Abstract
The spiral ganglion cell (SGC) is the target of electrical stimulation in cochlear implants. This study is designed to test the hypothesis that chronic electrical stimulation tends to preserve SGCs in implanted hearing-impaired ears. A total of 26 pairs of temporal bones were studied from 26 individuals who in life suffered bilateral profound hearing impairment that was symmetric (in degree of impairment and etiology) across ears and then underwent unilateral cochlear implantation. The subjects were divided in two groups by stimulus configuration: bipolar (n = 16) or monopolar (n = 10). The temporal bones were prepared for histological review by standard methods and two measures of SGC status were made by cochlear segment: count and maximal cross-sectional area. Within-subject comparison of the measures between the implanted-stimulated and the unimplanted ears showed: (1) for both stimulus configurations, the mean (across subjects and segments) of the count difference (implanted ear – unimplanted ear) was significantly less than zero; (2) the mean (across subject) count difference for cochlear segments I, II and III (segments with electrode contacts in the implanted ear) was significantly less negative than the mean difference for cochlear segment IV (no electrode in implanted ear) for bipolar but not for monopolar stimulation; (3) neither implantation-stimulation nor stimulus configuration significantly influenced the measures of maximum cross-sectional cell area. The SGC count results are consistent with the hypothesis that implantation results in a propensity across the whole cochlea for SGCs to degenerate and with chronic bipolar stimulation ameliorating this propensity in those cochlear segments with electrodes present.
1. Introduction
Intracochlear stimulation by cochlear implants (CIs) provides partial hearing restoration for profoundly hearing impaired patients. Because the presumed site of neural excitation is the spiral ganglion cell (SGC), the success of cochlear implantation should be dependent at least in part on the status of the surviving cells. SGCs gradually degenerate following inner hair cell (IHC) loss due to noise, trauma, ototoxicity or other causes of irreversible damage (Spoendlin, 1975; Webster et al., 1981; Xu et al., 1993). Inner hair cells provide the neural activity and neurotrophic support for SGCs, and reduction in this supportive role results in SGC degeneration (Fritzsch et al., 1999; Hartmann et al., 1984; Shepherd et al., 1997;Ylikoski et al., 1993). Therefore, if this supportive role is provided, it would be expected to preserve SGCs or at least slow the rate of degeneration. Reintroducing neurotrophic agents like the neurotrophins (e.g., brain derived neurotrophic factor (BDNF), and NT3) and glial derived neurotrophic factor (GDNF) in profoundly deafened cochleae has been shown to prevent or slow the loss of SGCs (Ernfors et al., 1996; Kanzaki et al., 2002; McGuinness et al., 2005;Miller et al., 1997; Wise et al., 2005). On the other hand, the efficacy of chronic electrical stimulation (CES) via cochlear implant by itself or in combination with neurotrophics in preserving SGCs in animal and human studies is less clear.
Several animal studies have reported that CES reduces the rate of degeneration of SGCs following aminoglycoside induced hair cell loss (Hultcrantz et al.,1991; Leake et al.,1999, 1991,1995; Lousteau,1987; Mitchell et al., 1997). However, in other animal studies no evidence for a CES trophic effect has been shown (Agterberg et al., 2010; Araki et al., 1998; Chatterjee, 1999; Coco et al., 2007; Shepherd et al., 1994;Xu et al., 1997). There are several methodological differences between these studies that could account for the difference in outcome, including differences in species, deafening method, electrode configuration, age at stimulation, stimulus intensity, rate and duration of stimulation, and stimulation current level.
The reported effect of CES on SGC survival is also mixed in studies of humans with cochlear implants. Fayad and Linthicum (2006) found no significant difference between counts in the implanted and non-implanted ears of eight subjects except in the most basal segment where significantly fewer cells were counted in the implanted ear. Khan et al. (2005) and Xu et al. (2012) also counted SGCs in implanted and non-implanted ears of eleven and three subjects respectively. The three more basal cochlear segments (in which electric stimuli were delivered) did not show significant count differences between the two ears; but in the apical segment, SGC counts in the implanted ears were significantly fewer than in the unimplanted ears. As pointed out by Khan et al. (2005), this result is consistent with the electrode insertion initiating a degeneration of SGCs that is minimized by CES in the more basal segments but not in the unstimulated apical segment of the implanted ear.
In this study we revisit the question of whether CES effects the survival of SGCs in humans with CIs using methods that control two variables not always controlled in previous human studies: the likelihood that within-subject SGC survival was similar across ears and stimulus configuration. Stimulus configuration (monopolar vs. bipolar) was controlled because Leake et al. (1999, 1995) suggest that bipolar stimulation preserves SGCs more effectively than monopolar stimulation. SGC status was evaluated using two measures: SGC count and maximum cross-sectional cell area. Temporal bones of profoundly hearing-impaired patients with symmetric hearing loss before implantation and, consequently, a similar number of surviving SGCs across ears (Seyyedi et al., 2011), were selected and segregated into two groups by the CI stimulus configuration: monopolar (MP) or bipolar (BP). Within each configuration group, comparisons of SGC status (count and cell size) were made between implanted and unimplanted ears.
2. Materials and methods
All temporal bones from the collections at the Massachusetts Eye and Ear infirmary (MEEI), House Research Institute (HRI) and University of Minnesota (UM) which met the following criteria were included in the study: (1) both right and left temporal bones of each subject available, (2) the etiology of hearing loss symmetric across ears in each subject, (3) bilaterally symmetric profound hearing impairment before implantation documented by audiometric test results (all subjects had bilateral pure tone average ≥ 90 dB with maximum 10 dB difference between ears at each test frequency before implantation) and (4) unilateral implantation with a multichannel CI. A total of 26 subjects were identified: 10 with devices configured for MP stimulation using Ineraid® or Nucleus®24 cochlear implants and 16 subjects with devices configured for BP stimulation all using Nucleus® 22 CIs.
The temporal bones were removed after death, fixed in Heidenhain Susa solution or 10% buffered formalin and decalcified in ethylene diamine tetra acetic acid (EDTA), and embedded in celloidin (Schuknecht, 1968). The temporal bones were sectioned at a thickness of 20 μm in the horizontal plane and every tenth section was stained with hematoxylin and eosin and mounted on a glass slide. Rosenthal’s canal and the cochlear duct were reconstructed in two dimensions (Fig. 1) by a method described by Schuknecht (1953) and Otte et al. (1978), and the length of Rosenthal’s canal and cochlear duct were calculated. All SGCs with visible nuclei were counted on every tenth section. SGC counts for the four cochlear segments identified in Fig. 1 were computed by adding all counts across slides from the same segment. Each segmental count was multiplied by ten to account for unmounted sections, and again multiplied by a correction factor of 0.68 to account for doubly counted SGCs (Konigsmark, 1970; Nadol, 1988; Seyyedi et al., 2011). The total spiral ganglion cell count was calculated as the sum of the four segmental counts (Nadol, 1988; Nadol et al., 1989).
Fig. 1.
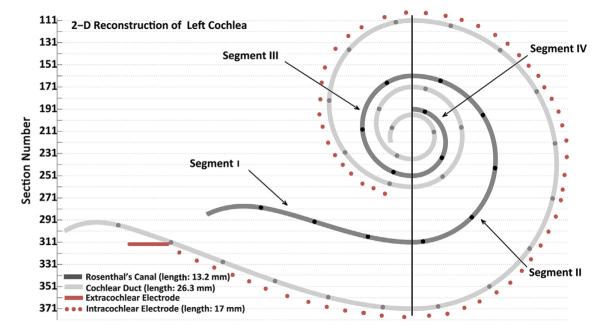
Two dimensional reconstruction of left cochlear duct, Rosenthal’s canal and path of electrode array. The vertical line divides Rosenthal’s canal to 4 segments. The black circles on Rosenthal’s canal and on the cochlear duct are 1 mm apart.
Because the maximum cross-sectional area of SGCs differ by location (segment) in Rosenthal’s canal with larger cells at the base and smaller cells at the apex in adults (Nadol et al., 1990), we targeted four positions (one in each segment) for SGC area measurements: 15, 50, 75 and 95% of total Rosenthal’s canal length. The maximum cross-sectional area of each SGC with a visible nucleolus in the segment of the mounted/stained section closest to each targeted position was measured (Chiong et al., 1993). In the case illustrated in Fig. 1 for example, the length of Rosenthal’s canal is 13.2 mm and 75% of that length is equal to 9.9 mm which corresponds to section 207 in the 2-D reconstruction. The closest mounted/stained section to 207 is section 211. Thus, the cross-sectional area of each SGC with a clear nucleolus and cell boundary in segment 3 of slide 211 was measured.
3. Results
Of the 26 subjects studied, the CI of 10 used MP stimulation (Table 1) and 16 used BP stimulation (Table 2). As shown in these tables, the subjects included 17 males and 9 females ranging in age from 42 to 92 years at the time of death. There was a wide range of duration of CI use (age at death minus age at implantation) which varied between 1 year in case 1 of the BP group to more than 21 years in case 1 of the MP group. Subjects were impaired postlingually by a variety of etiologies, but in 14 of 26 cases, the etiology was unknown. The progression of hearing loss in both ears of each subject was similar. All BP-group cases were implanted with the Nucleus® 22 device and the MP-group cases received either the Ineraid® or Nucleus® 24 devices.
Table 1.
MP subjects
| Case | Diagnosis | Gender | Age at death (years) | CI use (years) | Ear implanted | Device | Stimulus configuration |
|---|---|---|---|---|---|---|---|
| 1 | Idiopathic | F | 87 | 21 | AD | Ineraid | MP |
| 2 | Idiopathic | M | 55 | 15 | AD | Ineraid | MP |
| 3 | Otosclerosis | M | 83 | 12 | AS | Ineraid | MP |
| 4 | Idiopathic | M | 89 | 6 | AD | Nucleus 24 | MP |
| 5 | Meniere's | M | 70 | 3 | AD | Nucleus 24 | MP |
| 6 | Otosclerosis | M | 84 | 10 | AS | Ineraid | MP |
| 7 | Idiopathic | F | 80 | 9 | AD | Nucleus 24 | MP |
| 8 | Otosclerosis | F | 69 | 4 | AS | Nucleus 24 | MP |
| 9 | Idiopathic | F | 64 | 4 | AS | Nucleus 24 | MP |
| 10 | Idiopathic | M | 72 | 5 | AS | Nucleus 24 | MP |
CI: Cochlear implant; AD: Right; AS: Left; MP: Monopolar.
Table 2.
BP subjects
| Case | Diagnosis | Gender | Age at death (years) | CI use (years) | Ear implanted | Device | Stimulus configuration |
|---|---|---|---|---|---|---|---|
| 1 | Idiopathic | M | 42 | 1 | AD | Nucleus 22 | BP |
| 2 | Idiopathic | M | 78 | 4 | AS | Nucleus 22 | BP |
| 3 | Otosclerosis | M | 87 | 3 | AD | Nucleus 22 | BP |
| 4 | Idiopathic | F | 73 | 6 | AS | Nucleus 22 | BP |
| 5 | Idiopathic | F | 53 | 6 | AS | Nucleus 22 | BP |
| 6 | Meniere's | M | 67 | 7 | AS | Nucleus 22 | BP |
| 7 | Noise Trauma | M | 66 | 15 | AD | Nucleus 22 | BP |
| 8 | Viral Labyrinthitis | M | 67 | 2 | AD | Nucleus 22 | BP |
| 9 | Coch.Sac.Deg | F | 64 | 9 | AS | Nucleus 22 | BP |
| 10 | Idiopathic | M | 80 | 6 | AS | Nucleus 22 | BP |
| 11 | NFII | M | 70 | 11 | AD | Nucleus 22 | BP |
| 12 | Idiopathic | M | 75 | 12 | AD | Nucleus 22 | BP |
| 13 | Idiopathic | F | 77 | 18 | AS | Nucleus 22 | BP |
| 14 | Idiopathic | M | 92 | 12 | AD | Nucleus 22 | BP |
| 15 | Ototoxicity | F | 74 | 15 | AD | Nucleus 22 | BP |
| 16 | Meniere's | M | 92 | 11 | AS | Nucleus 22 | BP |
CI: Cochlear implant; AD: Right; AS: Left; BP: Bipolar.
Pre-implantation audiometric test results are presented in Tables 3 and 4. One of the case selection criteria was a symmetric hearing loss: a maximum 10 dB difference in hearing threshold between ears at each frequency eliciting a response. This criterion was easily applied to cases 14 and 15 of the BP group where response thresholds were measured at each frequency tested. Subjects who did not respond at the maximum acoustic intensity are identified by NR with a subscript indicating the maximum intensity delivered. In these cases both the response threshold and no-response entries were symmetric across ears, and the hearing loss was considered symmetric. For example, the thresholds measured at 250 Hz in case 11 of the BP group were within 10 dB across ears and at frequencies between 500 and 8000 Hz, no response was elicited in each ear for acoustic intensities up to 110 dB (NR110).
Table 3.
MP group preimplantation pure-tone audiometry
| Case | Left |
Right |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 4000 | 8000 | 250 | 500 | 1000 | 2000 | 4000 | 8000 | |
| 1 | – | NR90 | 85 | 95 | NR100 | – | – | NR90 | 85 | 95 | NR100 | – |
| 2 | NR105 | NR120 | NR120 | NR120 | NR120 | NR110 | NR105 | NR120 | NR120 | NR120 | NR120 | NR110 |
| 3 | 90 | 95 | 110 | NR115 | – | – | 90 | 95 | 110 | NR115 | – | – |
| 4 | – | 65 | 80 | NR110 | NR110 | NR100 | – | 65 | 80 | NR110 | NR110 | NR100 |
| 5 | – | 110 | 105 | 110 | 115 | NR110 | – | 120 | 115 | NR120 | NR120 | NR110 |
| 6 | – | NR110 | NR110 | NR120 | NR120 | NR110 | – | NR110 | NR110 | NR120 | NR120 | NR110 |
| 7 | – | 105 | 110 | NR120 | NR120 | NR110 | – | NR115 | 120 | NR120 | NR120 | NR110 |
| 8 | – | NR100 | NR100 | NR100 | NR100 | NR100 | – | NR100 | NR100 | NR100 | NR100 | NR100 |
| 9 | – | 90 | 90 | 95 | NR90 | – | – | 90 | 90 | 95 | NR90 | – |
| 10 | 80 | 90 | 105 | 115 | NR115 | NR115 | 90 | 95 | 110 | 105 | NR115 | NR115 |
NR: No response;“–”; no information available.
Table 4.
BP group preimplantation pure-tone audiometry
| Case | Left |
Right |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 4000 | 8000 | 250 | 500 | 1000 | 2000 | 4000 | 8000 | |
| 1 | NR100 | NR110 | NR110 | NR110 | NR110 | NR100 | NR100 | NR110 | NR110 | NR110 | NR110 | NR100 |
| 2 | 75 | 90 | 105 | NR115 | NR115 | NR100 | 85 | 100 | 110 | NR115 | NR115 | NR100 |
| 3 | NR100 | NR100 | NR120 | 110 | 115 | NR100 | NR100 | NR100 | NR120 | 110 | NR120 | NR100 |
| 4 | 105 | 115 | NR120 | NR120 | NR120 | NR110 | 105 | 110 | NR120 | NR120 | NR120 | NR110 |
| 5 | NR100 | NR115 | NR115 | NR115 | NR115 | NR100 | NR100 | NR115 | NR115 | NR115 | NR115 | NR100 |
| 6 | NR100 | NR115 | NR115 | NR115 | NR115 | NR100 | NR100 | NR115 | NR115 | NR115 | NR115 | NR100 |
| 7 | NR100 | NR115 | NR115 | NR115 | NR115 | NR100 | 90 | NR115 | NR115 | NR115 | NR115 | NR100 |
| 8 | – | NR120 | NR125 | NR125 | NR120 | NR110 | – | NR120 | NR125 | NR125 | NR120 | NR110 |
| 9 | 90 | NR110 | NR110 | NR110 | NR110 | NR100 | 90 | 100 | NR110 | NR110 | NR110 | NR100 |
| 10 | NR105 | NR120 | NR120 | NR120 | NR120 | NR110 | NR105 | NR120 | NR120 | NR120 | NR120 | NR110 |
| 11 | 90 | NR110 | NR110 | NR110 | NR110 | NR110 | 90 | NR110 | NR110 | NR110 | NR110 | NR110 |
| 12 | – | 85 | 85 | 115 | NR115 | – | – | 80 | 95 | 110 | NR115 | – |
| 13 | – | 110 | 110 | NR115 | NR115 | NR115 | – | 110 | 110 | NR115 | NR115 | NR115 |
| 14 | 80 | 85 | 90 | 90 | 80 | 90 | 70 | 90 | 95 | 95 | 90 | 95 |
| 15 | – | 90 | 90 | 90 | 90 | 90 | – | 90 | 90 | 90 | 90 | 90 |
| 16 | NR100 | NR105 | NR105 | NR105 | NR110 | NR110 | NR100 | NR105 | NR105 | NR105 | NR110 | NR110 |
NR: No response; “–”; no information available.
3.1. Spiral ganglion cell counts
Table 5 (MP) and 6 (BP) represent corrected segmental and total SGC counts for the implanted and unimplanted ears of each subject. In case 10 of the BP group (Table 6) and case 3 of the MP group (Table 5), portions of segments 2 and 4 in the unimplanted ears were lost at bone extraction. Therefore, these segmental counts and consequently total counts were not available for these two cases. There is a wide range in the total SGC count for the unimplanted ears (1068 to 15,334) and implanted ears (626–14,464). It should be mentioned that the counts represent the number of SGCs at the time of death (sometimes long after the preimplantation audiological measurements were made).
Table 5.
MP group corrected segmental and total SGC counts
| Case | Segment I |
Segment II |
Segment III |
Segment IV |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Im | Un | Im | Un | Im | Un | Im | Un | Im | Un | |
| 1 | 1958 | 1466 | 5896 | 3454 | 2978 | 2720 | 2781 | 2645 | 13613 | 10285 |
| 2 | 728 | 1897 | 5270 | 5392 | 3563 | 3679 | 2591 | 3543 | 12152 | 14511 |
| 3 | 1680 | 1136 | 4216 | – | 2264 | 3169 | 1825 | – | 9985 | – |
| 4 | 170 | 619 | 687 | 938 | 1455 | 2421 | 741 | 3516 | 3053 | 7494 |
| 5 | 517 | 1258 | 2700 | 2938 | 2216 | 2203 | 1075 | 1496 | 6508 | 7895 |
| 6 | 428 | 449 | 1442 | 3835 | 1374 | 2428 | 1448 | 2944 | 4692 | 9656 |
| 7 | 2258 | 1557 | 5229 | 5025 | 3271 | 2925 | 3706 | 3665 | 14464 | 13172 |
| 8 | 68 | 571 | 320 | 4604 | 163 | 3094 | 75 | 3346 | 626 | 11615 |
| 9 | 768 | 1099 | 3726 | 4447 | 1347 | 2312 | 1129 | 1142 | 6970 | 9000 |
| 10 | 333 | 428 | 2407 | 2468 | 2278 | 2298 | 2237 | 1932 | 7255 | 7126 |
Im: Implanted ears; Un: Unimplanted ears.
Table 6.
BP group corrected segmental and total SGC counts
| Case | Segment I |
Segment II |
Segment III |
Segment IV |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Im | Un | Im | Un | Im | Un | Im | Un | Im | Un | |
| 1 | 1251 | 3142 | 4903 | 5970 | 3318 | 3182 | 2863 | 3040 | 12335 | 15334 |
| 2 | 1754 | 1346 | 1714 | 2332 | 748 | 1285 | 1027 | 613 | 5243 | 5576 |
| 3 | 1040 | 1822 | 4257 | 4379 | 1319 | 2305 | 2897 | 2740 | 9513 | 11246 |
| 4 | 748 | 1047 | 1856 | 2754 | 700 | 1870 | 674 | 2142 | 3978 | 7813 |
| 5 | 619 | 415 | 4617 | 5243 | 3325 | 3631 | 2849 | 3427 | 11410 | 12716 |
| 6 | 1496 | 1564 | 5481 | 5392 | 3577 | 4379 | 3230 | 3815 | 13784 | 15150 |
| 7 | 88 | 292 | 2849 | 2693 | 2271 | 3237 | 1516 | 3387 | 6724 | 9608 |
| 8 | 1204 | 558 | 2876 | 2516 | 2074 | 1890 | 1618 | 2713 | 7772 | 7677 |
| 9 | 415 | 1000 | 2115 | 3570 | 1442 | 2550 | 1734 | 1952 | 5706 | 9072 |
| 10 | 721 | 326 | 3284 | – | 3176 | 1945 | 3046 | – | 10227 | – |
| 11 | 258 | 48 | 1060 | 422 | 544 | 265 | 197 | 333 | 2059 | 1067 |
| 12 | 843 | 734 | 898 | 748 | 1000 | 1061 | 1489 | 1904 | 4230 | 4447 |
| 13 | 1163 | 1197 | 1353 | 2482 | 680 | 1210 | 1136 | 1421 | 4332 | 6310 |
| 14 | 666 | 1027 | 1931 | 2434 | 1979 | 2094 | 2230 | 2496 | 6806 | 8051 |
| 15 | 1190 | 1285 | 2434 | 2624 | 1047 | 1401 | 1285 | 2060 | 5956 | 7371 |
| 16 | 524 | 462 | 2591 | 1604 | 1713 | 1557 | 1054 | 2285 | 5882 | 5909 |
Im: Implanted ears; Un: Unimplanted ears.
Table 7 (MP group) and 8 (BP group) show the differences between the implanted (Im) and unimplanted (Ui) SGC segmental counts for each subject by cochlear segment. The count differences (Im – Ui) ranged from −4284 (negative: implanted count < unimplanted count) to 2442 (positive: implanted count > unimplanted count) for MP stimulation and – 1891 to 1231 for BP. We tested whether the across-subject and across-segment variation in age at death and duration of cochlear implant (durCI) usage explained any of the variation in count difference using a least-squares fitting procedure to estimate the coefficients of the following linear model: cDiff = A*age + B *durCI + C, where cDiff is count difference, A is the coefficient associated with the age variable, B is the coefficient associated with the duration of cochlear implant usage and C is a constant. For both MP and BP stimulation, neither A (MP: 4.2, p = 0.82; BP: 12.2, p = 0.08) nor B (MP: 59.0, p = 0.11; BP: −12.9, p 0.46) were significantly different than zero indicating that neither age nor durCI significantly influenced the count difference. Thus, additional analyses proceeded assuming no effect of age at death or duration of cochlear implant use.
Table 7.
MP group segmental count differences
| Case | SGC count difference (implanted-unimplanted) |
|||
|---|---|---|---|---|
| Segment I | Segment II | Segment III | Segment IV | |
| 1 | 492 | 2442 | 258 | 136 |
| 2 | −1169 | −122 | −116 | −952 |
| 3 | 544 | – | −905 | – |
| 4 | −449 | −251 | −966 | −2775 |
| 5 | −741 | −238 | 13 | −421 |
| 6 | −21 | −2393 | −1054 | −1496 |
| 7 | 701 | 204 | 346 | 41 |
| 8 | −503 | −4284 | −2931 | −3271 |
| 9 | −331 | −721 | −965 | −13 |
| 10 | −95 | −61 | −20 | 305 |
| Segment mean | −157 | −603 | −634 | −938 |
The mean (across subjects) difference for each cochlear segment is negative for both MP and BP stimulation showing a tendency for counts to be lower in the implanted than in the unimplanted ear. Using the t-test, we found that the overall mean (across subjects and segments) of the count differences of both Table 7 (MP: −573) and Table 8 (BP: −322) were each significantly less than zero (MP: t = 2.87, df = 37, p = 0.003; BP: t = −3.96, df = 61, p < 0.0001) indicating fewer in the implanted/stimulated ears than in the unimplanted ears.
Table 8.
BP group segmental count differences
| Case | SGC count difference (implanted-unimplanted) |
|||
|---|---|---|---|---|
| Segment I | Segment II | Segment III | Segment IV | |
| 1 | −1891 | −1067 | 136 | −177 |
| 2 | 408 | −618 | −537 | 414 |
| 3 | −782 | −122 | −986 | 157 |
| 4 | −299 | −898 | −1170 | −1468 |
| 5 | 204 | −626 | −306 | −578 |
| 6 | −68 | 89 | −802 | −585 |
| 7 | −204 | 156 | −966 | −1871 |
| 8 | 646 | 360 | 184 | −1095 |
| 9 | −585 | −1455 | −1108 | −218 |
| 10 | 395 | – | 1231 | – |
| 11 | 210 | 638 | 279 | −136 |
| 12 | 109 | 150 | −61 | −415 |
| 13 | −34 | −1129 | −530 | −285 |
| 14 | −361 | −503 | −115 | −266 |
| 15 | −95 | −190 | −354 | −775 |
| 16 | 62 | 987 | 156 | −1231 |
| Segment mean | −143 | −282 | −309 | −569 |
Because the contacts of an electrode array typically extend from segment I through segment III in the implanted ears, the SGCs in segments I-III are likely to experience stronger stimulus excitation than SGCs in segment IV. We reasoned that if CES provides a trophic influence on SGCs, then the magnitude of the differences between the counts made in the implanted ear and those made in the unimplanted ear in segments I-III should be smaller than the magnitude differences of segment IV. This is consistent with the segment means shown in Table 7 (MP) and 8 (BP) where the magnitude of the individual segment mean differences for segments I-III are smaller (less negative) than those for segment IV. To test this hypothesis statistically, we considered the count differences for segments I-III as a stimulated group and tested whether the magnitudes of the means of these differences (MP: −460; BP: −244) were significantly smaller than the magnitudes of the mean segment-IV count differences (MP: −938; BP: −569) using a t-test. The magnitude of the mean difference was significantly lower in segments I-III than segment IV for BP stimulation (t = 1.75; df = 24; p = 0.046) but not for MP stimulation (t = 097; df = 12.45; p = 0.18). While the t-test results for MP stimulation did not reject the null hypothesis that the mean count difference for segments I-III was greater than or equal to the mean difference for segment IV, this result does not prove that the difference is zero. In fact, given the relatively low statistical power associated with these MP data, the 95% confidence limit of the mean difference between the count differences (Im-Ui) of the segments I-III and of the segment IV data is within the very broad range of ±1577 of zero.
3.2. Maximum cross-sectional cell area
The maximum cross-sectional area of 1422 cells was measured in the subject group experiencing MP stimulation: 647 in implanted and 775 in unimplanted ears. In the BP group, the cross-sectional area of 2276 cells was measured: 1095 in implanted and 1181 in unimplanted ears. The cell area ranges from 97 μm2 to 1296 μm2 in the MP group and from 76 μm2 to 1460 μm2 in the BP group. These ranges are roughly consistent with the results presented by Nadol (1990) for a normal-hearing temporal bone. The mean cell area was computed for each subject by segment and the results are listed in Table 9 (MP) and 10 (BP). Case 10 of the BP group was not included in the analysis because part of the unimplanted specimen was lost during bone removal. The mean cell area ranges from 241 μm2 to 812 μm2 in the MP group and from 340 μm2 to 864 μm2 in the BP group. These ranges are roughly consistent with the results presented by Nadol (1990) for a normal-hearing temporal bone.
Table 9.
MP group mean cell areas (μm2)
| Case | Segment I |
Segment II |
Segment III |
Segment IV |
||||
|---|---|---|---|---|---|---|---|---|
| Im | Un | Im | Un | Im | Un | Im | Un | |
| 1 | 466 | 569 | 467 | 618 | 569 | 434 | 524 | 503 |
| 2 | 497 | 505 | 621 | 424 | 491 | 372 | 522 | 382 |
| 3 | 500 | 556 | 514 | – | 512 | 601 | 603 | – |
| 4 | 812 | 693 | 595 | 544 | 679 | 639 | 718 | 693 |
| 5 | 443 | 345 | 282 | 249 | 241 | 290 | 355 | 248 |
| 6 | 731 | 642 | 556 | 578 | 710 | 649 | 530 | 568 |
| 7 | 453 | 415 | 432 | 318 | 507 | 316 | 476 | 329 |
| 8 | 452 | 618 | 418 | 583 | 510 | 701 | 390 | 713 |
| 9 | 624 | 658 | 510 | 569 | 617 | 472 | 573 | 568 |
| 10 | 464 | 445 | 504 | 583 | 470 | 483 | 517 | 415 |
Im: Implanted ears; Un: Unimplanted ears.
The difference (Im-Ui) in mean cell area by segment for each subject is shown in Table 11 (MP) and Table 12 (BP). We tested whether the across-subject and across-segment variation in age at death and duration of cochlear implant usage explained any of the variation in the mean cell area difference using a least-squares fitting procedure to estimate the coefficients of the following linear model: aDiff = A*age + B*durCI + C, where aDiff is the difference (Im-Ui) in mean cell area, A is the coefficient associated with the age variable, B is the coefficient associated with the duration of cochlear implant usage and C is a constant. For both MP and BP stimulation, neither A (MP: −0.7, p = 0.72; BP: 0.4, p = 0.75) nor B (MP: 3.1, p = 0.00.39; BP: 2.4, p = 0.48) were significantly different than zero indicating that neither age nor durCI significantly influenced the difference in mean cell area. Thus, additional analyses proceeded assuming no effect of age at death or duration of cochlear implant use.
Table 11.
MP group cell area (μm2) differences
| Case | Cell area difference (μm2, implanted-unimplanted) |
|||
|---|---|---|---|---|
| Segment I | Segment II | Segment III | Segment IV | |
| 1 | −104 | −151 | 135 | 21 |
| 2 | −8 | 197 | 118 | 141 |
| 3 | −57 | – | −89 | – |
| 4 | 119 | 52 | 40 | 25 |
| 5 | 98 | 33 | −49 | 107 |
| 6 | 89 | −21 | 61 | −38 |
| 7 | 38 | 113 | 191 | 147 |
| 8 | −166 | −166 | −191 | −323 |
| 9 | −35 | −59 | 145 | 5 |
| 10 | 19 | −79 | −13 | 101 |
| Segment mean | −1 | −9 | 35 | 21 |
Table 12.
BP group cell area (μm2) differences
| Case | Cell area difference (μm2, implanted-unimplanted) |
|||
|---|---|---|---|---|
| Segment I | Segment II | Segment III | Segment IV | |
| 1 | −24 | 111 | 77 | −100 |
| 2 | 68 | 13 | −2 | −127 |
| 3 | −56 | −5 | −171 | 94 |
| 4 | 18 | 30 | 190 | −80 |
| 5 | 53 | −63 | −18 | −87 |
| 6 | 112 | 58 | 10 | −72 |
| 7 | −253 | −348 | −101 | −116 |
| 8 | −230 | 46 | 61 | 130 |
| 9 | 82 | 19 | 156 | 19 |
| 11 | 258 | −110 | 243 | −76 |
| 12 | 222 | −116 | −1 | 85 |
| 13 | 3 | 175 | 230 | 104 |
| 14 | 111 | 13 | 130 | 71 |
| 15 | 47 | 61 | 146 | 108 |
| 16 | −12 | −15 | 4 | −108 |
| Segment mean | 27 | −9 | 64 | −10 |
Because the range of mean (across subjects and segments) cell area differences (Im-Ui) is quite large for both the MP ( −323 μm2 −197 μm2) and BP ( −348 μm2−258 μm2) groups while the segment mean differences are relatively small (−9 μm2−64 μm2), it is not surprising that a t-test shows that for both MP and BP stimulation, one is not able to reject the hypothesis that mean difference (Im-Ui) in cell area is zero (i.e. the same in the implanted ears as in the unimplanted ears; MP: t = 0.62; df = 37; p = 0.54; BP: t = 1.14; df = 59; p = 0.26). While this result not prove that the difference is zero, given the statistical power associated with the number of samples and the variance associated with these data, we are able to say with a likelihood of 95% that the mean difference is within ±57 μm2 of zero for BP stimulation and within ±70 μm2 of zero for MP stimulation.
Unlike the cell count results, one is also unable to reject the hypothesis by t-test that the mean difference (Im-Ui) in cell area for the segments receiving direct stimulation (segments I-II) (MP: 29 μm2; BP: 45 μm2) is equal to the mean cell area difference of unstimulated segment IV (MP: 9 μm2; BP: 15 μm2) for both MP (t = 0.22; df = 11; p = 0.83) and BP (t = 1.18; df 31; p = 0.25) stimulation. The 95% confidence limits for the difference = between = the mean cell area differences (Im-Ui) of the stimulated segments (I-III) and the unstimulated segment (IV) are relatively broad: within ±133 μm2 of zero for BP stimulation and within ±165 μm2 of zero for MP stimulation.
4. Discussion
4.1. Spiral ganglion cell counts
The total SGC counts for the unimplanted subjects group reported here tend to be lower than the counts by other investigators for other adult, profoundly hearing-impaired populations. For example, the total SGC count mean (8268 across all unimplanted subjects) of this study is significantly lower than those reported by Hinojosa and Marion ((1983); mean: 14,600, t: −3.69, df: 24, p: 0.0009) and Linthicum et al. ((1991); mean: 11,716, t: −2.54, p: 0.009) and lower (but not significantly) than Khan et al. ((2005),mean: 10,286, t: −1.69, df: 31, p: 0.051), Fayad and Linthicum ((2006); mean: 11,762, t: −1.48, df: 14, p: 0.080) and Xu et al. ((2012), mean: 9,906, t: −1.26, df: 9, p: 0.12). While it is possible that a methodological difference between the present study and the previous studies accounts for our lower counts, we are not able to identify a credible possibility and adopt the working hypothesis that our population’s SGC counts are lower than many other subject populations studied.
Analysis of the difference between the implanted and unimplanted SGC counts for each cochlear segment in each subject showed that the counts made in the implanted bones are significantly lower than the counts in the unimplanted bones. This finding is in agreement with the results reported by other studies where SGCs were counted in the implanted and unimplanted bones of each subject (e.g., Clark et al., 1988; Fayad et al., 2006; Khan et al., 2005; Marsh et al., 1992; Xu et al., 2012; Zappia et al., 1991). Taken together, these results are consistent with one or more factors associated with implantation and/or stimulation having a deleterious impact on SGC survival.
By separating our count differences into two groups (cochlear segments I-III and segment IV) we showed that the amount by which the implanted ear count is lower than the unimplanted ear in a subject is significantly smaller in the segments I-III group (mean: −249) than in the segment-IV group (mean: −569) for BP stimulation. This result, together with the result described in the previous paragraph leads us to a two part interpretation for profoundly-impaired adult ears: (1) implantation typically leads to degeneration of SGCs resulting in the mean SGC count in an implanted ear being lower than the count in an unimplanted ear and (2) BP stimulation promotes SGC survival relative to no stimulation and leads to less SGC degeneration in stimulated cochlear segments than in the unstimulated segment of the implanted ear. This interpretation is consistent with results reported by Khan et al. (2005) and Xu et al. (2012) where the SGC counts in the implanted ears tended to be less than the unimplanted ears in all cochlear segments but the only segment in which this difference was significant was the most apical segment that did not receive direct electric stimulation.
Our result suggesting that BP stimulation promotes survival of SGCs is consistent with data from a number of animal studies. Leake et al. (1999), in their experiment on 8 kittens with BP stimulation, showed 21% higher SGC density in implanted ears in comparison with unimplanted ears. The increase of SGC happened throughout the whole cochlea except the apical sector which showed no significant difference. Leake et al. (1991) in their earlier study on 4 neonate kittens with a shorter time of stimulation (12 vs. 32–56 weeks) and lower stimulation frequency (30 vs. 80–300 pps) also reported higher SGC density in implanted stimulated ears which was statistically significant and generally restricted to the segments adjacent to the stimulating electrode (basal segments) while the apical sector showed fewer cells but did not reach statistical significance. It should also be noted that a number of animal studies did not find a protective effect for CES (e.g., Araki et al., 1998; Coco et al., 2007; Xu et al., 1997).
MP stimulation did not show a significant protective effect on SGCs in the subject sample of this study. We suggest two possible explanations. First, the protective effect of MP stimulation may be weaker than for BP stimulation in human as has been observed by Leake et al. (1999, 1995) in neonatally-deafened cat. Second, the protective effect of MP stimulation is substantial but the statistical power associated with our MP-stimulation subject sample was not sufficient to find the effect of stimulation significant. For example, post-hoc power analyses showed a power of 0.26 for MP stimulation; about half the power (0.53) associated with our BP-stimulation sample. In order to distinguish between the two explanations, the power associated with the comparison of means would need to be increased (e.g., by increasing the number of subjects and/or decreasing the variance) to increase the likelihood that a significant effect would be detected if actually present.
4.2. Maximum cross-sectional cell area
There are two types of SGCs in Rosenthal’s canal: (1) larger type-I cells that innervate inner hair cells and constitute 90–95% of all SGCs and (2) smaller type-II cells that innervate outer hair cells and constitute 5–10% of all SGCs (Nadol et al., 1990). Nadol (1990) and Zimmerman et al. (1995) reported that the maximum cross-sectional area of SGCs from normal-hearing and hearing-impaired ears are distributed bimodally with distribution peaks at approximated 200 μm2 and 600 μm2 on either side of a minimum at 300 μm2. The cell area distributions of the current study did not fit this picture. The distributions (by subject or segment or stimulus configuration) were unimodal with a peak at approximately 520 μm2 and range from approximately 75–1460 μm2. Because of the unimodal distribution of cell area, we did not attempt to divide the population of cells measured into type I or type II categories.
Unlike the cell count data, the maximum cross-sectional cell area measures did not differ significantly between implanted/stimulated and unimplanted ears (see results section) for both MP and BP stimulus configurations. Also unlike the cell count data, the mean cell-area difference between stimulated cochlear segments (segments I-III) was not different than that for the unstimulated segment IV. Taken together, this means we see no evidence of implantation or CES influencing maximum cross-sectional cell area. This result is not consistent with animal studies where differences in cell size between implanted and unimplanted ears have been observed. For instance, Coco et al. (2007), Araki et al. (1998) and Leake et al. (1999) reported larger SGCs associated with BP stimulation Agterberg et al. (2010) also reported larger SGCs in ears with chronic or brief MP stimulation.
5. Conclusion
The SGC counts made in implanted ears were significantly lower than counts made in unimplanted ears for subjects who experienced either MP or BP stimulation. When the SGC count differences (implanted – implanted) in each subject were grouped by segments with stimulating electrodes (segments I-III) and segments without (segment IV), the mean difference for the stimulated segments were smaller than the unstimulated segment for BP stimulation but the effect of stimulation was not found to be significant for MP stimulation. These results are consistent with (1) implantation typically leading to degeneration of SGCs in adult humans and (2) BP stimulation (but not MP) promoting SGC survival relative to no implantation/stimulation. The measurements of maximum cross-sectional cell area did not show a significant influence of implantation or stimulation.
Table 10.
BP group mean cell areas (μm2)
| Case | Segment I |
Segment II |
Segment III |
Segment IV |
||||
|---|---|---|---|---|---|---|---|---|
| Im | Un | Im | Un | Im | Un | Im | Un | |
| 1 | 575 | 599 | 680 | 569 | 692 | 616 | 576 | 676 |
| 2 | 724 | 656 | 570 | 557 | 567 | 569 | 460 | 587 |
| 3 | 495 | 551 | 546 | 550 | 440 | 611 | 498 | 404 |
| 4 | 471 | 452 | 444 | 415 | 531 | 341 | 443 | 523 |
| 5 | 808 | 755 | 597 | 660 | 510 | 528 | 614 | 700 |
| 6 | 611 | 499 | 669 | 611 | 497 | 487 | 556 | 628 |
| 7 | 604 | 857 | 498 | 845 | 474 | 575 | 429 | 545 |
| 8 | 516 | 747 | 630 | 584 | 674 | 613 | 728 | 599 |
| 9 | 526 | 444 | 567 | 548 | 723 | 567 | 663 | 644 |
| 11 | 694 | 436 | 663 | 773 | 712 | 469 | 521 | 597 |
| 12 | 864 | 642 | 591 | 707 | 657 | 658 | 654 | 569 |
| 13 | 489 | 486 | 536 | 361 | 593 | 363 | 565 | 460 |
| 14 | 488 | 376 | 444 | 431 | 495 | 366 | 467 | 396 |
| 15 | 539 | 492 | 530 | 468 | 595 | 448 | 603 | 495 |
| 16 | 400 | 411 | 464 | 480 | 431 | 427 | 448 | 556 |
Im: Implanted ears; Un: Unimplanted ears.
Acknowledgment
This work was supported by grant R01-DC000152 from the National Institute of Deafness and Other Communication Disorders.
References
- Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear. Res. 2010;269:169–179. doi: 10.1016/j.heares.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Araki S, Kawano A, Seldon L, Shepherd RK, Funasaka S, Clark GM. Effects of chronic electrical stimulation on spiral ganglion neuron survival and size in deafened kittens. Laryngoscope. 1998;108:687–695. doi: 10.1097/00005537-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Chatterjee M. Effects of stimulation mode on threshold and loudness growth in multielectrode cochlear implants. The J. Acoust. Soc. America. 1999;105:850–860. doi: 10.1121/1.426274. [DOI] [PubMed] [Google Scholar]
- Chiong CM, Burgess BJ, Nadol JB., Jr. Postnatal maturation of human spiral ganglion cells: light and electron microscopic observations. Hear. Res. 1993;67:211–219. doi: 10.1016/0378-5955(93)90249-z. [DOI] [PubMed] [Google Scholar]
- Clark GM, Shepherd RK, Franz BK, Dowell RC, Tong YC, Blamey PJ, Webb RL, Pyman BC, McNaughtan J, Bloom DM, et al. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Correlation with psychophysics and speech perception results. Acta Otolaryngol. Suppl. 1988;448:1–65. doi: 10.3109/00016488809098972. [DOI] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear. Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat. Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell. Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear. Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann. N. Y Acad. Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Snyder R, Rebscher S, Leake P. Effects of neonatal deafening and chronic intracochlear electrical stimulation on the cochlear nucleus in cats. Hear. Res. 1991;54:272–280. doi: 10.1016/0378-5955(91)90121-o. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J. Comp. Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Damian D, Eddington DK, Nadol JB., Jr. Effect of cochlear implantation on residual spiral ganglion cell count as determined by comparison with the contralateral nonimplanted inner ear in humans. Ann. Otol. Rhinol. Laryngol. 2005;114:381–385. doi: 10.1177/000348940511400508. [DOI] [PubMed] [Google Scholar]
- Konigsmark BW. Methods for the counting of neurons. In: Nauta WJH, Ebbeson SOE, editors. Contemporary Research Methods in Neuroanatomy. Springer-Verlag; Berlin: 1970. pp. 315–338. [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J. Comp. Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear. Res. 1991;54:251–271. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Consequences of chronic extracochlear electrical stimulation in neonatally deafened cats. Hear. Res. 1995;82:65–80. doi: 10.1016/0378-5955(94)00167-o. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr., Anderson W. Cochlear implantation of totally deaf ears. Histologic evaluation of candidacy. Acta Oto-laryngologica. 1991;111:327–331. doi: 10.3109/00016489109137395. [DOI] [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–842. [PubMed] [Google Scholar]
- Marsh MA, Coker NJ, Jenkins HA. Temporal bone histopathology of a patient with a nucleus 22-channel cochlear implant. Am. J. Otol. 1992;13:241–248. [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int. J. Dev. Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear. Res. 1997;105:30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr. Quantification of human spiral ganglion cells by serial section reconstruction and segmental density estimates. Am. J. Otol. 1988;9:47–51. doi: 10.1016/s0196-0709(88)80007-3. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr., Burgess BJ, Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann. Otol. Rhinol. Laryngol. 1990;99:340–348. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. Temporal bone removal at autopsy. Preparation and uses. Arch. Otolaryngol. 1968;87:129–137. doi: 10.1001/archotol.1968.00760060131007. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Techniques for study of cochlear function and pathology in experimental animals; development of the anatomical frequency scale for the cat. AMA Arch. Otolaryngol. 1953;58:377–397. doi: 10.1001/archotol.1953.00710040399001. [DOI] [PubMed] [Google Scholar]
- Seyyedi M, Eddington DK, Nadol JB., Jr. Interaural comparison of spiral ganglion cell counts in profound deafness. Hear. Res. 2011;282:56–62. doi: 10.1016/j.heares.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear. Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens. Hear. Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Retrograde degeneration of the cochlear nerve. Acta Otolaryngol. 1975;79:266–275. doi: 10.3109/00016487509124683. [DOI] [PubMed] [Google Scholar]
- Webster M, Webster DB. Spiral ganglion neuron loss following organ of corti loss: a quantitative study. Brain Res. 1981;212:17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J. Comp. Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Xu HX, Kim GH, Snissarenko EP, Cureoglu S, Paparella MM. Multi-channel cochlear implant histopathology: are fewer spiral ganglion cells really related to better clinical performance? Acta Oto-laryngologica. 2012;132:482–490. doi: 10.3109/00016489.2011.647361. [DOI] [PubMed] [Google Scholar]
- Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hear. Res. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear. Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear. Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Zappia JJ, Niparko JK, Oviatt DL, Kemink JL, Altschuler RA. Evaluation of the temporal bones of a multichannel cochlear implant patient. Ann. Otol Rhinol Laryngol. 1991;100:914–921. doi: 10.1177/000348949110001111. [DOI] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB., Jr. Patterns of degeneration in the human cochlear nerve. Hear. Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]


