Abstract
Friendships and other rewarding affilliative bonds are associated with the actions of the nonapeptide hormone oxytocin (OT) in humans and many social mammals. We determined if OT itself is rewarding, and if that reward is dependent upon the presence of conspecifics. We evaluated the reinforcing effects of OT infusion in female mice on social (conditioned social preference, CSP), and non-social tests (conditioned place preference, CPP). Ovariectomised females received oestradiol implants and intracerebroventricular cannulas. During a pre-test, they were introduced to a 3-chamber apparatus for 10 minutes. Social and place apparatus were identical, except that each end-chamber contained a novel stimulus female for CSP, whereas they were distinguished by visual and tactile cues for CPP. For CSP, test females received OT (0, 100, 200 or 100ng) and were paired for 30 minutes with one stimulus female. On alternating days, they received saline vehicle and were paired with the opposite female, for a total of 4 pairings each. The final conditioned preference test was identical to the pre-test. OT induced CSP. Test mice that received 100ng OT increased their preference score from −67.4±22.1 seconds in pre-test to +55.7±35.1 seconds during the conditioned preference test (p<0.05). 200ng OT induced an increase in preference score from −162.7±47.3 to +74.3±23.7 seconds (p <0.001). There was no effect of 0 or 1000ng OT on CSP. An additional group of mice was tested for CPP at 200ng OT. Testing and pairings were identical to CSP. OT induced a small but significant CPP. Mice increased their preference score from −222.4±38.0 to −126.0±58.7 seconds (p<0.05). OT had no effect on anxiety or odor recognition as assessed by elevated plus maze and olfactory habituation/dishabituation tests, respectively. In conclusion, OT like other motivating stimuli (drugs, food) is rewarding when tested under solitary conditions, but is also reinforcing in a social setting.
Keywords: Oxytocin, Conditioned Social Preference, Conditioned Place Preference, Social Behaviour, Dose-Response Curve
Introduction
The majority of human social relationships are built among people who have neither family nor sexual connections. As diagnoses of diseases with social deficit phenotypes (eg. autism) rise precipitously (1), there is increasing interest in the neurobiology supporting these affiliative bonds. The nonapeptide hormone oxytocin (OT), most well-known for its role in parturition and milk letdown, is critical for the regulation of social behaviour and bonding in mammals, including humans, sheep, rats, voles and mice (2, 3). In humans, OT has calming effects similar to those of social support in stressful settings, reduces fear-related amygdala activity and increases both trust and generosity (4). In the female prairie vole, OT receptor expression in the nucleus accumbens is strongly correlated with the formation of partner preference (5), and OT disruption prevents the formation of social attachments (6). Moreover, social recognition is absent in mice without OT and is facilitated in rats that receive exogenous OT (7).
Social interaction is powerfully reinforcing for humans and other social animals, including rodents. Rats prefer an environment that is associated with social play (8), and prefer social over non-social play opportunities (ie. a ball; (9)). Dopamine activity underlies both social bonding and drug reward, thereby providing a neurobiologic link between attachment to a partner and addiction to a drug (10). Furthermore, OT is released during mating (10) as well as by ecstasy (11) and other drugs (12). Critical links emerge: that between socialization and reward, and that between OT, social behaviour and drug-reward. Therefore, we expect that OT contributes to social reward. The present study used conditioned social preference (CSP) to test reinforcing effects of OT in a social context. We also used conditioned place preference (CPP) to determine if females find OT rewarding in a non-social context.
Conditioned Place Preference (CPP) is a well-established behavioural paradigm for assessing stimulus reward (13). If a mouse is sufficiently motivated by an unconditioned stimulus (US; eg. food, drug) associated with a unique environment (conditioned stimulus; CS+), it will prefer that environment even in the absence of the US. This experimental design allows for behavioural testing in the absence of any acute effects of the US (eg. food-induced satiety, drug-induced immobility). However, CPP tests rodents in isolation, and thereby overlooks the social dimensions of many reinforcers.
We were interested in the potential for OT to be socially rewarding. Incorporating the presence of conspecifics converted the CPP experimental design to a Conditioned Social Preference (CSP) paradigm. A similar model has been used recently to demonstrate ethanol reward in a social context (14). Previously, CSP has been used to look at sexual reward in female rats (15). In the present study, test female mice were trained to associate a novel female mouse (CS+) with exogenous OT administration (US). The hypothesis was that if the test mice were sufficiently motivated by repeated OT administrations associated with a social partner, they would prefer the CS+ over another mouse (CS−) paired with saline, even in the absence of the US. Intracerebroventricular (ICV) administration ensured delivery to the brain.
To take advantage of the prosocial roles of both OT and oestrogen while controlling cycling oestrogen levels, the present study used an ovariectomized with replacement oestrogen (OVX+E) female mouse model. OT has long been known for its regulation of maternal behaviour (16), and it will induce mother-infant attachment in the absence of pregnancy (17). Furthermore, oestrogen increases social behaviours (18) and central OT receptors (19) in female rodents. OVX+E avoids the behavioural impact of fluctuating oestrogen levels across the ovarian cycles, as well as any potential interactions between varying oestrogen levels and OT. In view of the links between OT and oestrogen, whether OT could induce CSP in OVX females is unknown. Likewise, males are less sensitive than females to exogenous OT administration (20), and their CSP response is similarly hard to predict.
Materials and Methods
Subjects
Female C57Bl/6 mice 13-15 grams BW (Charles River Laboratories, Wilmington, MA, USA) were individually housed in a temperature-controlled room under a reversed 14L:10D photoperiod (lights on at 7PM). Mean temperature in the animal room was 24°C and mean humidity was 47%. Water and food were provided ad libitum. Daily training was conducted under dim illumination (50 lux, vs 400 lux with lights on) during the first 4 h of the dark phase when activity peaks. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Southern California Institutional Animal Care and Use Committee (IACUC).
Groups
Test mice were assigned to either CSP (n=34) or CPP (n=9). Stimulus mice for CSP were equivalent to test mice in strain, age and hormonal status. In CSP, test mice were trained to associate a novel stimulus female, the CS+, with an OT infusion, and a different stimulus female, the CS−, with a saline infusion. Mice were handled extensively prior to experimental onset to increase familiarity with researchers. Each group of test mice (n=7-9 each) was tested with repeated exposure to a singe dose of OT (0, 100, 200 or 1000ng). Based on the results of this initial study, a separate group of mice (n=9) was tested for CPP with 200ng OT, the dose that most effectively induced CSP. These mice were trained to associate novel environments differentiated by visual and tactile stimuli (CS+ and CS−), with OT and saline, respectively.
Surgical methods
Ovariectomy and Oestrogen Replacement (OVX+E)
To eliminate potential variability due to oestrous cyclicity, all mice were ovariectomised and oestrogen was replaced at constant physiological levels (21). Mice were anaesthetised by i.p. injection of a mixture of 130mg/kg Ketamine and 10mg/kg Xylazine. Ovariectomy was performed via bilateral dorsal flank incisions, and oestrogen was delivered by Silastic implant sc. The 5 mm implant (o.d. 2.16mm, i.d. 1.02 mm, Dow Corning, Midland, MI, USA), filled with a 1:1 mixture of 17β-estradiol and cholesterol (Sigma, St. Louis, MO, USA), sustains physiologic neuroendocrine and progesterone receptor function (21).
ICV Cannulation Implantation and Infusions
Test mice were secured in a stereotaxic instrument using non-traumatic ear bars (Stoelting Co, Wood Dale, IL, USA) with bregma and lambda in the same horizontal plane. An incision was made to expose the skull, and a hole was drilled over the right lateral ventricle [from bregma: −0.1 mm posterior and −0.9 mm lateral]. A 26-gauge guide cannula extending 1.8 mm below the pedestal (Plastics One, Roanoke, VA, USA) was implanted and cemented to the skull. The cannula was fitted with a 33-gauge dummy cannula. The mouse was allowed to recover for 4-7 days before undergoing behavioural testing and infusion.
OT was infused into the lateral ventricle with a programmable syringe pump (Harvard Apparatus, Holliston, MA, USA) delivering 2 ul over 2 minutes via a 33-gauge infusion cannula with a 0.5 mm protrusion beyond the guide cannula, connected by polyethylene tubing to a 50 μl Hamilton microsyringe (Hamilton Co, Reno, NV, USA). Tubing was of sufficient length as to allow mice to roam freely around a cage that contained only bedding. Mice did not respond to infusion in a way that indicated any procedure-related stress. For example, we observed no freezing or cowering behavior, but did observe typical exploratory and grooming behavior. To prevent backflow of the injectate through the guide cannula, the infusion cannula remained in place for at least 30 seconds after the infusion before being removed.
Behavioural methods
Conditioned Social Preference
CSP was conducted in a plastic rectangular apparatus containing three chambers separated by removable doors (Fig. 1A). The two end-chambers (16.5 × 30.5 × 9 cm) were separated by a smaller “neutral” chamber (12.5 × 15 × 9 cm), which was only used during pre-testing and conditioned preference testing. Time spent in each chamber was recorded by an experimenter blinded to group. A test mouse was considered to be inside an end-chamber only when all four paws were inside.
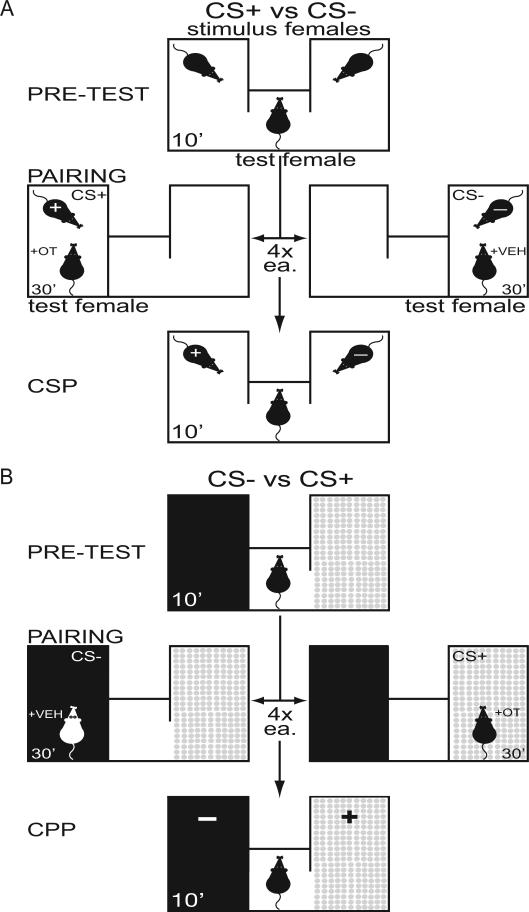
Fig. 1.
(A) Conditioned social preference (CSP) and (B) conditioned place preference (CPP) model for pre-test, pairing and conditioned preference test. Female test mice received oxytocin (OT) before pairing with the conditioned stimulus + (CS+) and received saline vehicle (VEH) before pairing with the conditioned stimulus – (CS−). See methods for details.
During a 10-minute pre-test, test mice were introduced into the neutral chamber, with one novel female stimulus mouse tethered in each end-chamber. Tethering restricted the stimulus mouse to one end-chamber but permitted investigation of her immediate surroundings, grooming and interaction with the test mouse. The least-preferred stimulus female was designated as the CS+. Twenty-four hours later, pairing began. Immediately following a saline infusion, the test female was paired for 30 minutes with the CS− female in one end-chamber. On alternate days, the test female received an OT infusion, and was immediately paired with the CS+ stimulus female in the opposite end-chamber. These pairings were repeated 4x each for a total of 8 days. During pairing, stimulus females were unrestrained to facilitate interaction with the test female. In a final 10-min conditioned preference test, test mice were re-introduced to the entire apparatus with both stimulus mice tethered in their respective end-chambers in a manner identical to the pre-test. Preference score (time with CS+ minus CS− in seconds) was determined for each test female during pre-test and conditioned preference test.
Conditioned Place Preference
The protocol for testing and pairing for CPP was identical to that for CSP, except that stimulus mice were omitted, and end-chambers were distinguished by unique cues (Fig 1B). One end-chamber had black walls and BioFresh bedding (Ferndale, WA, USA) while the other had white walls with pink polka-dots and Sani-Chip bedding (Harlan, Indianapolis, IN, USA). Because test mice preferred black chambers during the pre-test, these were designated as the CS−.
Habituation/Dishabituation
Following conditioned preference testing, all test mice were evaluated for olfactory habituation/dishabituation according to Yang and Crawley (22) to determine if OT enhances investigation and discrimination of odor cues. Mice received a one-time administration of the same dose of OT (0, 100, 200 or 1000ng) and were immediately introduced into an empty cage (17.5 × 28 × 12 cm). Briefly, after a 2-min acclimation, the mouse was presented with a series of 4 odorants in aqueous solution delivered via cotton swabs. During the 30-min test, each odor was presented on a fresh swab at the top of the cage for three consecutive 2-min trials. Water, lemon extract (diluted 1:100) and coconut extract (1:100) were presented first, followed by urinary odor acquired from an unfamiliar OVX+E female: urine (1:10). An observer blinded to treatment groups recorded investigation time. Habituation was measured as change in investigation time in seconds from the first to final odor presentation. Dishabituation was measured as change in investigation time from the third odor presentation to the first of the next series of odor presentations (eg. from the third coconut to the first urine presentation).
Elevated Plus Maze
Twenty-four hours after habituation/dishabituation testing, we determined if OT reduces anxiety on the elevated plus maze according to File et al. (23). The apparatus consisted of a plus-shaped maze with two arms (5 cm × 30 cm) closed by 15 cm clear Plexiglas sidewalls and two arms without walls. The maze was located 50 cm above the floor and visually isolated by a curtain enclosure. Immediately following the same dose of OT, mice were placed at the center of the maze facing an open arm and allowed to explore freely for 5 minutes. Exploratory activity was recorded on video camera and scored by an observer blinded to the treatment groups. The number of closed arm entries was scored as a measure of overall locomotor activity, and the percentage of time spent in the open arms was used as a measure of anxiety. An entry was recorded when all four paws entered the arm.
Confirmation of Cannula Placement
Twenty-four hours after final behavioural testing, mice were sacrificed. Following whole brain excision, the olfactory bulbs, cerebellum and brainstem were removed. The remainder of the brain was immersion-fixed in 4% paraformaldehyde for 1 week and cryoprotected in 20% sucrose for 24 hours. Tissue was sectioned at 40μm on a freezing microtome at −20°C, mounted on pig gel-subbed slides, cover-slipped and stained with cresyl violet. Microscopy confirmed cannula placement in the right lateral ventricle of each mouse.
Statistical Analysis
To determine the effect of pairing with OT and vehicle on CSP, pre-test and conditioned preference scores were evaluated using repeated measures analysis of variance (RM-ANOVA) (preference score before vs after pairing as the RM). To determine the specific effects of each dose of OT (0, 100, 200 and 1000ng), this was followed by paired t-tests. To determine the effect of OT on CPP, pre-test and conditioned preference scores were evaluated by paired t-tests. Change in investigation time on the olfactory habituation/dishabituation test was evaluated by RM-ANOVA (final odor investigation vs initial odor investigation as the RM) according to methods of Yang and Crawley (22). For elevated plus maze, entries into the closed arms and percentage of time spent in the open arms were evaluated by ANOVA. Mean data are reported alongside SEM.
Results
Conditioned Social Preference
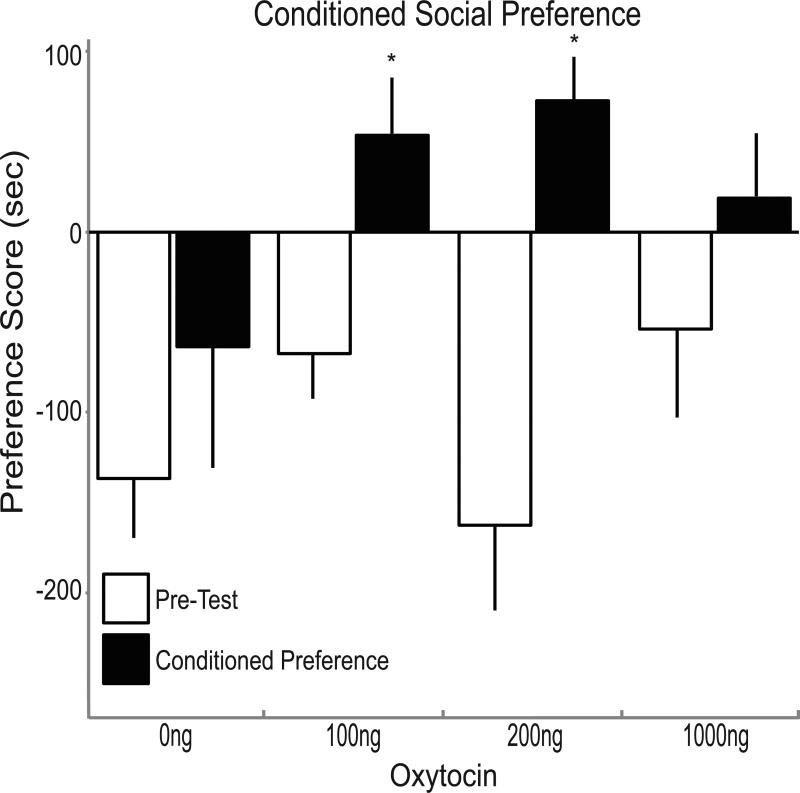
All pre-test preference scores are negative, because the less-preferred stimulus was designated at the CS+ (Fig. 2). RM-ANOVA on preference scores before and after pairing revealed an effect of pairing (F1.30 = 18.099, p<0.001), but no overall effect of dose (N.S. p>0.05), and no dose x pairing interaction (N.S. p>0.05). Preference scores were analyzed at each dose separately by paired t-tests. Repeated pairings with either 0ng or 1000ng OT did not induce a statistically significant increase in social preference score. After pairing, the preference score in females receiving 0ng OT was −63.6±67.2 seconds/10 minutes, and only 22% of the mice (2 of 9) showed positive preference scores (time with CS+ > time with CS−). Although the preference score in response to 1000ng OT was +19.6±35.3 seconds and 57% of the mice preferred the CS+ (positive preference scores), this was not significantly different from pre-test preference (−54.1±48.9 seconds; p>0.05). Likewise, total social interaction time with CS+ and CS− did not change significantly from pre-test to conditioned preference test. Mice that received 0ng OT spent 516.2±10.0 seconds/10 minutes in the end-chambers before pairing and 505.6±21.9 seconds after pairing. With OT treatment at 100, 200 or 1000ng, total interaction was similar (results not shown).
Fig. 2.
Preference score before and after oxytocin infusions and pairing for conditioned social preference. Oxytocin induces conditioned social preference at 100ng and 200ng. Preference score in seconds (mean±SEM) reflects time spent with CS+ minus time spent with CS–, before (pre-test, open bars) and after pairing (conditioned preference, filled bars) with oxytocin at 0 (n=9), 100 (n=9), 200 (n=9) and 1000ng (n=7). Asterisks indicate p<0.05.
In contrast, 100 and 200ng OT significantly increased CSP preference score. Mice that received 100ng OT increased their preference score from −67.4±22.1 seconds before pairing to +55.7±35.1 seconds during the CSP test (p<0.05). Mice that received 200ng OT increased their CSP preference score from −162.7±47.3 to +74.3±23.7 seconds (p<0.001). Furthermore, 78% (7 of 9 for each group) demonstrated positive preference scores after pairing.
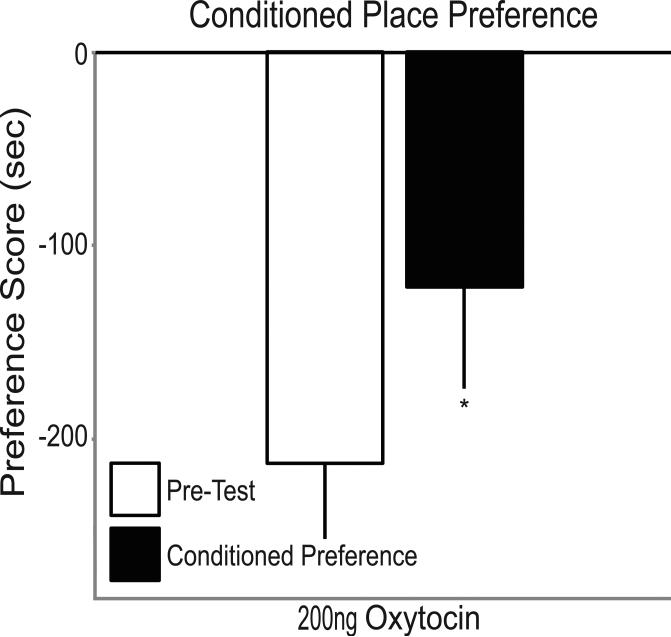
Conditioned Place Preference
OT significantly increased time spent with the CS+ for CPP (Fig. 3). 200ng OT increased preference scores from −222.4±38.0 seconds before pairing to −126.0±58.7 seconds after pairing (p<0.05). Although OT infusion increased time in the CS+ chamber, preference scores remained negative, and only 11% (1 of 9) showed a positive preference for the CS+ after CPP.
Fig. 3.
Preference score before and after oxytocin infusions and pairing for conditioned place preference. Oxytocin induces conditioned place preference at 200ng (n=9). Preference score in seconds (mean±SEM) reflects time spent with CS+ minus time spent with CS–, before (pre-test, open bars) and after pairing (conditioned preference, filled bars). Asterisks indicate p<0.05.
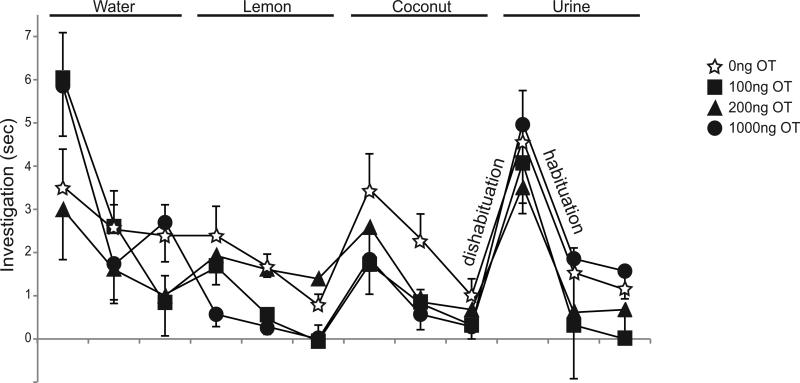
Olfactory Habituation/Dishabituation
OT had no effect on habituation or dishabituation to familiar and novel odors respectively (Fig. 4). As expected, mice decreased their investigation time upon repeated presentations of the same cue (habituation) and increased their investigation time with presentation of a novel odor (dishabituation). RM ANOVA revealed no effect of OT dose on habituation or dishabituation (N.S. p>0.05). As indicated by decreasing investigation time with repeated presentation, all mice significantly habituated to water, lemon, coconut, and urine odors (p<0.001). As indicated by increasing investigation time to a novel odor, mice did not significantly dishabituate to lemon (N.S. p>0.05), but did significantly dishabituate to coconut and urine (p<0.001).
Fig. 4.
Oxytocin (OT) does not enhance olfactory investigation and discrimination. Investigation time in seconds (mean ±SEM) for each presentation of an olfactory cue is represented by open stars for 0ng OT and by filled squares, triangles and circles for OT infusion at 100, 200 and 1000ng, respectively, over the course of three consecutive exposures to each odorant (water, lemon, coconut and urine). Labels illustrate decreases in investigation time from an initial to final odor presentation (habituation) and increases in investigation time from a repeat odor to a novel odor (dishabituation).
Elevated Plus Maze
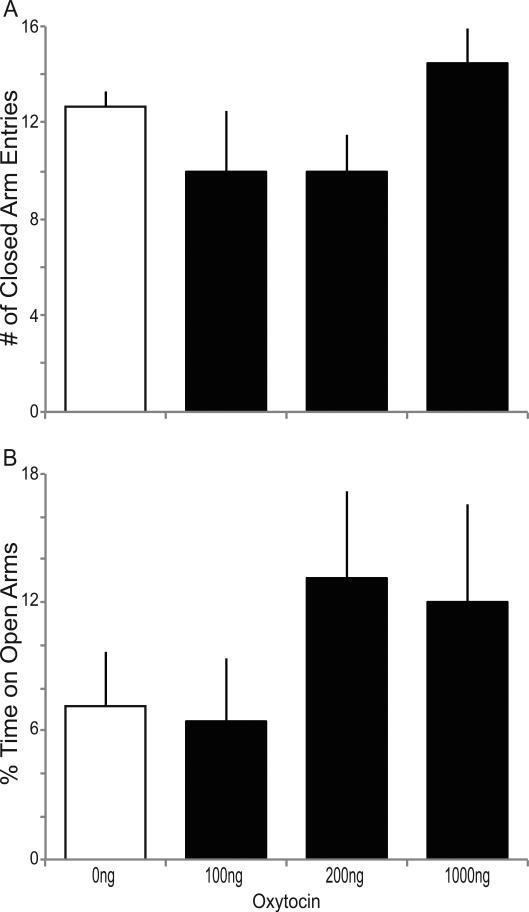
OT had no effect on anxiety as assessed by the elevated plus maze (Fig. 5). At 0ng OT, mice made an average of 12.7±0.7 closed arm entries, and OT had no effect on this measure of locomotor activity (Fig 5A, N.S. p>0.05). At 0ng OT, mice spent an average of 7.1±2.6% of their time in the open arms during 5 minutes of testing. Mice that received 100, 200 and 1000ng OT spent 4.9±2.0, 12.6±3.2 and 12.1±4.6% of their time in the open arms respectively, but ANOVA revealed no significant change on this measure of anxiety (Fig 5B, N.S. p>0.05).
Fig. 5.
Oxytocin (OT) does not reduce anxiety on the elevated plus maze. (A) The number of closed arm entries (mean ±SEM) in 5 minutes after ICV infusion of 0ng OT (open bars) or 100, 200 and 1000ng OT (closed bars) and (B) percentage of time spent in the open arms. There was no significant effect of OT on closed arm entries or percentage time in the open arms.
Discussion
In the present study, OT induced CSP in female mice. At the same dose, OT induced a modest CPP. CPP was used as a test of OT reward in isolation, while CSP tested for reward in the presence of a social partner. We also found that the reinforcing effect of OT was not due to enhanced olfaction or reduced anxiety. Together, these results suggest that OT is reinforcing to an animal in isolation and in the presence of a conspecific.
Although 200ng OT induced an increase in preference score in both CPP and CSP, the change in CSP resulted in a positive preference whereas the change in CPP only resulted in a less negative preference for the CS+. Only a single previous study in animals has looked at potential reinforcing effects of OT using CPP (24). In male rats, OT induced CPP when administered sc at 8 mg/kg (1000× the most effective 200ng dose in the present study).
There is a wide range of OT doses used in rodent research. Larger doses (5-20μg in rats) for studies of systemic administration are common (25, 26), and low dose effects of ICV OT (.001-1ng) have been found in oxytocin knock-out mice (OTKO) (27, 28). The present study tested OT at 100 and 200 ng because a dose of 100ng ICV OT has previously induced a partner preference in prairie voles (29), and we obtained pilot data in female mice to demonstrate CSP at 200ng. We tested 1000ng OT to deliberately probe the upper limits of drug-effect; 0ng served as vehicle control. While the only comparable dose-response studies for ICV OT administration assess social recognition in rats (30) or drug-response in OTKO mice (28), these and other data provide evidence of non-linear effects of OT administration (30, 31).
Numerous human studies have found prosocial actions of intranasal OT infusion, but have not observed euphoric effects (32). While there is evidence that OT does cross the blood-brain barrier (25), there are unanswered questions about how peripherally-administered OT gains access to the brain. It has been proposed that there are indirect means through which OT delivered by injection or intranasal infusion may impact the central nervous system. These include uptake by tissues surrounding the ventricles (26) and travel through the olfactory epithelium into the subarachnoid space (33). However, further studies directly comparing central versus peripheral delivery effects on OT reward are warranted.
It is possible that OT promotes CSP by accentuating interest in social odors. OT likely facilitates social recognition by promoting the processing of social odor cues in the medial amygdala (34). Socially-relevant odorants that have been studied experimentally include fox feces (35), cat skin and feces (36, 37) and bedding from a sexually receptive conspecific of the opposite sex (37). The present study conducted olfactory habituation/dishabituation using urine of a female conspecific, because it was directly related to the CSP experimental design. We found no effect of OT on either habituation or dishabituation and therefore no evidence that exogenous OT enhances olfactory perception. This finding further supports our conclusion that the actions of OT in the CSP paradigm are on the rewarding properties of the social interaction.
While there is some evidence to the contrary (38), often OT is regarded as anxiolytic in rodents (39) and is thereby responsible for increases in sociability (40). In a finding not entirely dissimilar from these and other studies (19), we saw a non-statistically significant trend toward spending more time in the open arms with increasing OT. Nonetheless, doses that were rewarding to test mice, as well as those that were not, did not significantly reduce anxiety. Differences in OT outcomes on anxiety measures may be explained by variations in species, sex and drug delivery profiles. In particular, mice are especially sensitive to any perturbations in their environment. Anxiety is modulated by oestrogen (41), and is reduced with chronic delivery of OT (39). Under these circumstances, it might be difficult to reduce anxiety in female mice used the present study, especially with acute OT delivery.
Because we did not find that OT non-specifically reduced anxiety or enhanced peripheral olfactory discrimination, we hypothesize that OT enhances the salience of social interaction via its effect on reward. We further found that OT had rewarding effects that may be enhanced by the presence of a social partner. 200ng OT induced a dramatic preference for the CS+ in CSP, and a moderate influence over preference score in CPP. There is some evidence that OT may modulate the rewarding properties of social interaction, thereby incentivizing the conspecific (42).
Dopamine (DA) may be facilitating the effect of OT on social reward. There is evidence that OT has direct impacts on DA reward-seeking systems during social interaction (43) that serve to reinforce social cues. For example, OT facilitates maternal grooming of pups (44), and maternal grooming of pups stimulates DA activity in the nucleus accumbens (45), thereby adding a reward component to social stimuli.
The hypothesis that DA serves as the link between OT and social reward has provoked a recent increase in research on the role of OT in addiction. Endogenous OT is responsible for acute prosocial effects of some drugs (i.e ecstasy) (46), while exogenous OT (47) as well as social bonding (48) can reduce DA response to others (i.e. methamphetamine, amphetamine), thereby blocking the drug's reinforcing properties. These opposing DA responses to OT may be mediated by different DA receptors. Pair-bonding in prairie voles is D2 DA receptor-dependent (49), while OT's potential to block drug reward is likely mediated by the D1 DA receptor (48). The present finding that socialization may heighten the rewarding properties of OT is likely DA-dependent and receptor specific.
Clinical trials are finding prosocial effects of intranasal OT in children with autism (50). Our data suggests OT is both inherently rewarding and enhances reward in social settings. These functions may serve to support the effects of OT in humans. Future studies addressing mechanism of action will deepen the understanding of the link between OT and social reward as well as the downstream pathway targets of exogenous administration.
Acknowledgments
We thank Erin McGillivray and Mary Rivas for assistance with surgical and behavioural protocols. This work was supported by a grant form the NIH (R21-AA020575 to RIW)
References
- 1.Investigators AaDDMNSYP, Prevention CfDCa. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50(4):506–17. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61(3):359–79. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- 5.Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29(5):1312–8. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008:170331–6. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 8.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51(4):667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 9.Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol Behav. 2012;105(3):749–56. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224(1):1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146(2):509–14. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Kovács GL, Sarnyai Z, Szabó G. Oxytocin and addiction: a review. Psychoneuroendocrinology. 1998;23(8):945–62. doi: 10.1016/s0306-4530(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 13.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3-4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood RI, Rice R. Ethanol-induced conditioned partner preference in female mice. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119(3):716–25. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- 16.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak R, Keller M, Lévy F. Mother-young relationships in sheep: a model for a multidisciplinary approach of the study of attachment in mammals. J Neuroendocrinol. 2011;23(11):1042–53. doi: 10.1111/j.1365-2826.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 18.Walf AA, Frye CA. Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport. 2008;19(7):789–92. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21(7):2546–52. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37(1):49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 21.Jacob DA, Temple JL, Patisaul HB, Young LJ, Rissman EF. Coumestrol antagonizes neuroendocrine actions of estrogen via the estrogen receptor alpha. Exp Biol Med (Maywood) 2001;226(4):301–6. doi: 10.1177/153537020122600406. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0824s48. Chapter 8Unit 8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004 doi: 10.1002/0471142301.ns0803s26. Chapter 8Unit 8.3. [DOI] [PubMed] [Google Scholar]
- 24.Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17(6):353–9. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 25.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262(1):143–9. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci. 1999;113(5):1071–9. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 30.Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28(4):251–5. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 31.Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007;52(2):274–9. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–26. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 34.Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Horm Behav. 2012;61(3):259–65. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Müller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res. 2006;167(1):57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard DC, Blanchard RJ, Griebel G. Defensive responses to predator threat in the rat and mouse. Curr Protoc Neurosci. 2005 doi: 10.1002/0471142301.ns0819s30. Chapter 8Unit 8.19. [DOI] [PubMed] [Google Scholar]
- 37.Markham CM, Blanchard DC, Canteras NS, Cuyno CD, Blanchard RJ. Modulation of predatory odor processing following lesions to the dorsal premammillary nucleus. Neurosci Lett. 2004;372(1-2):22–6. doi: 10.1016/j.neulet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B. Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58(1):56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 40.Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6(11):e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Lignières B, Vincens M. Differential effects of exogenous oestradiol and progesterone on mood in post-menopausal women: individual dose/effect relationship. Maturitas. 1982;4(1):67–72. doi: 10.1016/0378-5122(82)90021-4. [DOI] [PubMed] [Google Scholar]
- 42.Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol. 2011;23(11):1054–65. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bos PA, Panksepp J, Bluthé RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2012;33(1):17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61(3):331–9. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(6):441–8. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Young KA, Curtis JT, Aragona BJ, Wang Z. Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J Neurosci. 2011;31(22):7960–6. doi: 10.1523/JNEUROSCI.1006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci. 2000;114(1):173–83. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 50.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32(4):426–50. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]