Abstract
Bioactive glass is one of the widely used bone repair material due to its unique properties like osteoconductivity, osteoinductivity and biodegradability. In this study bioactive glass is prepared by the sol gel process and stabilized by a novel method that involves a solvent instead of the conventional calcinations process. This study represents the first attempt to use this method for the stabilization of bioactive glass. The bioactive glass stabilized by this ethanol washing process was characterized for its physicochemical and biomimetic property in comparison with similar composition of calcined bioactive glass. The compositional similarity of the two stabilized glass powders was confirmed by spectroscopic and thermogravimetric analysis. Other physicochemical characterizations together with the cell culture studies with L929 fibroblast cells and bone marrow mesenchymal stem cells proved that the stabilization was achieved with the retention of its inherent bioactive potential. However an increase in the surface area of the glass powder was obtained as a result of this ethanol washing process and this add up to the success of the study. Hence the present study exhibits a promising route for high surface area bioactive glass for increasing biomimicity.
Keywords: bioactive glass, bioactivity, hydroxyl carbonate apatite, sol gel synthesis, stabilization, bone regeneration, tissue engineering, scaffolds
Introduction
Bioactive glasses are certain glass compositions that are capable of inducing specific biological activity. In early 1970s, Larry L Hench reported on the bone bonding ability observed for the glass system based on SiO2-CaO-Na2O-P2O5 which was given the trade name Bioglass®.1 Today a wide range of bioactive glass compositions have been developed through different synthesis routes and found a variety of applications including soft tissue bonding,2-4 lung tissue engineering,5,6 inducing angiogenesis,7 stimulating the gene expression8 and growth factor production in osteoblasts etc.9
Bioactive glasses are now copiously synthesized by the sol gel method. Sol gel method is a chemical synthesis technique that involves the conversion of a sol which is a suspension of very small, colloidal particles to a three dimensional interconnected network termed gel. Six steps are involved in the preparation of bioactive glass by this method. The first step involves the mixing of precursors which form a low viscosity sol whose viscosity steadily increases as the network interconnect develops. Before the completion of the network formation, the sol is cast into a mold where gelation occurs as the third step.
The resulting three dimensional gel networks are completely filled with pore liquid. Aging is the fourth step, which involves holding the gel in its pore liquid for several hours. In step five, the gel is dried during which the pore liquid and the physically adsorbed water are completely eliminated from the pores. This involves heating at controlled rates at temperatures of 120–180°C.
Chemical stabilization of the gel, often called calcination is the sixth step which is necessary to remove the residual components and unwanted byproducts associated with the hydrolysis and condensation reactions. This stabilization step confers to the environmental stability and bioactivity. This step usually is a thermal treatment in the range of 500–900°C which desorbs surface silanols (Si-OH) and eliminates other residuals from the gel. Usually temperature of calcination for bioactive glasses is selected to be 600°C and it is quoted that maximum bioactivity is obtained with minimum stabilization temperature.8,10 Calcination also has its effect on increasing the strength and hardness of the gels and converts the network to a glass with network properties similar to the conventional melt derived glasses.11
Since the stabilization of the glass by conventional heat treatment alter the glass properties like particle size, density etc, similar to melt derived materials, stabilization of sol gel derived glasses by another means would be advantageous in this respect. Additionally, these properties are significant in the field of composites as well, where the bioactive glass serve as reinforcement in low elastic modulus polymeric matrix. Moreover it is well reported that the textural features like particle size distribution, specific surface area, porosity etc have a strong influence on bioactivity.12-16 This is for the reason that, during the bone bonding mechanism the rate of formation of hydroxyl carbonate apatite (HCA) layer, the interfacial layer that is structurally and chemically equivalent to the mineral phase of the bone, is influenced with the particle size range and powder volume fraction.
In the present paper a new approach is been taken up toward the processing of sol gel derived glasses wherein the glasses are not subjected to the high temperature chemical stabilization process. Hence this work presents an alternative method of stabilization of the gel glasses by ethanol washing. Although it is a common method to remove any impurities by washing the raw product with a solvent, to the author’s knowledge this is the first time to practice this method for bioactive glass.
Results and Discussion
Physicochemical characterizations
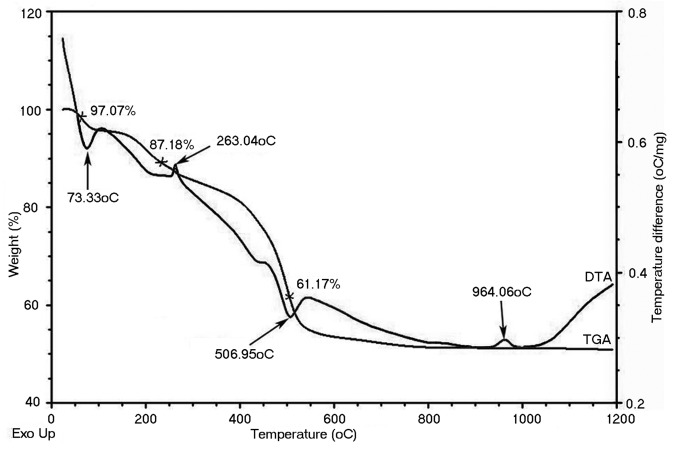
The much studied sol gel method has been employed to synthesize bioactive glass of composition SiO2 (67.12 mol%), CaO (28.5 mol%), and P2O5 (4.38 mol%) and the synthesized material was stabilized by a new route of alcohol washing. The comparison between bioactive glass obtained after stabilization by the new method (designated as BG-E) and that by conventional calcination method (designated as BG-C) is made through several characterization techniques. In order to prepare BG-C sample the calcination temperature for the raw glass was determined by TGA-DTA. In the TGA-DTA, the mass loss was found to occur in three stages (Fig. 1). The first mass loss occurred between 50°C and 130°C, corresponding to an endothermic peak at 73.3°C in the DTA trace. This is associated with the removal of physically absorbed water and pore liquor (water and alcohol byproducts of the condensation reactions) that were not removed during drying. Further mass loss that started from the end of first mass loss till about 270°C might be attributed to the removal of chemically absorbed water. This is correlated with an exothermic peak at 263C in the DTA curve. The third mass drop (onset at 270°C) till around 570°C is reflected by a sharp endothermic peak at 506.95°C in the DTA curve. This endothermic peak is due to the elimination of the residual nitrates and condensation of silanol groups. The second exothermic peak at around 960°C in the DTA curve corresponds to the crystallization of the glass.
Figure 1. TGA- DTA curve of raw bioactive glass powder.
From the TGA-DTA data it could be inferred that the residuals were removed within 600°C. Hence the synthesized raw bioactive glass powder was calcined at 600°C for 3 h at a heating rate of 3°C min−1 for comparison with the bioactive glass stabilized by ethanol washing.
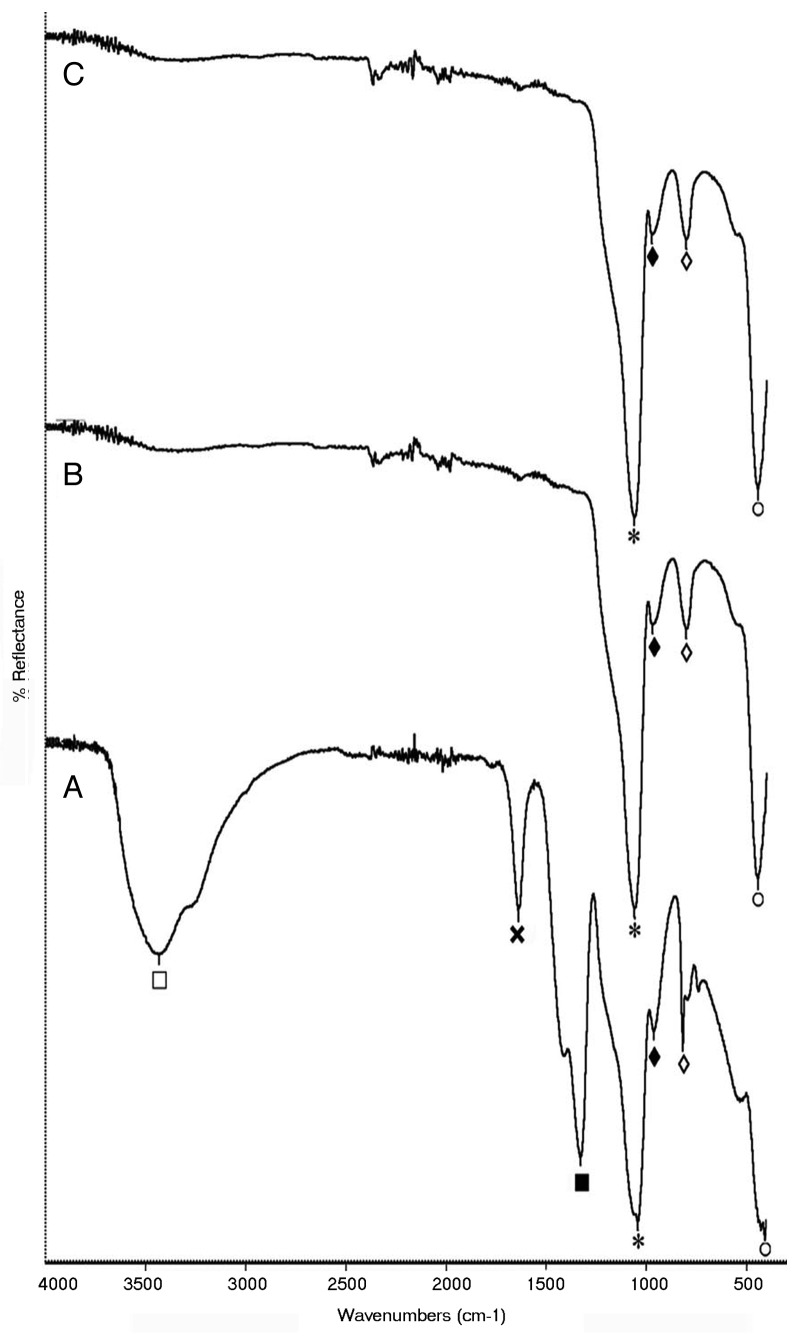
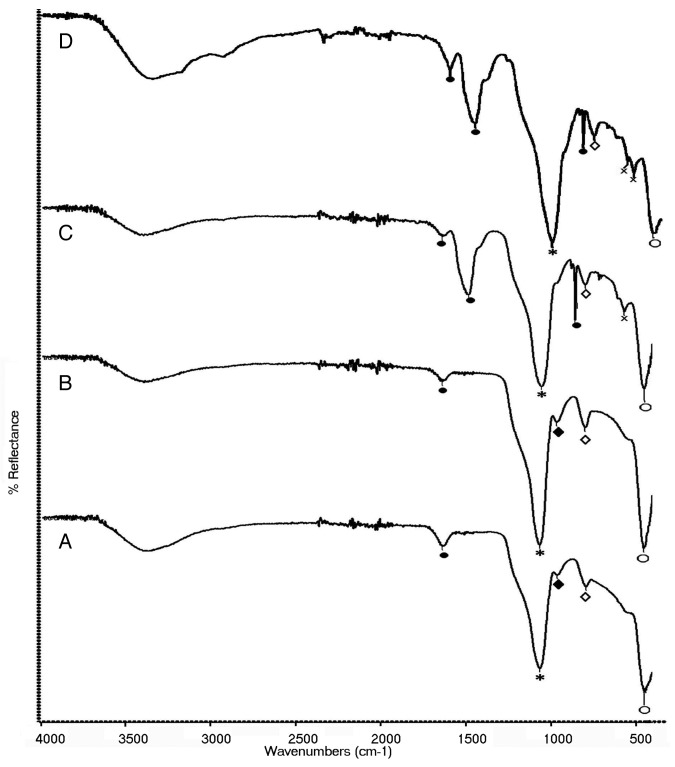
The FTIR spectrum of all the three bioactive glass samples shows the presence of characteristic peaks of bioactive glass (Fig. 2). In the raw bioactive glass sample, absorption peaks at 1042 cm−1 (*) and 446 cm−1 (○) corresponds to Si-O-Si stretching and bending vibrations respectively and that at 818 cm−1 (◊) corresponds to O-Si-O stretching. The absorption peak at 962 cm−1 (♦) is assigned to the typical P-O stretching. The presence of Ca creating the non-bridging oxygen (NBO) atom is also confirmed by the fact that when NBO are present, Si-O-Si absorption falls in the range 1040–940 cm−1 and never higher than 1100 cm−1. Here it is 1042 cm−1. Along with the characteristic peaks, the presence of residual nitrates and condensation by-products is also evident. The impurities that are expected to be in the unstabilized sample include ionic nitrates and alkoxides residues. In the raw powder, a broad absorption peak at 3426 cm−1 (⊕) corresponds to intermolecular hydrogen bonded OH (polymeric association) and the absorption peak at 1328 cm−1 (⊗) is assigned to NO3- stretching vibrations. The presence of molecular water is also evident by the sharp peak at 1637 cm−1 ( × ). However in the BG-E and BG-C samples only the characteristic peaks of bioactive glass are present confirming the stability of the glass samples. Hence it is observed in the IR spectroscopy that impurities are absent in the bioactive glass stabilized by ethanol washing process and that the spectrum is identical to that of the calcined sample confirming the compositional similarity.
Figure 2. IR spectra of (A) raw bioactive glass, (B) BG-E and (C) BG-C.
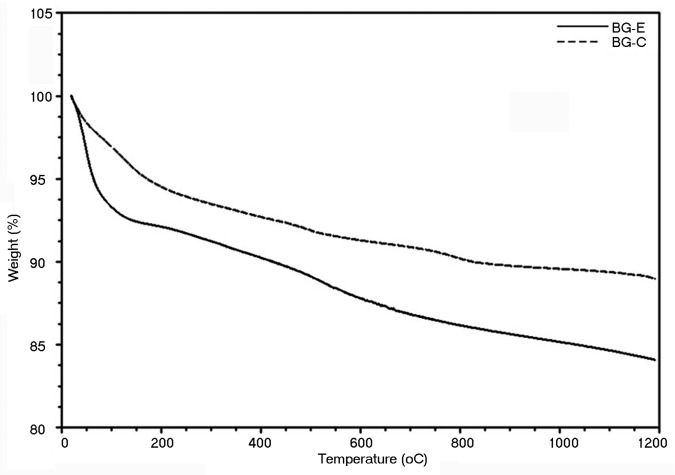
The stabilized glass powders (BG-E and BG-C) were then scanned for mass loss owing to the presence of any residual liquid, with a temperature range from 0 to 1200°C and the thermograms are shown in Figure 3. The thermograms of both the glass powder were identical with a slight difference in mass loss for BG-E at around 60°C. This initial endothermic process in the ethanol washed sample is attributed to the loss of physically absorbed water contributing the major share together with traces (since ethanol is volatile unlike the physisorbed water) of ethanol that retained in the pores during the washing process. The calcined sample hardly contains any ethanol and hence no considerable initial mass loss. A slight overall mass loss observed in both the sample types is attributed to the structural densification. Hence it is made clear from the TGA thermogram that there is hardly any impurity to be volatilized off after stabilization in either sample.
Figure 3. TGA thermo gram for bioactive glass stabilized by ethanol washing and calcination.
The shape and structural morphology of BG-E and BG-C as obtained by SEM are shown in Figure 4. Particles of BG-E are found to have a more flake like appearance with its angular corners clearly visible while BG-C is found to have a dense texture which appears covered by flakes of smaller particles. The absence of such a fused texture in BG-E may be owing to the washing procedure with ethanol during which the strong hydrogen bonding between the hydrous groups in gel particles were replaced by alcohol groups. However unlike the agglomerated structure of BG-C giving it a roughly spherical morphology, the irregular flake like particles of BG-E would be advantageous in developing polymer- bioactive glass comopsite scaffolds for bone tissue engineering. Scaffolds bearing an interconnected porous structure require a comparatively better interaction between the filler particles and the polymer matrix than in the case of bulk composite material used for tissue replacement. However this better interaction is accomplished well with particles having irregular shapes than the spherical ones since more matrix is engaged in wetting the particle surface in irregular particles owing to their larger surface area, resulting in a good bonding between the filler and the polymer. This wetting phenomenon has reported by Joseph et al. with respect to HAP.17
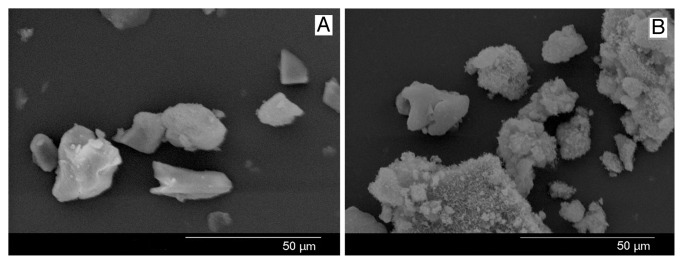
Figure 4. SEM image of (A) BG-E and (B) BG-C .
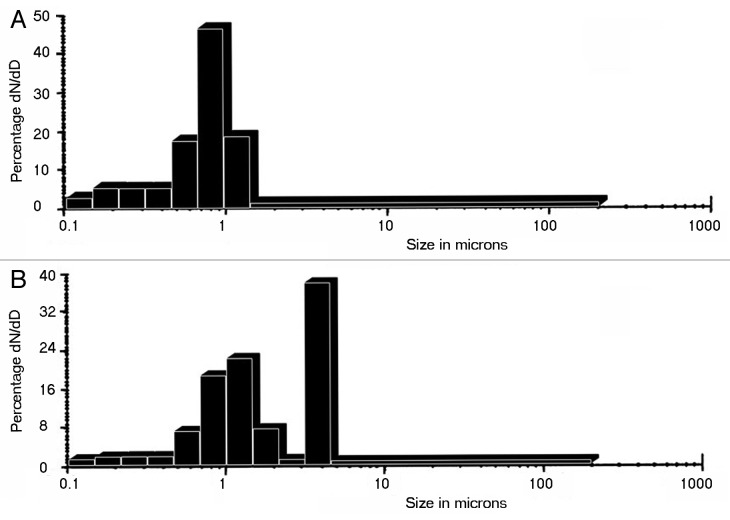
The average particle size values obtained for the samples were summarized in Table 1. It could be assessed from the particle size analysis that the greater part of particles of BG-C appear in the range 5–6 μ with few particles appearing in the range 1 μ (Fig. 6A). While in case of BG-E the average particle size come in the range < 1 μ (Fig. 6B). Hence it is found that BG-C is bigger in size compared with BG-E. In agreement to this result, the SEM image also depicts an agglomerated structure to BG-C which may be the reason for its slightly increased size.
Table 1. Characterization summary of bioactive glass samples.
| BG-C | BG-E | |
|---|---|---|
| Particle size (μ) |
5.88 ± 1.35 |
0.63 ± 0.22 |
| True density (g/cm3) |
3. 21 |
3.22 |
| Bulk density (g/cm3) |
0.27 ± 0.006 |
0.17 ± 0.002 |
| Total porosity (%) |
91.5 |
94.7 |
| Specific surface area (m2/g) |
94 |
290 |
| Average pore diameter (A0) |
57 |
100 |
| Total pore volume (cm3/g) | 0.006 | 0.012 |
Figure 6. Particle size distribution of (A) BG-E and (B) BG-C.
The true density or the absolute density which is the densities of the particles that make up the powder and that exclude the volume of the open and closed pores and the voids within the sample were determined and found to have no significant difference. The density of BG-E and BG-C obtained was 3.22 and 3.21 g/cm3 respectively. This result points out that the new washing procedure has no effect on altering the material chemistry and the structural densification achieved within 600°C is minor to cause any significant effect on the density of the material. However the bulk density expressed as the percentage of the true density of 3.21 g/cm3, is 5.3% and 8.5% for BG-E and BG-C respectively. As with the bulk density results the BG-E sample is more porous than BG-C as the total porosity obtained is 94.7% and 91.5% for the former and latter samples respectively.
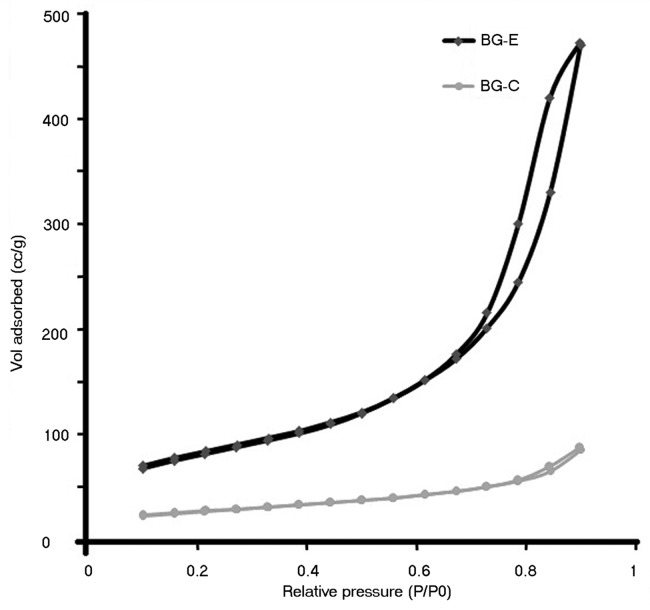
Specific surface area, average pore diameter and total pore volume of BG-E and BG-C were measured and the values are summarized in Table 1. Samples were degassed at 200°C for a period of 2 h in flowing nitrogen before making the BET measurements. At all relative pressures, BG-E sample adsorbed more volume of gas than BG-C which is indicative of the larger pore volume of the BG-E sample. The adsorption isotherms are shown in Figure 5. The specific surface area obtained from the adsorption isotherms is 290 m2/g for BG-E and 94 m2/g for BG-C. The reduced specific surface area of BG-C may be due to the pore collapse occurred during the calcination process. On evaporation of the pore fluid during the calcination process, a drying stress is exerted by the pore fluid which causes the pore collapse. And the pore collapse is more facilitated by high surface tension fluid like water. In case of BG-C water serves as the pore fluid which has more surface tension than ethanol which is the pore fluid of BG-E. During calcination the physisorbed water and the water of hydration acting as the pore fluid in BG-C exert a high drying stress on the pore walls due to its high surface tension and hence the subsequent reduction in pore diameter and pore volume. This in turn causes a reduction in specific surface area. While in the case of BG-E water is replaced with ethanol as the pore fluid to a larger extent during the repeated washing step and hence exerts a low drying stress that accounts for the high specific surface area. However this structural state leading to a higher pore diameter, pore volume and specific surface area are in phase with the higher total porosity value obtained for BG-E by the density measurements. Hence these results indicate that this proposed method of stabilization has its effect on keeping the specific surface area higher than that could be achieved by the conventional calcination method.
Figure 5. Adsorption isotherm of bioactive glass stabilized by ethanol washing and calcinations.
Cytotoxicity using fibroblasts
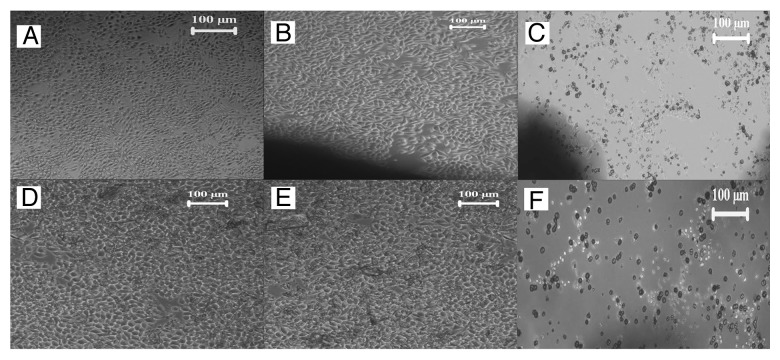
The in vitro cytotoxicity test by direct contact method was done as a preliminary screening test to assess the safety of the material. Since this method of stabilization involves the application of ethanol, there may be present traces of ethanol within the sample which can cause deleterious effect on the behavior of cells. However a feeble presence of physisorbed water and ethanol was observed from the previous TGA (Fig. 3). Yet, the results of direct contact test with L929 fibroblast cells, performed to determine the cytotoxicity that might result from its presence assures that the material is non-cytotoxic. The characteristic spindle shaped morphology of L929 mouse fibroblast cell line that is used for the study (Fig. 7A) is retained in both the bioactive glass samples (Fig. 7D and 7E) as in the negative sample (Fig. 7B). Hence it could be inferred that the washing procedure is adequate to effect the stabilization of bioactive glass and the removal of ethanol is satisfactory to attain a tolerable limit.
Figure 7. Microscopic image of (A) L929 fibroblast cells, L929 fibroblast cells in contact with (B) negative control HDPE, (C) positive control PVC, (D) BG-E, (E) BG-C and (F) raw bioactive glass powder.
Compatibility with stem cells
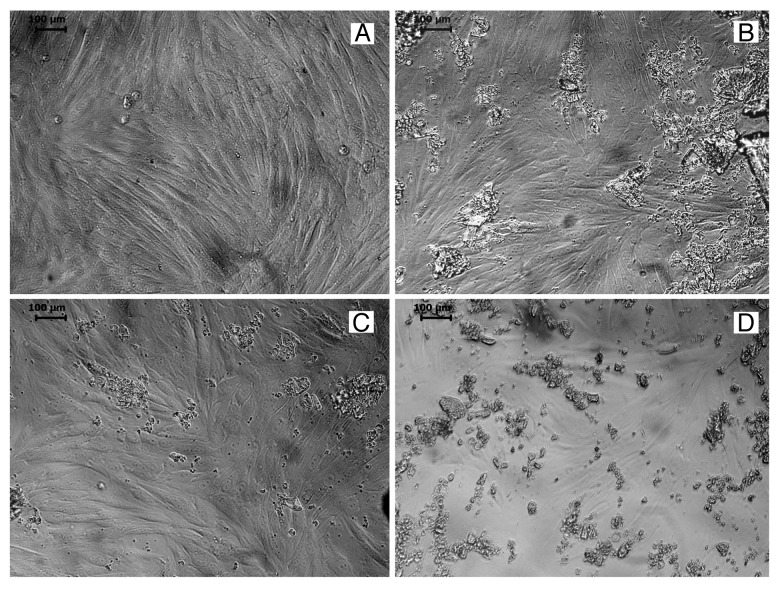
Having found that the material prepared by this novel method is non-toxic to L929 cells, its profound response to another cell type was studied using the bone marrow derived stem cells of rabbit. As observed in an inverted microscope the cells cultured with BG-E and BG-C exhibited its characteristic spindle shaped fibroblastic morphology, proliferated well and established a monolayer (Fig. 8). The result of in vitro cell culture using rMSC illustrates that the material is non-toxic and compatible to rMSC.
Figure 8. Microscopic image showing (A) rMSC, rMSC in contact with (B) BG-C, (C) BG-E and (D) raw bioactive glass powder.
Effect on cell viability
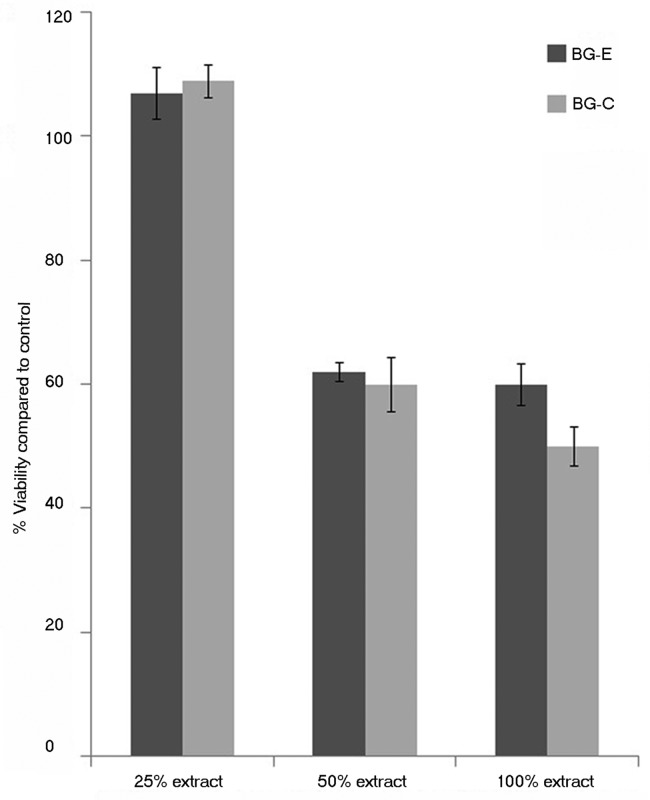
The metabolic acitivity of stem cells were evaluated by exposing the cells to extracts obtained from incubating the prepared bioglass powders in medium for 24 h by MTT assay. Figure 9 shows the cell viability assessed by carrying out the MTT test. The results showed that a 25% extract showed a percentage viability of higher than that of the control (p value ≤ 0.05) while 50% and 100% showed not much significant difference in the metabolic activity of cells when compared with untreated cells.
Figure 9. Percentage viability of rBMSC cultured with the extract of bioactive glass samples, as assessed by MTT assay.
From the results of cell culture studies with rMSC it is possible to assess that the cells cultured with the newly synthesized material are viable as with the conventionally stabilized material. Additionally, during the test on extract the metabolic activity of stem cells were seen increased than the control when cultured in a 25% extract of the proposed material suggesting the proliferative potential of bioactive glass which has been previously reported.18 In fact the metabolic activity levels are similar among the alcohol washed sample and calcined sample. This is indicative of the resemblance of the two stabilization methods. During the in vitro assessment the cell number was found increased and hence a high cellular proliferation occurred in the presence of bioactive glass at this concentration. Hence the cytocompatibility evaluation with rMSC suggests that the material is non-toxic to stem cells and could be used for biological applications and also points out its proliferative capability on bone marrow mesenchymal stem cells.
Bioactivity
The FTIR spectra of stabilized glasses after in vitro reaction in SBF for different time periods are shown in Figure 10 (A, B, C and D). The peaks characteristic to Si-O-Si stretching (*), Si-O-Si bending (○) and O-Si-O stretching (◊) are found in both the samples at all time periods. Along, the peaks characteristic to the P-O stretching (♦) vibrations in the [PO4] tetrahedra are present only in early stages of reaction. Hence in the samples reacted for 48 h, P-O stretching vibrations are absent and instead the P-O bending peak are seen. This is because with increase in immersion time the vibrations due to P-O stretching decrease in intensity. After 48 h of reaction, in BG-E sample the P-O bending peak is resolved into two peaks at 597 and 559 cm−1 and in BG-C the peak appears at 563 cm−1.19 The peak resolution in BG-E is indicative of the hydroxyapatite crystalline phase. Additionally, the peaks attributed to C-O bending (●) from CO3−2 ions are present at 1637 cm−1 for BG-E and BG-C after 24 h reaction. However in the samples after 48 h of reaction the peaks attributed to C-O bending (●) are present at 1627, 1479 and 853 cm−1 for BG-E and at 1637, 1482 and 853 cm-1 20 for BG-C. Moreover, the peak at 1627 cm−1 owing to this carbonate absorption in BG-E is of higher intensity than the corresponding peak at 1637 cm−1 in BG-C. This is also indicative of the early formation of HCA layer in BG-E glass sample.
Figure 10. FTIR spectra of (A) BG-C for 24 h, (B) BG-E for 24 h, (C) BG-C for 48 h, and (D) BG-E for 48 h of reaction in SBF.
As it is obvious that this study would not be ended without assessing the bioactive potential of the proposed material, the in vitro bioactivity studies were done in SBF and only a preliminary characterization was made by FTIR. And the results confirmed that the ethanol stabilized sample has the bioactive property as that of the classical bioactive glass. Both the sample types show peaks characteristic to HCA formation. Moreover, the conversion of single P-O bending mode into two in the BG-E sample, together with the detection of a higher intensity peak due to C-O bending vibrations of CO3−2 ions compared with that in BG-C indicates a higher potential for HCA formation by the BG-E sample. A reason for this may be due to the increased surface area of the BG-E sample since it is well accounted that higher the specific surface area faster is the HCA formation. However the higher bioactive capability of BG-E sample needs to be further studied and hence our future work involves the effect of this new stabilization process on bioactive glass properties like surface area and hence to its bioactivity. Hence the bioactive potential of our proposed material together with its anisotropic morphology suggests that it could also be used for increasing the biomimicity of the polymeric scaffolds by incorporating the particles in the scaffold and hence for the regeneration of hard tissues like bone.
Materials and Methods
Gel synthesis
Reagents used for the sol gel synthesis of bioactive glass are tetraethyl orthosilicate, Si(OC2H5)4, 98% (Aldrich), Triethyl phosphate, OP(OC2H5)3 (Merck), calcium nitrate tetra hydrate, (CaNO3)2.4H2O (Rankem), 0.1 M ammonia solution, 2 M nitric acid and ethanol (70%). All the materials were used as received without further purification. Bioactive glass of composition SiO2 (67.12 mol %), CaO (28.5 mol %), and P2O5 (4.38 mol %) was prepared by the sol gel method as reported elsewhere.21 Briefly, the synthesis procedure involves the preparation of sol by dispersing the precursors in ethanol. 2 M HNO3 was used as the catalyst to control the hydrolysis and condensation reactions. On completion of the reaction 2 M ammonia solution was added to reduce the gelling time. The resulting gel was kept in oven at 60°C for one day to remove the residual water and ethanol.
Alternate stabilization process
The raw bioactive glass powder was stabilized using a novel method of alcohol washing. The method involves repeated washing of bioactive glass with ethanol by agitating in a vortex mixer and the bioactive glass particles were collected on centrifugation. It was then dried at 60°C and pulverized in a mortar and sieved to collect particles < 150 µ size.
Physicochemical characterization
The bioactive glass stabilized by the alcohol washing method was characterized in comparison with the similar bioactive glass composition stabilized by the conventional calcination method. The temperature of calcination was determined by thermogravimetric analysis – differential thermal analysis (TGA-DTA) (SDT 2960, Simultaneous TGA-DTA, TA Instruments Inc.) analysis at a heating rate of 10°C min−1 from room temperature to 1200°C. After calcination the bioactive glass powder was pulverized and sieved as done previously for the ethanol stabilized sample. The stabilized bioactive glass powders BG-E and BG-C were analyzed by Fourier transform infrared (FTIR) spectroscopy (Nicolet 5700 FTIR spectrophotometer) for the characteristic absorption peaks of bioactive glass. The absorbance spectra were collected with a diffuse reflectance accessory in the frequency range of 400- 4000 cm−1. The two spectra were compared with that of the raw bioactive glass in order to confirm the absence of any impurities in the stabilized glasses. Further characterization was done by TGA to confirm the compositional similarity. The TGA analysis was performed in the same conditions as mentioned previously for the determination of calcination temperature. The particle size analysis of the two samples were performed using micro particle size analyzer (Ankersmid CIS-100, Israel) and the particle shape and morphology was compared by scanning electron microscopy (SEM) (Hitachi-S-2400). The true density of the two samples was measured using gas pycnometer (Micromeritics Accu Pyc 1330) and the bulk density of the samples by measuring the geometric dimensions and sample mass. The bulk densities were quoted as the percentage of the true density. The total porosity of the samples were found using the formula, Total porosity = 1- (bulk density / true density). The specific surface area was measured using the Brunauer-Emmett-Teller (BET) analysis (Micromeritics Gemini 2375 V5.01, USA). The average pore diameter and the total pore volume was calculated by the Barrett-Joyner-Halenda (BJH) method applied to the desorption branch of the nitrogen isotherm.
In vitro cytotoxicity tests by direct contact assay
The in vitro cytotoxic evaluation of BG-E, BG-C and raw sample was done by direct contact test using mammalian mouse fibroblast cell line (L929) according to ISO standards (ISO 10993-5, 1992). The bioactive glass samples, negative controls (high density polyethylene) and positive controls (zinc stabilized polyvinyl chloride disc) in triplicate were sterilized by ethylene oxide at 37°C for 5 h. These samples were put on a sub confluent monolayer of L929 mouse fibroblast cells. The culture plates were gently whirled to bring the glass powders which suspended in the medium to get in contact with the cell layer. Culture medium used was Dulbecco’s minimum essential medium (DMEM) supplemented with fetal bovine serum (FBS). After incubation of cell with test samples at 37°C for 24 h, cell culture was examined with an inverted phase contrast microscope (Leica, Generic) for cellular response around test samples. Morphology of the cells was assessed in comparison with the negative and positive control materials.
In vitro cell culture using rabbit bone marrow mesenchymal stem cells
Since bioactive glass is widely used in tissue engineering applications, it is crucial to check the compatibility of this novel material with other cell types. Hence the inherent potential of the bioactive glass stabilized by this method was explored using rabbit bone marrow mesenchymal stem cells (rMSC). Rabbit bone marrow was collected as per the guidelines of institute animal ethics committee from femoral bone under aseptic conditions and dispersed in to single cell suspension. Mononuclear cells were isolated by density gradient centrifugation using Friol- Lypague- plus solution using standard protocol. They were allowed to expand in vitro in DMEM – high glucose containing 20% FBS and 1% antibiotic/ antimycotic in 5% CO2 in a 37°C incubator. rMSC of third passage were used for the study. rMSC were cultured in presence of bioactive glass samples (0.01 g per 5 mL) for a period of 24 h. After the incubation time, cells surrounding the material were examined under microscope for cellular response.
Viability test on extract by MTT assay
The subsequent phase of biological evaluation with rMSC involves the test on extract for assessing the viability of the cells. For test on extract study, 0.1 g of respective powder was suspended in 2 mL of medium and kept at 37°C overnight to get the extract. rMSC were treated with this extract and the cell viability was evaluated using MTT assay. The in vitro tetrazolium based colorimetric assay is a rapid colorimetric method based on the cleavage of a yellow tetrazolium salt (3-{4, 5-dimethylthiazol- 2-yl}-2,5-diphenyl tetrazolium bromide) (MTT) to purple formozan crystals by mitochondrial esterase enzyme of metabolically active cells. The amount of formozan generated is directly proportional to the number of viable cells.22 rMSC (4000 cells in 100 µL DMEM (Gibco, Invitrogen, USA) supplemented with 10% FBS) were plated in 96 well plates. The monolayer of cells were treated with dilutions (25%, 50% and 100%) of the extract for a period of 24 h. Cells with medium alone served as control. After the plates were incubated for 24 h at 37°C in 5% carbon dioxide atmosphere, the extract was removed and 100 µL of MTT working solution (1 in 10 dilution, stock 5 mg mL−1 Sigma Aldrich) was introduced into each well and incubated at 95% humidified atmosphere at 37°C for 3 h. After incubation the reagent was removed and wells were gently rinsed with PBS. Then 100 µL of dimethyl sulfoxide was added to each well and incubated for 5 min at 37°C in a shaker for solubilising the formazan pigment produced. Dye absorbance was measured at 570 nm with background subtraction at 670 nm using an automated microplate reader (ASYS UVM- 340 Reader with Digi-Read/ScanPlus Software, Austria). Absorbance of cells cultured without extract (control) was taken as 100% viability and percentage viability of the test samples were calculated accordingly.
Bioactivity studies
The bone bonding ability or the bioactivity of any material is rated by its ability to induce HCA formation. Assessment of in vitro bioactivity in simulated body fluid (SBF) was also conducted for both the sample types. The bioactivity studies were performed by soaking the stabilized bioactive glass powders for 24 h and 48 h, in a clean polypropylene vial kept at 37°C under static conditions in SBF at a glass concentration of 0.005 g mL−1. SBF was prepared as reported elsewhere.23 The reacted powders were retrieved and freeze-dried to characterize for functional group absorption owing to HCA formation using a Nicolet 5700 FTIR Spectrometer.
Statistical analysis
Three samples were used for each of the quantitative experiments with each measurement done in duplicate to confirm the reproducibility. Each parameter was expressed as mean of all values and their standard deviations. Statistical analysis was performed using one way ANOVA test. Values of p < 0.05 were considered significant (*) and p < 0.0001 were considered very significant (**).
Conclusions
The present study confirms that the new processing route through alcohol washing offers an alternate way of stabilization to bioactive glass analogous to the conventional calcinations method. Bioactive glass stabilized by this ethanol washing method was found identical to the calcined sample in composition. However the structure of the glass sample is varied owing to this procedure. A higher pore diameter and pore volume was resulted by this ethanol treatment unlike the high temperature calcination process and subsequently acquired a higher surface area. The surface area appears to increase three times and a value as high as 290 m2/g could be achieved. However the effect of this washing procedure is found to be only physical and hence unsound to the density of the material. There seems a slight difference in the particle size and morphology as well. The anisotropic flake like morphology of the synthesized material would be utilized to achieve good interaction of the matrix and filler to fabricate polymer ceramic composite scaffolds. Although it was confirmed by the spectroscopic and thermogravimetric analysis that the washing procedure is adequate to effect the stabilization of bioactive glass, the biological evaluation with L929 fibroblast cells and rabbit bone marrow mesenchymal stem cells also supports the same. Moreover both the glass samples were found to exert a proliferative capability on bone marrow mesenchymal stem cells. The retention of the innate bioactive capability of the glass sample was also affirmed by bioactivity studies in SBF. Hence the results confirms that the stabilization has been achieved with a boosting up of surface area by this proposed method of ethanol washing and hence recommendable to improve the biomimmicity of various scaffolds used for soft and hard tissue engineering applications.
Acknowledgments
The authors acknowledge Director SCTIMST, Head BMT Wing for the facilities and Dept. of Biotechnology, COE Program in Tissue Engineering for the financial support.
Glossary
Abbreviations:
- HCA
hydroxyl carbonate apatite
- FTIR
fourier transform infrared
- NBO
non-bridging oxygen
- BG-E
bioactive glass stabilized by ethanol washing
- BG-C
bioactive glass stabilized by calcinations
- TGA-DTA
thermogravimetric analysis – differential thermal analysis
- SEM
scanning electron microscopy
- BET
Brunauer-Emmett-Teller
- MTT
3-{4, 5-dimethylthiazol- 2-yl}-2,5-diphenyl tetrazolium bromide
- rMSC
rabbit bone marrow mesenchymal stem cells
- SBF
simulated body fluid
- DMEM
Dulbecco’s minimum essential medium
- FBS
foetal bovine serum
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24288
References
- 1.Hench LL, Splinter RJ, Allen WC. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5:117–41. doi: 10.1002/jbm.820050611. [DOI] [Google Scholar]
- 2.Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–28. doi: 10.1111/j.1151-2916.1998.tb02540.x. [DOI] [Google Scholar]
- 3.Wilson J, Nicolletti D. Bonding of soft tissues to bioglass. In: Yamamuro T, Hench LL, Wilson J, ed. Handbook of bioactive ceramics: bioactive glasses and glass ceramics. Boca Raton FL:CRC Press, 1990:283-302. [Google Scholar]
- 4.Wilson J, Pigott GH, Schoen FJ, Hench LL. Toxicology and biocompatibility of bioglasses. J Biomed Mater Res. 1981;15:805–17. doi: 10.1002/jbm.820150605. [DOI] [PubMed] [Google Scholar]
- 5.Tan A, Romanska HM, Lenza R, Jones J, Polak JM, Bishop AE. The effect of 58S bioactive sol- gel derived foams on the growth of murine lung epithelial cells. Key Eng Mater. 2003;240-242:719–24. doi: 10.4028/www.scientific.net/KEM.240-242.719. [DOI] [Google Scholar]
- 6.Verrier S, Blaker JJ, Maquet V, Hench LL, Boccaccini AR. PDLLA/Bioglass composites for soft-tissue and hard-tissue engineering: an in vitro cell biology assessment. Biomaterials. 2004;25:3013–21. doi: 10.1016/j.biomaterials.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Day RM. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005;11:768–77. doi: 10.1089/ten.2005.11.768. [DOI] [PubMed] [Google Scholar]
- 8.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55:151–7. doi: 10.1002/1097-4636(200105)55:2<151::AID-JBM1001>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic dissolution products of bioactive glass increase proliferation of human osteoblasts and induce insulin like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000;276:461–5. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 10.Jones JR, Ehrenfried LM, Hench LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73. doi: 10.1016/j.biomaterials.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Hench LL, Wilson J. An introduction to bioceramics. In: Hench LL, Wilson, ed. Advanced series in ceramics, USA:World Scientific Publishing Co, 1993:1-24. [Google Scholar]
- 12.Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol-gel processing. J Appl Biomater. 1991;2:231–9. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Clark AE, Hench LL. Effects of structure and surface area on bioactive powders by sol- gel process. In: Hench LL, West J, ed. Chem. Processing of Advanced Materials. New York:John Wiley, 1992:627-633. [Google Scholar]
- 14.Kokubo T, Cho SB, Nakanishi K, Soga N, Ohtsuki C, Kitsugi T, et al. Dependence of bone like apatite formation on structure of silica gel. In: Andersson, Happonen, YliUrpo, ed. Bioceramics. New York:Butterworth- Heinemann, 1994:49-54. [Google Scholar]
- 15.Li PJ, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Nakamura T, et al. Apatite formation induced by silica gel in a simulated body fliud. J Am Ceram Soc. 1992;75:2094–7. doi: 10.1111/j.1151-2916.1992.tb04470.x. [DOI] [Google Scholar]
- 16.Greenspan DC, Zhong JP, Latorre GP. The evaluation of surface structure of bioactive glasses in vitro. In: Wilson J, Hench LL, Greenspan DC, ed. Bioceramics. New York:Elsevier Science, 1995:477-482. [Google Scholar]
- 17.Joseph R, McGregor WJ, Martyn MT, Tanner KE, Coates PD. Effect of hydroxyapatite morphology/surface area on the rheology and processability of hydroxyapatite filled polyethylene composites. Biomaterials. 2002;23:4295–302. doi: 10.1016/S0142-9612(02)00192-8. [DOI] [PubMed] [Google Scholar]
- 18.Xynos ID, Hukkanen MV, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67:321–9. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 19.Peirera MM, Clark AE, Hench LL. Calcium phosphate formation on sol-gel-derived bioactive glasses in vitro. J Biomed Mater Res. 1994;28:693–8. doi: 10.1002/jbm.820280606. [DOI] [PubMed] [Google Scholar]
- 20.Rehman I, Karsh M, Hench LL, Bonfield W. Analysis of apatite layers on glass-ceramic particulate using FTIR and FT-Raman spectroscopy. J Biomed Mater Res. 2000;50:97–100. doi: 10.1002/(SICI)1097-4636(200005)50:2<97::AID-JBM1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Xia W, Chang J. Preparation and characterization of nano-bioactive-glasses by a quick alkali mediated sol-gel method. Mater Lett. 2007;61:3251–3. doi: 10.1016/j.matlet.2006.11.048. [DOI] [Google Scholar]
- 22.Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993;14:359–64. doi: 10.1016/0142-9612(93)90055-7. [DOI] [PubMed] [Google Scholar]
- 23.Saravanapavan P, Hench LL. Low-temperature synthesis, structure, and bioactivity of gel-derived glasses in the binary CaO-SiO2 system. J Biomed Mater Res. 2001;54:608–18. doi: 10.1002/1097-4636(20010315)54:4<608::AID-JBM180>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]