Abstract
Mitochondrial dynamics play a crucial role in the pathobiology underlying Alzheimer’s disease (AD) and Parkinson’s disease (PD). Although a complete scientific understanding of these devastating conditions has yet to be realized, alterations in mitochondrial fission and fusion, and in the protein complexes that orchestrate mitochondrial fission and fusion, have been well established in AD- and PD-related neurodegeneration. Whether fission/fusion disruption in the brain is a causal agent in neuronal demise or a product of some other upstream disturbance is still a matter of debate; however, in both AD and PD, the potential for successful therapeutic amelioration of degeneration via mitochondrial protection is high. We here discuss the role of mitochondrial dynamics in AD and PD and assess the need for their therapeutic exploitation.
Keywords: Alzheimer’s disease, DLP-1, Drp-1, Fis1, fission, fusion, Mfn1, Mfn2, mitochondrial dynamics, neurodegeneration, Parkinson’s disease, therapeutics
INTRODUCTION
The tremendous complexity that characterizes eukaryotic cell activity fundamentally relies on healthy mitochondria. Oxidative phosphorylation, the process by which the electrons extracted from glucose metabolism establish a proton gradient for the synthesis of cellular ATP, occurs within the mitochondrial inner-membrane via protein machinery partly encoded for by mitochondrial DNA (mtDNA). The multitude of cellular interactions driven by the energy stored in the phosphate bonds of ATP therefore depend on an intact oxidative phosphorylation program, and ergo, competent mitochondria. Calcium (Ca2+) buffering, a process that both complements and stimulates oxidative phosphorylation [1] and permits cytosolic calcium signaling [2], is largely mediated by mitochondrial calcium transport mechanisms, and thus also relies on adequate mitochondrial function.
In regard to the stimulation of oxidative phosphorylation, the three key metabolic enzymes, pyruvate, ketoglutarate, and isocitrate dehydrogenases, have been demonstrated to be activated by Ca2+ ions, with pyruvate dehydrogenase undergoing a Ca2+-dependent dephosphorylation step and ketoglutarate and isocitrate dehydrogenases becoming directly activated upon Ca2+ binding [3]. An activated cell, which typically exhibits waves of Ca2+ increase (i.e., as a ubiquitous method of signal cascade propagation), thus becomes able to compensate for the extra activity by increasing the generation of ATP within its mitochondria. The cytosolic rises in Ca2+ concentrations that characterize such cell stimulation are also partly buffered by mitochondria to optimize intracellular signaling and to ensure its correct spatiotemporal patterning, especially at places where the endoplasmic reticulum (ER) is lacking [2]. Notably, the ER is the primary mediator of intracellular Ca2+ signaling/ buffering, acting as a high-affinity, low-capacity reserve that buffers Ca2+ at concentrations below 0.5 µM [4, 5]. In the presynaptic terminal of a neuron, however, where ER is lacking, the Ca2+ influx generated by action potential-triggered opening of voltage sensitive Ca2+ channels elicits vesicular release of neurotransmitters; mitochondria in the nerve ending act as a Ca2+ sink to remove the signaling ion from the terminal to enable subsequent activation [6]. Mitochondria located near microdomains that are especially sensitive to the presence of Ca2+ ions, such as those surrounding Ca2+ channels, ensure adequate Ca2+ homeostasis such that the optimal functioning of those areas may be reached [7].
Apoptosis is also critically regulated and mediated by mitochondria. Following intrinsic apoptosis-triggering events, such as DNA damage or ER stress, the mitochondrial outer membrane undergoes permeabilization via a specific set of signaling proteins, the result of which is mitochondrial release of the electron transport protein cytochrome c (see [8–10]). Cytochrome c then induces an apoptotic cascade involving caspases 9, 3, and 7, which ultimately destroys the affected cell [8, 11]. Although the release of cytochrome c and subsequent apoptotic cell death has been revealed to be a reversible event (i.e., in NGF-deprived sympathetic neurons), in most cases, the outer membrane permeabilization of mitochondria, as induced by the above-noted triggers, represent an apoptotic point of no return [12].
The importance of mitochondria in cell metabolism is reflected not only in their adequate functioning, but also in their proper intracellular distribution. Cells maintain a largely heterogeneous interior, with different reactions and processes being catalyzed in discrete, sometimes compartmentalized regions, and the demand for ATP is not uniform throughout the cell. This notion is particularly evident in post-mitotic neurons, where ion channels, ion pump activity, axonal/dendritic transport, and synaptic transmission all necessitate great quantities of ATP in very specific intracellular domains. In such cells, the appropriate distribution of mitochondria is equally vital for maximum cellular efficacy as is mitochondrial integrity. The transport mechanisms of mitochondria, consisting mostly of microtubule networks [13], are critical in this respect.
The importance of mitochondria in cell vitality is emphasized by conditions of mitochondrial dysfunction. The electron transport process, which involves sequential oxidation-reduction reactions between transition-metal-containing protein complexes, is very vulnerable to disruption: aberrant distribution of electrons yields free radicals, such as the hydroxyl radical or superoxide anion, and redox-active compounds like hydrogen peroxide and peroxy nitrate, which diffuse through the cell and incur damage to macromolecules [14]. Oxidative stress resulting from oxidative phosphorylation errors is the focus of a leading theory of aging [15, 16], whereby free radicals generated from within mitochondria gradually corrode cellular machinery to the point of complete impairment and death. As discussed below, similar perturbations are a key factor of both AD and PD, and mitochondrial therapeutics may offer substantial relief.
Errors in mitochondrial Ca2+/apoptotic signaling also have severe consequences for cellular activity and are the underlying progenitors of many devastating conditions [2, 8]. Fortunately, evolution has provided the eukaryotic cell with a means to prevent mitochondrial catastrophe from wreaking havoc within its interior. In AD and PD, however, the system of mitochondrial dynamics, by which mitochondria maintain their reliability and appropriate cellular distribution, is compromised and may be responsible for much of the degenerative pathology that characterizes each disease.
MITOCHONDRIAL DYNAMICS: FISSION, FUSION, AND CELLULAR PROTECTION
Mitochondria exist in the cell as highly dynamic entities that divide and fuse with each other as the metabolic environment demands [17]. Depending on physiological conditions, mitochondria can form giant tubular networks to allow the rapid exchange of mitochondrial content or they can divide into individual, rod-like organelles with the ability to penetrate deep within the narrow confines of neuronal extensions [17]. In the metabolically heterogeneous environment of the neuron, where specific regions necessitate varying amounts of ATP or Ca2+ buffering, such malleability of mitochondria is invaluable. These dynamics have, in fact, proven to be essential for cellular functioning [17].
Mitochondrial fission and fusion, the two processes that mediate the dynamic morphology of mitochondria, provide another vital aspect to functioning cells: the protection of mitochondrial integrity. Specifically, mitochondrial fusion permits the exchange of mitochondrial contents, such as lipid membranes, mtDNA, and oxidative phosphorylation complexes, such that the percentage of mitochondria containing defective components remains at a minimum [18, 19]. Fusion is orchestrated by three large GTPases that tether neighboring mitochondria together and merge their innerand outer-membranes. These proteins are mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy protein 1 (OPA-1) [17, 20, 21]. The mitofusins are transmembrane proteins that span the outer mitochondrial membrane; they form homo- or hetero-dimers upon fusion initiation and utilize their GTPase activity to fuse the membranes together [22, 23]. OPA-1 faces the inter-membrane space and corresponds with Mfn1 to enable inner-membrane fusion [22, 24]. This latter process, however, is incompletely understood.
Mitochondrial fission, conversely, enables the sequestration and elimination of irreversibly damaged mitochondria and mitochondrial content [25]. The two primary proteins involved in fission are dynamin-like protein 1 (DLP-1, sometimes Drp-1), a large GTPase that inhabits the cytoplasm until it is recruited for fission [21], and Fis1, a small mitochondrial surface receptor that recruits DLP-1 complexes to permit fission [26]. Fission thus occurs when DLP-1 oligomerizes into large ring-like complexes and binds to Fis1 proteins, which are evenly distributed along the mitochondrial outer surface [21, 26]. Upon binding, DLP-1 begins to hydrolyze GTP to constrict and twist tubules, thereby severing the mitochondria into two daughter organelles [27]. The mechanisms of the remodeling of cristae and other mitochondrial internal structures are not entirely clear.
A third method of mitochondrial regulation, termed mitophagy, is the process by which defective or irreversibly damaged mitochondria are sequestered and eliminated within the cell. Mitophagy has been demonstrated in yeast and mammalian cells and involves distinct protein complexes that escort the metabolic organelles to lysozomes for degradation (reviewed in depth in [28]). Notably, two proteins that have recently been demonstrated to orchestrate mitophagy under normal conditions, the phosphatase-tensin homologue- induced kinase-1 (PINK-1) and Parkin, are known proteins involved in the pathogenesis of familial PD (see below). Such a relationship is undoubtedly of high therapeutic value.
The cellular pathways directing fission and fusion are only beginning to be understood. Posttranslational modification of fission/ fusion proteins, such as their phosphorylation, ubiquitinization, nitrosylation, and SUMOylation, are believed to play a large role [29–34], and studies focusing on DLP-1 have yielded tremendous insights (for a detailed review, see [35]). Specifically, phosphorylation of human DLP-1 at Ser637 (Ser656 in the conserved GTPase effector domain in rats) has been demonstrated to inhibit DLP-1 activity, thereby reducing mitochondrial fission [36]. This event is mediated by a cyclic AMP-dependent kinase (PKA) and the scaffolding protein AKAP1; the latter moves PKA to the mitochondrial outer membrane where it phosphorylates and inactivates DLP-1 [37]. Phosphorylation of DLP-1 on Ser585 by Cdk1/cyclin B has conversely been shown to increase its activity during mitosis [34, 38], and rises in endogenous potassium concentration seems to trigger translocation of DLP-1 from cytoplasm to mitochondria for increased activity [38].
The cell signaling compounds nitric oxide (NO) and calcium are also implicated in regulation of mitochondrial dynamics machinery. NO, a neurotransmitter tied to learning and memory [39], reacts with superoxide anion to form the highly neurotoxic compound peroxynitrate [40, 41]. Recent data show that NO rapidly induces mitochondrial fragmentation and strongly suggests NOrelated fission to involve DLP-1, Mfn1, and Fis1 [42]. The precise mechanisms have not been elucidated. Calcium signaling is similarly linked to mitochondrial morphology. Using the ER calcium- ATPase thapsigargin, Hom et al. demonstrated a marked increase in mitochondrial fragmentation following intracellular calcium increases [43]. These changes were prevented by inhibition of DLP-1 and Fis1, suggesting their involvement in the calcium-induced mitochondrial fission. Additionally, mitochondrial depolarization, which may occur via sustained cytosolic Ca2+ increases, has been demonstrated to elicit excessive mitochondrial fragmentation, presumably due to phosphorylation of DLP-1 [38, 44].
The processes of mitochondrial fission and fusion are also sensitive to the physiological conditions within the cell. That is, cellular ion homeostasis, oxidative stress conditions, and genetic integrity affect mitochondrial fission and fusion to a considerable extent. For instance, increases in oxidative stress and corresponding inhibition of complex II in the electron transport chain have been demonstrated to yield excess mitochondrial fission [45], and short exposure of low levels of reactive oxidative species (ROS) causes significant alterations in mitochondrial morphology and fine structure through modulation of fission/fusion proteins [46].
Perhaps most striking are the results from a recent report by Ichishita et al. in which the authors knocked down 719 genes predicted to code for mitochondrial proteins [47]. In their study, more than 80% of genetic knockdowns caused abnormal mitochondrial morphology, including fragmentation and elongation. Other genetic defects, such as deficiency of the phosphatase-tensin homologueinduced kinase-1 (PINK-1) protein, have been shown to elicit abnormal mitochondrial fission via impaired mitochondrial membrane potential [48]. It is thus quite clear that mitochondrial dynamics are pertinent to cell viability and functioning; disruptions in their integrity are devastating to affected cells and account for a significant fraction of pathological inclusions in AD and PD.
MITOCHONDRIAL DYNAMICS IN AD
AD is an age-related neurobiological disorder characterized by distinct losses of cognitive function, including memory, language and visuo-spatial skills, and impairments in behavior [49]. It is an incurable, chronic condition that eventually results in patient death. Despite advances in our understanding of the disease over the last twenty years, the tremendous complexity that underlies AD has made any certain understanding of it beyond reach. Particularly elusive is an explanation for the onset of sporadic AD, known to affect 95% of sufferers [49] (familial AD is linked to several auto somal dominant mutations that affect the processing of the amyloid-β (Aβ) peptide and drop the average age of onset from approximately 65 years old to 40 – it is pathologically similar to sporadic AD, and it is very rare [49]).
Among the known pathological infarctions that exist in AD brains, mitochondrial morphology has become a compelling focus of attention. AD neurons, for instance, are known to contain a significantly higher percentage of mitochondria with broken cristae compared to age-matched controls [50]. Alterations in mitochondrial size and number have been demonstrated in vulnerable neuronal populations in AD (i.e., those of the entorhinal cortex, the dentate gyrus, and the CA1/CA3 regions of the hippocampus [51, 52]), suggesting these changes to be an upstream purveyor of neurodegeneration in AD. Along these lines, fibroblasts from AD patients demonstrate abnormal mitochondrial dynamics compared to age-matched, healthy controls [53]. The overall expression levels of the fission/fusion proteins DLP-1, OPA-1, Mfn1 and 2, and Fis1 are significantly altered in AD, as demonstrated by immunohistochemistry, Western blotting, immunofluorescence, time lapse imaging, and electron microscopy [54]. Finally, mitochondrial distribution has been observed to be deficient in the axonal/dendritic processes of AD neurons.
As indicated earlier, these disruptions in mitochondrial dynamics have severe repercussions for cellular viability. The adequate functioning of neuronal ion pumps, signal transmission/neurotransmitter uptake, and ion homeostasis definitively rests on mitochondrial integrity, and damage to the processes of fission and fusion drastically compromises this integrity and as well as that of the cell. It therefore comes as no surprise that many well-established aspects of AD pathogenesis are directly linked to mitochondria. Oxidative stress, resulting from impairment of electron transport machinery and/or ROS-sequestering protein complexes, and abnormal Ca2+ signaling and homeostasis have long been known to participate in AD-related neurodegeneration [2, 55], although their pathological origins are still unclear. Given the dramatic effects that mitochondrial fission/fusion aberration has on the cell, specifically in reference to oxidative stress and Ca2+ homeostasis, it is quite likely that mitochondrial dynamics play an upstream role in AD.
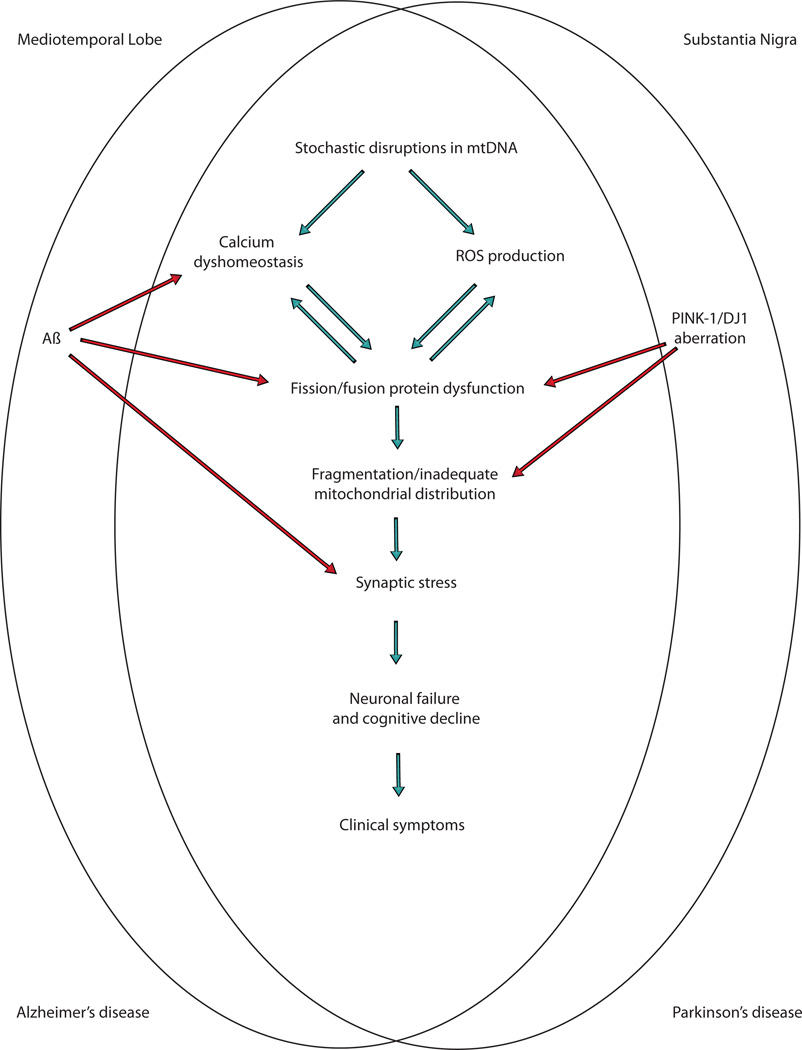
Interestingly, mitochondrial morphology also acts as a downstream target of AD pathology, and Aβ seems to be particularly important in this regard (Fig. 1). Aβ, the cleaved protein product of the amyloid-β protein precursor (AβPP), has been the central focus of AD research since its link to the familial form of the disease [56]. Specifically, mutations in AβPP, presenilin 1, and presenilin 2, proteins that orchestrate the cleavage of AβPP into the varying Aβ species, definitively cause familial AD [49], and Aβ is therefore traditionally considered the ultimate underlying factor of the disease [57]. The resulting “amyloid cascade hypothesis”, however, has yielded no translational benefits in clinical trials so far and its long-time certitude has come into question [58]. Regardless of its primacy, however, the cleaved protein products of AβPP are likely involved in AD.
Fig. (1).
Mitochondrial abnormalities are a common feature of both Alzheimer's disease and Parkinson's disease and thus may be a potential target for therapeutic intervention.
In reference to mitochondrial morphology, several findings are of note. First, Aβ is known to localize to mitochondria and to interact with mitochondrial proteins, thereby potentiating mitochondrial, neuronal, and synaptic stress [59]. Aβ has been demonstrated to induce acute impairment in mitochondrial axonal transport [60, 61] as well as striking fragmentation and synaptic dysfunction [42, 54]. Moreover, over-expression of AβPP, and that of the familial ADlinked AβPP Swedish mutation, induce mitochondrial fragmentation and abnormal distribution [54, 62] and the overexpression of either DLP1 or OPA1 can partially correct the distributional or morphological abnormalities and subsequent functional consequences.
Other AD-related pathologies, such as sporadic mtDNA mutation, GSK3β aberration, and neurofibrillary tangle deposition, negatively affect mitochondria and mitochondrial dynamics and emphasize the role of the metabolic organelle in disease [21, 63, 64].
It becomes clear that the processes of mitochondrial fission and fusion are central to mitochondrial dysfunction and the pathogenesis of AD [65]. Whether or not their aberration is causal in disease or further downstream has yet to be elucidated; however, a complex interplay is likely involved in which stochastic alterations in any number of mitochondrial proteins/DNA occurs and propagates a cycle of destruction throughout the cell until synaptic dysfunction and cell death result. Interestingly, PD, an entirely different neurodegenerative disorder involving unrelated brain regions, is also described by damages to mitochondrial dynamics. Such coincidental pathology may be no coincidence at all; mitochondria are likely key to the pathogenesis of both diseases.
MITOCHONDRIAL DYNAMICS IN PD
PD is the second most common neurodegenerative disease next to AD and is characterized by progressive loss of dopaminergic neurons in the substantia nigra of the brain. Patients exhibit motor impairments involving resting tremor, progressive rigidity, bradykinesia, and postural instability. As in AD, the majority of PD cases are sporadic and unpredictable; the small percentage of cases attributed to familial mutations have been linked to mutations in 16 genes, including α-synuclein, Parkin, PINK-1 (mentioned above), DJ-1, Omi/Htra2, and Leucine-rich repeat kinase 2 (LRRK2) [66, 67]. Interestingly, mitochondrial aberration is long considered to be a central factor underlying PD pathogenesis, and more recent studies has indicated that mitochondrial dynamics and quality control play a large role. Briefly, implications of mitochondria in PD stem from the following: i) substantia nigra cells and blood platelets from PD patients, as well as from cybrid cell lines, exhibit significant deficits in the activity and number of mitochondrial respiratory chain complex I [68]; ii) substantia nigra neurons from PD patients exhibit accumulations of mtDNA deletions [69]; iii) mitochondrial complex I inhibitors, such as the synthetic heroine metabolite MPP+ and the toxin rotenone, produce a form of PD indistinguishable from sporadic PD [70, 71]; and iv) PINK-1, DJ-1, and Parkin, mutants of which are linked to familial forms of PD, are localized to and involved in mitochondrial functioning [68] (Fig. 1).
As noted above, mounting evidence indicates alterations in mitochondrial dynamics as the mediators of PD-type neurological dysfunction. Application of rotenone to CVI-4A cells, for instance, induces rapid, DLP-1-dependent mitochondrial fragmentation that is reversible upon removal of the toxin; this phenomenon has been repeated in neuronal cells and shown to be preventable by overexpression of Mfn1 or via dominant negative mutation of DLP-1 [42]. In both cases, excessive but reversible fragmentation preceded cell death. Similarly, 6-OHDA application induces DLP-1-dependent mitochondrial fission that precedes and mediates apoptosis [72] and further suggests complex I dysfunction in PD to yield mitochondrial fragmentation and cell death. Our more detailed time lapse study in MPP+-treated SH-SY5Y neuroblastoma cells and rat primary dopaminergic midbrain neurons indicated that MPP+-induced DLP1-dependent mitochondrial fragmentation is an early and upstream event that exacerbates MPP+-induced bioenergetic impairments and plays a critical role in mediating other downstream adverse effects. These include increased ROS production, decreased MMP, calcium disturbance, increased mitophagy and cell death in neuronal cells [73]. A crosstalk between ROS and calcium disturbance and mitochondrial fragmentation form a mitochondrial fission-initiated downward spiral that augments these adverse effects. Indeed, attenuation of mitochondrial fragmentation by DLP1 knockdown reduces or even completely prevents these downstream events induced by MPP+, suggesting that prevention of mitochondrial fragmentation is not only an attractive target to prevent MPP+-induced deficits but may also have significant relevance to the treatment of PD.
The mutant genotypes associated with PD have also been demonstrated to affect mitochondrial dynamics. DJ-1 knockout mouse embryonic fibroblasts, for example, exhibit reduced mitochondrial connectivity due to fragmentation—this phenotype can be reversed by overexpression of wildtype DJ-1 [73]. PINK-1 deficient drosophila demonstrate mitochondria with fragmented cristae and hollow appearing mitochondria, or enlarged, disintegrated mitochondria; these conditions can each be rescued by Parkin overexpression [74– 76]. In studies manipulating the expression of fission/fusion proteins in PINK-1 or Parkin-deficient flies, these results were confirmed (i.e., affected cells exhibited increased fission/fusion ratio) and the emerging consensus is that a PINK-1/Parkin pathway relates mitochondrial dynamics and neurodegeneration in PD [77, 78]. Interestingly, as stated above, PINK-1 and Parkin are involved in the process of mitophagy, by which defective mitochondria are removed from the cell [28], and their mutation in familial forms of PD may certainly contribute to the characteristic neurodegeneration of the disease [79, 80].
THERAPEUTIC CONSIDERATIONS AND CONCLUDING REMARKS
Aberrant mitochondrial dynamics likely represent a common mediator in the neuropathogenesis of AD and PD (Fig. 1). These two strikingly dissimilar disorders, which involve mutually different brain regions, genetic mutations, and cognitive/behavioral phenotypes, are governed largely by the integrity of the mitochondria that exist within the neuronal environment. Given the vital roles mitochondria play within eukaryotic cells, especially within neurons, the centrality of mitochondrial disruption in these disorders perhaps comes as no surprise. It thus begs the question as to what extent the therapeutic upholding of proper mitochondrial fission and fusion might benefit AD and PD patients. With little progress being made toward an adequate treatment of either of these diseases, both of which are projected to increase substantially throughout this century [85, 86], we must strongly consider the therapeutic potential of mitochondrial dynamics protection as a high priority.
The development of compounds aiming at changing mitochondrial dynamics is still at its infancy and Mdivi-1 is among the first of them. Mdivi-1 is an inhibitor of mitochondrial fission that acts by selectively preventing DLP-1 assembly and thus inhibiting its GTPase activity [87]. Cells treated with mdivi-1 are resistant to apoptosis upon apoptotic challenges (presumably due to an inhibition of mitochondrial membrane permeabilization). In vivo treatment of mice during renal ischemia/reperfusion and cisplatininduced nephrotoxicity attenuated tubular cell apoptosis and acute kidney injury via a prevention of mitochondrial fragmentation [88]. These benefits warrant further investigations into the therapeutic benefits of mdivi-1 in AD or PD. PKCδ interacts and phosphorylates DLP1 which promotes DLP1 translocation to mitochondria and mitochondrial fragmentation. Importantly, inhibition of PKCδ, using a selective PKCδ peptide inhibitor (δV1-1), reduced mitochondrial fission and fragmentation and conferred neuronal protection in vitro and in the brains of hypertensive rats with hypertension-induced brain injury, suggesting upstream signaling molecules may also be a good candidate [89].
PGC-1α has most recently been shown to sustain adequate mitochondrial distribution in neurites [90]. Coupled with its wellestablished role as a transcriptional co-activator and regulator of mitochondrial biogenesis and respiration [91–93], PGC-1α represents an excellent therapeutic target for mitochondrial dysfunction in AD and PD. Indeed, overexpression of PGC-1α protects neurons from degeneration induced by oxidative stress or mutant huntingtin [93, 94].
Although there is much to be done in the search for a viable treatment regime for the devastating neurodegenerative conditions here described, these compounds represent significant milestones toward the ultimate goal. It is becoming increasingly clear that brain mitochondria are largely involved in the cognitive/behavioral deterioration of both AD and PD and it thus seems likely that an agent that attenuates mitochondrial dysfunction will yield positive results. Given the complexity that underlies and regulates mitochondrial dynamics, there are likely numerous points of access for therapeutic intervention that may yield beneficial results. A successful treatment regime must take into account this complexity to i) ensure an adequate balance of mitochondrial fission and fusion that can adapt as the neuronal environment demands and ii) to attenuate these aspects of neurodegeneration without producing unwanted side effects (see, for instance, [95]). Regardless, with the aging population rapidly increasing worldwide, forward movement in the search for a cure is greatly anticipated.
ACKNOWLEDGMENTS
Work in the authors’ laboratories was supported by the National Institutes of Health (AG031852 and NS071184) and Alzheimer’s Association (IIRG-10-173358). The funding source had no involvement in data collection, analysis or interpretation of the study.
REFERENCES
- 1.Jouaville LS, Pinton P, Bastianutto C, et al. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celsi F, Pizzo P, Brini M, et al. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim Biophys Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denton RM, McCormack JG. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- 4.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 5.Werth JL, Usachev YM, Thayer SA. Modulation of calcium efflux from cultured rat dorsal root ganglion neurons. J Neurosci. 1996;16:1008–1015. doi: 10.1523/JNEUROSCI.16-03-01008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509(Pt 1):59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcke M, Hudson JL, Camacho P, Lechleiter JD. Impact of mitochondrial Ca2+ cycling on pattern formation and stability. Biophys J. 1999;77:37–44. doi: 10.1016/S0006-3495(99)76870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 9.Chipuk JE, Moldoveanu T, Llambi F, et al. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 11.Li K, Li Y, Shelton JM, et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 12.Haraguchi M, Torii S, Matsuzawa S, et al. Apoptotic protease activating factor 1 (Apaf-1)-independent cell death suppression by Bcl-2. J Exp Med. 2000;191:1709–1720. doi: 10.1084/jem.191.10.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 14.Bonda DJ, Wang X, Perry G, et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 16.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 17.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su B, Wang X, Zheng L, et al. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 24.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 27.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 30.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meuer K, Suppanz IE, Lingor P, et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi N, Ishihara N, Jofuku A, et al. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 35.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han XJ, Tomizawa K, Fujimura A, et al. Regulation of mitochondrial dynamics and neurodegenerative diseases. Acta Med Okayama. 2011;65:1–10. doi: 10.18926/AMO/43824. [DOI] [PubMed] [Google Scholar]
- 37.Merrill RA, Dagda RK, Dickey AS, et al. Mechanism of Neuroprotective Mitochondrial Remodeling by PKA/AKAP1. PLoS Biol. 2011;9:e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han XJ, Lu YF, Li SA, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holscher C. Nitric oxide, the enigmatic neuronal messenger: its role in synaptic plasticity. Trends Neurosci. 1997;20:298–303. doi: 10.1016/s0166-2236(97)01065-5. [DOI] [PubMed] [Google Scholar]
- 40.Boje KM. Nitric oxide neurotoxicity in neurodegenerative diseases. Front Biosci. 2004;9:763–776. doi: 10.2741/1268. [DOI] [PubMed] [Google Scholar]
- 41.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 42.Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. Embo J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hom JR, Gewandter JS, Michael L, et al. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- 44.Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liot G, Bossy B, Lubitz S, et al. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jendrach M, Mai S, Pohl S, et al. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion. 2008;8:293–304. doi: 10.1016/j.mito.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Ichishita R, Tanaka K, Sugiura Y, et al. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem. 2008;143:449–454. doi: 10.1093/jb/mvm245. [DOI] [PubMed] [Google Scholar]
- 48.Sandebring A, Thomas KJ, Beilina A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol. 1986;20:472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- 52.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Su B, Lee HG, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Schellenberg GD, Bird TD, Wijsman EM, et al. The genetics of Alzheimer's disease. Biomed Pharmacother. 1989;43:463–468. doi: 10.1016/0753-3322(89)90106-6. [DOI] [PubMed] [Google Scholar]
- 57.Hardy J. Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9:151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 58.Castellani RJ, Lee HG, Siedlak SL, et al. Reexamining Alzheimer's disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis. 2009;18:447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manczak M, Anekonda TS, Henson E, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 60.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Su B, Siedlak SL, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonda DJ, Wang X, Perry G, et al. Mitochondrial dynamics in Alzheimer's disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181–192. doi: 10.2165/11532140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva DFF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer's disease. Curr Alzheimer Res. 2011;8:563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 65.Santos RX, Correia SC, Wang X, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 2):S401–S412. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 67.Abeliovich A, Flint Beal M. Parkinsonism genes: culprits and clues. J Neurochem. 2006;99:1062–1072. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- 68.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 69.Bender A, Krishnan KJ, Morris CM, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 70.Fiskum G, Starkov A, Polster BM, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson's disease. Ann N Y Acad Sci. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 71.Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson's disease. IUBMB Life. 2001;52:135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Lazaro M, Bonekamp NA, Galindo MF, et al. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med. 2008;44:1960–1969. doi: 10.1016/j.freeradbiomed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Su B, Liu W, et al. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson's disease. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark IE, Dodson MW, Jiang C, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 75.Park J, Lee SB, Lee S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, Gehrke S, Imai Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Ouyang Y, Yang L, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mortiboys H, Thomas KJ, Koopman WJ, et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pogson JH, Ivatt RM, Whitworth AJ. Molecular Mechanisms of PINK1-Related Neurodegeneration. Curr Neurol Neurosci Rep. 2011;11:283–290. doi: 10.1007/s11910-011-0187-x. [DOI] [PubMed] [Google Scholar]
- 80.Santos RX, Correia SC, Carvalho C, et al. Mitophagy in Neurodegeneration: An Opportunity for Therapy? Curr Drug Targets. 2011 doi: 10.2174/138945011795528813. [DOI] [PubMed] [Google Scholar]
- 81.Park J, Lee G, Chung J. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun. 2009;378:518–523. doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 82.Lutz AK, Exner N, Fett ME, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–22951. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Exner N, Treske B, Paquet D, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grunewald A, Gegg ME, Taanman JW, et al. Differential effects of PINK1 nonsense and missense mutations on mitochondrial function and morphology. Exp Neurol. 2009;219:266–273. doi: 10.1016/j.expneurol.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 85.Colantuoni E, Surplus G, Hackman A, et al. Web-based application to project the burden of Alzheimer's disease. Alzheimers Dement. 2010;6:425–428. doi: 10.1016/j.jalz.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Calabrese VP. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;69:223–224. doi: 10.1212/01.wnl.0000271777.50910.73. author reply 224. [DOI] [PubMed] [Google Scholar]
- 87.Cassidy-Stone A, Chipuk JE, Ingerman E, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qi X, Disatnik MH, Shen N, et al. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22:256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wareski P, Vaarmann A, Choubey V, et al. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 92.Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 94.Cui L, Jeong H, Borovecki F, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 95.Waterham HR, Koster J, van Roermund CW, et al. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]