buffer solution was measured periodically with a calibrated pH meter and the buffer solutions were changed every two days at the beginning of the test series, due to fast drug release and the fact that we wanted to avoid drug accumulation in the buffer solution. After five weeks, the buffer solution was changed twice a week and after ten weeks, once a week. Test samples were withdrawn at predetermined time points, which were 2, 4, 6, 8, 12, 16, 20, 26, 30 and 52 weeks.
Methods of analysis
Residual monomer
Ramboll Analytics Oy performed the determination of residual L-lactide and ε-caprolactone monomer contents. The ε-caprolactone and L-lactide contents were measured after chloroform extraction of the samples using gas chromatography (DC8000, CE Instruments) and an FI-detector after chloroform dilution. The measuring resolution was 0.02%.
Initial drug content
Five samples of about 150 mg were weighed from each manufactured composition and dissolved in 50 ml of chloroform (J.T. Baker). The amount of ciprofloxacin in the chloroform solution was determined using a Unicam UV 540 Spectrophotometer (ThermoSpectronic) at a maximum absorption wavelength of 284 nm. The concentrations of ciprofloxacin in the solutions were calculated with the help of a standard curve prepared with known concentrations of ciprofloxacin.
Molecular structure
The molecular structures of the PLCL copolymer and ciprofloxacin antibiotic during degradation test series were studied measuring and analyzing the proton NMR spectra of the samples. The NMR equipment used was a Varian Mercury 300 MHz NMR Spectrometer (Varian Associates Inc.) and the measurements were done at room temperature in standard 5 mm tubes in deuterochloroform. Tetramethylsilane (TMS) was used as an internal standard, and chemical shifts were measured relative to TMS. The data were acquired until the quality of the spectrum was sufficient and the number of scans was about 400. The proton NMR spectra of the samples were processed and analyzed using SpinWorks 3.1 software. Phase correction and baseline correction were applied to all spectra.
Molecular weights
The molecular weights (number average, Mn, and weight average, Mw, molecular weights) and polydispersity (PD) of the copolymer during the in vitro degradation test series were determined at room temperature by size exclusion chromatography (SEC) (Waters Associates system was equipped with a Waters 717plus autosampler, a Waters 510 HPLC solvent pump, four linear PL gel columns (104, 105, 103 and 100 Å) connected in series, and a Waters 2414 differential refractometer). Chloroform (Riedel-de Haën Ag, stabilized with 1% ethanol) was used as a solvent and eluent. Before injection, the samples were filtered through a 0.5 μm Millex SR filter. The injected volume was 200 μl and the flow rate was 1.0 ml/min. Monodisperse polystyrene standards were used for primary calibration. One to two parallel samples were measured at each time point for each composite.
Mass loss and water absorption
For the water absorption analysis, the test samples were withdrawn from the incubator shaker and rinsed twice with distilled water. The surfaces of the test samples were then wiped carefully with tissue paper and the test samples were weighed immediately after wiping. The weighed test samples were dried for at least three days at ambient conditions and for one week in vacuum. After vacuum drying, the test samples were weighed again to obtain the dry masses for the mass loss calculations. The dried test samples were stored in a desiccator for further analysis.
The mass loss was calculated as the difference between the initial mass of the test sample and the mass of the dried test sample divided by the initial mass of the test sample. The water absorption was calculated as the difference between the mass of the wet test sample and the mass of the dried test sample divided by the mass of the dried test sample
Ceramic content
The β-TCP content of the test samples was measured using thermogravimetric analysis (TGA Q500, TA Instruments). Samples of approximately 20 mg were used and they were heated at a rate of 20°C/min up to 700°C. Universal Analysis Software was used for the analysis of the results. Five parallel samples of each composition were analyzed at each time point and the results were calculated as averages and standard deviations.
Thermal properties
Thermal analysis was performed using a differential scanning calorimeter DSC Q1000 (TA Instruments). Nitrogen was used as the sweeping gas. To ensure the samples had a similar thermal history, they were heated twice and cooled rapidly in between. The heating rate was 20°C/min, the cooling rate was 50°C/min and the temperature range was -60°C to +200°C. Glass transition temperatures (Tg) were obtained from the second heating and melting temperatures and enthalpies (Tm and ΔHf respectively) were obtained from the first heating cycle. Although absolute crystallinity values could not be calculated because the theoretical value of 100% crystalline poly(L-lactide-co-ε-caprolactone) 70/30 was not available, relative changes in the crystallinity were observed. Two to five parallel samples were tested for each composite material and time point. The results were analyzed using Universal Analysis Software and averages and standard deviations were calculated.
Microstructure of the samples
The microstructure of the composites was observed using scanning electron microscopy (Philips XL-30 SEM equipped with a LaB6 filament, Philips) with an acceleration voltage of 12.0 kV. The micrographs were taken both on the surface of the samples and on cryogenically fractured samples, which were coated with gold (Edwards S150 Sputter Coater) prior to microstructure examination.
Acknowledgments
Research collaboration with Bioretec Ltd. and financial support from the Finnish Funding Agency for Technology and Innovation (TEKES) and the National Graduate School of Musculoskeletal Disorders and Biomaterials (N.A and M.V) are gratefully appreciated. Raija Reinikainen, Kaija Honkavaara, Eija Ahonen and Vuokko Heino are warmly thanked for their technical assistance. Peter Heath is thanked for checking the language of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/23162
References
- 1.Galanakis N, Giamarellou H, Moussas T, Dounis E. Chronic osteomyelitis caused by multi-resistant Gram-negative bacteria: evaluation of treatment with newer quinolones after prolonged follow-up. J Antimicrob Chemother. 1997;39:241–6. doi: 10.1093/jac/39.2.241. [DOI] [PubMed] [Google Scholar]
- 2.Chihara S, Segreti J. Osteomyelitis. Dis Mon. 2010;56:6–31. doi: 10.1016/j.disamonth.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Parsons B, Strauss E. Surgical management of chronic osteomyelitis. Am J Surg. 2004;188(Suppl):57–66. doi: 10.1016/S0002-9610(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 4.Soundrapandian C, Datta S, Sa B. Drug-eluting implants for osteomyelitis. Crit Rev Ther Drug Carrier Syst. 2007;24:493–545. doi: 10.1615/CritRevTherDrugCarrierSyst.v24.i6.10. [DOI] [PubMed] [Google Scholar]
- 5.Farhad R, Roger PM, Albert C, Pélligri C, Touati C, Dellamonica P, et al. Six weeks antibiotic therapy for all bone infections: results of a cohort study. Eur J Clin Microbiol Infect Dis. 2010;29:217–22. doi: 10.1007/s10096-009-0842-1. [DOI] [PubMed] [Google Scholar]
- 6.Haidar R, Der Boghossian A, Atiyeh B. Duration of post-surgical antibiotics in chronic osteomyelitis: empiric or evidence-based? Int J Infect Dis. 2010;14:e752–8. doi: 10.1016/j.ijid.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Gupta Y, Agrawal R, Khare P, Jain SK. Biofilms--a microbial life perspective: a critical review. Crit Rev Ther Drug Carrier Syst. 2007;24:393–443. doi: 10.1615/CritRevTherDrugCarrierSyst.v24.i5.10. [DOI] [PubMed] [Google Scholar]
- 8.Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury. 2006;37(Suppl 2):S3–14. doi: 10.1016/j.injury.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Esposito S, Leone S. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents. 2008;32:287–93. doi: 10.1016/j.ijantimicag.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–15. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Garvin KL, Miyano JA, Robinson D, Giger D, Novak J, Radio S. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J Bone Joint Surg Am. 1994;76:1500–6. doi: 10.2106/00004623-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Mäkinen TJ, Veiranto M, Lankinen P, Moritz N, Jalava J, Törmälä P, et al. In vitro and in vivo release of ciprofloxacin from osteoconductive bone defect filler. J Antimicrob Chemother. 2005;56:1063–8. doi: 10.1093/jac/dki366. [DOI] [PubMed] [Google Scholar]
- 13.Koort JK, Suokas E, Veiranto M, Mäkinen TJ, Jalava J, Törmälä P, et al. In vitro and in vivo testing of bioabsorbable antibiotic containing bone filler for osteomyelitis treatment. J Biomed Mater Res A. 2006;78:532–40. doi: 10.1002/jbm.a.30766. [DOI] [PubMed] [Google Scholar]
- 14.Koort JK, Makinen TJ, Suokas E, Veiranto M, Jalava J, Tormala P, et al. Sustained release of ciprofloxacin from an osteoconductive poly(DL)-lactide implant. Acta Orthop. 2008;79:295–301. doi: 10.1080/17453670710015111. [DOI] [PubMed] [Google Scholar]
- 15.Mäkinen TJ, Veiranto M, Knuuti J, Jalava J, Törmälä P, Aro HT. Efficacy of bioabsorbable antibiotic containing bone screw in the prevention of biomaterial-related infection due to Staphylococcus aureus. Bone. 2005;36:292–9. doi: 10.1016/j.bone.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Miyai T, Ito A, Tamazawa G, Matsuno T, Sogo Y, Nakamura C, et al. Antibiotic-loaded poly-ε-caprolactone and porous β-tricalcium phosphate composite for treating osteomyelitis. Biomaterials. 2008;29:350–8. doi: 10.1016/j.biomaterials.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Lucke M, Wildemann B, Sadoni S, Surke C, Schiller R, Stemberger A, et al. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone. 2005;36:770–8. doi: 10.1016/j.bone.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez H, Castro C, Moujir L, Perera A, Delgado A, Soriano I, et al. Efficacy of ciprofloxacin implants in treating experimental osteomyelitis. J Biomed Mater Res B Appl Biomater. 2008;85:93–104. doi: 10.1002/jbm.b.30921. [DOI] [PubMed] [Google Scholar]
- 19.Tiainen J, Knuutila K, Veiranto M, Suokas E, Törmälä P, Kaarela O, et al. Pull-out strength of multifunctional bioabsorbable ciprofloxacin-releasing polylactide-polyglycolide 80/20 tacks: an experimental study allograft cranial bone. J Craniofac Surg. 2009;20:58–61. doi: 10.1097/SCS.0b013e318190df48. [DOI] [PubMed] [Google Scholar]
- 20.Waknis V, Jonnalagadda S. Novel poly-DL-lactide-polycaprolactone copolymer based flexible drug delivery system for sustained release of ciprofloxacin. Drug Deliv. 2011;18:236–45. doi: 10.3109/10717544.2010.528070. [DOI] [PubMed] [Google Scholar]
- 21.Castro C, Évora C, Baro M, Soriano I, Sánchez E. Two-month ciprofloxacin implants for multibacterial bone infections. Eur J Pharm Biopharm. 2005;60:401–6. doi: 10.1016/j.ejpb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Castro C, Sánchez E, Delgado A, Soriano I, Núñez P, Baro M, et al. Ciprofloxacin implants for bone infection. In vitro-in vivo characterization. J Control Release. 2003;93:341–54. doi: 10.1016/j.jconrel.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Koort JK, Mäkinen TJ, Suokas E, Veiranto M, Jalava J, Knuuti J, et al. Efficacy of ciprofloxacin-releasing bioabsorbable osteoconductive bone defect filler for treatment of experimental osteomyelitis due to Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1502–8. doi: 10.1128/AAC.49.4.1502-1508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mäkinen T. Osteomyelitis and orthopedic implant infections, PhD thesis, University of Turku, 2005. [Google Scholar]
- 25.Paakinaho K, Ellä V, Syrjälä S, Kellomäki M. Melt spinning of poly(l/d)lactide 96/4: Effects of molecular weight and melt processing on hydrolytic degradation. Polym Degrad Stabil. 2009;94:438–42. doi: 10.1016/j.polymdegradstab.2008.11.010. [DOI] [Google Scholar]
- 26.Daculsi G, Goyenvalle E, Cognet R, Aguado E, Suokas EO. Osteoconductive properties of poly(96L/4D-lactide)/beta-tricalcium phosphate in long term animal model. Biomaterials. 2011;32:3166–77. doi: 10.1016/j.biomaterials.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Duvvuri S, Gaurav Janoria K, Mitra AK. Effect of polymer blending on the release of ganciclovir from PLGA microspheres. Pharm Res. 2006;23:215–23. doi: 10.1007/s11095-005-9042-6. [DOI] [PubMed] [Google Scholar]
- 28.Zamoume O, Thibault S, Regnié G, Mecherri MO, Fiallo M, Sharrock P. Macroporous calcium phosphate ceramic implants for sustained drug delivery. Mater Sci Eng C. 2011;31:1352–6. doi: 10.1016/j.msec.2011.04.020. [DOI] [Google Scholar]
- 29.Kankilic B, Bayramli E, Kilic E, Dağdeviren S, Korkusuz F. Vancomycin containing PLLA/β-TCP controls MRSA in vitro. Clin Orthop Relat Res. 2011;469:3222–8. doi: 10.1007/s11999-011-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luginbuehl V, Ruffieux K, Hess C, Reichardt D, von Rechenberg B, Nuss K. Controlled release of tetracycline from biodegradable β-tricalcium phosphate composites. J Biomed Mater Res B Appl Biomater. 2010;92:341–52. doi: 10.1002/jbm.b.31520. [DOI] [PubMed] [Google Scholar]
- 31.Baker R. Controlled Release of Biologically Active Agents. New York: John Wiley & Sons, 1987. [Google Scholar]
- 32.Pitt CG. Poly- ε-caprolactone and its copolymers In: Chasin M, Langer R, eds. Biodegradable Polymers as Drug Delivery Systems. New York: Marcel Dekker Inc., 1990:71-120. [Google Scholar]
- 33.Pitt CG, Jeffcoat AR, Zweidinger RA, Schindler A. Sustained drug delivery systems. I. The permeability of poly(ε-caprolactone), poly(DL-lactic acid), and their copolymers. J Biomed Mater Res. 1979;13:497–507. doi: 10.1002/jbm.820130313. [DOI] [PubMed] [Google Scholar]
- 34.Bowker KE, Wootton M, Rogers CA, Lewis R, Holt HA, MacGowan AP. Comparison of in-vitro pharmacodynamics of once and twice daily ciprofloxacin. J Antimicrob Chemother. 1999;44:661–7. doi: 10.1093/jac/44.5.661. [DOI] [PubMed] [Google Scholar]
- 35.Mouriño V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7:209–27. doi: 10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holden MTG, Ram Chhabra S, de Nys R, Stead P, Bainton NJ, Hill PJ, et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol. 1999;33:1254–66. doi: 10.1046/j.1365-2958.1999.01577.x. [DOI] [PubMed] [Google Scholar]
- 37.Jeong SI, Kim BS, Lee YM, Ihn KJ, Kim SH, Kim YH. Morphology of elastic poly(L-lactide-co-ε-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules. 2004;5:1303–9. doi: 10.1021/bm049921i. [DOI] [PubMed] [Google Scholar]
- 38.Fernández J, Etxeberria A, Sarasua JR. Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J Mech Behav Biomed Mater. 2012;9:100–12. doi: 10.1016/j.jmbbm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Herbert IR. Statistical analysis of copolymer sequence distribution. In: Ibbet RN, ed. NMR Spectroscopy of Polymers. London: Blackie Academic & Professional, 1993:50-79. [Google Scholar]
- 40.Lemmouchi Y, Schacht E, Lootens C. In vitro release of trypanocidal drugs from biodegradable implants based on poly(ε-caprolactone) and poly(D,L-lactide) J Control Release. 1998;55:79–85. doi: 10.1016/S0168-3659(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 41.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res. 1999;48:342–53. doi: 10.1002/(SICI)1097-4636(1999)48:3<342::AID-JBM20>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed J, Zhang J, Song Z, Varshney SK. Thermal properties of polylactides - effect of molecular mass and nature of lactide isomer. J Therm Anal Calor. 2008:1–8. [Google Scholar]
- 43.Li S, Garreau H, Vert M. Structure-property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous media - part 3 influence of the morphology of poly(l-lactic acid) J Mater Sci Mater Med. 1990;1:198–206. doi: 10.1007/BF00701077. [DOI] [Google Scholar]
- 44.Sarasua J. -, Prud'homme RE, Wisniewski M, Le Borgne A, Spassky N. Crystallization and melting behavior of polylactides. Macromolecules. 1998;31:3895–905. doi: 10.1021/ma971545p. [DOI] [Google Scholar]
- 45.Shore EC, Holmes E. Porous hydroxyapatite. In: Hench LL, Wilson J, eds. An Introduction to Bioceramics. Singapore: World Scientific Publishing, 1993:181-198. [Google Scholar]
- 46.ISO 15814. Implants for surgery – copolymers and blends based in polylactide – in vitro degradation testing.
- 47.Su L, Jia W, Hou C, Lei Y. Microbial biosensors: a review. Biosens Bioelectron. 2011;26:1788–99. doi: 10.1016/j.bios.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Galluzzi L, Karp M. Whole cell strategies based on lux genes for high throughput applications toward new antimicrobials. Comb Chem High Throughput Screen. 2006;9:501–14. doi: 10.2174/138620706777935351. [DOI] [PubMed] [Google Scholar]
- 49.Moir DT, Ming Di, Opperman T, Schweizer HP, Bowlin TL. A high-throughput, homogeneous, bioluminescent assay for Pseudomonas aeruginosa gyrase inhibitors and other DNA-damaging agents. J Biomol Screen. 2007;12:855–64. doi: 10.1177/1087057107304729. [DOI] [PMC free article] [PubMed] [Google Scholar]