Abstract
In all mammalian species studied so far, sperm capacitation correlates with an increase in protein tyrosine (Tyr) phosphorylation mediated by a bicarbonate-dependent cAMP/protein kinase A (PKA) pathway. Recent studies in mice revealed, however, that a Src family kinase (SFK)-induced inactivation of serine/threonine (Ser/Thr) phosphatases is also involved in the signaling pathways leading to Tyr phosphorylation. In view of these observations and with the aim of getting a better understanding of the signaling pathways involved in human sperm capacitation, in the present work we investigated the involvement of both the cAMP/PKA and SFK/phosphatase pathways in relation to the capacitation state of the cells. For this purpose, different signaling events and sperm functional parameters were analyzed as a function of capacitation time. Results revealed a very early bicarbonate-dependent activation of PKA indicated by the rapid (1 min) increase in both phospho-PKA substrates and cAMP levels (P < 0.05). However, a complete pattern of Tyr phosphorylation was detected only after 6-h incubation at which time sperm exhibited the ability to undergo the acrosome reaction (AR) and to penetrate zona-free hamster oocytes. Sperm capacitated in the presence of the SFK inhibitor SKI606 showed a decrease in both PKA substrate and Tyr phosphorylation levels, which was overcome by exposure of sperm to the Ser/Thr phosphatase inhibitor okadaic acid (OA). However, OA was unable to induce phosphorylation when sperm were incubated under PKA-inhibitory conditions (i.e. in the absence of bicarbonate or in the presence of PKA inhibitor). Moreover, the increase in PKA activity by exposure to a cAMP analog and a phosphodiesterase inhibitor did not overcome the inhibition produced by SKI606. Whereas the presence of SKI606 during capacitation produced a negative effect (P < 0.05) on sperm motility, progesterone-induced AR and fertilizing ability, none of these inhibitions were observed when sperm were exposed to SKI606 and OA. Interestingly, different concentrations of inhibitors were required to modulate human and mouse capacitation revealing the species specificity of the molecular mechanisms underlying this process. In conclusion, our results describe for the first time the involvement of both PKA activation and Ser/Thr phosphatase down-regulation in functional human sperm capacitation and provide convincing evidence that early PKA-dependent phosphorylation is the convergent regulatory point between these two signaling pathways.
Keywords: capacitation, human sperm, phosphatase, PKA, signaling pathway
Introduction
Reversible phosphorylation of proteins has a crucial role for proper cellular functioning in eukaryotic organisms, providing an efficient and rapid system to initiate or cease a biological response. This mechanism also operates in sperm cells, which lack the transcription and translation machineries and thus, mainly rely on post-translational modifications such as protein phosphorylation to control maturational processes.
During the last years, the phosphorylation of proteins in tyrosine (Tyr) residues has emerged as a key mechanism involved in the mandatory process by which mammalian sperm become competent to fertilize the oocyte. This process known as capacitation (Chang, 1951; Austin, 1952) involves a series of changes in both the head and tail of sperm while they transit through the female reproductive tract, and prepares the cells to undergo the acrosome reaction (AR), express hyperactivated motility and fertilize an oocyte. Although an increase in Tyr phosphorylation of proteins during sperm capacitation has been described in all the mammalian species studied so far (Visconti et al., 1995a; Leclerc et al., 1996; Galantino-Homer et al., 1997; Osheroff et al., 1999; Visconti et al., 1999; Da Ros et al., 2004), the molecular mechanisms controlling this time-precise process are still under investigation. Research in the field sheds light on sperm minimal requirements to support in vitro capacitation with molecules such as bicarbonate, calcium and albumin being crucial for this process. Sperm entering the female reproductive tract are exposed to high concentrations of bicarbonate, which directly stimulate a testis-specific soluble adenylyl cyclase (Adcyc10, also known as sAC; Chen et al., 2000), shown to be essential for sperm motility and male fertility (Esposito et al., 2004; Hess et al., 2005; Xie et al., 2006). It is well accepted that cAMP, produced as a consequence of a counterbalance between sAC and phosphodiesterases (PDEs), stimulates protein kinase A (PKA), which phosphorylates proteins in serine/threonine (Ser/Thr) residues. Ablation of the PKA catalytic subunit (PKA-Cα2) produces mouse sterility with a broader phenotype than sAC null animals, suggesting the involvement of PKA in additional stages of sperm production and maturation (Nolan et al., 2004). As a consequence of the activation of this bicarbonate-dependent cAMP/PKA signaling pathway, a set of proteins is phosphorylated in Tyr residues (Visconti et al., 1995b). In this regard, non-receptor Tyr kinases belonging to different families such as ABL, CSK, SRC or TEC have been identified in mouse (Baker et al., 2006; Baker et al., 2009; Kierszenbaum et al., 2009), bull (Lalancette et al., 2006) and human (Naz, 1998; Mitchell et al., 2008) sperm. Specifically, c-SRC, a member of the Src family kinase (SFK) has been postulated as one of the intermediate Tyr kinases promoting capacitation-associated Tyr phosphorylation in human sperm (Lawson et al., 2008; Mitchell et al., 2008; Varano et al., 2008). However, a recent study in mouse demonstrated that SFK members are not directly involved in the observed capacitation-associated increase in Tyr phosphorylation but rather participate the upstream of this process by regulating the activity of Ser/Thr phosphatases (Krapf et al., 2010). The Ser/Thr phosphatases described so far in sperm are those belonging to the families PP1γ (Smith et al., 1996), PP2A (Vijayaraghavan et al., 1996) and the Ca2+/calmodulin-dependent PP2B (Tash et al., 1988). Whereas the roles of PP2A and PP2B in sperm physiology are still under investigation, PP1γ is essential for mouse fertility as PP1γ null males exhibit impaired spermatogenesis (Varmuza et al., 1999).
Based on these observations, in the present work we investigated the signaling pathways involved in human sperm capacitation. We present evidence showing that both the cAMP/PKA and SFK/phosphatase pathways are required for functional human sperm capacitation and that PKA-induced Ser/Thr phosphorylation is the convergent regulatory point between these two signaling pathways. In addition, the analysis of the temporal correlation between these signaling events and sperm function parameters revealed that in spite of a very early PKA activation, human sperm need to undergo other late signaling events in order to reach a functional capacitation state.
Materials and Methods
Culture media
The media used in this study contains 25 mM NaHCO3 (Sigma-Aldrich Co., St. Louis, MO, USA), 1.7 mM Cl2Ca (Sigma), 10 mM HEPES (Sigma) and 2.6% p/v bovine serum albumin (BSA; Sigma), and was described by Biggers, Whitten and Whittingham (BWW; Biggers et al., 1971). For some experiments, BWW medium was prepared without the addition of bicarbonate or with 3.5% p/v HSA (Sigma) instead of BSA. In all the cases, pH was adjusted to 7.2–7.4.
Chemical compounds
A cAMP analog (dbcAMP: N6, 2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate; Sigma) and an inhibitor of PDE (IBMX: 3-isobutyl-1-methylxanthine; Sigma) were used for PKA activation. The following compounds were used to inhibit specific enzymatic activity: KH7 (Cayman Chemical Co., Ann Arbor, MI, USA) for sAC, H89 (Calbiochem, EMD Biosciences Inc., La Jolla, CA, USA) for PKA, SKI606 (Bosutinib; Selleck Chemical, Houston, TX, USA) for SFKs and okadaic acid (OA; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for Ser/Thr phosphatases.
Ethical approval
The study protocol was approved by the Bioethics Committee of the Institute of Biology and Experimental Medicine from the National Research Council (CONICET, Buenos Aires, Argentina), and by the Institutional Review Board of Amherst Faculty from the University of Massachusetts. Human donors were provided with written information about the study prior to giving an informed consent. Experiments involving animals were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the NIH.
Human sperm capacitation
Experiments involved the use of 60 semen samples obtained from 20 healthy donors (aged 20–35) with no known fertility problems. Semen samples were obtained by masturbation into a sterile plastic container after 2–3 days of sexual abstinence and analyzed following World Health Organization recommendations (WHO, 2010). The semen parameters (total fluid volume, sperm concentration, motility and morphology) of all the samples fell within the WHO normality criteria. After complete liquefaction, semen samples were processed as previously described (Cohen et al., 2001). Briefly, sperm ejaculates were allowed to swim-up in a bicarbonate-free BWW medium and motile spermatozoa were then incubated in either bicarbonate-free or bicarbonate-containing BWW media at 37°C for different time periods. Sperm suspensions incubated in media with bicarbonate were maintained in 5% CO2 in air, whereas those incubated without the anion were placed in air tight vials to avoid the production of bicarbonate from the CO2 of the air.
To evaluate the relevance of kinases or phosphatases for capacitation, motile sperm were pre-incubated for 10 min with different enzyme inhibitors in bicarbonate-free media and then incubated in capacitating media containing the same inhibitors. The effect of the inhibitors on sperm viability was assessed by dye exclusion using 0.5% (v/v) Eosin Y (Sigma) and the percentage of viable sperm was calculated as the number of sperm that did not incorporate the dye over the total number of sperm counted under the light microscope (×400).
At the end of each incubation, sperm were recovered and analyzed for either PKA substrate phosphorylation, cAMP content, Tyr phosphorylation, motility, acrosomal status or their ability to penetrate zona-free hamster oocytes.
Protein extracts and western blotting analysis
Total proteins from 1 × 106 spermatozoa were solubilized in Laemmli sample buffer (Laemmli, 1970) and the phosphorylated proteins were assessed by SDS–PAGE and western blot as previously described (Da Ros et al., 2004). The monoclonal primary antibodies used were the anti-phospho-PKA substrate (anti-pPKAs; 1:1000; clone 100G7E, Cell Signaling, Danvers, MA, USA) or anti-phospho-Tyr (anti-pTyr; 1:3000; clone 4G10, Millipore Corporation, Temecula, CA, USA) and the corresponding secondary antibodies were coupled to peroxidase (1:4000; Vector Laboratories, Burlingame, CA, USA). In all the cases, the reactive bands were detected by enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA) and the blotted membranes were stripped (100 mM β-mercaptoethanol, 2% SDS, 62.5% Tris pH 6.7, 15–30 min at 50°C) and subjected to western blot using a different primary antibody. Loading control was confirmed by β-tubulin immunoblot using the specific anti-β-tubulin antibody (1:5000; clone D66, Sigma).
Indirect immunofluorescence
Human sperm were fixed in 2% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature, thoroughly washed with PBS, and then incubated with FACS™ permeabilizing solution (BD Biosciences, San Jose, CA, USA) for 10 min. Sperm were then exposed to an anti-pPKAs antibody (1:40 in PBS) or a purified rabbit IgG (control) for 1 h at 4°C, washed three times in PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:100 in PBS; Sigma) for 30 min at room temperature. Finally, sperm were air dried on poly-l-lysine (0.01 mg/ml; Sigma) coated slides, mounted in 90% (v/v) glycerol in PBS and examined under a Nikon Optiphot microscope (Nikon, Tokyo, Japan) equipped with epifluorescence optics (×1250). A minimum of 400 cells were analyzed in each sample.
Determination of cAMP content
Intracellular sperm cAMP concentrations were determined using a PKA radioactivity assay. Sperm samples were lysed with Triton X-100 buffer (40 mM HEPES, pH 7.4, 0.1 mM IBMX, EDTA-free protease inhibitor cocktail and 1% Triton X-100), boiled for 10 min to inactivate sperm enzymes, centrifuged for 5 min at 8000g and the supernatants used for determination of cAMP. PKA activity was measured as previously described (Visconti et al., 1997). Briefly, the amount of 32P incorporated into the Kemptide (Leu–Arg–Arg–Ala–Ser–Leu–Gly; Sigma) specific substrate was quantified and cAMP concentrations calculated based on standard curves (dbcAMP concentration versus PKA activity) made for each independent experiment.
Computer-assisted sperm analysis
Sperm suspensions were loaded on chamber slides with a depth of 20 µm (Leja Slide, Spectrum Technologies, Healdsburg, CA, USA) pre-warmed at 37°C. Sperm movements were examined at 37°C using the CEROS computer-assisted sperm analysis (CASA) system (Hamilton Thorne, Inc., Beverly, MA, USA; Mortimer et al., 1998). At least, 20 microscopy fields corresponding to a minimum of 200 sperm were analyzed (30 frames acquired at 60 Hz for each measurement) per sample. The following parameters were measured: average path velocity (VAP, µm/s), curvilinear velocity (VCL, µm/s), straight line velocity (VSL, µm/s), linearity (LIN, %), amplitude of lateral head displacement (ALH, µm) and straightness (STR, %).
AR assays
Human sperm were exposed to different concentrations of progesterone (Sigma) 30 min before the end of incubation for the assessment of acrosomal status as previously described (Cohen et al., 2001). Briefly, sperm were fixed in 1% (w/v) paraformaldehyde in PBS, methanol permeabilized, stained with FITC-labeled Pisum sativum agglutinin (PSA; Sigma) and observed under a Nikon Optiphot microscope equipped with epifluorescence optics (×1250). Sperm were scored as acrosome intact when a bright staining was observed in the acrosome, or as acrosome reacted when either fluorescent staining was restricted to the equatorial segment or no labeling was observed.
Zona-free hamster oocyte penetration test
Hamster oocyte penetration test (HOPT) was performed as previously described (Cohen et al., 2001). Briefly, cumulus–oocyte complexes were collected from superovulated immature hamster (Mesocricetus aureatus) females maintained with food and water ad libitum in a temperature-controlled room with 14:10 light:dark cycle. The collected cumulus were treated with hyaluronidase and trypsin (Sigma) to remove cumulus cells and the zona pellucida, respectively, and zona-free oocytes thoroughly washed in capacitation medium and finally distributed among treatment groups.
Drops containing 15–20 zona-free hamster oocytes were inseminated with 3 × 105 human motile sperm and gametes co-incubated for 2.5 h at 37°C in an atmosphere of 5% CO2. The oocytes were then thoroughly washed, fixed in 2.5% glutaraldehyde, mounted on slides, stained with 1% aceto-carmine solution and observed under the microscope (×400). The number of oocytes presenting either decondensing sperm heads or pronuclei and sperm tails in the ooplasm were recorded.
Calculations and statistical analysis
The percentages of sperm viability, acrosome-reacted sperm and penetrated oocytes were analyzed by the χ2 test. The time-dependent AR levels were analyzed by one-way analysis of variance. Cyclic AMP levels and motility values were analyzed by the Student's t-test. Calculations were performed using the Prism 3.0 software (GraphPad Software, La Jolla, CA, USA). ‘n’ refers to the number of independent experiments carried out using different donors in each case. Results were considered to be significantly different at P < 0.05.
Results
Temporal correlation between PKA-dependent signaling events and the sperm functional state
As a first approach to investigate the signaling pathways involved in human sperm capacitation, we performed a series of studies aimed to further characterize the cAMP/PKA pathway leading to Tyr phosphorylation. These studies were conducted using a wide range of incubation times (1 min–18 h) in order to investigate the temporal correlation between signaling events and the functional capacitation state of human cells.
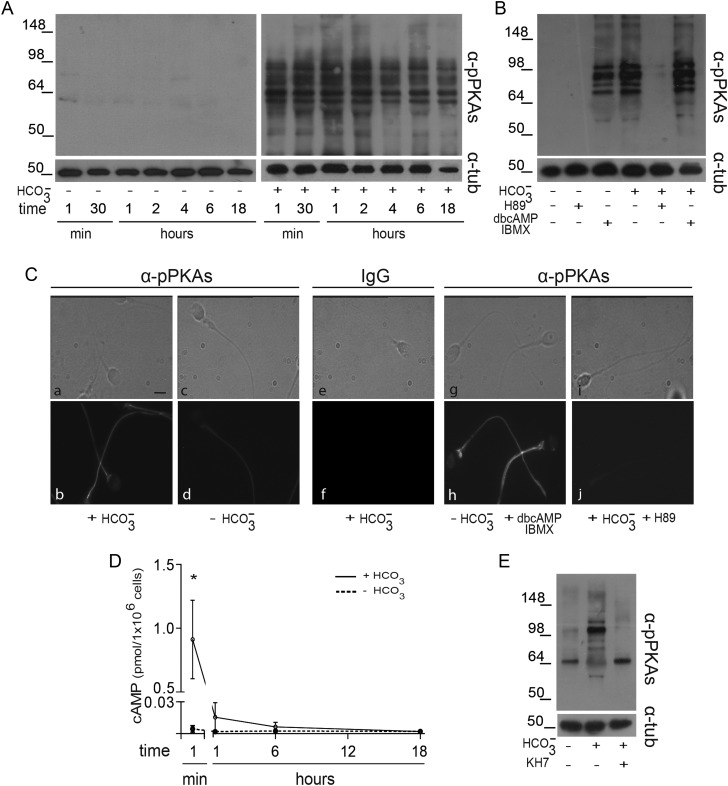
PKA activation was studied through the analysis of specific substrate phosphorylation by western blot using an anti-pPKAs antibody that recognizes the consensus PKA-phosphorylated motif (Arg-Arg-X-pSer/pThr). Whereas sperm incubated in the absence of bicarbonate did not show phosphorylation of PKA substrates at any time assayed (Fig. 1A, left panel), those incubated in a bicarbonate-containing medium exhibited numerous reactive bands (with a molecular weight <100 kDa), as early as 1-min incubation (Fig. 1A, right panel). This phosphorylation was specific for PKA as judged by the facts that exposure of sperm to both dbcAMP and IBMX-induced phosphorylation in the absence of bicarbonate, and inhibition of PKA activity by H89 prevented the bicarbonate-induced phosphorylation (Fig. 1B).
Figure 1.
Evaluation of PKA activity during human sperm capacitation. (A) Sperm were incubated in media with (right panel) or without (left panel) 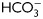 for different time periods (1–18 h). Aliquots were removed at different intervals and sperm proteins were analyzed for PKA substrate phosphorylation by western blotting using α-pPKAs as the first antibody. β-tubulin was used as control of loading (n = 8). (B) Sperm were incubated for 18 h in media with or without
for different time periods (1–18 h). Aliquots were removed at different intervals and sperm proteins were analyzed for PKA substrate phosphorylation by western blotting using α-pPKAs as the first antibody. β-tubulin was used as control of loading (n = 8). (B) Sperm were incubated for 18 h in media with or without 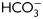 and containing either H89 (30 µM) or dbcAMP/IBMX (5 mM/0.2 mM). Protein extracts were analyzed for PKA substrate phosphorylation by western blotting (n = 5). (C) Phase-contrast (upper) and fluorescent (bottom) images of sperm incubated for 1 min in media with (a, b, e, f) or without (c, d)
and containing either H89 (30 µM) or dbcAMP/IBMX (5 mM/0.2 mM). Protein extracts were analyzed for PKA substrate phosphorylation by western blotting (n = 5). (C) Phase-contrast (upper) and fluorescent (bottom) images of sperm incubated for 1 min in media with (a, b, e, f) or without (c, d) 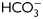 , in media without
, in media without 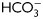 but containing dbcAMP and IBMX (g, h), or in complete media with H89 (i, j). The IIF was performed using α-pPKAs (a–d, g–j) or purified IgG as first antibodies (e, f) (n = 4). The scale bar is 5 μm. The same images were observed for sperm incubated for 1 or 18 h. (D) Cell extracts from sperm incubated in media with (solid line) or without (dotted line)
but containing dbcAMP and IBMX (g, h), or in complete media with H89 (i, j). The IIF was performed using α-pPKAs (a–d, g–j) or purified IgG as first antibodies (e, f) (n = 4). The scale bar is 5 μm. The same images were observed for sperm incubated for 1 or 18 h. (D) Cell extracts from sperm incubated in media with (solid line) or without (dotted line) 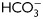 for different times (1 min to 18 h) were analyzed for total cAMP intracellular levels. Values represent the mean ± SEM of four independent experiments. *P < 0.05. (E) Sperm were incubated in media with or without
for different times (1 min to 18 h) were analyzed for total cAMP intracellular levels. Values represent the mean ± SEM of four independent experiments. *P < 0.05. (E) Sperm were incubated in media with or without 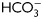 in the absence or presence of KH7 (75 µM), and aliquots were removed at 1-min incubation and analyzed for PKA substrate phosphorylation by western blotting (n = 3).
in the absence or presence of KH7 (75 µM), and aliquots were removed at 1-min incubation and analyzed for PKA substrate phosphorylation by western blotting (n = 3).
Indirect immunofluorescence (IIF) assays carried out in cells fixed at different incubation times (1 min, 1 or 18 h) and then exposed to the anti-pPKAs antibody revealed that most (82%) sperm incubated under capacitating conditions for 1 min already exhibited a clear fluorescent labeling in the flagellum (Fig. 1C, a, b), which did not differ from that observed in sperm incubated for longer periods (data not shown). In contrast, a faint labeling was observed in most (81%) sperm incubated under non-capacitating conditions (Fig. 1C, c, d), and no fluorescence was detected in control capacitated sperm incubated with only purified IgG as the first antibody (Fig. 1C, e, f). In agreement with western blotting observations, sperm incubated in the absence of bicarbonate but exposed to dbcAMP and IBMX exhibited fluorescent labeling in the tail (Fig. 1C, g, h), whereas those incubated under capacitating conditions in the presence of H89 were unlabeled (Fig. 1C, i, j).
As another approach to study PKA activation, the kinetics of cAMP steady-state levels was evaluated by using a PKA radioactivity assay. As expected, whereas sperm incubated under non-capacitating conditions contained undetectable levels of cAMP at all the time points assayed (Fig. 1D), those incubated in a bicarbonate-containing medium showed high levels of cAMP at only 1 min of incubation (Fig. 1D). Interestingly, under these conditions, cAMP levels markedly declined within the first hour and remained at this level for the rest of the capacitation period (Fig. 1D). The early (1 min) cAMP increase seems to be due to sAC activation as indicated by the fact that exposure of sperm to sAC inhibitor KH7 led to both undetectable cAMP levels (data not shown) and lack of PKA-induced phosphorylation (Fig. 1E).
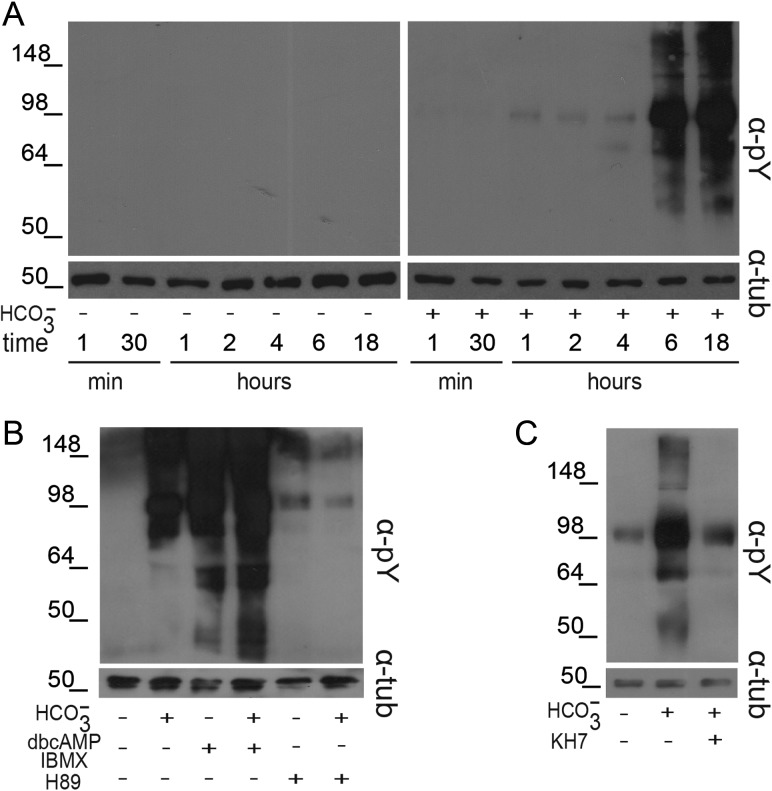
To study the downstream Tyr phosphorylation event, the same protein extracts prepared for PKA studies were subjected to western blot using an anti-pTyr antibody. As shown in Fig. 2A, whereas the absence of bicarbonate in the media prevented Tyr phosphorylation at all the time points assayed (left panel), sperm incubated under capacitating conditions began to show Tyr phosphorylation signals at 1 h, which increased as a function of time reaching maximum levels at 6-h incubation (right panel). In agreement with previous reports, Tyr phosphorylation was exacerbated by exposure of sperm to dbcAMP and IBMX and it was clearly inhibited when H89 was present in the media (Fig. 2B). A significant decrease in Tyr phosphorylation was also observed when sperm were exposed to KH7, indicating the contribution of sAC to protein Tyr phosphorylation during capacitation (Fig. 2C).
Figure 2.
Analysis of Tyr phosphorylation during human sperm capacitation. (A) Sperm were incubated in media with (right panel) or without (left panel) 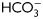 for different time periods (1–18 h). Aliquots were removed at different intervals and sperm proteins were analyzed for Tyr phosphorylation by western blotting using α-pY as the first antibody. β-tubulin was used as control of loading (n = 8). (B) Sperm were incubated for 18 h in media with or without
for different time periods (1–18 h). Aliquots were removed at different intervals and sperm proteins were analyzed for Tyr phosphorylation by western blotting using α-pY as the first antibody. β-tubulin was used as control of loading (n = 8). (B) Sperm were incubated for 18 h in media with or without 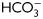 and containing either H89 (30 µM) or dbcAMP/IBMX (5 mM/0.2 mM). Protein extracts were analyzed for Tyr phosphorylation by western blotting (n = 6). (C) Sperm were incubated for 18 h in media with or without
and containing either H89 (30 µM) or dbcAMP/IBMX (5 mM/0.2 mM). Protein extracts were analyzed for Tyr phosphorylation by western blotting (n = 6). (C) Sperm were incubated for 18 h in media with or without 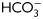 in the absence or presence of KH7 (75 µM), and aliquots were removed at the end of incubation and analyzed for Tyr phosphorylation by western blotting (n = 3).
in the absence or presence of KH7 (75 µM), and aliquots were removed at the end of incubation and analyzed for Tyr phosphorylation by western blotting (n = 3).
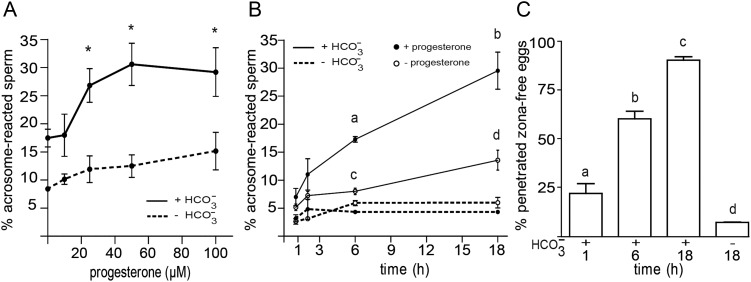
To study the temporal correlation between the described cAMP/PKA signaling events and the functional capacitation state of human sperm, the ability of the cells to undergo the spontaneous and the progesterone-induced AR as well as to fertilize the oocyte was examined as a function of the capacitation time. As different progesterone concentrations have been reported to induce human AR under different capacitating conditions (Bedu-Addo et al., 2005; Varano et al., 2008; Sagare-Patil et al., 2012), we first evaluated the percentage of AR as a function of progesterone concentration under our experimental conditions. Results revealed that 25 µM was the minimal concentration of hormone required for inducing a significant increase in the percentage of acrosome-reacted cells (Fig. 3A). At this concentration, the kinetics of progesterone-induced AR significantly increased at 6 h and continued increasing during overnight incubation (Fig. 3B). Although with significantly lower values, a similar kinetics of response was observed for the spontaneous AR, which also reached levels significantly higher than the controls at 6-h incubation (Fig. 3B). As expected, in the absence of bicarbonate in the medium, baseline levels for both spontaneous and progesterone-induced AR were observed throughout incubation (Fig. 3A, B).
Figure 3.
Evaluation of the sperm functional state during capacitation. (A) Sperm were incubated for 18 h in media with (solid lines) or without (dotted lines) 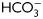 , exposed to different concentrations of progesterone (1–100 µM) during the last 30 min of incubation, and evaluated for the occurrence of the AR by staining sperm with FITC-PSA. Results represent the mean value ± SEM of three independent experiments. *P < 0.01 versus all concentrations assessed. (B) Sperm were incubated for different times (1–18 h) in media with (solid lines) or without (dotted lines)
, exposed to different concentrations of progesterone (1–100 µM) during the last 30 min of incubation, and evaluated for the occurrence of the AR by staining sperm with FITC-PSA. Results represent the mean value ± SEM of three independent experiments. *P < 0.01 versus all concentrations assessed. (B) Sperm were incubated for different times (1–18 h) in media with (solid lines) or without (dotted lines) 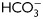 , exposed to progesterone (25 µM) (full circle) or vehicle (0.05% v/v DMSO) (open circle) during the last 30 min of incubation, and evaluated for the occurrence of AR by staining sperm with FITC-PSA. Results represent the mean ± SEM of three independent experiments. a, b, d versus all time assessed, P < 0.05; c versus all times assessed except 2 h, P < 0.05. (C) Sperm were incubated in media with or without
, exposed to progesterone (25 µM) (full circle) or vehicle (0.05% v/v DMSO) (open circle) during the last 30 min of incubation, and evaluated for the occurrence of AR by staining sperm with FITC-PSA. Results represent the mean ± SEM of three independent experiments. a, b, d versus all time assessed, P < 0.05; c versus all times assessed except 2 h, P < 0.05. (C) Sperm were incubated in media with or without 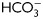 for different time periods (1, 6 or 18 h), co-incubated with zona-free hamster oocytes in capacitating media for 2.5 h, and the percentage of penetrated oocytes determined. Results represent the mean ± SEM of three independent experiments. Bars with different letters are significantly different, P < 0.05.
for different time periods (1, 6 or 18 h), co-incubated with zona-free hamster oocytes in capacitating media for 2.5 h, and the percentage of penetrated oocytes determined. Results represent the mean ± SEM of three independent experiments. Bars with different letters are significantly different, P < 0.05.
To test the development of human sperm fertilizing ability during capacitation, sperm incubated under capacitating conditions for different periods of times (1, 6 or 18 h) were co-incubated with zona-free hamster oocytes for additional 2.5 h, and the percentage of fertilized oocytes was determined. Results showed that whereas sperm incubated for 1 h were capable of penetrating a low percentage of oocytes comparable with that observed for non-capacitated cells, those incubated for 6 h already exhibited high fertilization rates (60%), which further increased (90%) for overnight incubated cells (Fig. 3C).
Together, comparison of the kinetics of cAMP steady-state levels, PKA-dependent phosphorylation, Tyr phosphorylation, induction of the AR and sperm fertilizing ability indicates that in spite of the early onset (1 min) of cAMP and PKA-mediated phosphorylation, human sperm may require at least 6 h of incubation under capacitating conditions to reach a functional capacitation state.
Involvement of SFK/phosphatase signaling pathway during human sperm capacitation
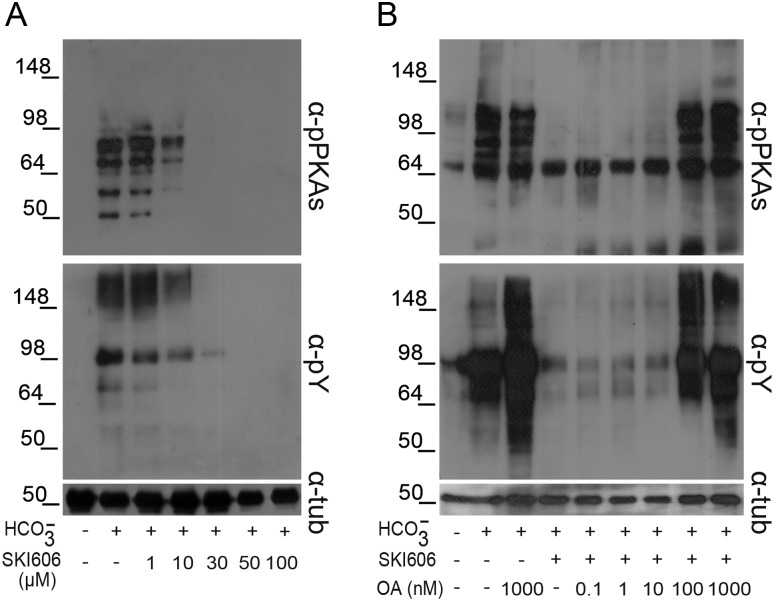
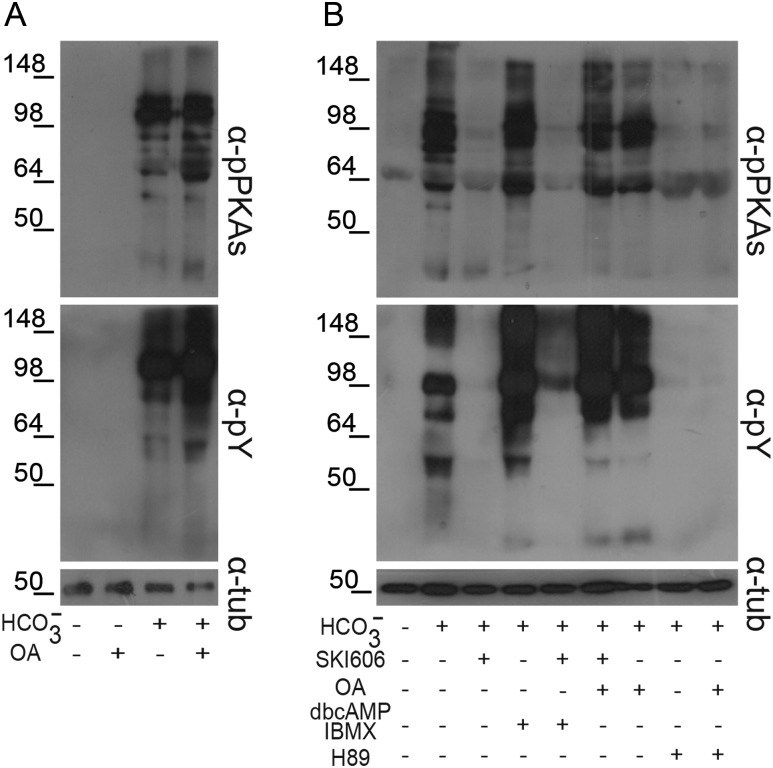
To further investigate the signaling events leading to human sperm capacitation, the involvement of SFK members during this process was investigated by analyzing the phosphorylation of both PKA substrates and Tyr residues in the presence or absence of SKI606, a specific SFK inhibitor (Bantscheff et al., 2007). Protein extracts were obtained from sperm incubated overnight under capacitating conditions to ensure that a high proportion of the cells had already attained a functional capacitation state. Western blotting results showed that both PKA-dependent and Tyr phosphorylations negatively correlated with increasing concentrations of SKI606 (Fig. 4A) without affecting sperm viability (control: 84.6% ± 3.5; 30 μM SKI606: 79.4% ± 4.9, NS, n = 4). Interestingly, the IC50 for this inhibitor (∼10 µM) was five times lower than the one observed for mouse sperm (Krapf et al., 2010).
Figure 4.
Effect of SFK and Ser/Thr phosphatase inhibitors on both PKA substrate and Tyr phosphorylations. Sperm were incubated for 18 h under capacitating conditions in the presence of either different concentrations of SKI606 (1–100 µM; n = 7) (A) or both SKI606 (30 µM) and different concentrations (0.1–1000 nM) of OA (n = 5) (B). In all the cases, sperm protein extracts were analyzed for phosphorylation by western blotting using α-pPKAs or α-pY as first antibodies.
To investigate whether the participation of SFKs in human sperm capacitation was mediated by the inactivation of Ser/Thr phosphatases as previously observed for murine sperm (Krapf et al., 2010), both PKA substrate and Tyr phosphorylations were analyzed by capacitating sperm in the presence of 30 μM SKI606 and increasing concentrations of OA, a well-known specific inhibitor of Ser/Thr phosphatases (Ishihara et al., 1989). Differently from the very low (0.1 nM) OA concentration needed to prevent the SKI606 inhibitory effects in mice, exposure of human sperm to 100 nM OA was required to overcome the decrease in both PKA substrate and Tyr phosphorylations produced by SKI606 (Fig. 4B).
Overall, data suggest that although the signaling pathways controlling mouse and human sperm capacitation are conserved, the identity of the SFK members and Ser/Thr phosphatases involved are likely to be different in both species.
Cross talk between cAMP/PKA and SFK/phosphatase signaling pathways
In order to identify the potential convergent regulatory point between the cAMP/PKA and SFK/phosphatase signaling pathways, sperm were incubated under capacitating conditions in which one of the two pathways were down-regulated, and then both PKA substrate and Tyr phosphorylations were analyzed. Results revealed that inhibition of the cAMP/PKA pathway by incubating sperm in either the absence of bicarbonate (Fig. 5A) or in the presence of H89 (Fig. 5B) in the medium, abrogated both PKA substrate and Tyr phosphorylations regardless of the activation of the SFK/phosphatase pathway by OA-mediated down-regulation of Ser/Thr phosphatases. Similarly, inactivation of the SFK/phosphatase pathway by SKI606 prevented both PKA-dependent and Tyr phosphorylations even when the cAMP/PKA pathway was activated by addition of dbcAMP and IBMX (Fig. 5B). These data indicate that the activation of both cAMP/PKA and SFK/phosphatase signaling pathways are necessary for human sperm capacitation-associated Tyr phosphorylation and that the crossroads between both pathways is located at the PKA-induced phosphorylation level.
Figure 5.
Cross talk between the cAMP/PKA and the SFK/phosphatase signaling pathways. (A) Sperm were incubated for 18 h in media with or without 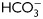 in the absence or presence of OA (100 nM) and sperm extracts were analyzed by western blotting using α-pPKAs or α-pY as first antibodies (n = 4). (B) Sperm were incubated for 18 h in media with or without
in the absence or presence of OA (100 nM) and sperm extracts were analyzed by western blotting using α-pPKAs or α-pY as first antibodies (n = 4). (B) Sperm were incubated for 18 h in media with or without 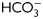 in the presence or absence of SKI606 (30 µM), OA (100 nM), dbcAMP/IBMX (5 mM/0.2 mM) or H89 (30 µM). At the end of incubation, sperm extracts were analyzed by western blotting using α-pPKAs or α-pY as first antibodies (n = 4).
in the presence or absence of SKI606 (30 µM), OA (100 nM), dbcAMP/IBMX (5 mM/0.2 mM) or H89 (30 µM). At the end of incubation, sperm extracts were analyzed by western blotting using α-pPKAs or α-pY as first antibodies (n = 4).
Relevance of the SFK/phosphatase pathway for sperm function
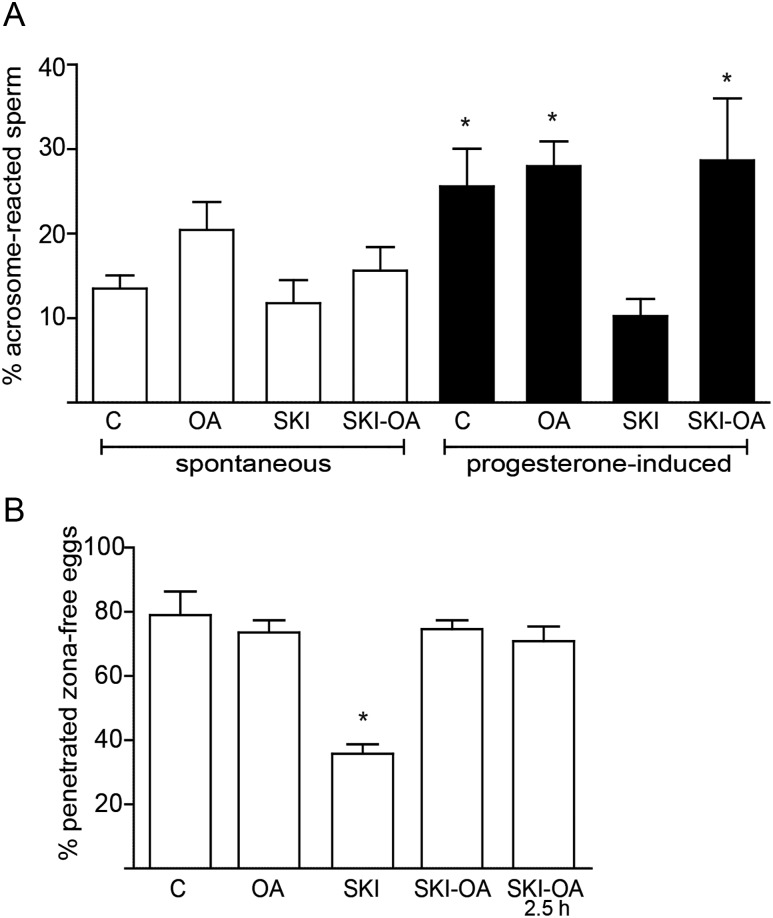
To evaluate the relevance of the SFK/phosphatase pathway for human sperm function, sperm were capacitated for 18 h in the presence of SKI606 and/or OA, and different functional parameters such as sperm motility and sperm ability to undergo the AR and penetrate zona-free hamster oocytes were analyzed. Motility assessment by CASA (Table I) revealed that sperm capacitated in the presence of SKI606 exhibited a clear decrease in all the motility parameters evaluated (i.e. VAP, VCL, VSL, ALH, LIN and STR), reaching the levels observed for non-capacitated sperm. Exposure of the cells to both SKI606 and OA prevented the decrease in all the motility parameters assayed.
Table I.
Relevance of SFK/phosphatase pathway for sperm motility.
| VAP | VCL | VSL | ALH | LIN | STR | |
|---|---|---|---|---|---|---|
| NC | 28.5 ± 5.0* | 57.0 ± 6.9* | 19.0 ± 5.0* | 3.6 ± 0.5 | 21.3 ± 4.4* | 61.5 ± 5.7* |
| C | 55.0 ± 1.8 | 83.0 ± 3.7 | 48.3 ± 1.4 | 4.5 ± 0.1 | 54.3 ± 1.3 | 85.0 ± 1.7 |
| OA | 40.2 ± 6.0 | 66.3 ± 5.7 | 34.1 ± 5.7 | 4.7 ± 0.8 | 46.5 ± 4.9 | 77.8 ± 3.1 |
| SKI | 23.7 ± 2.2* | 50.0 ± 2.6* | 14.5 ± 2.1* | 3.8 ± 0.1* | 28.5 ± 2.9* | 59.3 ± 2.4* |
| SKI-OA | 40.1 ± 4.7 | 65.9 ± 5.2 | 34.2 ± 5.0 | 3.9 ± 0.3 | 47.8 ± 4.3 | 78.3 ± 3.3 |
CASA analysis was performed on sperm incubated under non-capacitating (NC) or capacitating conditions in the absence (C) or presence of 30 μM SKI606 and/or 100 nM OA (SKI, OA or SKI-OA) for 18 h. Values represent the mean ± SEM of four independent experiments. VAP, average path velocity; VCL, curvilinear velocity; VSL, straight line velocity; ALH, amplitude of lateral head displacement; LIN, linearity; STR, straightness.
*versus C, P < 0.05.
Whereas no significant differences among treatments were observed for the spontaneous AR rates (Fig. 6A), sperm exposed to SKI606 did not exhibit the ability to acrosome react in response to progesterone induction as observed for control sperm or sperm incubated with both SKI606 and OA (Fig. 6A). The ability of human sperm to penetrate zona-free hamster oocytes was also significantly reduced by the presence of SKI606 during capacitation and showed levels not different from the controls when sperm were incubated in a medium containing both SKI606 and OA (Fig. 6B). Normal oocyte penetration levels were observed when these compounds were added only during the gamete co-incubation period (2.5 h), indicating that the effects produced by the inhibitors were exerted at the capacitation stage (Fig. 6B). Altogether, these observations support the functional relevance of the SFK/phosphatase pathway for human sperm capacitation.
Figure 6.
Functional relevance of the SFK/phosphatase pathway. Sperm were capacitated for 18 h in control media (C) or in media containing either OA (100 nM), SKI606 (30 µM) or both inhibitors together (SKI-OA). (A) Spontaneous and progesterone (25 µM)-induced AR were evaluated by staining sperm with FITC-PSA. Results represent the mean ± SEM of four independent experiments. No significant differences among groups were observed in spontaneous AR levels. *P < 0.05. (B) Sperm were incubated under capacitating conditions in control media (C) or in media containing either SKI606 (30 µM), OA (100 nM) or both inhibitors (SKI-OA) for 18 h, then co-incubated with zona-free hamster oocytes in fresh capacitating media for 2.5 h, and finally the percentage of penetrated oocytes determined. As a control, sperm were capacitated in control media and then co-incubated with zona-free oocytes in the presence of the inhibitors for 2.5 h (SKI-OA/2.5 h). Results represent the mean ± SEM of three independent experiments. *P < 0.05.
Discussion
Sperm capacitation is characterized by a series of biochemical and physiological changes requiring transmembrane and intracellular signal transduction. With the aim of getting a better understanding of the molecular mechanisms involved in this key reproductive process, in the present work we investigated the signaling pathways operating during human sperm capacitation and their temporal correlation with the functional state of the cells.
As described for all the mammalian species studies so far (Visconti et al., 2011), human capacitation also involves a cAMP/PKA-dependent induction of protein Tyr phosphorylation (Leclerc et al., 1996; Osheroff et al., 1999). However, due to the high heterogeneity of incubation media and times employed to achieve human sperm capacitation (O'Flaherty et al., 2004; Brenker et al., 2012; Orta et al., 2012; Itach et al., 2012; Li et al., 2013), there is no clear information on the temporal occurrence of the described signaling events. Thus, as a first approach to this question, we examined the kinetics of PKA activation by analyzing PKA substrate phosphorylation during a wide range of incubation times (1 min to 18 h) and using a medium known to support capacitation and the development of sperm fertilizing ability (Cohen et al., 2001; and present work). Results from both western blot and IIF assays revealed an immediate (1 min) bicarbonate-dependent increase in PKA-induced phosphorylation that remained constant throughout incubation. The detected phosphorylated bands and tail fluorescent labeling corresponded to specific PKA targets as judged by their increase or decrease in response to PKA modulators. Interestingly, the flagella localization of PKA-phosphorylated proteins is consistent with the tail localization of PKA regulatory subunit (Pariset and Weinman, 1994), sAC (Hess et al., 2005) and phospho-Tyr proteins (Carrera et al., 1996; Ficarro et al., 2003).
In agreement with PKA-induced phosphorylation studies, evaluation of the kinetics of cAMP steady-state levels demonstrated an absolute requirement of bicarbonate for the increase in this second messenger levels, which reached a maximum at 1 min. Moreover, the rapid rise in cAMP and PKA-dependent phosphorylation observed in our study appeared to be sAC dependent as judged by the fact that KH7 inhibited both events. Interestingly, the early increase in the cAMP concentration was followed by a rapid decrease to control levels at 1-h incubation. Similar results were reported by Brenker et al. (2012). Different mechanisms could explain the decrease in the cAMP concentration observed in our study such as the release of the messenger from sperm during incubation as observed in human leukemia cells (Copsel et al., 2011), a possible negative feedback of active PKA on sAC activity (Nolan et al., 2004; Burton and McKnight, 2007) or the activation of sperm PDE.
Of note, the discrepancy between the decrease in cAMP and the sustained PKA-induced phosphorylation levels throughout capacitation indicates that the intracellular cAMP concentration remaining in capacitated cells could be sufficient to sustain PKA activation. In addition, PKA substrate phosphorylation levels could be maintained by a yet non-described mechanism such as phosphatase inactivation.
As observed for PKA substrate phosphorylation, protein Tyr phosphorylation was completely dependent on the presence of bicarbonate in the medium and induced by dbcAMP and IBMX. Moreover, Tyr phosphorylation was also inhibited by KH7 supporting the proposed involvement of sAC in the occurrence of this event. However, differently from the very rapid detection of both PKA substrate phosphorylation and cAMP production, Tyr phosphorylation increased gradually as a function of time, reaching maximum levels only at 6 h incubation.
Similarly to Tyr phosphorylation, the percentage of cells capable of undergoing either the spontaneous or progesterone-induced AR increased gradually during capacitation reaching levels significantly different from controls at 6 h, which did not increase with further incubation. These studies were carried out using 25 µM concentration of progesterone as this was the minimum hormone concentration required to significantly induce the AR. This concentration is much higher than that reported to induce the calcium channel Catsper (0.5 µM) in the human sperm (Lishko et al., 2011; Strunker et al., 2011), suggesting that progesterone might have different targets in sperm in addition to Catsper. The kinetics of AR is consistent with the HOPT results showing that sperm require at least 6-h incubation under capacitating conditions to be able to penetrate the most zona-free hamster oocytes. Together, these observations indicate that in spite of the occurrence of very early events such as cAMP production and PKA-dependent phosphorylation, sperm need to undergo the activation of other slower signaling pathways in order to reach a functional capacitation state.
Although the link between PKA-dependent Ser/Thr phosphorylation and the subsequent Tyr phosphorylation is still unknown, evidence supports that a phospho-PKA substrate is involved. In the human sperm, SRC, a member of the SFK, has been postulated as this key intermediate by analyzing the effects of its inhibition on Tyr phosphorylation, hyperactivation and progesterone-induced AR (Lawson et al., 2008; Mitchell et al., 2008; Varano et al., 2008). However, our results, showing that SKI606 produced a dose-dependent inhibition of both PKA-dependent and Tyr phosphorylations revealed the involvement of SFK upstream PKA-mediated phosphorylation. Interestingly, the SKI606 concentration needed to block PKA substrate and Tyr phosphorylations was at least five times lower than the one required for blocking this pathway in mouse sperm (Krapf et al., 2010), suggesting that different SFK members would be involved in mouse and human sperm capacitation. Moreover, the finding that SKI606 inhibition occurred at IC50 of ∼10 μM points towards the participation of SRC and/or ABL kinases in this pathway as these enzymes are more sensitive to SKI606 than other kinases (Bantscheff et al., 2007). In this regard, it is worthy to note that both SRC and ABL have already been described in human sperm (Naz 1998; Lawson et al., 2008; Mitchell et al., 2008; Varano et al., 2008).
Our results showing that exposure of sperm to OA prevented the effects of SKI606 on PKA-dependent and Tyr phosphorylations support the role of SFK in human sperm capacitation through the inactivation of Ser/Thr phosphastases (i.e. PP1γ and/or PP2A). These results are in agreement with those previously reported in mice (Krapf et al., 2010) as well as with recent results obtained by our group in rats (unpublished data). However, human gametes require a much higher concentration of OA than rodent sperm (100 and 0.1 nM, respectively) to overcome the SKI606 inhibitory effect suggesting the involvement of different OA-sensitive phosphatases in each species. In this regard, whereas PP2A has been proposed to mediate mouse capacitation (Krapf et al., 2010), the fact that PP1γ is ∼100-fold less sensitive to OA than PP2A (Ishihara et al., 1989) supports the participation of PP1γ in the human SFK/phosphatase pathway. More specifically, evidence supports a role for PP1γ2 in human capacitation as this sperm predominant spliced variant (Smith et al., 1996) exhibits predicted Tyr phosphorylation sites and it is essential for mouse fertility (Sinha et al., 2012). Nevertheless, as high OA concentrations can affect PP2A, and this enzyme was shown to be inactivated by SFK phosphorylation in other experimental models (i.e. Hu et al., 2009), a possible role of PP2A in conjunction with PP1γ2 in human sperm capacitation cannot be excluded. In addition to this, other phosphatases reported to be present in the testes (i.e. PP4, PP5, PP6 or PP7; Fardilha et al., 2011a) but not yet described in sperm, might also be involved in human capacitation. The identification of sperm-specific phosphatases involved in human capacitation might provide an important information for the future development of male contraceptives.
Our results argue against the possible participation of SFKs and OA-sensitive phosphatases upstream of PKA activation as the addition of cAMP agonists and IBMX did not overcome the inhibitory effects of SKI606 on both PKA substrate and Tyr phosphorylations. Accordingly, the inactivation of phosphatases was not sufficient to induce phosphorylation when the cAMP/PKA pathway was not stimulated. Altogether, these results support that the crossroads between the cAMP/PKA and SFK/phosphatase pathways is located at the PKA substrate phosphorylation step. According to this, the steady-state levels of PKA substrate phosphorylation would result from a fine-tuned balance between the activation of PKA and the inactivation of Ser/Thr phosphatases by SFK activity. Supporting this notion, PKA substrates (present work) and PP1γ2 (Fardilha et al., 2011b) are both localized in the sperm flagella.
Our studies aimed to investigate the relevance of the SFK/phosphatase pathway for human capacitation showed that modulation of the phosphatase activity by SKI606 and OA had a significant impact on different functional capacitation-dependent events. Whereas previous reports showed that human sperm motility parameters were not modified by SFK inhibition (Mitchell et al., 2008; Varano et al., 2008), our results revealed a significant decrease in several CASA motility parameters in those sperm exposed to SKI606 as previously described in mouse (Krapf et al., 2010). This discrepancy might be due to the different SFK inhibitors (SU6656 versus SKI606) and the different incubations periods (0.5–3 h versus 18 h) employed. Our data also indicate that exposure of human sperm to SKI606 throughout incubation prevented the progesterone-induced AR, suggesting a role for SFK during capacitation and/or AR as previously described (Varano et al., 2008). In this regard, it is important to note that our motility and AR studies using not only SKI606 but also OA revealed the novel involvement of SFK in human capacitation through the Ser/Thr phosphatase inactivation. Considering that the development of sperm fertilizing ability reflects the occurrence of a functional capacitation process, our results showing that SKI606 inhibits the sperm ability to penetrate zona-free hamster oocytes and that the addition of OA overcomes this negative effect further support the functional relevance of the SFK/phosphatase pathway for human sperm capacitation.
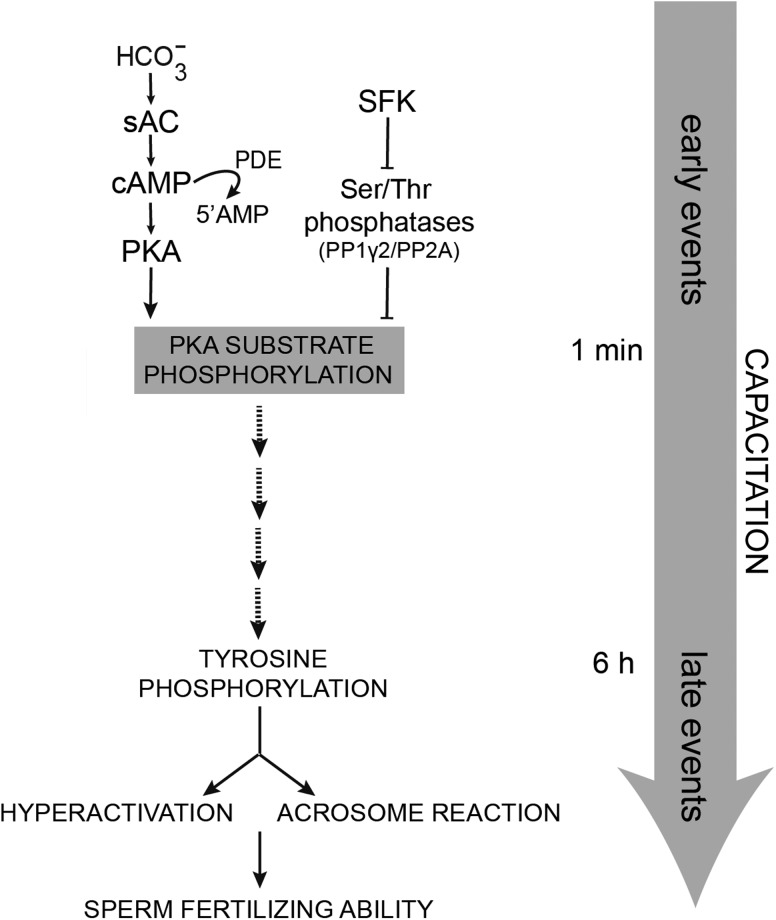
In conclusion, our results strongly indicate the need of both PKA activation and Ser/Thr phosphatase down-regulation for achieving a functional human sperm capacitation state and provide convincing evidence that both signaling pathways converge at the early PKA substrate phophorylation level (Fig. 7). Interestingly, whereas these results support the idea that the main signaling pathways involved in sperm capacitation are evolutionarily conserved, the SFK members and Ser/Thr phosphatases involved in human sperm capacitation appear to be different from those required for mouse sperm capacitation revealing the species specificity of the molecular mechanisms underlying this key reproductive process.
Figure 7.
Signaling pathways involved in human sperm capacitation. Two pathways are required to promote the functional human sperm capacitation. One corresponds to the 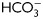 -dependent activation of PKA by sAC/cAMP and the other involves the down-regulation of Ser/Thr phosphatases (i.e. PP1γ2 and/or PP2A) by SFK. The crossroads between both signaling pathways is located at the PKA-Ser/Thr phosphorylation step. In spite of the early onset (1 min) of cAMP and PKA-dependent phosphorylation, human sperm require at least 6 h incubation under capacitating conditions to reach a functional capacitation state (i.e. AR and sperm fertilizing ability).
-dependent activation of PKA by sAC/cAMP and the other involves the down-regulation of Ser/Thr phosphatases (i.e. PP1γ2 and/or PP2A) by SFK. The crossroads between both signaling pathways is located at the PKA-Ser/Thr phosphorylation step. In spite of the early onset (1 min) of cAMP and PKA-dependent phosphorylation, human sperm require at least 6 h incubation under capacitating conditions to reach a functional capacitation state (i.e. AR and sperm fertilizing ability).
Finally, considering the variable capacitating conditions used to study human sperm capacitation, our results analyzing the temporal correlation between the two signaling pathways and the functional state of human sperm (Fig. 7) will contribute to a better understanding of the mechanisms leading to the acquisition of human sperm fertilizing ability as well as to human infertility.
Authors' roles
M.A.B. carried out the main experiments. M.A.B., V.G.D.R., P.E.V. and P.S.C. designed experiments, analyzed the data and wrote the article. D.K., P.E.V. and P.S.C. conceived the study. V.G.D.R., A.M.S. and F.A.N. collaborated with experiments. A.M.S., F.A.N. and D.K. helped in drafting the article.
Funding
This study was partially supported by WHO RMG grant (H9/TSA/037), the National Research Council of Argentina (CONICET) grant (PIP 2009-290) and the National Agency for Scientific and Technological Promotion (ANPCyT) grant (PICT 2012-2023) to P.S.C., by Amherst Faculty Research Grant/Healey Endowment Grant (University of Massachusetts) to A.M.S. and by the Eunice Kennedy Shriver grants (National Institute of Child Health and Human Development: NIH HD38082 and HD44044) to P.E.V.
Conflict of interest
None declared.
Acknowledgements
The authors thank members of Cuasnicu's laboratory for helpful comments.
References
- Austin CR. The ‘capacitation’ of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–3192. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Curry B, Aitken RJ. Phosphorylation and consequent stimulation of the tyrosine kinase c-Abl by PKA in mouse spermatozoa: its implications during capacitation. Dev Biol. 2009;333:57–66. doi: 10.1016/j.ydbio.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Lefievre L, Moseley FL, Barratt CL, Publicover SJ. Bicarbonate and bovine serum albumin reversibly ‘switch’ capacitation-induced events in human spermatozoa. Mol Hum Reprod. 2005;11:683–691. doi: 10.1093/molehr/gah226. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittingham DG. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian Embrology. San Francisco, CA: Freeman and Co. Press; 1971. pp. 86–116. [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strunker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 2012;31:1654–1665. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KA, McKnight GS. PKA, germ cells, and fertility. Physiology. 2007;22:40–46. doi: 10.1152/physiol.00034.2006. [DOI] [PubMed] [Google Scholar]
- Carrera A, Moos J, Ning XP, Gerton GL, Tesarik J, Kopf GS, Moss SB. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–296. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Ellerman DA, Busso D, Morgenfeld M, Piazza A, Hayashi M, Young ET, Kasahara M, Cuasnicu PS. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human oocyte. Biol Reprod. 2001;65:1000–1005. doi: 10.1095/biolreprod65.4.1000. [DOI] [PubMed] [Google Scholar]
- Copsel S, Garcia C, Diez F, Vermeulem M, Baldi A, Bianciotti LG, Russel FG, Shayo C, Davio C. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. 2011;286:6979–6988. doi: 10.1074/jbc.M110.166868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ros VG, Munuce MJ, Cohen DJ, Marin-Briggiler CI, Busso D, Visconti PE, Cuasnicu PS. Bicarbonate is required for migration of sperm epididymal protein DE (CRISP-1) to the equatorial segment and expression of rat sperm fusion ability. Biol Reprod. 2004;70:1325–1332. doi: 10.1095/biolreprod.103.022822. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, Van Duin M, Conti M, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardilha M, Esteves SL, Korrodi-Gregorio L, Pelech S, da Cruz E Silva OA, da Cruz e Silva E. Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility. Mol Hum Reprod. 2011a;17:466–477. doi: 10.1093/molehr/gar004. [DOI] [PubMed] [Google Scholar]
- Fardilha M, Esteves SL, Korrodi-Gregorio L, Vintem AP, Domingues SC, Rebelo S, Morrice N, Cohen PT, da Cruz E Silva OA, da Cruz e Silva EF. Identification of the human testis protein phosphatase 1 interactome. Biochem Pharmacol. 2011b;82:1403–1415. doi: 10.1016/j.bcp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, Kalab P, Marto JA, Shabanowitz J, Herr JC, Hunt DF, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–11589. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol Reprod. 1997;56:707–719. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wu X, Xu J, Zhou J, Han X, Guo J. Src kinase up-regulates the ERK cascade through inactivation of protein phosphatase 2A following cerebral ischemia. BMC Neurosci. 2009;10:74. doi: 10.1186/1471-2202-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, et al. Calyculin A and okadaic acid:inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Itach SB, Finklestein M, Etkovitz N, Breitbart H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev Biol. 2012;362:154–161. doi: 10.1016/j.ydbio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL, Rivkin E, Talmor-Cohen A, Shalgi R, Tres LL. Expression of full-length and truncated Fyn tyrosine kinase transcripts and encoded proteins during spermatogenesis and localization during acrosome biogenesis and fertilization. Mol Reprod Dev. 2009;76:832–843. doi: 10.1002/mrd.21049. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavege of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalancette C, Faure RL, Leclerc P. Identification of the proteins present in the bull sperm cytosolic fraction enriched in tyrosine kinase activity: a proteomic approach. Proteomics. 2006;6:4523–4540. doi: 10.1002/pmic.200500578. [DOI] [PubMed] [Google Scholar]
- Lawson C, Goupil S, Leclerc P. Increased activity of the human sperm tyrosine kinase SRC by the cAMP-dependent pathway in the presence of calcium. Biol Reprod. 2008;79:657–666. doi: 10.1095/biolreprod.108.070367. [DOI] [PubMed] [Google Scholar]
- Leclerc P, De Lamirande E, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Li K, Ni Y, He Y, Chen WY, Lu JX, Cheng CY, Ge RS, Shi QX. Inhibition of sperm capacitation and fertilizing capacity by adjudin is mediated by chloride and its channels in humans. Hum Reprod. 2013;28:47–59. doi: 10.1093/humrep/des384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod. 2008;14:235–243. doi: 10.1093/molehr/gan007. [DOI] [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod. 1998;13:2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- Naz RK. c-Abl proto-oncoprotein is expressed and tyrosine phosphorylated in human sperm cell. Mol Reprod Dev. 1998;51:210–217. doi: 10.1002/(SICI)1098-2795(199810)51:2<210::AID-MRD11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty C, De Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10:355–363. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol. 2012;590:2659–2675. doi: 10.1113/jphysiol.2011.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Pariset C, Weinman S. Differential localization of two isoforms of the regulatory subunit RII alpha of cAMP-dependent protein kinase in human sperm: biochemical and cytochemical study. Mol Reprod Dev. 1994;39:415–422. doi: 10.1002/mrd.1080390410. [DOI] [PubMed] [Google Scholar]
- Sagare-Patil V, Galvankar M, Satiya M, Bhandari B, Gupta SK, Modi D. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int J Androl. 2012;35:633–644. doi: 10.1111/j.1365-2605.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Sinha N, Pilder S, Vijayaraghavan S. Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PLoS One. 2012;7:e47623. doi: 10.1371/journal.pone.0047623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, Vijayaraghavan S. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod. 1996;54:719–727. doi: 10.1095/biolreprod54.3.719. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988;106:1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Hum Reprod. 2008;23:2652–2662. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, Shipp EB. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Dev Biol. 1999;205:98–110. doi: 10.1006/dbio.1998.9100. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, Greengard P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod. 1996;54:709–718. doi: 10.1095/biolreprod54.3.709. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995a;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995b;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Johnson LR, Oyaski M, Fornes M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol. 1997;192:351–363. doi: 10.1006/dbio.1997.8768. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezon JG. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod. 1999;61:76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Krapf D, De La Vega-Beltran JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13:395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Geneva, Switzerland: World Health Organization Press; 2010. [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, Van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]