Abstract
We aimed to determine whether the ACE A11860G genotype is associated with small for gestational age babies (SGA) and to determine whether the association is affected by environmental factors and fetal sex. Overall, 3234 healthy nulliparous women with singleton pregnancies, their partners and babies were prospectively recruited in Adelaide, Australia and Auckland, New Zealand. Data analyses were confined to 2121 Caucasian parent–infant trios, among which 216 were pregnancies with SGA infants and 1185 were uncomplicated pregnancies. Women with the ACE A11860G GG genotype in the combined and Adelaide cohorts had increased risk for SGA [odds ratios (OR) 1.5, 95% confidence interval (CI) 1.1–2.1 and OR 2.0, 95% CI 1.3–3.3, respectively) and delivered lighter babies (P = 0.02; P = 0.007, respectively) compared with those with AA/AG genotypes. The maternal ACE A11860G GG genotype was associated with higher maternal plasma ACE concentration at 15 weeks' gestation than AA/AG genotypes (P < 0.001). When the Adelaide cohort was stratified by maternal socio-economic index (SEI) and pre-pregnancy green leafy vegetable intake, the ACE A11860G GG genotype was only associated with an increased risk for SGA (OR 4.9, 95% CI 1.8–13.4 and OR 3.3, 95% CI 1.6–7.0, respectively) and a reduction in customized birthweight centile (P = 0.006 and P = 0.03) if superimposed on maternal SEI <34 or pre-pregnancy green leafy vegetable intake <1 serve/day. Furthermore, the associations of maternal ACE A11860G with customized birthweight centile observed among Adelaide women with SEI <34 or pre-pregnancy green leafy vegetable intake <1 serve/day were female specific. The current study identified a novel association of maternal ACE A11860G with SGA. More interestingly, this association was modified by environmental factors and fetal sex, suggesting ACE A11860G–environment–fetal sex interactions.
Trial Registry Name: Screening nulliparous women to identify the combinations of clinical risk factors and/or biomarkers required to predict pre-eclampsia, SGA babies and spontaneous preterm birth.
URL: http://www.anzctr.org.au.
Registration number: ACTRN12607000551493.
Keywords: ACE A11860G, small for gestational age, socio-economic index, pre-pregnancy green leafy vegetable intake, fetal sex
Introduction
Small for gestational age (SGA) babies are usually defined as those with birthweight less than the 10th centile for gestational age (Alkalay et al., 1998; Lee et al., 2003). Being born SGA not only increases the risk of morbidity and mortality in the perinatal period (de Courcy-Wheeler et al., 1995; McCowan and Horgan, 2009), but is also associated with an increased risk of the metabolic syndrome in later life, in particular type II diabetes mellitus, cardiovascular disease and hypertension (Barker et al., 1989, 1990, 2007; McKeigue et al., 1998). To date, the exact cause of SGA pregnancies remains largely unknown; however, impairments in maternal plasma volume expansion (Duvekot et al., 1995; Salas et al., 2006) and spiral artery remodelling in the uterus (Khong et al., 1986) have been implicated.
The renin–angiotensin system (RAS), well-known for its role in regulating blood pressure, fluid and electrolyte balance (Griendling et al., 1993), is one of the first hormone systems to ‘recognize’ pregnancy (Broughton Pipkin, 1992). Its activation is thought to be induced by decreased systemic vascular resistance and contributes to the plasma volume expansion by conserving sodium (Schrier and Niederberger, 1994). The existence of a local RAS in the uteroplacental unit has been documented by several studies (Morgan et al., 1998; Cooper et al., 1999; Anton et al., 2009; Marques et al., 2011). Evidence from in vitro and genetic association studies has suggested that angiotensin II, one of the main effectors of the RAS, in the uteroplacental unit inhibits trophoblast invasion and spiral artery remodelling (Morgan et al., 1997, 1999; Xia et al., 2002).
Given the role of the RAS in plasma volume expansion and spiral artery remodelling, genetic polymorphisms in the RAS component genes, which modulate encoded RAS protein level or activity, are likely to modulate women's risk for SGA. One of such polymorphisms is ACE A11860G (rs4343), located in exon 16 of the angiotensin-converting enzyme (ACE) gene on chromosome 17. It has been shown to be in near perfect linkage disequilibrium with the well-known 287 bp insertion/deletion polymorphism (i.e. ACE I/D) (Abdollahi et al., 2008) and appears to be a stronger predictor of plasma ACE concentration than ACE I/D (McKenzie et al., 2005). Furthermore, ACE A11860G has also been shown to associate with plasma ACE activity in hypertensive cohorts (Chung et al., 2010).
The current study aimed to determine the association of ACE A11860G in mothers, fathers and babies with SGA in a nested case–control study of the prospective Screening for Pregnancy Endpoints (SCOPE) cohort. Paternal genotypes were considered because epidemiological evidence suggests that fathers also contribute to the risk for SGA. Specifically, fathers who were born SGA, have a 3.5-fold greater risk of fathering an SGA pregnancy (Jaquet et al., 2005) and we have previously shown that paternal obesity is an independent risk factor for SGA (McCowan et al., 2010a). In addition, since identifying gene–environment interactions is becoming an increasingly important aspect of genetic association studies (Wang et al., 2002; Tsai et al., 2008; Roberts, 2010), we also aimed to establish whether any association of ACE A11860G with SGA is affected by common risk factors for SGA. Furthermore, given sex differences in fetal growth and hence in risk for intrauterine growth restriction (Clifton, 2010), we also determined whether any association of ACE A11860G with SGA is affected by fetal sex.
Materials and Methods
Participants
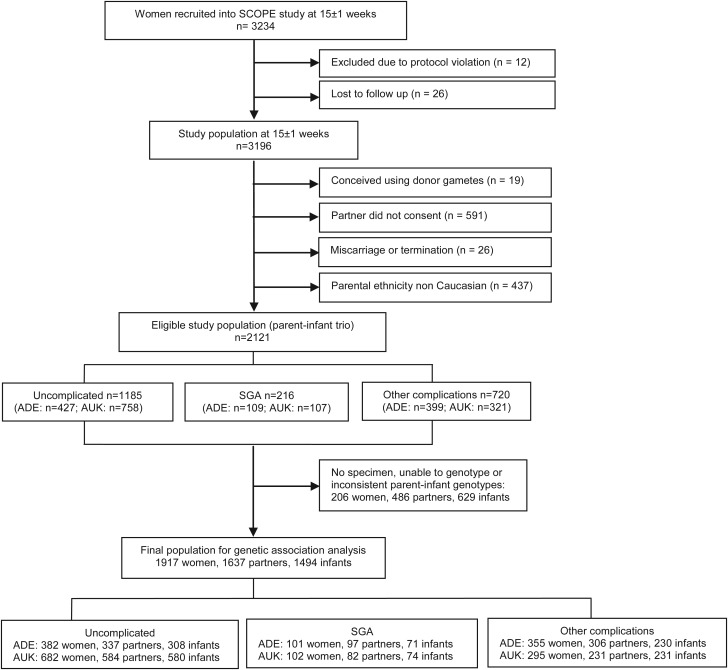
The current study is a nested case–control study within a large prospective multi-centre study, SCOPE. Healthy nulliparous women with singleton pregnancies were prospectively recruited to the SCOPE study between November 2004 and September 2008 in Adelaide, Australia and Auckland, New Zealand (McCowan et al., 2007). The main aim of the SCOPE study is to develop screening tests to predict pre-eclampsia, SGA infants and spontaneous preterm birth. Overall, 3196 women, their partners and babies participated in the study. The study population for the current genetic study was confined to the 2121 Caucasian parent–infant trios (66%) (Fig. 1).
Figure 1.
Flow chart of participant recruitment. ADE, Adelaide; AUK, Auckland.
Participants were recruited to the SCOPE study prior to 15 weeks' gestation through hospital antenatal clinics, obstetricians, general practitioners, community midwives and self-referral in response to advertisements or recommendations of friends. Women were not eligible if they were considered at high risk of pre-eclampsia, SGA babies or spontaneous preterm birth due to underlying medical conditions, gynaecological history, three or more previous miscarriages or three or more terminations of pregnancy or those who had received interventions that might affect pregnancy outcome (McCowan et al., 2007).
Women were interviewed and examined by research midwives at 15 ± 1 weeks' gestation. Maternal demographic and dietary information, including ethnicity, age, height, weight, birthweight, gestational age at birth, socio-economic index (SEI: the New Zealand SEI of occupational status, a number between 10 and 90; it is a validated measure of the individual's socioeconomic status and derived from the specific occupation of the women. A higher score indicates higher socioeconomic status) (Davis et al., 1999), smoking status at 15 weeks' gestation and pre-pregnancy green leafy vegetable intake among other variables as previously reported (McCowan et al., 2010b), were recorded.
In addition, women's blood pressure at 15 weeks' gestation was measured twice consecutively. Paternal information, including age, birthweight, height and weight, was also recorded. Newborn parameters, including infant's gestational age at birth, body length, head circumference, mid-arm circumference, birthweight and customized birthweight centile, were measured and recorded by research midwives usually within 72 h of birth.
Sample collection
Whole blood was collected into EDTA tubes from women at 15 ± 1 weeks' gestation, from partners at some time during the woman's pregnancy and umbilical cord after delivery. Plasma and buffy coat were separated via centrifugation within 3 h of collection. Buccal swabs or saliva samples were collected from partners, who were unwilling to undergo venepuncture, and babies whose cord blood was not obtained at delivery. The buccal swabs were applied to Whatman FTA cards (Whatman, USA) immediately following sample collection and saliva was collected using Oragene kits (DNA Genotek, USA).
Pregnancy outcome definitions
SGA was defined as birthweight less than the 10th customized centile, adjusted for maternal height, booking weight, ethnicity, parity and infant gestation and sex (Gardosi et al., 1995). Uncomplicated pregnancies were those without any pregnancy disorder and with delivery of an appropriately grown baby at term.
Genotyping assays
Maternal, paternal and neonatal DNA was extracted from buffy coats isolated from peripheral or cord blood (QiAamp 96 DNA blood kit), Whatman FTA cards or from saliva (Oragene® DNA kits) following the manufacturers' instructions. Genotyping was conducted by the Australian Genome Research Facility using the Sequenom MassARRAY system. Two quality controls were performed to ensure the accuracy of the genotyping data: (i) each sample was genotyped for Amelogenin, a sex-determinant gene (Sullivan et al., 1993) and (ii) parental and neonatal genotyping data were checked for a Mendelian pattern of inheritance. Samples were excluded if an inconsistency between the sex of the sample and the corresponding Amelogenin genotype and/or non-Mendelian pattern of inheritance was observed. In addition, some samples were excluded due to inadequate blood samples, low quality of DNA or failure to genotype.
Plasma ACE concentration
A plasma ACE concentration was measured in 451 women with a range of pregnancy outcomes, who were selected from the Adelaide cohort, using a commercial ELISA kit (ACE duoset, R&D Systems, Minneapolis, USA). The sensitivity of the assay was 62.5 pg/ml. Optical density was determined using a microplate reader (BioRad, Benchmark) at 450 nm and corrected at 570 nm.
Statistics
The χ2 test was used to test the ACE A11860G genotypes for Hardy–Weinberg equilibrium and to compare the categorical variables. Odds ratios (OR) were calculated by logistic regression to quantify the effects of genotypes and environmental factors on risk for SGA. The student's t-test or one-way analysis of variance with Dunnett's post hoc adjustment was performed to compare continuous variables. Covariate analysis was used to adjust for the effect of gestational age on baby length, birthweight and head circumference. The Mann–Whitney test was performed to compare the plasma ACE concentration. All data analyses were performed using PASW (SPSS, Chicago), version 17.02. P of <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the participants
Combined Adelaide and Auckland SCOPE
In the combined cohort, women who later delivered an SGA baby had a higher BMI (P = 0.003, Table I), higher systolic blood pressure (sBP) at 15 weeks' gestation (P < 0.001, Table I) and they weighed less at birth (P < 0.001, Table I) than those without any complication. The proportion of women with a socioeconomic index <34, smoking at 15 weeks' gestation and with pre-pregnancy green leafy vegetable intake <1 serve/day was significantly higher in pregnancies complicated by SGA than those in uncomplicated pregnancies (P < 0.001, P < 0.001 and P = 0.012, respectively, Table I). Partners, who fathered an SGA baby, on average were shorter (P < 0.001, Table I) and weighed less at birth (P < 0.001, Table I) than those who fathered an uncomplicated pregnancy. All newborn measurements were lower in SGA pregnancies compared with those in uncomplicated pregnancies (Table I).
Table I.
Demographic characteristics of the study population.
| Combined SCOPE |
Adelaide SCOPE |
Auckland SCOPE |
ADE versus AUK |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uncomplicated | SGA | P | Uncomplicated | SGA | P | Uncomplicated | SGA | P | Uncomplicated | SGA | |
| n = 1185 | n = 216 | n = 427 | n = 109 | n = 758 | n = 107 | P | P | ||||
| Maternal characteristics | |||||||||||
| Age (years)a | 28.2 (5.6) | 28.5 (6.2) | 0.5 | 23.6 (5.0) | 24.3 (5.3) | 0.1 | 30.8 (4.1) | 32.7 (3.8) | <0.001 | <0.001 | <0.001 |
| BMI (kg/m2)a | 24.9 (4.5) | 26.1 (5.5) | 0.003 | 26.0 (5.6) | 27.5 (6.2) | 0.01 | 24.3 (3.7) | 24.7 (4.3) | 0.3 | <0.001 | <0.001 |
| sBP (mmHg)a | 106.2 (9.9) | 109.5 (11.1) | <0.001 | 107.9 (8.9) | 111.6 (11.2) | 0.002 | 105.3 (10.3) | 107.5 (10.8) | 0.05 | <0.001 | 0.006 |
| dBP (mmHg)a | 63.3 (7.6) | 66.0 (8.9) | <0.001 | 63.5 (7.5) | 66.0 (8.9) | 0.007 | 63.2 (7.7) | 66.1 (8.9) | 0.002 | 0.5 | 1.0 |
| Socioeconomic index (%) | |||||||||||
| ≥34 | 735 (62.0) | 104 (48.1) | <0.001 | 80 (18.7) | 9 (8.3) | 0.009 | 655 (86.4) | 95 (88.8) | 0.7 | <0.001 | <0.001 |
| <34 | 450 (38.0) | 112 (51.9) | 347 (81.3) | 100 (91.7) | 103 (13.6) | 12 (11.2) | |||||
| PPGLV (%) | |||||||||||
| ≥1 serve/day | 615 (51.9) | 92 (42.6) | 0.012 | 120 (28.1) | 25 (22.9) | 0.3 | 495 (65.3) | 67 (62.6) | 0.6 | <0.001 | <0.001 |
| <1 serve/day | 570 (48.1) | 124 (57.4) | 307 (71.9) | 84 (77.1) | 263 (34.7) | 40 (37.4) | |||||
| Smoking (%)a | |||||||||||
| Non-smokers | 1074 (90.6) | 166 (76.9) | <0.001 | 337 (78.9) | 61 (56.0) | <0.001 | 737 (97.2) | 105 (98.1) | 1.0 | <0.001 | <0.001 |
| Smokers | 111 (9.4) | 50 (23.1) | 90 (21.1) | 48 (44.0) | 21 (2.8) | 2 (1.9) | |||||
| Gestational age at birth (weeks)b | 39.9 (1.9) | 39.7 (2.1) | 0.4 | 39.5 (1.9) | 39.4 (2.3) | 0.6 | 40.0 (1.8) | 40.1 (1.7) | 0.9 | <0.001 | 0.02 |
| Maternal birthweight (g)c | 3335 (530) | 3167 (527) | <0.001 | 3287 (549) | 3136 (544) | 0.01 | 3361 (517) | 3197 (511) | 0.003 | 0.02 | 0.4 |
| Paternal characteristics | |||||||||||
| Age (years) | 30.7 (6.3) | 31.1 (6.7) | 0.5 | 26.4 (6.1) | 27.6 (6.3) | 0.054 | 33.2 (4.9) | 34.6 (5.0) | 0.005 | <0.001 | <0.001 |
| Height (cm)d | 179.6 (6.7) | 177.6 (6.9) | <0.001 | 178.5 (6.7) | 176.3 (7.2) | 0.002 | 180.3 (6.7) | 179.0 (6.4) | 0.06 | <0.001 | 0.005 |
| BMI (kg/m2)d | 26.6 (4.0) | 27.2 (4.6) | 0.07 | 26.9 (5.0) | 27.3 (5.0) | 0.4 | 26.4 (3.3) | 27.1 (4.1) | 0.1 | 0.07 | 0.7 |
| Paternal birthweight (g)e | 3488 (571) | 3314 (521) | <0.001 | 3462 (593) | 3347 (558) | 0.09 | 3502 (559) | 3283 (485) | <0.001 | 0.2 | 0.4 |
| Newborn characteristics | |||||||||||
| Gestational age at birth (days) | 280.7 (8.1) | 271.6 (24.5) | <0.001 | 280.6 (8.0) | 271.9 (23.2) | <0.001 | 280.7 (8.1) | 271.4 (25.9) | <0.001 | 0.8 | 0.9 |
| Body lengthf (cm) | 51.0 (2.2) | 47.1 (4.5) | <0.001 | 50.0 (1.8) | 46.2 (3.9) | <0.001 | 51.5 (2.2) | 48.0 (4.8) | <0.001 | <0.001 | 0.003 |
| Head circumferencef (mm) | 35.2 (1.3) | 32.9 (2.5) | <0.001 | 34.9 (1.3) | 32.8 (2.3) | <0.001 | 35.3 (1.4) | 32.9 (2.8) | <0.001 | 0.001 | 0.7 |
| Mid-arm circumferencef (mm) | 11.0 (0.9) | 9.4 (1.1) | <0.001 | 11.0 (0.9) | 9.2 (1.2) | <0.001 | 11.1 (0.9) | 9.5 (1.0) | <0.001 | 0.2 | 0.2 |
| Birthweightf (g) | 3591 (394) | 2587 (560) | <0.001 | 3575 (390) | 2554 (536) | <0.001 | 3600 (396) | 2621 (583) | <0.001 | 0.3 | 0.4 |
| Customized birthweight centile | 53.7 (25.0) | 4.6 (2.9) | <0.001 | 53.9 (24.9) | 4.0 (2.8) | <0.001 | 53.6 (25.1) | 5.1 (2.9) | <0.001 | 0.9 | 0.004 |
| Sex of babies (%) | |||||||||||
| Males | 601 (50.7) | 107 (49.5) | 0.8 | 210 (49.2) | 49 (45.0) | 0.4 | 391 (51.6) | 58 (54.2) | 0.7 | 0.4 | 0.2 |
| Females | 584 (49.3) | 109 (50.5) | 217 (50.8) | 60 (55.0) | 367 (48.4) | 49 (45.8) | |||||
Data are presented as the mean (SD) or n (%). sBP, systolic blood pressure; the second measurement; dBP, diastolic blood pressure; the second measurement; PPGLV, pre-pregnancy green leafy vegetable intake; 15 weeks, 15 weeks' gestation; ADE, Adelaide; AUK, Auckland. Italic values indicate significant difference.
aMeasurements were taken at 15 weeks' gestation.
bNumbers for women's gestational age at birth were 1166, 208, 419, 105, 747 and 103, for each group, respectively.
cNumbers for women's birthweight were 1153, 208, 411, 104, 742 and 104, for each group, respectively.
dNumbers for partner's height or BMI were 1157, 210, 422, 108, 735 and 102 for each group, respectively.
eNumbers for partner's birthweight were 1107, 197, 385, 94, 722 and 103 for each group, respectively.
fAdjusted for gestational age at birth.
Adelaide SCOPE versus Auckland SCOPE
In both uncomplicated and SGA pregnancies, Adelaide women were younger, had higher BMI and higher sBP at 15 weeks' gestation than Auckland women (Table I). The proportions of women who continued to smoke cigarettes at 15 weeks' gestation and those with SEI <34 and pre-pregnancy green leafy vegetable intake <1 serve/day were also significantly higher in the Adelaide cohort than those in the Auckland cohort (Table I). In both uncomplicated and SGA pregnancies, Adelaide fathers were shorter and younger than Auckland fathers (Table I). The characteristics of neonates were similar between the Adelaide and Auckland cohort except that Adelaide neonates were shorter than Auckland neonates (Table I).
Association of ACE A11860G with SGA and newborn growth parameters
Maternal, paternal and neonatal ACE A11860G genotype distributions in uncomplicated and SGA pregnancies were in Hardy–Weinberg equilibrium in the combined, Adelaide and Auckland SCOPE pregnancies (data not shown). In the combined SCOPE cohort, the genotype distribution of maternal ACE A11860G is: AA: 21.8% (uncomplicated) and 21.7% (SGA), AG: 51.3% (uncomplicated) and 42.9% (SGA) and GG: 26.9% (uncomplicated) and 35.5% (SGA); paternal ACE A11860G is: AA: 20.2% (uncomplicated) and 17.3% (SGA), AG: 50.8% (uncomplicated) and 54.2% (SGA) and GG: 29.0% (uncomplicated) and 28.5% (SGA); neonatal ACE A11860G is AA: 21.5% (uncomplicated) and 20.7% (SGA), AG: 48.9% (uncomplicated) and 48.3% (SGA) and GG: 29.60% (uncomplicated) and 31.0% (SGA).
In the combined and Adelaide cohorts, the maternal ACE A11860G GG genotype was associated with an increased risk for SGA (OR 1.5, 95% confidence interval (CI) 1.1–2.1 and OR 2.0, 95% CI 1.3–2.3, respectively, Table II). No associations of paternal or neonatal ACE A11860G with SGA were found in the combined, Adelaide and Auckland SCOPE cohorts (Table II).
Table II.
Maternal, paternal and neonatal ACE A11860G genotype distribution in uncomplicated pregnancies and SGA pregnancies, stratified by cohorts.
| Combined SCOPE |
Adelaide SCOPE |
Auckland SCOPE |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncomplicated (%) | SGA (%) | OR (95% CI) | Uncomplicated (%) | SGA (%) | OR (95% CI) | Uncomplicated (%) | SGA (%) | OR (95% CI) | |
| Maternal ACE A11860G | n = 1064 | n = 203 | n = 382 | n = 101 | n = 682 | n = 102 | |||
| AA/AG | 778 (73.1) | 131 (64.5) | Ref | 295 (77.2) | 63 (62.4) | Ref | 483 (70.8) | 68 (66.7) | Ref |
| GG | 286 (26.9) | 72 (35.5) | 1.5(1.1–2.1) | 87 (22.8) | 38 (37.6) | 2.0 (1.3–3.3) | 199 (29.2) | 34 (33.3) | 1.2 (0.8–1.9) |
| Paternal ACE A11860G | n = 921 | n = 179 | n = 337 | n = 97 | n = 584 | n = 82 | |||
| AA/AG | 654 (71.0) | 128 (71.5) | Ref | 237 (70.3) | 72 (74.2) | Ref | 417 (71.4) | 56 (68.3) | Ref |
| GG | 267 (29.0) | 51 (28.5) | 1.0(0.7–1.4) | 100 (29.7) | 25 (25.8) | 0.8 (0.5–1.4) | 167 (28.6) | 26(31.7) | 1.2 (0.7–1.9) |
| Neonatal ACE A11860G | n = 888 | n = 145 | n = 308 | n = 71 | n = 580 | n = 74 | |||
| AA/AG | 625 (70.4) | 100 (69.0) | Ref | 224 (72.7) | 48 (67.6) | Ref | 401 (69.1) | 52 (70.3) | Ref |
| GG | 263 (29.6) | 45 (31.0) | 1.1(0.7–1.6) | 84 (27.3) | 23 (32.4) | 1.3 (0.7–2.2) | 179 (30.9) | 22 (29.7) | 1.0 (0.6–1.6) |
Data are presented as n (%). Ref, reference. Italic values indicate significant difference.
In the combined and Adelaide cohorts, the maternal ACE A11860G GG genotype was associated with a mean 50 and 94 g reduction, respectively, in birthweight adjusted for gestational age (−1.5%, P = 0.02 and −3%, P = 0.007, respectively, Table III). Maternal ACE A11860G GG genotype in the Adelaide cohort was also associated with a 5.6% reduction in customized birthweight centile (P = 0.02, Table III).
Table III.
The association of maternal ACE A11860G with newborn growth parameters, stratified by cohorts.
| Combined maternal ACE A11860G |
Adelaide maternal ACE A11860G |
Auckland maternal ACE A11860G |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AA/AG | GG | P | AA/AG | GG | P | AA/AG | GG | P | |
| Body lengtha (cm) | n = 1371 | n = 529 | 0.2 | n = 616 | n = 219 | 0.4 | n = 755 | n = 310 | 0.08 |
| 50.1 (3.2) | 49.9 (3.2) | 49.1 (3.5) | 48.9 (3.0) | 50.9 (2.8) | 50.6 (3.2) | ||||
| Birthweighta (g) | n = 1383 | n = 532 | 0.02 | n = 617 | n = 219 | 0.007 | n = 766 | n = 313 | 0.5 |
| 3394 (607) | 3344 (584) | 3363 (644) | 3269 (626) | 3419 (574) | 3399 (546) | ||||
| Customized birthweight centile | n = 1382 | n = 531 | 0.2 | n = 617 | n = 219 | 0.02 | n = 765 | n = 312 | 0.9 |
| 47.6 (28.1) | 45.5 (29.3) | 47.8 (28.7) | 42.3 (30.4) | 47.4 (27.6) | 47.7 (28.3) | ||||
Data are presented as the mean (SD). Italic values indicate significant difference.
aAdjusted for gestational age at birth.
Gene–environment and gene-fetal sex interactions
Since the association of maternal ACE A11860G with SGA and neonatal growth parameters was observed in the Adelaide cohort but not in the Auckland cohort, subsequent gene–environment and gene–fetal sex interaction analyses were only performed for the Adelaide cohort.
The factors selected included maternal age (<29 years versus ≥29 years), BMI (<25 versus ≥25 kg/m2), pre-pregnancy green leafy vegetable intake (<1 serve/day versus ≥1 serve/day), SEI (<34 versus ≥34) and smoking status at 15 weeks' gestation (no smoking versus smoking). All these factors have previously been shown to affect risk for SGA and are also significantly different between Adelaide and Auckland SCOPE pregnancies in the current study. The selection of cut-off points for maternal age, pre-pregnancy green leafy vegetable intake and SEI was based on the fact that these points are at or close to the medians in the combined cohort.
Among these selected factors, only maternal SEI and pre-pregnancy green leafy vegetable intake were found to modulate the associations of interest. Specifically, the maternal ACE A11860G GG genotype was only associated with increased risk for SGA and reduction in customized birthweight centile if superimposed on maternal SEI <34 or pre-pregnancy green leafy vegetable intake (Table IV).
Table IV.
The association of maternal ACE A11860G with SGA and customized birthweight centile in the Adelaide SCOPE, stratified by maternal SEI and PPGLV.
| n | Uncomplicated (%) | SGA (%) | OR (95% CI) | n | Customized birthweight centile | P | ||
|---|---|---|---|---|---|---|---|---|
| Maternal SEI | Maternal ACE A11860G | |||||||
| SEI ≥ 34 | — | 74 | 66 (89.2) | 8 (10.8) | Ref | 181 | 50.1 (27.6) | Ref |
| SEI < 34 | — | 409 | 316 (77.3) | 93 (22.7) | 2.4 (1.1–5.2) | 754 | 45.9 (29.6) | 0.09 |
| SEI ≥ 34 | AA/AG | 54 | 49 (90.7) | 5 (9.3) | Ref | 112 | 52.1 (27.4) | Ref |
| SEI ≥ 34 | GG | 20 | 17 (85.0) | 3 (15.0) | 1.7 (0.4–8.0) | 42 | 46.4 (29.6) | 0.6 |
| SEI < 34 | AA/AG | 304 | 246 (80.9) | 58 (19.1) | 2.3 (0.9–6.1) | 505 | 46.9 (29.0) | 0.2 |
| SEI < 34 | GG | 105 | 70 (66.7) | 35 (33.3) | 4.9 (1.8–13.4) | 177 | 41.3 (30.5) | 0.006 |
| Maternal PPGLV | Maternal ACE A11860G | |||||||
| ≥1 serve/day | — | 127 | 107 (84.3) | 20 (15.7) | Ref | 237 | 47.6 (29.5) | Ref |
| <1 serve/day | — | 356 | 275 (77.2) | 81 (22.8) | 1.6 (0.9–2.7) | 698 | 46.4 (29.2) | 0.6 |
| ≥1 serve/day | AA/AG | 94 | 82 (87.2) | 12 (12.8) | Ref | 149 | 49.7 (29.2) | Ref |
| ≥1 serve/day | GG | 33 | 25 (75.8) | 8 (24.2) | 2.2 (0.8–5.9) | 63 | 44.9 (29.6) | 0.6 |
| <1 serve/day | AA/AG | 264 | 213 (80.7) | 51 (19.3) | 1.6 (0.8–3.2) | 468 | 47.3 (28.6) | 0.7 |
| <1 serve/day | GG | 92 | 62 (67.4) | 30 (33.3) | 3.3 (1.6–7.0) | 156 | 41.3 (30.7) | 0.03 |
The data are presented as n (%) or mean (SD). SEI, socioeconomic index; PPGLV, pre-pregnancy green leafy vegetable intake; Ref, reference. Italic values indicate significant difference.
We further stratified the Adelaide cohort by fetal sex to investigate gene–fetal sex interactions. When the cohort was stratified by fetal sex, the associations of maternal ACE A11860G GG genotype with SGA and reduction in customized birthweight centile were specific to female-bearing pregnancies (Table V).
Table V.
The association of maternal ACE A11860G with SGA and customized birthweight centile in the Australian SCOPE cohort, stratified by maternal SEI, PPGLV and fetal sex.
| Female babies |
Male babies |
||||||
|---|---|---|---|---|---|---|---|
| Maternal ACE A11860G | n | SGA (%) | P | n | SGA (%) | P | |
| AA/AG | 188 | 33 (17.6) | 170 | 30 (17.6) | |||
| GG | 62 | 23 (37.1) | 0.003 | 63 | 15 (23.8) | 0.4 | |
| Maternal ACE A11860G | n | Customized birthweight centile | n | Customized birthweight centile | |||
| AA/AG | 323 | 48.1 (28.7) | 294 | 47.6 (28.8) | |||
| GG | 110 | 38.9 (29.5) | 0.004 | 109 | 45.7 (31.0) | 0.6 | |
| Maternal SEI | Maternal ACE A11860G | n | Customized birthweight centile | n | Customized birthweight centile | ||
| SEI ≥ 34 | AA/AG | 63 | 52.9 (27.0) | Ref | 49 | 50.9 (28.2) | Ref |
| SEI ≥ 34 | GG | 18 | 39.8 (31.4) | 0.2 | 24 | 51.4 (27.8) | 1 |
| SEI < 34 | AA/AG | 260 | 46.9 (29.1) | 0.3 | 245 | 46.9 (28.9) | 0.7 |
| SEI < 34 | GG | 92 | 38.8 (29.3) | 0.008 | 85 | 44.1 (31.8) | 0.4 |
| Maternal PPGLV | Maternal ACE A11860G | n | Customized birthweight centile | n | Customized birthweight centile | ||
| ≥1 serve/day | AA/AG | 74 | 49.7 (28.8) | Ref | 75 | 49.7 (29.8) | Ref |
| ≥1 serve/day | GG | 35 | 42.5 (30.1) | 0.5 | 28 | 47.9 (29.2) | 1.0 |
| <1 serve/day | AA/AG | 249 | 47.6 (28.7) | 0.9 | 219 | 46.8 (28.4) | 0.8 |
| <1 serve/day | GG | 75 | 37.3 (29.3) | 0.03 | 81 | 44.9 (31.7) | 0.6 |
The data are presented as mean (SD) or n (%). SEI, socioeconomic index; PPGLV, pre-pregnancy green leafy vegetable intake; Ref, reference. Italic values indicate significant difference.
Plasma ACE concentration
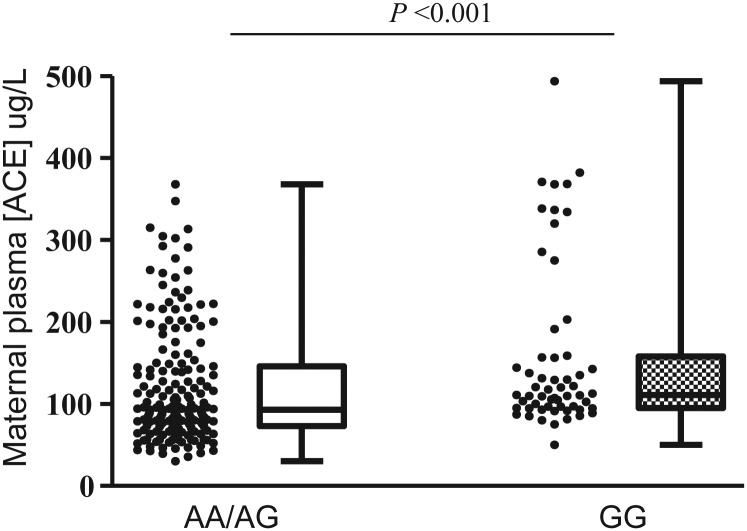
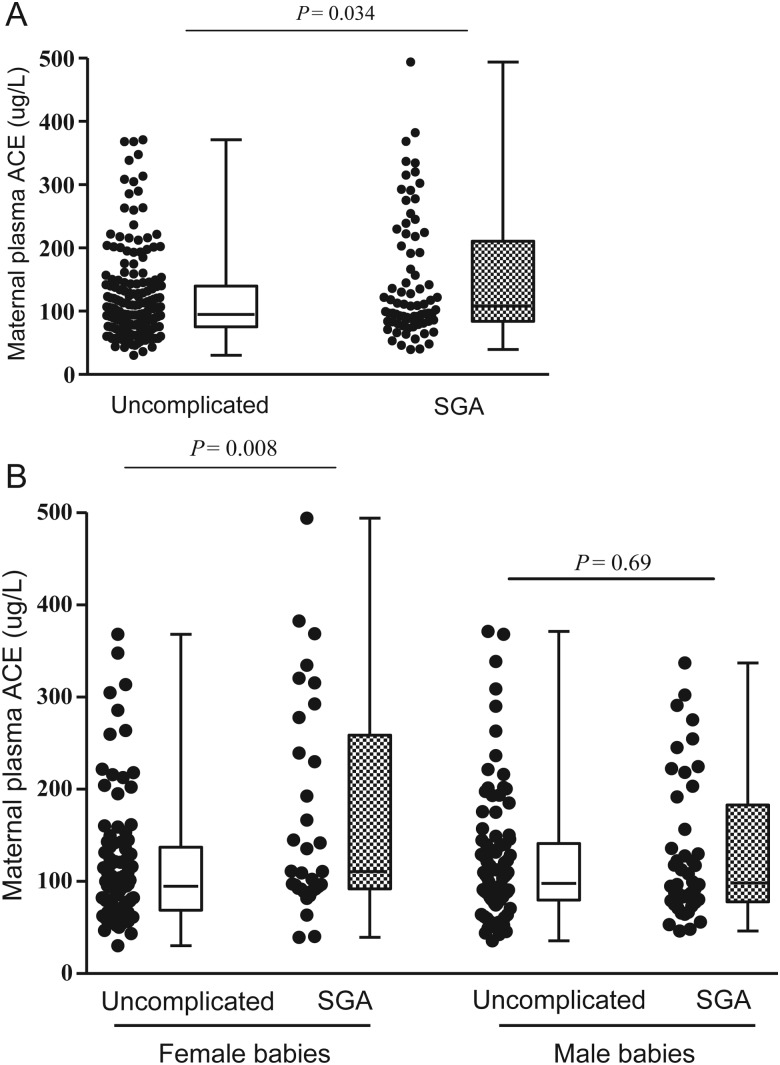
In the uncomplicated and SGA pregnancies combined, women bearing the ACE A11860G GG genotype had higher circulating ACE concentration at 15 weeks' gestation than those bearing AA or AG genotypes (P < 0.001, Fig. 2). Subgroup analysis of women without any complication showed the same association (P = 0.015). In addition, women who delivered SGA infants had higher plasma ACE concentrations at 15 weeks' gestation than women with uncomplicated pregnancies (P = 0.034) and this relationship was specific to pregnancies bearing females (P = 0.008) (Fig. 3).
Figure 2.
The association of maternal ACE A11860G with maternal plasma ACE concentration at 15 weeks' gestation in the Adelaide cohort. Mann–Whitney U-test, P < 0.001. nAA/AG = 183, nGG = 57.
Figure 3.
Maternal plasma ACE concentration at 15 weeks' gestation in Adelaide uncomplicated and SGA pregnancies. (A) All pregnancies irrespective of infant sex, Mann–Whitney U-test, P = 0.034, nuncomplicated = 190, nSGA = 77. (B) Pregnancies stratified by the sex of babies. For pregnancies bearing females, Mann–Whitney U-test, P = 0.008, nuncomplicated = 96, nSGA = 33; for pregnancies bearing males, Mann–Whitney U-test, P = 0.690, nuncomplicated = 94, nSGA = 44.
Discussion
In the current study, we have identified a novel association of maternal ACE A11860G with SGA in the combined Adelaide and Auckland cohorts. Specifically, women with homozygous ACE A11860G GG had increased risk for delivering an SGA infant. Consistent with this, we also found that babies delivered by women with the ACE A11860G GG genotype weighed less at birth, corrected for gestational age, than those born to women with ACE A11860G AA or AG genotypes.
The ACE A11860G, located in exon 16 of the ACE gene, has previously been shown to associate with plasma ACE concentration (McKenzie et al., 2005) and activity (Chung et al., 2010). Consistent with this, in our pregnant cohort, mothers bearing the ACE A11860G GG genotype had a higher plasma ACE concentration at 15 weeks' gestation than those bearing AA or AG genotypes.
The ACE A11860G GG genotype has been shown to associate with various disease phenotypes, including nephropathy (Ahluwalia et al., 2009), Alzheimer's disease (Zhu et al., 2001; Meng et al., 2006; Bruandet et al., 2008; Helbecque et al., 2009), coronary artery disease (Freitas et al., 2008), hypertension (Alvi and Hasnain, 2009) and left ventricular hypertrophy (Wakahara et al., 2007). To the best of our knowledge, no previous studies have investigated its association with SGA. For the first time we have demonstrated that ACE A11860G is associated with SGA, suggesting the involvement of the RAS in the pathogenesis of impaired fetal growth.
The mechanism behind the association of maternal ACE A11860G with SGA is yet to be elucidated. We speculate that arterial stiffening in the maternal systemic vasculature may be involved. Specifically, women with the ACE A11860G GG genotype, who have a higher plasma ACE concentration, may chronically have stiffer arteries than those bearing AA or AG genotypes. As a result, they would be more likely to experience a deficit in plasma volume expansion and hence be at a higher risk of delivering an SGA baby. It has been shown that the DD genotype of ACE I/D, which is in linkage disequilibrium with ACE A11860G (Abdollahi et al., 2008), is associated with higher arterial stiffness (Mattace-Raso et al., 2004). In addition, increased arterial stiffness has previously been linked to a significant reduction in birthweight centile, potentially via diminished maternal plasma volume expansion and uteroplacental blood flow (Elvan-Taspinar et al., 2005).
The Adelaide and Auckland cohorts have a similar distribution in maternal ACE A11860G genotypes, but striking differences in environmental risk factors for SGA. Of particular note, the Adelaide women were younger, ate fewer green leafy vegetables prior to pregnancy, had a higher BMI and lower socioeconomic status and were more likely to smoke during pregnancy than the Auckland women. In post hoc analyses when we analysed the data on the Adelaide and Auckland cohorts separately, the associations of maternal ACE A11860G with SGA and neonatal birthweight were only observed in the Adelaide SCOPE (i.e. the disadvantaged population), suggesting a gene–environment interaction.
We further investigated gene–environment interactions in the Adelaide cohort and observed that maternal SEI and pre-pregnancy green leafy vegetable intake modulated the associations of maternal ACE A11860G with SGA and customized birthweight centile. Specifically, the maternal ACE A11860G GG genotype was apparently harmless among women with SEI ≥34 or pre-pregnancy green leafy vegetable intake ≥1 serve/day, and it only became a risk factor for SGA and led to a reduction in customized birthweight centile when it combined with SEI <34 or pre-pregnancy green leafy vegetable intake <1 serve/day. It is worth noting that the combined effects of maternal ACE A11860G GG genotype with maternal SEI < 34 or pre-pregnancy green leafy vegetable intake < 1 serve/day were more profound than the effect of maternal SEI < 34 or pre-pregnancy green leafy vegetable intake vegetable < 1 serve/day alone. These data may indicate that the adverse effect of maternal ACE A11860G GG genotype on its own is subtle and can only put women at risk for SGA if superimposed on adverse environments. The mechanisms behind the observed gene–environment interactions are unclear. Vascular stiffness, which as proposed earlier, may be associated with maternal ACE A11860G, may well be involved, since both low socio-economic status and green vegetable consumption have previously been shown to associate with vascular stiffness (Thurston and Matthews, 2009; Aatola et al., 2010).
Importantly, the current study has also demonstrated that the association of maternal ACE A11860G GG genotype with SGA and reduction in customized birthweight centile was specific to female-bearing pregnancies. If one considers a reduction in customized birthweight centile as a response to the adverse effect of ACE A11860G GG genotype, our data suggest that only female fetuses respond to this effect. Given the central role of the placenta in regulating fetal growth and survival (Clifton, 2010), the female-specific response observed in the current study may be attributable to sex-specific placental function. A study is under way to examine the sex differences in placentas from women with the ACE A11860G GG genotype. A female-specific response has previously been reported in pregnancies complicated by maternal asthma (Murphy et al., 2003). Studies have also shown that placental global gene expression (Osei-Kumah et al., 2011), microRNA expression (Clifton, 2010), proteomic profile (Osei-Kumah et al., 2008) and placental structure (Mayhew et al., 2008) vary between female and male placentas from pregnancies complicated by maternal asthma, indicating the underlying role of the placenta in the female-specific response. In summary, our data, together with those of others, suggest that there is a need for new investigations to define the molecular differences between the male versus female placenta.
Previously published genetic association studies for pregnancy complications have proved difficult to replicate. The gene–environment and gene–fetal sex interactions observed in the current study may shed light on this and suggest that conflicting results in the literature may be, in part, due to differences in environmental factors between study populations and failure to appreciate the significance of fetal sex.
Finally, in the current study, women who delivered an SGA baby had a significantly higher plasma ACE concentration at 15 weeks' gestation than those with an uncomplicated pregnancy. Interestingly, when the cohort was stratified by fetal sex, this SGA-related elevation of the plasma ACE concentration only remained in pregnancies bearing females. These results together provide functional support for the association of maternal ACE A11860G (a functional variant) with SGA and the interaction of this variant with fetal sex.
The strength of the current study is its large multi-centre prospective design. In addition, the outcome data of these cases were reviewed by highly skilled SCOPE clinicians to ensure the accuracy and consistency of diagnoses. The weakness of the study is the missing genotypes of some participants, which reduced our sample size and may potentially introduce bias into our results. However, there are no any systematic reasons for missing genotypes. Furthermore, the current study performed multiple comparisons. As a result, the likelihood of our significant results being false positive is increased. However, since our data were supported by functional data on plasma ACE concentration, it is unlikely we have made a false discovery. Finally, although our results on gene–environment and gene–fetal sex interactions were intriguing, they were derived from analyses of subgroups with low sample sizes. Further studies with larger sample size are required to validate these results.
In summary, we have shown that the maternal ACE A11860G was associated with SGA. More interestingly, the association was modulated by modifiable environmental factors (maternal SEI and pre-pregnancy green leafy vegetable consumption), as well as fetal sex, suggesting ACE A11860G–environment–fetal sex interactions. We recommend future genetic association studies should take into consideration clinical and lifestyle factors, as well as fetal sex, in order to elucidate the genetic associations more clearly.
Ethical approval
Ethical approval was gained from local ethics committees (Australia REC 1712/5/2008 and New Zealand AKX/02/00/364) and all women provided written informed consent.
Authors' roles
G.A.D., C.T.R. and A.Z. had full access to all the required data and took responsibility for the integrity of the data and the accuracy of the data analysis. G.A.D., L.M.E.M. and C.T.R. had roles in SCOPE study conception and design. A.Z., G.A.D. and C.T.R. were involved in candidate gene association study conception and design. G.A.D., E.R.L., C.T.R. and A.Z. interpreted the data. G.A.D., E.R.L., L.M.E.M., C.T.R. and A.Z. took part in drafting and critical revision of the manuscript for important intellectual content. G.A.D., L.M.E.M. and C.T.R. obtained funding for the study. S.L., G.H. and S.D.T. provided statistical and technical support. G.A.D. and L.M.E.M. supervised the clinical study.
Funding
The Adelaide SCOPE study was funded by the Premier's Science and Research Fund, Government of South Australia. The Auckland SCOPE study was funded by New Enterprise Research Fund, Foundation for Research Science and Technology; Health Research Council; Evelyn Bond Fund, Auckland District Health Board Charitable Trust. Genotyping and data analyses were funded by a project grant from the National Health and Medical Research Council Australia awarded to CTR and ERL (#565320) and University of Adelaide. CTR is supported by an NHMRC Senior Research Fellowship APP1020749. None of the study sponsors had a role in study design, data analysis and interpretation or in writing this report. Funding to pay the Open Access publication charges for this article was provided by the University of Adelaide.
Conflict of interest
None of the authors have any conflicts of interest to declare.
Acknowledgements
The authors would like to thank the families who participated in the SCOPE study. We would also like to thank Denise Healy and Rennae Taylor for coordinating the Adelaide and Auckland cohorts, respectively. We thank MedSciNet (Sweden), Eliza Chan and SCOPE midwives for support with the database.
References
- Aatola H, Koivistoinen T, Hutri-Kahonen N, Juonala M, Mikkila V, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Lifetime fruit and vegetable consumption and arterial pulse wave velocity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:2521–2528. doi: 10.1161/CIRCULATIONAHA.110.969279. [DOI] [PubMed] [Google Scholar]
- Abdollahi MR, Huang S, Rodriguez S, Guthrie PA, Smith GD, Ebrahim S, Lawlor DA, Day IN, Gaunt TR. Homogeneous assay of rs4343, an ACE I/D proxy, and an analysis in the British Women's Heart and Health Study (BWHHS) Dis Markers. 2008;24:11–17. doi: 10.1155/2008/813679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, Bhansali A, Sud K, Khullar M. ACE variants interact with the RAS pathway to confer risk and protection against type 2 diabetic nephropathy. DNA Cell Biol. 2009;28:141–150. doi: 10.1089/dna.2008.0810. [DOI] [PubMed] [Google Scholar]
- Alkalay AL, Graham JM, Jr., Pomerance JJ. Evaluation of neonates born with intrauterine growth retardation: review and practice guidelines. J Perinatol. 1998;18:142–151. [PubMed] [Google Scholar]
- Alvi FM, Hasnain S. ACE I/D and G2350A polymorphisms in Pakistani hypertensive population of Punjab. Clin Exp Hypertens. 2009;31:471–480. doi: 10.1080/10641960902825479. [DOI] [PubMed] [Google Scholar]
- Anton L, Merrill DC, Neves LA, Diz DI, Corthran J, Valdes G, Stovall K, Gallagher PE, Moorefield C, Gruver C, et al. The uterine placental bed renin–angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150:4316–4325. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Maternal and social origins of hypertension. Hypertension. 2007;50:565–571. doi: 10.1161/HYPERTENSIONAHA.107.091512. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F. The renin angiotensin aldosterone system and pregnancy. Fetal Matern Med Rev. 1992;4:59–71. [Google Scholar]
- Bruandet A, Richard F, Tzourio C, Berr C, Dartigues JF, Alperovitch A, Amouyel P, Helbecque N. Haplotypes across ACE and the risk of Alzheimer's disease: the three-city study. J Alzheimers Dis. 2008;13:333–339. doi: 10.3233/jad-2008-13310. [DOI] [PubMed] [Google Scholar]
- Chung CM, Wang RY, Chen JW, Fann CS, Leu HB, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, et al. A genome-wide association study identifies new loci for ACE activity: potential implications for response to ACE inhibitor. Pharmacogenomics J. 2010;10:537–544. doi: 10.1038/tpj.2009.70. [DOI] [PubMed] [Google Scholar]
- Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl.):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cooper AC, Robinson G, Vinson GP, Cheung WT, Broughton Pipkin F. The localization and expression of the rennin–angiotensin system in the human placenta throughout pregnancy. Placenta. 1999;20:467–474. doi: 10.1053/plac.1999.0404. [DOI] [PubMed] [Google Scholar]
- Davis P, McLeod K, Ransom M, Ongley P, Pearce N, Howden-Chapman P. The New Zealand Socioeconomic Index: developing and validating an occupationally-derived indicator of socio-economic status. Aust N Z J Public Health. 1999;23:27–33. doi: 10.1111/j.1467-842x.1999.tb01201.x. [DOI] [PubMed] [Google Scholar]
- de Courcy-Wheeler RH, Wolfe CD, Warburton F, Goodman J, Reynolds F, Gamsu H. The association between small size for gestational age and perinatal and neonatal death in a UK Regional Health Authority. Paediatr Perinat Epidemiol. 1995;9:431–440. doi: 10.1111/j.1365-3016.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Schouten HJ, Peeters LL. Maternal volume homeostasis in early pregnancy in relation to fetal growth restriction. Obstet Gynecol. 1995;85:361–367. doi: 10.1016/0029-7844(94)00417-C. [DOI] [PubMed] [Google Scholar]
- Elvan-Taspinar A, Franx A, Bots ML, Koomans HA, Bruinse HW. Arterial stiffness and fetal growth in normotensive pregnancy. Am J Hypertens. 2005;18:337–341. doi: 10.1016/j.amjhyper.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Freitas AI, Mendonca I, Brion M, Sequeira MM, Reis RP, Carracedo A, Brehm A. RAS gene polymorphisms, classical risk factors and the advent of coronary artery disease in the Portuguese population. BMC Cardiovasc Disord. 2008;8:15. doi: 10.1186/1471-2261-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–174. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin–angiotensin system. Circulation. 1993;87:1816–1828. doi: 10.1161/01.cir.87.6.1816. [DOI] [PubMed] [Google Scholar]
- Helbecque N, Codron V, Cottel D, Amouyel P. An age effect on the association of common variants of ACE with Alzheimer's disease. Neurosci Lett. 2009;461:181–184. doi: 10.1016/j.neulet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Swaminathan S, Alexander GR, Czernichow P, Collin D, Salihu HM, Kirby RS, Levy-Marchal C. Significant paternal contribution to the risk of small for gestational age. BJOG. 2005;112:153–159. doi: 10.1111/j.1471-0528.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24–October 1, 2001. Pediatrics. 2003;111:1253–1261. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Pringle KG, Conquest A, Hirst JJ, Markus MA, Sarris M, Zakar T, Morris BJ, Lumbers ER. Molecular characterization of renin–angiotensin system components in human intrauterine tissues and fetal membranes from vaginal delivery and cesarean section. Placenta. 2011;32:214–221. doi: 10.1016/j.placenta.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Sayed-Tabatabaei FA, van Popele NM, Asmar R, Schalekamp MA, Hofman A, van Duijn CM, Witteman JC. Angiotensin-converting enzyme gene polymorphism and common carotid stiffness. The Rotterdam study. Atherosclerosis. 2004;174:121–126. doi: 10.1016/j.atherosclerosis.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Jenkins H, Todd B, Clifton VL. Maternal asthma and placental morphometry: effects of severity, treatment and fetal sex. Placenta. 2008;29:366–373. doi: 10.1016/j.placenta.2008.01.011. [DOI] [PubMed] [Google Scholar]
- McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–793. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McCowan LM, North R, Taylor R. Australian New Zealand Clinical Trials Registry. 2007. wwwanzctrorgau/trialSearchaspx . [Google Scholar]
- McCowan LM, North RA, Kho EM, Black MA, Chan EH, Dekker GA, Poston L, Taylor RS, Roberts CT. Paternal contribution to small for gestational age babies: a multicenter prospective study. Obesity (Silver Spring) 2010a;19:1035–1039. doi: 10.1038/oby.2010.279. [DOI] [PubMed] [Google Scholar]
- McCowan LM, Roberts CT, Dekker GA, Taylor RS, Chan EH, Kenny LC, Baker PN, Moss-Morris R, Chappell LC, North RA. Risk factors for small-for-gestational-age infants by customised birthweight centiles: data from an international prospective cohort study. BJOG. 2010b;117:1599–1607. doi: 10.1111/j.1471-0528.2010.02737.x. [DOI] [PubMed] [Google Scholar]
- McKeigue PM, Lithell HO, Leon DA. Glucose tolerance and resistance to insulin-stimulated glucose uptake in men aged 70 years in relation to size at birth. Diabetologia. 1998;41:1133–1138. doi: 10.1007/s001250051042. [DOI] [PubMed] [Google Scholar]
- McKenzie CA, Sinsheimer JS, Adeyemo AA, Cox RD, Southam L, Hugill A, Bouzekri N, Lathrop M, Forrester TE, Cooper RS, et al. SNP haplotypes in the angiotensin I-converting enzyme (ACE) gene: analysis of Nigerian family data using gamete competition models. Ann Hum Genet. 2005;69:227–232. doi: 10.1046/j.1529-8817.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Meng Y, Baldwin CT, Bowirrat A, Waraska K, Inzelberg R, Friedland RP, Farrer LA. Association of polymorphisms in the angiotensin-converting enzyme gene with Alzheimer disease in an Israeli Arab community. Am J Hum Genet. 2006;78:871–877. doi: 10.1086/503687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T, Craven C, Nelson L, Lalouel JM, Ward K. Angiotensinogen T235 expression is elevated in decidual spiral arteries. J Clin Invest. 1997;100:1406–1415. doi: 10.1172/JCI119661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T, Craven C, Ward K. Human spiral artery rennin–angiotensin system. Hypertension. 1998;32:683–687. doi: 10.1161/01.hyp.32.4.683. [DOI] [PubMed] [Google Scholar]
- Morgan T, Craven C, Lalouel JM, Ward K. Angiotensinogen Thr235 variant is associated with abnormal physiologic change of the uterine spiral arteries in first-trimester decidua. Am J Obstet Gynecol. 1999;180:95–102. doi: 10.1016/s0002-9378(99)70156-0. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- Osei-Kumah A, Hodyl N, Clifton VL. Proteomics in asthma. Expert Rev Clin Immunol. 2008;4:713–721. doi: 10.1586/1744666X.4.6.713. [DOI] [PubMed] [Google Scholar]
- Osei-Kumah A, Smith R, Jursca I, Caniggia I, Clifton VL. Sex-specific differences in placental global gene expression in pregnancies complicated by asthma. Placenta. 2011;32:570–578. doi: 10.1016/j.placenta.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Roberts CT. IFPA Award in Placentology Lecture: complicated interactions between genes and the environment in placentation, pregnancy outcome and long term health. Placenta. 2010;31(Suppl.):S47–S53. doi: 10.1016/j.placenta.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–208. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Niederberger M. Paradoxes of body fluid volume regulation in health and disease. A unifying hypothesis. West J Med. 1994;161:393–408. [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Mannucci A, Kimpton CP, Gill P. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques. 1993;15:636–638. 40–1. [PubMed] [Google Scholar]
- Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68:807–813. doi: 10.1016/j.socscimed.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, Pearson C, Ortiz K, Zuckerman B, Bauchner H, et al. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on GxE interactions and pathogenic pathways. Hum Genet. 2008;123:359–369. doi: 10.1007/s00439-008-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahara S, Konoshita T, Mizuno S, Motomura M, Aoyama C, Makino Y, Kato N, Koni I, Miyamori I. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148:2453–2457. doi: 10.1210/en.2006-1287. [DOI] [PubMed] [Google Scholar]
- Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem. 2002;277:24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bouzekri N, Southam L, Cooper RS, Adeyemo A, McKenzie CA, Luke A, Chen G, Elston RC, Ward R. Linkage and association analysis of angiotensin I-converting enzyme (ACE)-gene polymorphisms with ACE concentration and blood pressure. Am J Hum Genet. 2001;68:1139–1148. doi: 10.1086/320104. [DOI] [PMC free article] [PubMed] [Google Scholar]