Summary
Galectin-3 is expressed and secreted by immune cells and has been implicated in multiple aspects of the inflammatory response. It is a glycan binding protein which can exert its functions within cells or exogenously by binding cell surface ligands, acting as a molecular bridge or activating signaling pathways. In addition, this lectin has been shown to bind to microorganisms. In this study we investigated the interaction between galectin-3 and Neisseria meningitidis, an important extracellular human pathogen, which is a leading cause of septicaemia and meningitis. Immunohistochemical analysis indicated that galectin-3 is expressed during meningococcal disease and co-localises with bacterial colonies in infected tissues from patients. We show that galectin-3 binds to N. meningitidis and we demonstrate that this interaction requires full length, intact lipopolysaccharide molecules. We found that neither exogenous nor endogenous galectin-3 contributes to phagocytosis of N. meningitidis; instead exogenous galectin-3 increases adhesion to monocytes and macrophages but not epithelial cells. Finally we used galectin-3 deficient (Gal-3−/−) mice to evaluate the contribution of galectin-3 to meningococcal bacteraemia. We found that Gal3−/−mice manifested significantly lower levels of bacteriaemia compared with wild-type mice after challenge with live bacteria, indicating that galectin-3 confers an advantage to N.meningitidis during systemic infection.
INTRODUCTION
Galectins are a family of carbohydrate binding lectins defined by a β-sandwich fold, a signature binding site (Gabius et al., 2011) and affinity for β-galactose-containing molecules. They are key regulators of the immune system, expressed by almost all immune cells and have roles in fundamental biological processes including cell adhesion, chemotaxis, growth regulation and inflammation (Di Lella et al., 2011). Galectin effector functions are exerted within cells and extracellularly, where they cross-link cell surface or matrix glycoconjugates, leading to adhesion and/or activation of signalling cascades to modulate cell activities and survival (Boscher et al., 2011). Each galectin has a characteristic profile of ligand specificity which can be influenced by clustering of glycans and their structural context (Kopitz et al., 2010; Krzeminski et al., 2011). In addition to cellular ligands, galectins also target sites on the surface of microorganisms (reviewed in (Vasta, 2009)) and have thus attracted attention for investigation into their roles as potential pathogen receptors and regulators of responses to infection.
Galectin-3 (also known as MAC-2 antigen) is widely expressed in lymphoid and non lymphoid tissues (Flotte et al., 1983). It is present intracellulary (Wang et al., 2004) and can be secreted via a non-classical pathway (Hughes, 1999). Endogenous galectin-3 is required for efficient phagocytosis of opsonised erythrocytes and apoptotic thymocytes (Sano et al., 2003), although the precise mechanism by which it contributes to this process is not known. Galectin-3 is a monovalent glycan binding protein, however, upon binding to multivalent ligands, it oligomerizes and forms stable complexes. Galectin-3 can target glycans in microdomains with high affinity (Kopitz et al., 2010) and has been shown to have important roles in adhesion or biosignalling. For example, it can directly enhance adhesion of neutrophils to laminin (Kuwabara et al., 1996) and endothelial cells (Sato et al., 2002).
Galectin-3 production and secretion is highly up-regulated in response to inflammatory stimuli (Henderson et al., 2009), indicating that this protein is important in immune responses to infection. Consistent with this, galectin-3 is a key molecule in host defense against Streptococcus pneumoniae as it enhances recruitment, adhesion and function of neutrophils in the lungs of mice during pneumococcal infection to aid in phagocytosis and clearance of the pathogen (Sato et al., 2002; Farnworth et al., 2008; Nieminen et al., 2008). Interestingly, intracellular galectin-3 is recruited to phagosome membranes of cells infected with Shigella and Listeria (Paz et al., 2010) and accumulates around vacuoles containing Mycobacteria (Beatty et al., 2002), however, in each case, the precise function of galectin-3 recruitment and the effects on bacterial survival have not been established.
Several microorganisms display surface carbohydrates that contain epitopes which are known ligands for galectin-3. For example, through recognition of a unique pathogen-associated glycan, galectin-3 has direct fungicidal activity against Candida albicans by as yet undefined mechanisms (Kohatsu et al., 2006). Alternatively, galectin-3 can act as a cell surface receptor, as shown for Proteus mirabilis on MDCK cells (Altman et al., 2001), Helicobacter pylori on gastric epithelial cells (Fowler et al., 2006) and Pseudomonas aeruginosa on corneal epithelia (Gupta et al., 1997), while addition of exogenous galectin-3 to cultures of Trypanosoma cruzi increases its adhesion to smooth muscle cells (Kleshchenko et al., 2004). Interaction with galectin-3 may thus prove beneficial for pathogens, by acting as a cell surface docking site or as a cross-linking molecule which promotes adhesion. Several of the microbial ligands for galectin-3 have been identified. These include the fimbrial adhesin from P. mirabilis (Altman et al., 2001), glycosylphosphatidylinositols from Toxoplasma gondii (Debierre-Grockiego et al., 2010), phosphatidylinositol mannosides from Mycobacteria (Beatty et al., 2002) and lipopolysaccharide (LPS) from Klebsiella pneumoniae, Salmonella (Mey et al., 1996), H. pylori (Fowler et al., 2006), P. aeruginosa (Gupta et al., 1997), Escherichia coli (Stowell et al., 2010) and Neisseria gonorrhoeae (John et al., 2002).
Neisseria meningitidis colonises the human respiratory tract and is an important Gram-negative human pathogen, able to cause septicaemia and meningitis (Lo et al., 2009). Along with type four pili, the polysaccharide capsule and LPS are important virulence factors expressed by this bacterium. These key surface structures can influence interaction with host cells and the differences in the composition of the capsule and the LPS can be used to classify strains into serogroups (Craven et al., 1978) and immunotypes (Scholten et al., 1994). Meningococcal invasion activates the complement and coagulation cascades and provokes an excessive inflammatory response, which is largely due to LPS activation via Toll like receptor 4 (TLR4) (Brandtzaeg et al., 1989). In light of the emerging roles of galectins as modulators of inflammatory responses and the evidence for galectin-3 interaction with pathogen surfaces, the goal of this work was to investigate whether galectin-3 could bind to N. meningitidis and to examine whether this could have consequences during meningococcal infection.
RESULTS
Galectin-3 is expressed during meningococcal infection
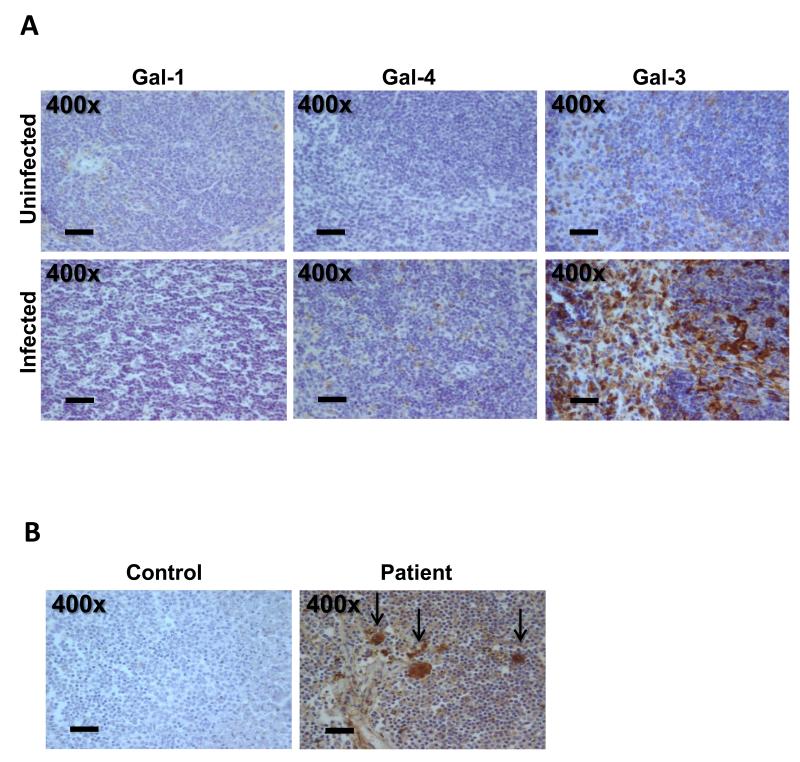
Meningococcal infection is characterised by a marked inflammatory response that contributes to the severity of the disease (Stephens et al., 2007). We therefore investigated the presence of different galectins in tissues of mice challenged with N. meningitidis. Based on their structure and composition, galectins have been classified into three groups: proto-type, tandem-repeat and chimera-type galectins (Kasai et al., 1996). We chose a representative galectin from each of these three subgroups and therefore using specific antibodies (Kaltner et al., 2002; Lohr et al., 2007; Lohr et al., 2008) and immunohistochemistry we analysed the presence of the proto-type galectin-1, the tandem-repeat-type galectin-4 and chimera-type galectin-3 in spleen tissue of mice infected with serogroup B isolate MC58 for 24 hrs, compared to tissues from uninfected animals. Galectin-1 and galectin-4 were detected only at low levels in the spleen from both uninfected and infected animals (Fig. 1A). In contrast, while low levels of galectin-3 were present in tissue of control mice, intense staining for galectin-3 was detected in tissue from infected mice (Fig. 1A). Similar results were obtained in other organs such as the heart, lungs, kidneys and the meninges (data not shown).
Fig. 1. Immunohistochemical detection of different galectins in murine and human tissue.
(A) Detection of galectin-1, galectin-4 and galectin-3 in spleens of mice 24 hrs after infection with N. meningitidis serogroup B strain MC58 (infected) and in spleens of control mice (uninfected). Galectins were detected with rabbit anti-human galectin-1, -3 and -4 antibodies which cross-react with the respective galectins in other mammals (Kaltner et al., 2002; Lohr et al., 2007). Galectin-1 and galectin-4 are only detected at very low levels (light brown staining) in uninfected or infected tissues. Galectin-3 presence is seen in a few cells of control tissue, while marked staining (dark brown) is observed in cells of the tissues from infected mice. Images are representative of five infected and five control mice. (B) Detection of galectin-3 in tissue from a patient with meningococcal infection and control. Sections of spleen tissue were incubated with a rabbit anti-human galectin-3 antibody (brown staining). Galectin-3 staining is detected in the patient tissue but not observed in control tissue. Similar results were obtained from staining of tissue from an additional patient (not shown). The level of magnification is shown in each panel.
To determine whether galectin-3 is also detected in human disease, we performed immunohistochemical analysis of spleen tissue from a patient with meningococcal disease (Fig. 1B). As a control, spleen tissue from an uninfected patient was examined. Galectin-3 could be observed within cells and extracellularly in splenic tissue from infected patients but not in control tissue. The distribution and shape of the galectin-3 positive cells indicates that they are most likely macrophages or monocytes.
Galectin-3 binds to N. meningitidis
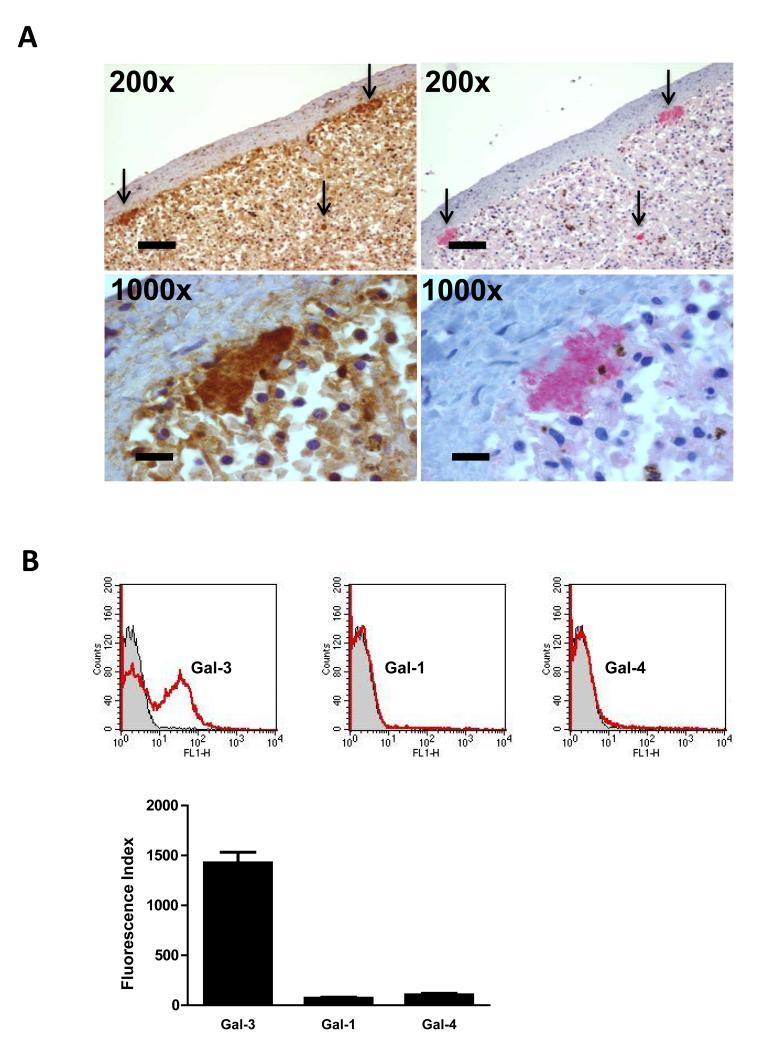
Since galectin-3 can be secreted by phagocytes and is able to bind to galactoside-containing molecules on the surface of cells or of microorganisms, we sought to establish whether galectin-3 can bind to N. meningitidis. Closer inspection of galectin-3 staining in sections of spleen tissue from patients with meningococcal disease revealed co-localisation of galectin-3 staining with bacterial colonies (Fig. 2A). Serial sections of tissue were examined for presence of galectin-3 or for meningococci which were detected using a polyclonal antibody against N. meningitidis. Accumulation of galectin-3 in foci corresponding to bacterial colonies was evident, suggesting that galectin-3 could be bound to N. meningitidis within the tissues.
Fig. 2. Galectin-3 binding to N. meningitidis.
(A) Immunohistochemical staining of spleen tissue from a patient with meningococcal infection showing co-localisation of bacterial colonies and galectin-3. Serial sections of spleen tissue were processed with a rabbit anti-human galectin-3 antibody (brown staining) or a polyclonal antibody against N. meningitidis (red staining). Intense accumulation of brown staining corresponding to galectin-3 is seen (arrows) and red staining corresponding to N. meningitidis occurs in the same area of the tissue (arrows). The higher magnification images (1000x) show co-localisation of galectin-3 and a bacterial colony. The level of magnification is shown in each panel. (B) Representative flow cytometry traces and quantification of recombinant galectin-3 (Gal-3), galectin-1 (Gal-1) and galectin-4 (Gal-4) binding to N. meningitidis following incubation of fixed MC58 with galectins (3.3 μM). Flow cytometry traces show binding of galectins to N. meningitidis (red) with the negative control (no galectin) shown in grey. Quantification of flow cytometry analysis shows that N. meningitidis specifically binds galectin-3. Binding is expressed as the Fluorescence Index. Data are from four different experiments and error bars show the standard deviation.
We therefore analysed the binding of galectin-3 to serogroup B N. meningitidis strain MC58 using fixed, whole bacteria and recombinant human galectin-3. For comparison, we also tested human galectin-1 and galectin-4 that belong to other galectin subgroups, have overlapping but not identical specificities/affinities, and which did not show strong staining in our immunohistochemical analysis of N. meningitidis-infected murine tissue. Flow cytometry analysis revealed that galectin-3 binds to N. meningitidis (Fig. 2B). The lack of detectable binding of the other galectins tested indicates this is a particular feature of galectin-3 and prompted us to further characterise the interaction.
Full length galectin-3 is required for interaction with N. meningitidis
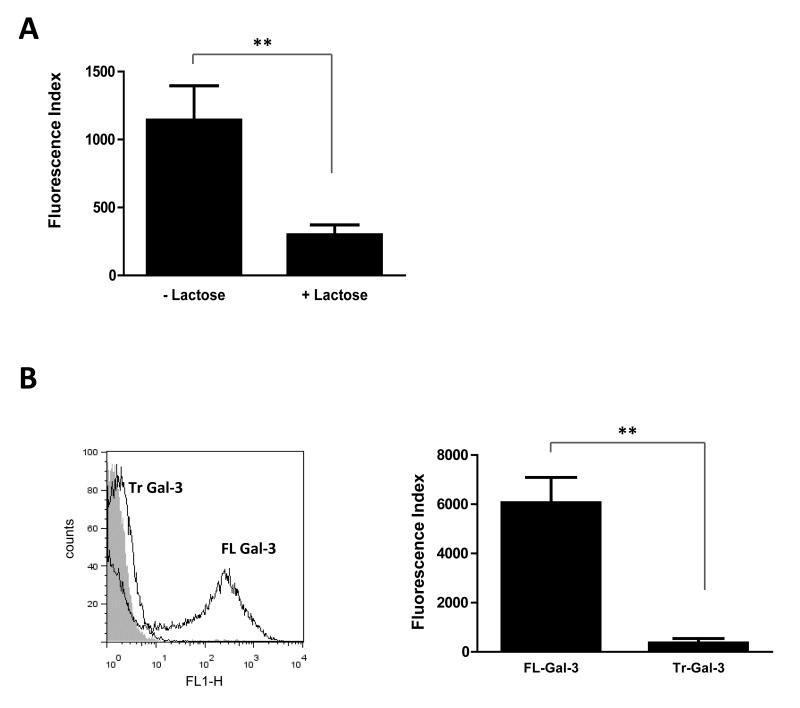
The biological activities of galectin-3 are mainly dependent on its interaction with various ligands via its C-terminal carbohydrate recognition domain (CRD) (Ochieng et al., 2004). In addition, the N-terminal domain of galectin-3 can interact with non-carbohydrate ligands (Mey et al., 1996) and can mediate oligomerisation and the formation of pentamers in presence of multivalent ligands (Hsu et al., 1992; Ahmad et al., 2004). To characterise the interaction of N. meningitidis and galectin-3, we first analysed the binding in presence of lactose, a pan-galectin ligand which acts as a competitive inhibitor of galectin-carbohydrate interactions via the CRD (Sparrow et al., 1987; Seetharaman et al., 1998). Consistent with a carbohydrate-dependent binding of galectin-3 to N. meningitidis we showed that the interaction is inhibited by lactose. Fixed bacteria were incubated with galectin-3 in absence or presence of 100mM lactose. Addition of lactose partially inhibited lectin association (Fig. 3A and Fig. S1), resulting in a reduction of up to 75% in galectin-3 binding (p= 0.0062), while addition of sucrose, a disaccharide that does not inhibit galectin-carbohydrate interactions, had no inhibitory effect (Fig. S1).
Fig. 3. Full length galectin-3 is required for binding to N. meningitidis.
(A) Quantification of flow cytometry analysis of galectin-3 binding to N. meningitidis in absence or presence of 100 mM lactose. Lactose significantly reduces galectin-3 binding to N. meningitidis (**, p=0.0062). Data are from four different experiments performed in duplicate and error bars show the standard deviation. (B) Representative flow cytometry traces showing binding of full length galectin-3 to N. meningitidis (black trace, FL Gal-3) and no binding of proteolytically truncated galectin-3 consisting of the CRD alone (black trace, Tr Gal-3), with the negative control (no gal-3) shown in grey. Quantification of flow cytometry analysis of binding shows Tr Gal-3 binding to N. meningitidis to be significantly reduced (**, p=0.0016). The binding is expressed as the Fluorescence Index. Data are from four different experiments and error bars show the standard deviation.
In addition, to identify which domains of galectin-3 are required for interaction, we examined the binding of truncated galectin-3 which lacks the N-terminal regions following proteolytic treatment with collagenase D, as described previously (Kopitz et al., 2001). This leaves the CRD of the protein intact and still able to bind to endogenous ligands on cell surfaces (Kopitz et al., 2001). Flow cytometry was performed using the full length protein (FL Gal-3) and the truncated protein (Tr Gal-3) and binding to N. meningitidis strain MC58 was analysed. As shown in Fig. 3B, the proteolytic removal of the N-terminal regions of galectin-3 almost completely abolished binding to MC58, indicating that the CRD is insufficient to support galectin-meningococcal interaction and that the N-terminal domain of the protein is also required.
Full length lipopolysaccharide is required for galectin-3 binding to N. meningitidis
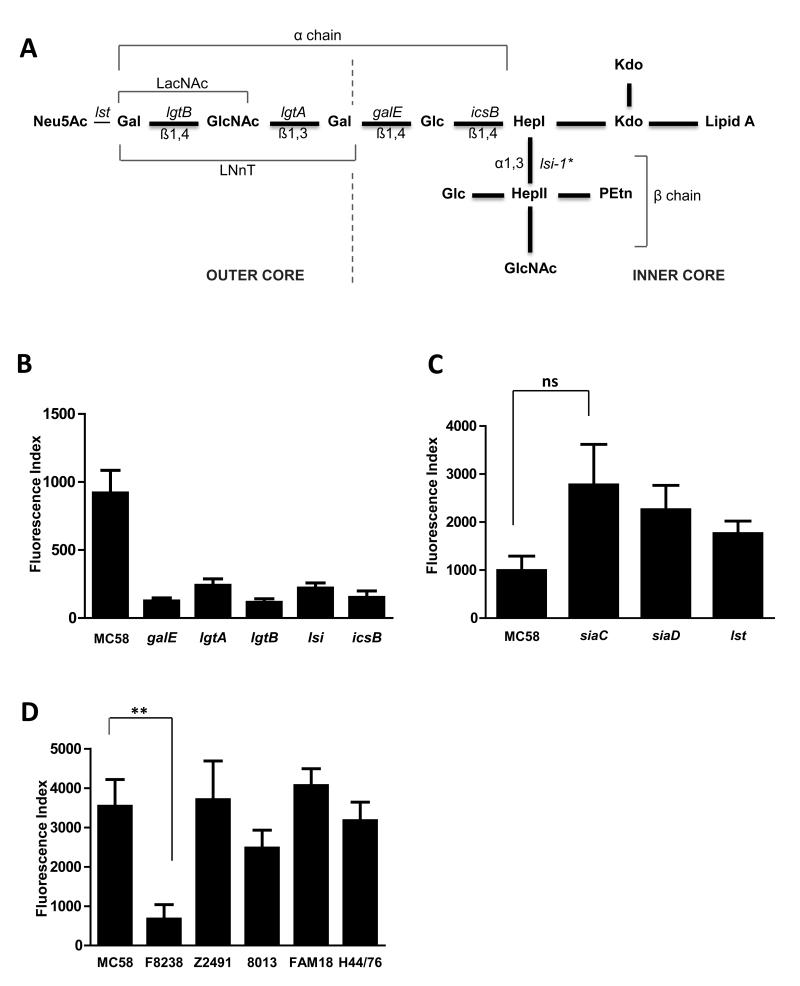
In almost all Gram-negative bacteria which have been reported to bind galectin-3, the target ligand has been identified as LPS (reviewed in (Vasta, 2009)). We therefore examined whether meningococcal LPS is involved in the attachment of galectin-3 to the bacterial surface. Meningococcal LPS (Fig. 4A) consists of lipid A containing a disaccharide of glucosamine residues, which anchors the molecule in the outer membrane. The lipid A region of the LPS molecule is linked via two 2-keto-3-deoxy-octulosonic acid residues (Kdo) to a core oligosaccharide with an inner core di-heptose-N-acetylglucosamine backbone, comprising two heptose residues (HepI and HepII). This backbone provides a point of attachment for a variety of outer core oligosaccharides which leads to expression of different immunotypes of LPS (Jennings et al., 1999). Furthermore, N-acetylneuraminic acid (Neu5Ac, Fig. 4A) can be attached to LPS via the terminal galactose of specific LPS immunotypes. We investigated the contribution of N. meningitidis LPS to galectin-3 binding using meningococcal strains with defined truncations of LPS in order to assess the importance of the saccharide units to galectin recruitment. We examined binding to wild-type strain MC58 and isogenic strains carrying mutations in genes encoding glycosyltransferases involved in LPS biosynthesis (Fig 4A). The lgtA and lgtB mutants lack enzymes responsible for the biosynthesis of the N-acetyl-lactosamine (LacNAc), while the galE mutant lacks the lacto-N-neo-tetraose (LNnT) structure (Jennings et al., 1995b); icsB lacks the enzyme responsible for the addition of glucose to HepI (van der Ley et al., 1997) and the lsi-1 mutant lacks the enzyme responsible for the addition of HepII to HepI but has also been shown to lack the oligosaccharide portion of the LPS molecule except the first heptose (Jennings et al., 1995a). Analysis of galectin-3 binding to N. meningitidis by flow cytometry demonstrated that all mutants expressing a truncated LPS molecule showed a significant reduction in the binding of galectin-3 compared to the wild-type strain (Fig.4B) demonstrating that full length LPS is necessary for galectin-3 attachment to the meningococcal surface. The fact that mutation of lgtB alone was sufficient to abolish galectin-3 binding indicates that the terminal galactose and an intact LacNAc moiety are required for interaction.
Fig. 4. Galectin-3 binds to meningococci expressing LPS with a terminal LacNAc.
(A) The structure of L3,7,9 immunotype of meningococcal LPS. Neu5Ac, N-acetylneuraminic acid; Kdo, keto-deoxyoctulonic acid; Hep, heptose; Glc, glucose; GlcNAc, N-acetyl-glucosamine; Gal, galactose; PEtn, phospho-ethanolamine, LacNAc, N-acetyl-lactosamine; LNnT, Lacto-N-neo-tetraose. The inner and outer cores of the structure are indicated. Genes encoding enzymes responsible for the addition of sugars and the glycosidic linkages are indicated; *of note loss of lsi-1 results in loss of the β-chain and also the oligosaccharide chain apart from the first heptose (Jennings et al., 1995a). (B) Flow cytometric quantification of the binding of galectin-3 (3.3 μM) to strains of N. meningitidis with full length (MC58) or truncated versions of LPS (MC58ΔgalE, MC58ΔlgtA, MC58ΔlgtB, MC58Δlsi and MC58ΔicsB). There is a significant reduction in the binding of galectin-3 to all strains with truncated LPS (MC58ΔgalE p=0.0032, MC58ΔlgtA p=0.0076, MC58ΔlgtB p=0.0003, MC58Δlsi-1 p=0.0062, MC58ΔicsB p=0.0043). Data are from four different experiments and error bars show the standard deviation. (C) Influence of sialic acid on binding of galectin-3 to N. meningitidis. Quantification of flow cytometric analysis of galectin-3 binding shows no significant difference in binding to the wild-type strain (MC58) or strains lacking LPS sialylation (MC58Δlst), capsule (MC58ΔsiaD) or both (MC58ΔsiaC). A tendency to increased galectin-3 binding was detected with the strain lacking sialic acid but this was not significant (ns, p=0.0671). Data are from four different experiments performed in duplicate and error bars show the standard deviation. (D) Binding of galectin-3 to serogroups A, B and C N. meningitidis. Fixed bacteria were incubated with 3.3 μM galectin-3 for 1 hr and binding was detected by flow cytometry. Galectin-3 binding to N. meningitidis strain Z2491 (serogroup A), MC58 and H44/76 (serogroup B), 8013 and FAM18 (serogroup C) demonstrates that binding is independent of serogroup. Interaction of the strain F8238 with galectin-3 is significantly reduced (**, p=0.0098) compared to strain MC58. Binding is expressed as the Fluorescence Index. Data are from four independent experiments and error bars show the standard deviation.
Meningococcal strain MC58 expresses an L3,7,9 LPS immunotype which can be modified by the addition of an α2,3-linked sialic acid to the terminal galactose of the alpha-chain through the activity of a specific sialyltransferase enzyme (Lst) (Mandrell et al., 1990; Mandrell et al., 1991; Gilbert et al., 1997). The presence of sialic acid on terminal residues of glycans can modulate binding of some galectins, although this was shown to have minor effect on galectin-3; in fact galectin-3 is known to bind to LacNAc repeats with terminal α2,3-sialylation and α2,6-sialylation (Ahmad et al., 2002; Stowell et al., 2008). To examine whether sialylation of LPS alters galectin-3 binding to the surface of N. meningitidis we compared the binding to wild-type strain MC58 and an isogenic mutant lacking lst. In addition, we examined the effect of the presence of the polysaccharide capsule which is expressed on the bacterial surface and protects the bacterium from complement mediated lysis and phagocytosis (Hill et al., 2010). The gene siaD encodes the α2,8-polysialyltransferase required for chain elongation during capsule synthesis and siaC is necessary for sialic acid biosynthesis (Edwards et al., 1994); therefore, MC58ΔsiaC is unable to produce a capsule or sialylate LPS without exogenous 5′cytidine monophospho-N-acetyl-neuramic acid (CMP-NANA). We analysed galectin-3 binding to MC58 and the isogenic mutant strains MC58ΔsiaC, MC58ΔsiaD, and MC58Δlst (Exley et al., 2005). All three mutants exhibited elevated levels of galectin-3 binding, although these increases were not significantly different to the parental strain MC58 (Fig. 4C). These data indicate that the presence of surface sialic acids (capsule and terminal sialylation of LPS) does not affect galectin-3 association with the bacterial surface.
Finally, to ascertain whether galectin-3 binding was restricted to MC58 we analysed the binding to other strains of N. meningitidis. We chose a small representative panel of disease causing isolates that belong to different serogroups and have different LPS immunotypes (Table 1). Analysis by flow cytometry demonstrated that, while there was some variation in the relative level of binding, strains belonging to serogroups A, B and C were able to bind galectin-3; only one strain, F8238, had a significantly reduced level of galectin-3 binding compared to MC58 (Fig. 4D). Interestingly, all the strains which bind galectin-3 are able to express an LPS immunotype containing a terminal LacNAc; Z2491 is a serogroup A isolate which expresses an L9 immunotype (Choudhury et al., 2008) while serogroup B strains MC58 and H44/76 and serogroup C strains 8013 and FAM18 (Plested et al., 1999; Geoffroy et al., 2003) and this study, data not shown) all express L3,7,9 immunotype. F8238 has an LPS immunotype L10 (Maslanka et al., 1997) that does not include the LacNAc and LNnT moieties (Kim et al., 1994), consistent with these structures being required for galectin-3 binding.
Table 1.
N. meningitidis strains used in this study
| Strain | Description | Immunotype | References |
|---|---|---|---|
| MC58 | Serogroup B clinical isolate | L3,7,9 | (Tettelin et al., 2000), (Plested et al., 1999) |
| F8238 | Serogroup A clinical isolate | L10 | (Maslanka et al., 1997) |
| Z2491 | Serogroup A clinical isolate | L9 | (Parkhill et al., 2000), (Choudhury et al., 2008) |
| H44/76 | Serogroup B clinical isolate | L3 | (Holten, 1979); (Plested et al., 1999) |
| 8013 | Serogroup C clinical isolate | L3,7,9 | (Geoffroy et al., 2003; Rusniok et al., 2009) |
| FAM18 | Serogroup C clinical isolate | L3,7,9 | (Bentley et al., 2007) (This study) |
| MC58ΔsiaC | Insertionally inactivated siaC | - | (Exley et al., 2005) |
| MC58ΔsiaD | Insertionally inactivated siaD | - | (Exley et al., 2005) |
| MC58Δlst | Insertionally inactivated lst | - | (Exley et al., 2005) |
| MC58ΔlgtA | Truncated LPS mutant | - | (Jennings et al., 1995b) |
| MC58ΔlgtB | Truncated LPS mutant | - | (Jennings et al., 1995b) |
| MC58ΔgalE | Truncated LPS mutant | - | (Jennings et al., 1995b) |
| MC58Δlsi-1 | Truncated LPS mutant | - | (Jennings et al., 1995a) |
| MC58ΔicsB | Truncated LPS mutant | - | (van der Ley et al., 1997) |
Taken together these data demonstrate that full length galectin-3 is able to bind to N. meningitidis and that LPS molecules on the meningococcal surface are required for this interaction. We therefore chose to examine the impact of galectin-3 binding to N. meningitidis on bacterial interaction with cells.
Endogenous galectin-3 does not alter the interaction of N. meningitidis with phagocytes
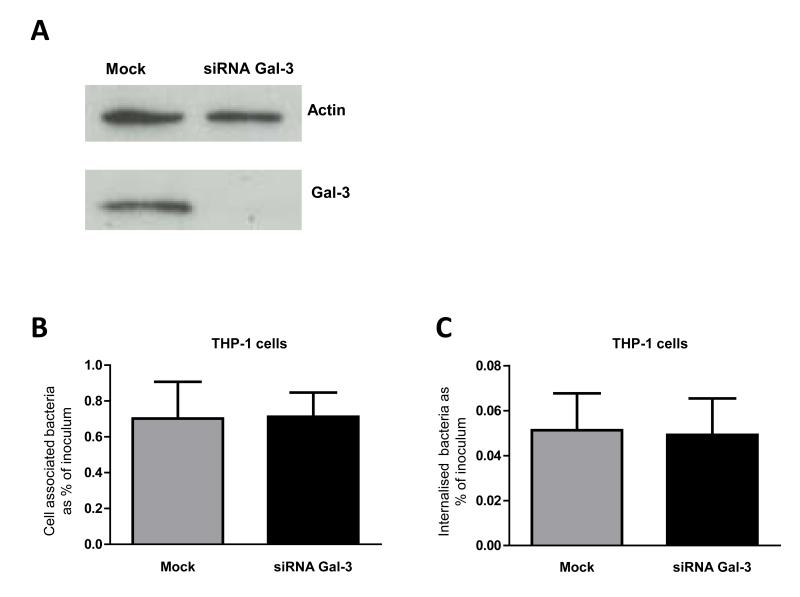
Galectin-3 is highly expressed by immune cells such as activated macrophages (Liu et al., 1995) and endogenous galectin-3 has been shown to be important for phagocytosis of opsonised erythrocytes and apoptotic cells in vitro and in vivo (Sano et al., 2003). In the first instance we examined whether endogenous galectin-3 is important for binding and phagocytosis of N. meningitidis. As a relevant cell model, human THP-1 cells were differentiated into macrophages using phorbol 12-myristate 13-acetate (PMA) and transfected with small interfering RNA (siRNA) designed to impair galectin-3 expression. Knock-down was confirmed by Western blot analysis (Fig. 5A). Cells were challenged with N. meningitidis strain MC58 at a multiplicity of infection (MOI) of 30 and bacteria were recovered one hour later. Internalisation of N. meningitidis was analysed after gentamicin treatment to kill extracellular bacteria. In parallel we analysed the phagocytosis of N. meningitidis by THP-1 macrophages subjected to transfection in the same conditions as the siRNA treated cells but in absence of siRNA oligonucleotides as controls. Neither the total number of cell-associated bacteria (Fig. 5B) nor the number of internalised bacteria (Fig. 5C) (each expressed as percentage of the inoculum) was affected by knock-down of galectin-3. These data suggest that endogenous galectin-3 is not required for the binding and uptake of N. meningitidis by macrophages.
Fig. 5. Effect of endogenous galectin-3 on the interaction of N. meningitidis with phagocytes.
(A) Confirmation of siRNA transfection and galectin-3 knock down by Western blot. Similar levels of actin protein are observed in both wild-type (Control) and galectin-3 siRNA treated cells, while galectin-3 protein is detected only in wild-type cells. (B) Bacterial association with THP-1 macrophages after 1 hour of infection. The association of meningococci with THP-1 macrophages is shown as percentage of the total bacteria in the inoculum recovered from wild-type (grey bar) and galectin-3 siRNA treated cells (black bar). The percentage of cell-associated bacteria is similar in wild-type and galectin-3 siRNA treated cells. Results are the mean of five different experiments and error bars show standard deviation. (C) The percentage of bacteria which are internalised by THP-1 macrophages after 1 hour of infection and following gentamicin treatment (100 μg/ml for 15 min) is shown as a percentage of the bacteria in the inoculum. There is no significant difference in the internalisation of N. meningitidis by wild-type or galectin-3 siRNA treated cells. Results are the mean of five different experiments and error bars show the standard deviation.
Association of meningococci with phagocytic cells is increased in presence of exogenous galectin-3
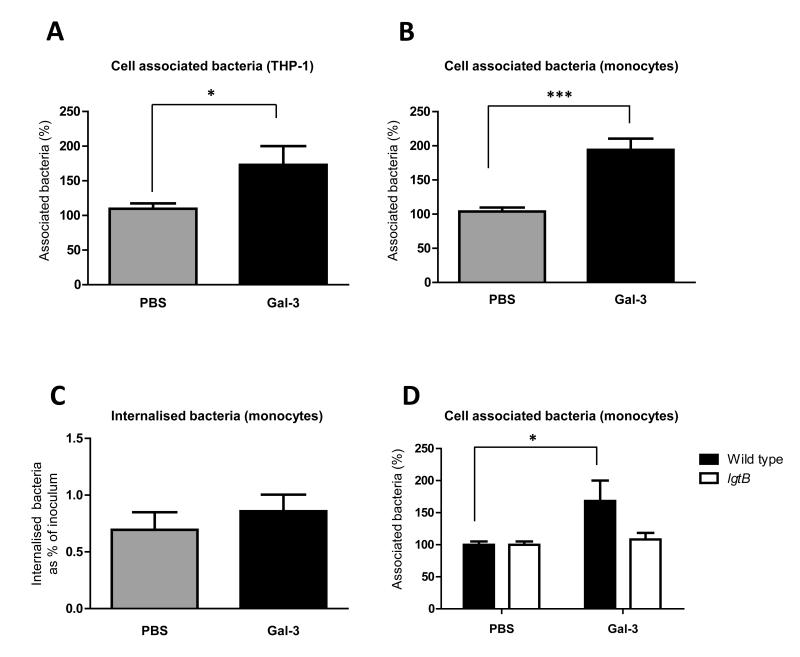
Our initial analysis of the role of galectin-3 in interaction with phagocytes showed that depletion of endogenous galectin-3 from THP-1 macrophages did not affect the adhesion or uptake of meningococci, however, phagocytes also secrete galectin-3 upon activation, and inflammation is characterised by galectin-3 in the microenvironment. We therefore examined whether the binding of exogenous galectin-3 to N. meningitidis had an impact on the bacterial-phagocyte interaction. We analysed the effect of pre-incubating N. meningitidis with galectin-3 on association with and uptake into THP-1 macrophages and primary human monocytes obtained from whole blood of healthy donors. Wild-type THP-1 cells were differentiated into macrophages using PMA for 72 hrs and infected at an MOI of 30 for one hour with wild-type N. meningitidis pre-incubated with 3.3 μM galectin-3 or PBS. Pre-incubation of meningococci with recombinant galectin-3 resulted in a significant increase (1.7 fold, p=0.0201) in the total number of bacteria associated with THP-1 cells (expressed as a percentage of the inoculum, Fig. 6A). Similar results were obtained after infection for 6 hrs (Fig. S2) and with primary human monocytes (2 fold increase, p=0.0002) (Fig. 6B). On the other hand, we observed that the percentage of bacteria which are internalised by monocytes and THP-1 cells is comparable for bacteria pre-incubated with PBS or galectin-3 (Fig. 6C and Fig. S3), suggesting that exogenously added galectin-3 increases the adhesion of bacteria to these cells, with no net effect on bacterial internalisation.
Fig. 6. Influence of exogenous galectin-3 on the interaction of N. meningitidis with phagocytes.
(A) Association of meningococci to PMA-differentiated THP-1 macrophages analysed by bacterial CFU recovery following infection with bacteria pre-incubated with 3.3 μM galectin-3 (black bars) or PBS (grey bars) for 1 hr. Percentage of cell-associated bacteria pre-incubated with galectin-3 was normalised to the percentage of cell-associated bacteria pre-incubated with PBS. The association of bacteria pre-incubated with galectin-3 is significantly increased compared to bacteria pre-incubated with PBS (*, p=0.0201). Data are from six different experiments performed in duplicate. (B) Association of meningococci to primary human monocytes 90 min after infection following pre-incubation of MC58 with galectin-3 (3.3 μM) or PBS prior to infection. The percentage of cell-associated bacteria pre-incubated with galectin-3 was normalised to the percentage of cell-associated bacteria pre-incubated with PBS. The number of bacteria recovered from cells after pre-incubation with galectin-3 is significantly higher than the number of bacteria recovered following pre-incubation with PBS (***, p=0.0002). Data are normalised from five different experiments performed in duplicate. (C) Uptake of meningococci by primary monocytes shown as the percentage of internalised bacteria relative to the total number of bacteria in the inoculum, 90 min post inoculation. The uptake of bacteria by primary monocytes is unaltered following pre-incubation of bacteria with galectin-3 or PBS. (D) Association of meningococci to primary human monocytes following pre-incubation of MC58 and MC58ΔlgtB strains with galectin-3 or PBS prior to infection for 90 min. Association of MC58ΔlgtB strain is unaltered in presence or absence of galectin-3 in contrast to the wild-type strain which shows a significantly increased adhesion (*, p=0.0249) to monocytes when pre-incubated with galectin-3. The percentage of cell-associated bacteria pre-incubated with galectin-3 is normalised to the percentage of cell-associated bacteria pre-incubated with PBS. Data are from nine different experiments performed in duplicate. The actual percentage of association of the wild-type and the lgtB mutant to primary monocytes is shown in Fig. S5.
Finally, we examined whether presence of LacNAc in the bacterial LPS was required for the galectin-3-mediated enhanced association to monocytes, using the lgtB mutant that fails to bind galectin-3 (Fig. 4B). Addition of exogenous galectin-3 had no effect on the adhesion of the lgtB mutant (Fig. 6D), indicating that a full length LPS is required for the galectin-3 enhanced interaction of N. meningitidis with phagocytes.
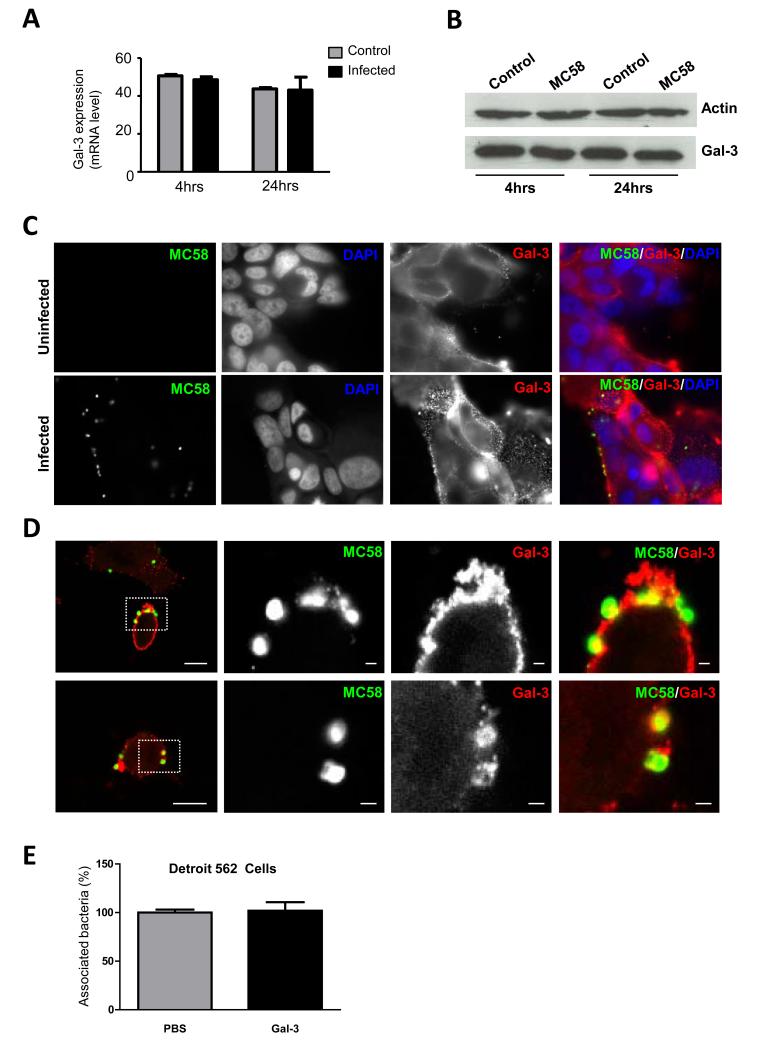
Galectin-3 expression in epithelial cells is unaltered upon infection with N. meningitidis
Several pathogens can induce the upregulation and secretion of galectin-3 from epithelial surfaces. For example, this protein is released in soluble form into the alveolar fluid during pneumococcal infection in mice (Sato et al., 2002) and H. pylori induces galectin-3 upregulation in and release from human gastric epithelial cells (Fowler et al., 2006). The first cells that N. meningitidis encounters in the human host are nasopharyngeal epithelial cells (Carbonnelle et al., 2009). We therefore analysed galectin-3 mRNA level in the human pharyngeal cell line, Detroit 562, with or without infection with N. meningitidis (Fig. 7A). Levels of galectin-3 mRNA were not altered 4 hrs or 24 hrs after challenge and there was no obvious change in galectin-3 protein production as shown by Western blot (Fig. 7B). Using fluorescence microscopy we also assessed whether galectin-3 is expressed on the cell surface, where it could potentially serve as a receptor for N. meningitidis, as it is known that other bacteria bind to galectin-3 on epithelial cell surfaces (Gupta et al., 1997; Fowler et al., 2006). We were able to detect surface galectin-3 on uninfected cells or cells infected with MC58 (Fig. 7C). Whilst we did not observe any striking change in the intensity of staining of surface galectin-3 or localisation in infected cells, in some instances we could detect co-localisation of galectin-3 with adherent meningococci (Fig. 7D). Finally, given that pre-incubation of N. meningitidis with galectin-3 led to a higher number of bacteria associated to the surface of phagocytes we examined whether this was also observed upon interaction with epithelial cells; however, the preincubation of bacteria with galectin-3 did not affect their association with Detroit 562 cells (Fig. 7E).
Fig. 7. Galectin-3 expression in epithelial cells during infection in vitro.
(A) Relative amount of galectin-3 transcripts in infected (infected, black bars) and uninfected (control, grey bars) Detroit 562 epithelial cells, measured by relative RT-PCR. Galectin-3 mRNA expression levels are comparable in infected and uninfected cells following 4 and 24 hrs of infection with N. meningitidis MC58 strain (MOI of 30). Values obtained from the PCR analysis were normalized to results for expression of the β-actin housekeeping gene. (B) Western blot analysis showing galectin-3 protein levels compared to the expression of actin in infected (MC58) and uninfected (Control) cells following 4 or 24 hrs of infection. Levels of galectin-3 are similar in all samples when normalised to the amount of actin. (C) Fluorescence microscopy showing non-permeabilised Detroit 562 cells stained with an anti-galectin-3 antibody (red) and meningococci detected with an anti-LPS antibody (green). Cell surface galectin-3 expression is observed in both infected and uninfected control cells. (D) Confocal microscopy showing non-permeabilised Detroit 562 cells stained for surface galectin-3 (red) infected with GFP-MC58. Extracellular galectin-3 co-localises with meningococci. Scale bars correspond to 10 μm in first panel and 1 μm in subsequent panels. Enlargements correspond to boxed regions. (E) Association of meningococci to Detroit 562 epithelial cells infected at an MOI of 30 for 1 hr following pre-incubation of bacteria with 3.3 μM galectin-3 (black bars) or PBS (grey bars). Bacterial interaction was measured as a percentage of cells associated bacteria pre-incubated with galectin-3 normalised to the cells associated bacteria pre-incubated with PBS. No significant difference in the percentage of bacterial association was observed following pre-incubation with galectin-3 or with PBS. Data are from three different experiments; error bars indicate the standard deviation.
Meningococcal bacteraemia is reduced in Galectin-3 deficient mice
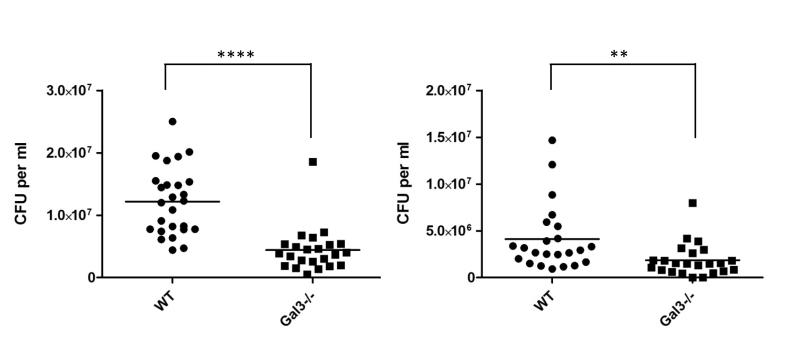
To gain insight into the role of galectin-3 in live meningococcal infection we compared the levels of bacteraemia using wild-type and galectin-3 deficient mice, both in a C57BL/6 genetic background (Fig. 8). Although N. meningitidis is a human specific pathogen, murine galectin-3 binds the meningococcus (Fig. S5) and the murine model has been used in previous studies to examine virulence (Exley et al., 2005), assess efficacy of vaccine antigens (Li et al., 2009) and analyse the contribution of innate immune components to bacterial survival (Pluddemann et al., 2009a). Groups of 8-week old mice were challenged by intraperitoneal injection of N. meningitidis and bacteraemia was measured at seven and 24 hrs post challenge by counting the number of bacteria in blood after serial dilution and plating. At seven hrs, the average bacteraemia from wild-type and Gal3−/− mice was 1.2×107 CFU/ml of blood and 4.4×106 CFU/mL of blood, respectively (Fig. 8. p <0.0001). A significant difference in levels of bacteraemia between the wild-type and Gal3−/− mice was also evident at 24 hrs (Fig. 8. p=0.087). This demonstrates that galectin-3 promotes bacteraemic meningococcal disease in this model.
Fig.8. Galectin-3 increases levels of bacteraemia in mice.
Wild-type and galectin-3 deficient (Gal3−/−) mice were infected with N. meningitidis strain MC58 and bacterial levels in blood were determined at 7 and 24 hrs post challenge. At both time points, significantly fewer bacteria were recovered from Gal3−/− mice (**** p<0.0001; ** p=0.0087). Data are from two independent experiments. Counts for individual mice in each group are shown and lines represent mean values. Results were analysed using a two-tailed, unpaired t test.
DISCUSSION
N. meningitidis is a frequent commensal of the upper respiratory tract, but can also cause serious blood and central nervous system infections manifested as septicaemia and meningitis. A characteristic of meningococcal disease is the profound inflammatory response that is largely responsible for the often devastating outcomes of this infection (Stephens et al., 2007). Galectin-3 production and secretion is highly upregulated in response to inflammatory stimuli (Liu et al., 1995), thus meningococci are likely to encounter this molecule in several sites where cells have been activated during invasive disease. Here we demonstrate for the first time that galectin-3 binds to N. meningtidis in vitro and show a striking colocalisation of meningococci and galectin-3 in tissue from patients with meningococcal infection by immunohistochemistry. This interaction relies on the presence of full length LPS molecules containing LacNAc and we show that galectin-3 binding enhances the association of meningococci to the surface of monocytes and macrophages.
Galectin-3 expression is associated with inflammatory responses to different parasites and bacterial pathogens. It is found in lung tissue from mice infected with S. pneumoniae (Farnworth et al., 2008) and in patients with disseminated C. albicans infection (Kohatsu et al., 2006). Consistent with this, by immunohistochemistry we found that galectin-3 is present in spleen tissue from mice and patients infected with N. meningitidis, and is evident within cells and in the extracellular compartment. Interestingly, we did not detect a similar presence of proto-type galectin-1, which has been shown to share some functions with galectin-3 in inflammatory responses (Almkvist et al., 2004), or tandem-repeat type galectin-4 which has been proposed to contribute to the innate immune response to bacterial infection (Stowell et al., 2010).
Similar to findings with the gonococcus (John et al., 2002), we showed that human galectin-3 binds to N. meningitidis and that this is dependent on the presence of LacNAc in the outer core of the alpha-chain of LPS. Although the lsi-1 mutant showed a significant reduction in galectin-3 binding, this could be due to the interdependence of synthesis of the alpha- and beta-chains; there is no discernible oligosaccharide extension in the lsi-1 mutant (Jennings et al., 1995a). It is also possible that the inner core is important for binding, by allowing appropriate positioning or presentation of the molecule and/or by supporting multiple interactions and permitting galectin-3 oligomerisation, as was recently described for glycoprotein ligands of galectin-3 (Ahmad et al., 2004; Krzeminski et al., 2011). Importantly, since galectin-1 and galectin-4 did not react, the mere presence of the LacNAc residue which is recognised by all three galectins with similar affinity (Dam et al., 2005) is not sufficient for binding and highlights the specific nature of the interaction between galectin-3 and N. meningitidis.
The ability of lactose to inhibit binding of galectin-3 to N. meningitidis shows that the interaction relies largely on the CRD of galectin-3, although we also observed some residual binding to N. meningitidis in the presence of lactose. In addition, the CRD is not sufficient for interaction as we also found that there was almost complete abolition of binding between meningococci and the truncated form of galectin-3 devoid of the N-terminal section. This may reflect a requirement for both the N- and C-terminal regions of galectin-3 to bind different bacterial targets, for example, the N-terminal domain of galectin-3 has been shown to bind to lipid A of S. minnesota in a manner which is not inhibited by lactose (Mey et al., 1996). In addition, this could also reflect that oligomerisation of galectin-3 is important for binding. It has been shown that the association via the non-CRD region of galectin-3 leads to concentration- and time-dependent formation of oligomers, increasing the affinity of this protein compared to the monomeric form and leading to positive cooperativity (Hsu et al., 1992; Massa et al., 1993; Ahmad et al., 2004). We propose that binding of galectin-3 to N. meningitidis is achieved through multivalent interactions with bacterial LPS molecules and with itself, a hypothesis which is supported by recent evidence that LPS from E. coli can induce galectin-3 oligomerisation (Fermino et al., 2011).
The presence of terminal sialic acid on cell surface glycans can modulate and even block galectin interactions, although it is thought to have little effect on galectin-3 binding (Stowell et al., 2008). Consistent with this, we found that removal of the sialic acid capsule and/or the terminal sialylation of LPS did not significantly increase the binding of galectin-3 to N. meningitidis. We observed that the propensity of different strains of N. meningitidis to bind galectin-3 was associated with their LPS immunotype. Meningococcal LPS can be classified into different immunotypes based on variations in composition that alter its structure and antigenic properties (Tsai et al., 1983; Plested et al., 1999). All the strains which bind galectin-3 are able to express an LPS immunotype containing LNnT (and LacNAc); the only strain which did not show similar levels of binding was F8238, a serogroup A isolate which expresses LPS immunotype L10 (Maslanka et al., 1997) that is proposed to lack this structure (Kim et al., 1994). Interestingly, through phase variation, individual meningococcal strains have the potential to express alternative outer core structures (Jennings et al., 1999; Berrington et al., 2002). For example, phase variation leading to switching off expression of the lgtA gene results in loss of LacNAc and complete LnNT epitopes and a switch from immunotype L3 to L8 (Jennings et al., 1995b). Our results using a defined LPS mutant lacking lgtA suggest that this phase variation event would abolish galectin-3 binding. Of note, expression of an L3,7,9 immunotype is associated with invasive disease isolates (Mackinnon et al., 1993). To date this has been largely associated with the ability of this structure to undergo sialylation which enhances serum resistance (Moran et al., 1994) but further elucidation of the consequences of galectin-3 binding may reveal that the capacity to bind this lectin also confers a survival advantage to the meningococcus during infection.
Galectin-carbohydrate interactions have been proposed as a mechanism of pathogen recognition and attachment (Sato et al., 2009; Vasta, 2009). Binding of galectin-3 to several microorganisms has been described (Altman et al., 2001; John et al., 2002; Fowler et al., 2006; Stowell et al., 2010) and cell surface or extracellular galectin-3 can be involved in pathogen binding. N. meningitidis is largely an extracellular pathogen but a key event in meningococcal infection is the interaction with host cells (Carbonnelle et al., 2009). N. meningitidis encounters different cell types from the state of colonisation to invasion and the onset of systemic disease. These include epithelial cells in the nasopharynx to which the bacteria must bind in order to establish carriage, resident macrophages in the submucosa during the early invasive process, endothelial cells during traversal and infiltration of the systemic circulation and monocytes in the bloodstream. To investigate the possible effects of galectin-3 binding to N. meningitidis, we used siRNA knock down and relevant human cell infection models in vitro to investigate the consequences of the interaction on the association of bacteria with different cell types.
Other pathogens such as H. pylori and P. aeruginosa (Gupta et al., 1997; Fowler et al., 2006) bind galectin-3 on the surface of epithelial cells and it has been proposed as a receptor for N. gonorrhoeae as it is constitutively expressed at the apical side of the non-ciliated fallopian tube epithelial cells which selectively bind gonococcus (John et al., 2002). Galectin-3 expression has also been demonstrated in human nasopharyngeal epithelial tissues (Saussez et al., 2008). We found that Detroit 562 human pharyngeal epithelial cells express galectin-3 and expression levels are unchanged upon infection with N. meningitidis. We also found that the protein is detectable on the surface of Detroit 562 cells and confocal microscopy indicated that in some instances galectin-3 co-localises with meningococci. It is therefore possible that galectin-3 on the surface of these cells could contribute to meningococcal attachment, similar to other pathogens. Although our experiments did not address this directly, we found that pre-incubation of bacteria with galectin-3 did not result in a reduction in the association of the bacteria, which might be expected if surface galectin was a direct target for meningococcal LPS binding. To conclusively address the role of epithelial cell surface galectin-3 as a receptor for N. meningitidis, more detailed studies are required.
In order to investigate the role of galectin-3 in meningococcal interactions with phagocytes, we used differentiated THP-1 cells as a source of macrophage-like cells and employed siRNA technology to gain insights into galectin-3 function in adhesion/uptake. Galectin-3 appears to be important for phagocytosis of erythrocytes and thymocytes (Sano et al., 2003), however, we found that siRNA knock-down of galectin-3 in THP-1 macrophages did not alter the capacity of these cells to bind or phagocytose N. meningitidis. This indicates that neither surface expressed nor intracellular galectin-3 is necessary for meningococcal recognition and uptake by macrophages in vitro. Galectin3 accumulations have been observed in macrophages infected with Shigella, Listeria and Mycobacteria (Beatty et al., 2002; Paz et al., 2010), but using macrophages from galectin-3 null mice, no role for intracellular galectin-3 in mycobacterial or Salmonella uptake and survival was identified (Beatty et al., 2002; Li et al., 2008). In fact, we are not aware of evidence for endogenous galectin-3 having a role in phagocytosis of any bacterium by macrophages.
Interestingly, pre-incubation of N. meningitidis with exogenous galectin-3 led to a significant increase in bacterial adhesion to monocytes and macrophages but did not alter internalisation. This increased adhesion was dependent upon expression of full length meningococcal LPS molecules, which permit galectin-3 binding. Recent data has shown that pre-incubation of exogenous galectin-3 with purified LPS dramatically increases LPS binding to neutrophil surfaces (Fermino et al., 2011) and our observations with whole bacteria and macrophages or monocytes are consistent with this. The increased attachment of the meningococcus to the surface of macrophages and monocytes but not to epithelial cells suggests this role is restricted to phagocyte interactions and may be related to different repertoires of receptors or the different outcomes of interaction with these cell types. Previous work has shown that human macrophages can bind, phagocytose and at least partially kill N. meningitidis (Read et al., 1996) and receptors that have been implicated in meningococcal interaction with phagocytes include TLRs, MARCO, scavenger receptors (Pluddemann et al., 2009b) and siglecs (Jones et al., 2003). How pre-incubation of N. meningitidis with galectin-3 leads to increased adhesion without affecting uptake remains to be elucidated. Taking into account the known properties of this protein, we speculate that decoration of the bacterial surface with galectin-3 could enhance adhesion through cross-linking of surface glycans, receptor rearrangement, or modification of signaling pathways, in a way that leads to bacteria being maintained on the surface.
Our data from in vivo infection experiments are consistent with galectin-3 being beneficial for meningococcal survival, as we found that the bacterial load in the blood was significantly lower in mice lacking galectin-3. Given the pleiotropic roles of both endogenous and exogenous galectin-3 in response to infection (Vasta, 2009), there are several potential explanations for this finding. One possibility is that the increased association to macrophages and monocytes we observed in vitro could lead to enhanced meningococcal survival, for example by allowing escape from phagocytosis by neighbouring cells, enhancing dissemination or affecting the activity of the phagocyte through altered TLR ligand presentation. Gal3−/− mice have been shown to be more susceptible to LPS-induced shock yet are relatively resistant to infection with Salmonella (Li et al., 2008). Bacteria may therefore use galectin-3 to mask LPS on the surface, promoting bacterial replication. We are currently analysing macrophage and monocyte cytokine responses to N. meningitidis in presence or absence of galectin-3 to elucidate how this interaction affects the LPS induction of host immune response during infection.
Overall, our data reveal an interaction between N. meningitidis and galectin-3. We propose that this interaction could occur during meningococcal infection at sites where local concentrations of galectin-3 secreted by activated immune cells could accumulate and bind to the surface of bacteria. As a consequence of this interaction, bacterial adhesion to the surface of phagocytes is increased; this could eventually lead to enhanced meningococcal survival, for example by allowing escape from phagocytosis by neighbouring cells, affecting the activity of the phagocyte through altered TLR ligand presentation or enhancing dissemination. Further elucidation of the roles of galectin-3 during meningococcal infection will provide important insights into the contribution of this protein to bacterial survival and disease progression.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions
N. meningitidis was grown on Brain Heart Infusion (BHI) agar (1.5% wt/vol, Oxoid) at 37°C in the presence of 5% CO2. MC58 is a serogroup B isolate of N. meningitidis. The mutants MC58ΔsiaD, MC58ΔsiaC and MC58Δlst, MC58ΔlgtA, MC58ΔlgtB, MC58ΔgalE, MC58Δlsi-1, MC58ΔicsB have been described previously (Table 1).
Galectin-3 and antibodies
The lectins used in this work were either purchased as recombinant purified protein from R&D Systems (human galectin-3) or purified after recombinant production by affinity chromatography on lactosylated Sepharose 4B. LPS contamination was removed by a further chromatographic step as previously described (Sarter et al., 2009). Purity was ascertained by one- and two-dimensional gel electrophoreses and mass spectrometry, which was also performed after proteolytic truncation with collagenase at Tyr106/Ile107 and Glu229/Ile230 peptide bonds and biotinylation was carried out under activity preserving conditions (Kubler et al., 2008). Activity controls were run by solid-phase and cell assays using asialofetuin as a matrix as described in (Andre et al., 2009; Leyden et al., 2009). Polyclonal antibodies against full length galectin-1, galectin-3 and galectin-4 were made and checked for specificity and absence of cross-reactivity to other galectins as previously described (Kaltner et al., 2002; Lohr et al., 2007). Rabbit poly-clonal anti-human galectin-3 antibody was also purchased from Santa Cruz Biotechnology.
Challenge of mice with live Neisseria meningitidis
All animal experiments were carried out under protocols reviewed and approved by the Home Office, United Kingdom. For immunohistochemistry, groups of ten 6-week old female BALB/c mice (Harlan) were housed under pathogen-free conditions, acclimatised for one week and randomly distributed in two groups. Animals were challenged by intraperitoneal injection of N. meningitidis MC58. Bacteria were grown overnight on BHI plates and then harvested into phosphate-buffered saline (PBS). The number of CFU was estimated by measuring the absorbance at 260 nm of a lysate of the suspension in P2 lysis buffer (Qiagen), and the number of viable bacteria was confirmed by plating to solid media. Each animal in the challenge group received 1×106 CFU of MC58 in 0.5 ml of BHI medium containing 0.5% (vol/vol) iron dextran (Sigma, Poole, United Kingdom). Control groups consisted of mice given BHI medium with iron dextran alone (uninfected). After 24 or 48 hrs, the animals were sacrificed and multiple organs were collected, fixed in 10% formalin for one week and embedded in paraffin using routine methods. Five micrometer thickness sections were cut from each block for analysis.
Bacteraemia was assessed in wild-type and galectin-3 deficient (Gal3−/−) C57BL/6 mice which were generated by targeted disruption of the galectin-3 gene (Hsu et al., 2000). Groups of eight or ten 8-week old mice were challenged by intraperitoneal injection of N. meningitidis MC58 grown overnight at 37°C in 5% CO2 on BHI plates. Each animal received 1×108 CFU in 0.5 ml of BHI medium containing 0.5% (vol/vol) iron dextran and bacterial dose was verified by serial dilution and plating to BHI at the time of infection. Blood was obtained from mice 7 and 24 hrs after challenge, and serial dilutions were plated to BHI agar to quantify bacteria after overnight growth at 37°C, 5% CO2. Experiments were performed on two independent occasions. Counts for individual animals were plotted and results were analysed using a two-tailed, unpaired t-test.
Tissue immunostaining
The presence of galectins-1, -3, and -4 was assessed in different organs from mice challenged with N. meningitidis strain MC58 or uninfected animals and presence of galectin-3 was assessed in spleen tissue sections from patients with systemic meningococcal infection and control tissue from patients without bacterial infection. Tissues were obtained during a research study in 1991-1992 (Lucas et al., 1993). Paraffin-embedded sections were deparaffinised in xylene, and rehydrated in serially diluted ethanol (100%, 95%, 75%, 50% and 0%). Following washes with distilled water for 5 min, sections were incubated with 3% methanol for 10 min to quench endogenous peroxidase. For antigen retrieval specimens were boiled in citrate buffer under full vapour pressure for 2 min, and washed in distilled water and in PBS/0.1% Tween-20. Following incubation with 150 μl of protein block (Dako) for 5 min, samples were incubated overnight at 4°C with 200 μl of polyclonal anti-galectin-3 antibody (2 μg/ml) or 200 μl of a 1/500 dilution of polyclonal antibody against N. meningitidis (Fitzgerald Industries International). The following day, sections were washed with PBS/0.1% Tween-20 and incubated with 100 μl of goat anti-rabbit antibody conjugated with horseradish peroxidase (HRP; Dako) or alkaline phosphatase (AP; Dako) for 30 min. After washing with PBS/0.1% Tween-20, galectins in the tissues were visualized by incubation in diaminobenzidine (DAB) for HRP or permanent red for AP. Finally tissue sections were counterstained with Mayer’s hematoxylin, mounted and analysed with a light microscope. The immunohistochemical analysis for different galectins was repeated at least three times.
Cell cultures
The monocytic cell line THP-1 and the nasopharyngeal epithelial cell line Detroit 562 were purchased from ATCC. THP-1 cells were grown in RPMI 1640 medium (Gibco, Invitrogen) with 2 mM glutamine (Invitrogen) supplemented with 10% foetal calf serum (PAA Laboratories), 10 mM HEPES, 1 mM sodium pyruvate, 1.25 g D-glucose, 0.05 mM β-mercaptoethanol. THP-1 cells (1 × 106) were differentiated into macrophages in 24 well-plates containing 1 ml RPMI 1640 medium with 100 ng/ml PMA for 72 hrs prior to experiments. Detroit 562 epithelial cells were cultured in Minimum Essential Medium (Eagle) with non essential amino acids (Invitrogen). Detroit 562 medium was supplemented with 2 mM Glutamax™ (Invitrogen), 10% foetal calf serum, 1 mM sodium pyruvate and lactalbumin hydrolysate (0.1%). Cells were cultured to a density of 1-5 × 105 cells/ml and maintained at 37°C with 5% CO2.
siRNA procedures
Transfection of THP-1 cells was performed using Thermo Scientific Dharmacon On Target plus SMARTpool, a four si-RNA oligo system designed specifically for galectin-3. The RNA sequences targeted are: GGAGAGUCAUUGUUUGCAA, GUACAAUCAUCGGGUUAAA, GGCCACUGAUUGUGCCUUA, CGGUGAAGCCCAAUGCAAA. Briefly, 1×106 cells were transfected with 3 μg of siRNA using Nucleofector solution V (Dharmacon) according to manufacturer’s instructions. Negative controls were carried out using only the Nucleofector buffer and electroporation was carried out with a Nucleofector machine (Amaxa) using the program V-01 / V-001 (for high transfection efficiency). Cells were then cultured in culture medium supplemented with PMA for 24 hrs to allow attachment. The medium was then replaced with fresh medium to remove dead cells and the cells were cultured for other 48 hrs to allow differentiation into macrophages. To confirm silencing of galectin-3, Western blot analysis was performed on the cells 72 hrs after the initial transfection. All the experiments were performed on cells with no detectable galectin-3.
Preparation of human monocytes
Peripheral blood was extracted from healthy consenting volunteers in accordance with local research committee guidelines and density centrifugation of blood-EDTA on Histopaque 1077 gradient was performed as per the manufacturer’s instructions (Sigma Aldrich). Peripheral blood mononuclear cells (PBMCs) were gently washed off and seeded at 3×105 cells/ml in 24 well plates in RPMI medium without serum and positively selected by plastic adherence for 3 hrs at 37°C, after which residual non adherent B and T cells were washed away. Cell viability was estimated by Trypan blue exclusion and was higher than 95%. Monocytes were infected with N. meningitidis as described for adhesion/internalisation assays.
Flow cytometry
For analysis by flow cytometry, bacteria were grown overnight on BHI agar and then harvested into PBS. The number of bacteria was estimated by measuring the OD at 260 nm of an aliquot of the bacterial suspension lysed in 200 mM NaOH/1% SDS (v/w). Bacteria (2×108 CFU) were fixed in 3% paraformaldehyde for 2 hrs at room temperature, washed three times in PBS, and stored at −80°C in PBS/15% glycerol. To measure galectin binding, 2×107 fixed bacteria were incubated with 30 μl of label free or biotinylated galectins (Andre et al., 2006) at a final concentration of 3.3 μM (100μg/ml) for 1 hr at 37°C and when required lactose was added at a concentration of 100 mM. After two washes with PBS/Tween 0.1% binding was detected following incubation with a rabbit anti-human galectin antibody (Lohr et al., 2008) for 45 min at 4°C or a mouse anti-biotin antibody. The cells were washed twice in PBS/Tween 0.1%, then resuspended in 50 μl of PBS with a polyclonal donkey anti-rabbit IgG-Cy2 conjugate (1/200 dilution in PBS; Jackson ImmunoResearch Laboratories) or a polyclonal donkey anti-mouse IgG FITC conjugate (1/200 dilution; Jackson ImmunoResearch Laboratories) and incubated for 30 min on ice. Galectin binding was quantified using a flow cytometer (Calibur FACScan; BD Biosciences), and at least 4×104 events were recorded. Galectin-3 binding was expressed by calculating the Fluorescence Index (% positive gated bacteria multiplied by the geometric mean fluorescence (Findlow et al., 2006)). Unless otherwise stated, data shown is the mean and standard deviation and the number of experiments is specified in the figure legends.
RNA procedures and RT-PCR
RNA samples were isolated by using RNeasy mini kit (QIAGEN) and genomic DNA was removed by treatment with DNase (QIAGEN). 400 ng RNA was reverse transcribed to cDNA using the QuantiTect reverse transcription kit (QIAGEN). Transcript levels were measured by qrtRT-PCR using QuantiFast with SYBR green detection (QIAGEN) on a thermal cycler (Rotor-Gene 3000; Corbett Research). Results for the transcription of galectin-3 were normalized with levels for the housekeeping gene ß-actin (primers 5′-CTCTTCCAGCCTTCCTTCCT-3′ and 5′-GCACTGTGTTGGCGTACAG-3′). The following primers were used to monitor transcript levels: forward 5′-TGTGCCTTATAACCTGCCTTT-3, reverse 5′-TTAAAGTGGAAGGCAACATCA-3′. Data were analysed using the Comparative Quantitation method by Rotor-Gene software (version 6.0; Corbett Research). Controls included reactions with no template and samples of RNA that had not been treated with reverse transcriptase (RT). qrtRT-PCR was performed in triplicate on cDNA samples derived from three independent assays.
Western blot analysis
Cell pellets 1×106 were resuspended in SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) loading buffer (50 mM Tris-HCl, 2% SDS, 100 μM β-mercaptoethanol, 10% glycerol, 0.1% bromophenol blue), protein samples were separated under reducing conditions by 12% SDS-PAGE, then transferred to PVDF (polyvinylidene fluoride) membranes (Millipore) for 1 hr in cold transfer buffer (25 mM Tris, 190 mM glycine). After blocking overnight in 5% milk in PBS, galectin-3 was detected by incubation of the membrane with anti-galectin-3 (Santa Cruz Biotechnology; 300 ng/ml) or anti-actin (Sigma) for 1 hour at room temperature. Binding of the primary antibody was detected using an anti-rabbit-IgG Horse Radish Peroxidase-conjugated secondary antibody (1:1000 dilution; Santa Cruz Biotechnology), and ECL detection kit (GE Healthcare).
Fluorescence and confocal microscopy
For fluorescence and confocal microscopy, Detroit cells were seeded on glass coverslips and infected with MC58 at a MOI of 30 for 4 hrs at 37°C. Samples were washed twice with PBS, fixed for 20 min at room temperature in 3% paraformaldehyde in PBS, and then washed three times in PBS. For labelling of bacteria the cells were incubated for 1 hr with an anti-lipoplysaccharide (LPS) antibody (α-L3,7,9) and with an anti-galectin-3 antibody (Santa Cruz Biotechnology), after which the cells were washed twice in PBS then incubated for 30 min with an anti-mouse Alexa Fluor 488-conjugated antibody and anti-human Alexa Fluor 555-conjugated antibody (Invitrogen) to label bacteria and cells respectively. DAPI staining was also performed to visualise nuclei. After labelling, cells were washed twice in PBS and then double distilled water, mounted in Aqua Poly/Mount (Polysciences Inc), and analysed by fluorescence microscopy (BX40, Olympus) or by laser-scanning confocal microscopy (Zeiss Axiovert LSM510).
Adhesion and internalisation assays
For adhesion and internalisation assays Detroit 562 cells, THP-1 cells differentiated with PMA and human primary monocytes from healthy volunteers were used. Bacteria were harvested following overnight culture on solid media and re-suspended in 400 μl of PBS. The concentration of the bacterial suspension was adjusted in culture media to give the desired MOI. PMA differentiated THP-1 and Detroit 562 were infected at a MOI of 30 for 1 hr at 37°C, while human primary monocytes were infected at a MOI of 50 for 90 min at 37°C, in each case following pre-incubation of bacteria with 30 μl of recombinant galectin-3 (3.3 μM) or PBS for 1 hr at 37°C. For adhesion assays, cells were washed extensively with PBS to eliminate non-adherent bacteria, then lysed with 1% saponin for 10 min at 37°C to recover cell associated bacteria. For internalisation assays, cells were washed with PBS to eliminate non-adherent bacteria and exposed to gentamicin (100 μg/ml) for 15 min to kill extracellular bacteria. Cells were washed extensively with PBS after gentamicin treatment to remove the antibiotic and dead extracellular bacteria, and subsequently lysed or reincubated in medium for 1 or 2 hrs. In both adhesion and internalisation assays serial dilutions of bacteria were plated following cell lysis or gentamicin treatment and CFU were counted the following day. Bacteria recovered from cells without gentamicin treatment represented the total number of bacteria associated with cells; bacteria recovered from cells treated with gentamicin represent the number of internalised bacteria.
Statistical analysis
The statistical significance of galectin-3 binding or interaction with cells was determined with the Graph Pad Prism 5 Software by using the Student’s unpaired t-test for flow cytometry data analysis, the one-tailed, paired Student’s t-test for cell adhesion or internalisation assay data analysis and a two-tailed, unpaired t-test for analysis of levels of bacteraemia. Statistical significance is indicated with asterisks where *=p <0.05, **=p<0.01 and ***=p<0.001 and ****=p<0.0001 Exact p values are shown in the figure legends.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Holden and his group for helpful comments and are grateful to Antonio Santos for assistance with confocal microscopy. MH was supported by the DFG (SFB643), the Interdisciplinary Center of Clinical Research (IZKF) at the University Hospital of the University of Erlangen-Nuremberg and the K&R Wucherpfennigstiftung. HJG wishes to acknowledge the generous funding by the EC research program GlycoHIT and inspiring discussions with Dr. B. Friday. Work in CT’s lab is funded by the MRC and Wellcome Trust.
REFERENCES
- Ahmad N, Gabius HJ, Kaltner H, Andre S, Kuwabara I, Liu FT, et al. Thermodynamic binding studies of cell surface carbohydrate epitopes to galectins-1, -3 and -7. Evidence for differential binding specificities. Can. J. Chem. 2002;80:1096–1104. [Google Scholar]
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–581. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- Altman E, Harrison BA, Latta RK, Lee KK, Kelly JF, Thibault P. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem Cell Biol. 2001;79:783–788. [PubMed] [Google Scholar]
- Andre S, Pei Z, Siebert HC, Ramstrom O, Gabius HJ. Glycosyldisulfides from dynamic combinatorial libraries as O-glycoside mimetics for plant and endogenous lectins: their reactivities in solid-phase and cell assays and conformational analysis by molecular dynamics simulations. Bioorg Med Chem. 2006;14:6314–6326. doi: 10.1016/j.bmc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Andre S, Specker D, Bovin NV, Lensch M, Kaltner H, Gabius HJ, Wittmann V. Carbamate-linked lactose: design of clusters and evidence for selectivity to block binding of human lectins to (neo)glycoproteins with increasing degree of branching and to tumor cells. Bioconjug Chem. 2009;20:1716–1728. doi: 10.1021/bc900152w. [DOI] [PubMed] [Google Scholar]
- Beatty WL, Rhoades ER, Hsu DK, Liu FT, Russell DG. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002;4:167–176. doi: 10.1046/j.1462-5822.2002.00183.x. [DOI] [PubMed] [Google Scholar]
- Bentley SD, Vernikos GS, Snyder LA, Churcher C, Arrowsmith C, Chillingworth T, et al. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 2007;3:e23. doi: 10.1371/journal.pgen.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington AW, Tan YC, Srikhanta Y, Kuipers B, van der Ley P, Peak IR, Jennings MP. Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol Med Microbiol. 2002;34:267–275. doi: 10.1111/j.1574-695X.2002.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23:383–392. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sorensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Carbonnelle E, Hill DJ, Morand P, Griffiths NJ, Bourdoulous S, Murillo I, et al. Meningococcal interactions with the host. Vaccine. 2009;27(Suppl 2):B78–89. doi: 10.1016/j.vaccine.2009.04.069. [DOI] [PubMed] [Google Scholar]
- Choudhury B, Kahler CM, Datta A, Stephens DS, Carlson RW. The structure of the L9 immunotype lipooligosaccharide from Neisseria meningitidis NMA Z2491. Carbohydr Res. 2008;343:2971–2979. doi: 10.1016/j.carres.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven DE, Frasch CE, Robbins JB, Feldman HA. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J Clin Microbiol. 1978;7:410–414. doi: 10.1128/jcm.7.5.410-414.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TK, Gabius HJ, Andre S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- Debierre-Grockiego F, Niehus S, Coddeville B, Elass E, Poirier F, Weingart R, et al. Binding of Toxoplasma gondii glycosylphosphatidylinositols to galectin-3 is required for their recognition by macrophages. J Biol Chem. 2010;285:32744–32750. doi: 10.1074/jbc.M110.137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lella S, Sundblad V, Cerliani JP, Guardia CM, Estrin DA, Vasta GR, Rabinovich GA. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry. 2011;50:7842–7857. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards U, Muller A, Hammerschmidt S, Gerardy-Schahn R, Frosch M. Molecular analysis of the biosynthesis pathway of the alpha-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol Microbiol. 1994;14:141–149. doi: 10.1111/j.1365-2958.1994.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Exley RM, Shaw J, Mowe E, Sun YH, West NP, Williamson M, et al. Available carbon source influences the resistance of Neisseria meningitidis against complement. J Exp Med. 2005;201:1637–1645. doi: 10.1084/jem.20041548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermino ML, Polli CD, Toledo KA, Liu FT, Hsu DK, Roque-Barreira MC, et al. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS One. 2011;6:e26004. doi: 10.1371/journal.pone.0026004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlow J, Taylor S, Aase A, Horton R, Heyderman R, Southern J, et al. Comparison and correlation of neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infection and immunity. 2006;74:4557–4565. doi: 10.1128/IAI.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983;111:112–124. [PMC free article] [PubMed] [Google Scholar]
- Fowler M, Thomas RJ, Atherton J, Roberts IS, High NJ. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006;8:44–54. doi: 10.1111/j.1462-5822.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- Gabius HJ, Andre S, Jimenez-Barbero J, Romero A, Solis D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci. 2011;36:298–313. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Geoffroy MC, Floquet S, Metais A, Nassif X, Pelicic V. Large-scale analysis of the meningococcus genome by gene disruption: resistance to complement-mediated lysis. Genome Res. 2003;13:391–398. doi: 10.1101/gr.664303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Cunningham AM, Watson DC, Martin A, Richards JC, Wakarchuk WW. Characterization of a recombinant Neisseria meningitidis alpha-2,3-sialyltransferase and its acceptor specificity. Eur J Biochem. 1997;249:187–194. doi: 10.1111/j.1432-1033.1997.t01-1-00187.x. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Masinick S, Garrett M, Hazlett LD. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect Immun. 1997;65:2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Griffiths NJ, Borodina E, Virji M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin Sci (Lond) 2010;118:547–564. doi: 10.1042/CS20090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167–14174. [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- Jennings MP, Bisercic M, Dunn KL, Virji M, Martin A, Wilks KE, et al. Cloning and molecular analysis of the Isi1 (rfaF) gene of Neisseria meningitidis which encodes a heptosyl-2-transferase involved in LPS biosynthesis: evaluation of surface exposed carbohydrates in LPS mediated toxicity for human endothelial cells. Microb Pathog. 1995a;19:391–407. doi: 10.1006/mpat.1995.0074. [DOI] [PubMed] [Google Scholar]
- Jennings MP, Hood DW, Peak IR, Virji M, Moxon ER. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol Microbiol. 1995b;18:729–740. doi: 10.1111/j.1365-2958.1995.mmi_18040729.x. [DOI] [PubMed] [Google Scholar]
- Jennings MP, Srikhanta YN, Moxon ER, Kramer M, Poolman JT, Kuipers B, van der Ley P. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology. 1999;145(Pt 11):3013–3021. doi: 10.1099/00221287-145-11-3013. [DOI] [PubMed] [Google Scholar]
- John CM, Jarvis GA, Swanson KV, Leffler H, Cooper MD, Huflejt ME, Griffiss JM. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell Microbiol. 2002;4:649–662. doi: 10.1046/j.1462-5822.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- Kaltner H, Seyrek K, Heck A, Sinowatz F, Gabius HJ. Galectin-1 and galectin-3 in fetal development of bovine respiratory and digestive tracts. Comparison of cell type-specific expression profiles and subcellular localization. Cell Tissue Res. 2002;307:35–46. doi: 10.1007/s004410100457. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hirabayashi J. Galectins: a family of animal lectins that decipher glycocodes. J Biochem. 1996;119:1–8. doi: 10.1093/oxfordjournals.jbchem.a021192. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Phillips NJ, Gibson BW, Griffiss JM, Yamasaki R. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect Immun. 1994;62:1566–1575. doi: 10.1128/iai.62.5.1566-1575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleshchenko YY, Moody TN, Furtak VA, Ochieng J, Lima MF, Villalta F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect Immun. 2004;72:6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- Kopitz J, von Reitzenstein C, Andre S, Kaltner H, Uhl J, Ehemann V, et al. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem. 2001;276:35917–35923. doi: 10.1074/jbc.M105135200. [DOI] [PubMed] [Google Scholar]
- Kopitz J, Bergmann M, Gabius HJ. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life. 2010;62:624–628. doi: 10.1002/iub.358. [DOI] [PubMed] [Google Scholar]
- Krzeminski M, Singh T, Andre S, Lensch M, Wu AM, Bonvin AM, Gabius HJ. Human galectin-3 (Mac-2 antigen): Defining molecular switches of affinity to natural glycoproteins, structural and dynamic aspects of glycan binding by flexible ligand docking and putative regulatory sequences in the proximal promoter region. Biochim Biophys Acta. 2011;1810:150–161. doi: 10.1016/j.bbagen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Kubler D, Hung CW, Dam TK, Kopitz J, Andre S, Kaltner H, et al. Phosphorylated human galectin-3: facile large-scale preparation of active lectin and detection of structural changes by CD spectroscopy. Biochim Biophys Acta. 2008;1780:716–722. doi: 10.1016/j.bbagen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939–3944. [PubMed] [Google Scholar]
- Leyden R, Velasco-Torrijos T, Andre S, Gouin S, Gabius HJ, Murphy PV. Synthesis of bivalent lactosides based on terephthalamide, N,N’-diglucosylterephthalamide, and glycophane scaffolds and assessment of their inhibitory capacity on medically relevant lectins. J Org Chem. 2009;74:9010–9026. doi: 10.1021/jo901667r. [DOI] [PubMed] [Google Scholar]
- Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu FT, Springall T, Xu D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- Li Y, Wooldridge KG, Javed MA, Tang CM, Ala’aldeen DA. Secreted proteins of Neisseria meningitidis protect mice against infection. Vaccine. 2009;27:2320–2325. doi: 10.1016/j.vaccine.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis. 2009;9:418–427. doi: 10.1016/S1473-3099(09)70132-X. [DOI] [PubMed] [Google Scholar]
- Lohr M, Lensch M, Andre S, Kaltner H, Siebert HC, Smetana K, Jr., et al. Murine homodimeric adhesion/growth-regulatory galectins-1, -2 and -7: comparative profiling of gene/promoter sequences by database mining, of expression by RT-PCR/immunohistochemistry and of contact sites for carbohydrate ligands by computational chemistry. Folia Biol (Praha) 2007;53:109–128. [PubMed] [Google Scholar]
- Lohr M, Kaltner H, Lensch M, Andre S, Sinowatz F, Gabius HJ. Cell-type-specific expression of murine multifunctional galectin-3 and its association with follicular atresia/luteolysis in contrast to pro-apoptotic galectins-1 and -7. Histochem Cell Biol. 2008;130:567–581. doi: 10.1007/s00418-008-0465-0. [DOI] [PubMed] [Google Scholar]
- Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N’Gbichi JM, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- Mackinnon FG, Borrow R, Gorringe AR, Fox AJ, Jones DM, Robinson A. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microbial pathogenesis. 1993;15:359–366. doi: 10.1006/mpat.1993.1085. [DOI] [PubMed] [Google Scholar]
- Mandrell RE, Lesse AJ, Sugai JV, Shero M, Griffiss JM, Cole JA, et al. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990;171:1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell RE, Kim JJ, John CM, Gibson BW, Sugai JV, Apicella MA, et al. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Cooper DN, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- Mey A, Leffler H, Hmama Z, Normier G, Revillard JP. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J Immunol. 1996;156:1572–1577. [PubMed] [Google Scholar]
- Moran EE, Brandt BL, Zollinger WD. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infection and immunity. 1994;62:5290–5295. doi: 10.1128/iai.62.12.5290-5295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J Immunol. 2008;180:2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Achtman M, James KD, Bentley SD, Churcher C, Klee SR, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 2010;12:530–544. doi: 10.1111/j.1462-5822.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- Plested JS, Makepeace K, Jennings MP, Gidney MA, Lacelle S, Brisson J, et al. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect Immun. 1999;67:5417–5426. doi: 10.1128/iai.67.10.5417-5426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluddemann A, Hoe JC, Makepeace K, Moxon ER, Gordon S. The macrophage scavenger receptor A is host-protective in experimental meningococcal septicaemia. PLoS Pathog. 2009a;5:e1000297. doi: 10.1371/journal.ppat.1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluddemann A, Mukhopadhyay S, Sankala M, Savino S, Pizza M, Rappuoli R, et al. SR-A, MARCO and TLRs differentially recognise selected surface proteins from Neisseria meningitidis: an example of fine specificity in microbial ligand recognition by innate immune receptors. J Innate Immun. 2009b;1:153–163. doi: 10.1159/000155227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RC, Zimmerli S, Broaddus C, Sanan DA, Stephens DS, Ernst JD. The (alpha2-->8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusniok C, Vallenet D, Floquet S, Ewles H, Mouze-Soulama C, Brown D, et al. NeMeSys: a biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis. Genome Biol. 2009;10:R110. doi: 10.1186/gb-2009-10-10-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, et al. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter K, Andre S, Kaltner H, Lensch M, Schulze C, Urbonaviciute V, et al. Detection and chromatographic removal of lipopolysaccharide in preparations of multifunctional galectins. Biochem Biophys Res Commun. 2009;379:155–159. doi: 10.1016/j.bbrc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- Saussez S, Decaestecker C, Mahillon V, Cludts S, Capouillez A, Chevalier D, et al. Galectin-3 upregulation during tumor progression in head and neck cancer. Laryngoscope. 2008;118:1583–1590. doi: 10.1097/MLG.0b013e31817b0718. [DOI] [PubMed] [Google Scholar]
- Scholten RJ, Kuipers B, Valkenburg HA, Dankert J, Zollinger WD, Poolman JT. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J Med Microbiol. 1994;41:236–243. doi: 10.1099/00222615-41-4-236. [DOI] [PubMed] [Google Scholar]
- Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem. 1998;273:13047–13052. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- Sparrow CP, Leffler H, Barondes SH. Multiple soluble beta-galactoside-binding lectins from human lung. J Biol Chem. 1987;262:7383–7390. [PubMed] [Google Scholar]
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 2010;16:295–301. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- Tsai CM, Boykins R, Frasch CE. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983;155:498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P, Kramer M, Martin A, Richards JC, Poolman JT. Analysis of the icsBA locus required for biosynthesis of the inner core region from Neisseria meningitidis lipopolysaccharide. FEMS Microbiol Lett. 1997;146:247–253. doi: 10.1111/j.1574-6968.1997.tb10201.x. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.