Abstract
Objective
To describe the association between six-month weight gain on antiretroviral therapy (ART) and subsequent clinical outcomes.
Design
A retrospective analysis of a large programmatic cohort in Lusaka, Zambia.
Methods
Using Kaplan-Meier analysis and Cox Proportional Hazards models, we examined the association between six-month weight gain and the risk of subsequent death and clinical treatment failure. Because it is a known effect modifier, we stratified our analysis according to body mass index (BMI).
Results
27,915 adults initiating ART were included in the analysis. Patients in the lower BMI categories demonstrated greater weight gain. In the post-six month analysis, absolute weight loss was strongly associated with mortality across all BMI strata, with the highest risk observed among those with BMI <16 kg/m2 (adjusted hazard ratio 9.7; 95%CI 4.7–20.0). There appeared to be an inverse relationship between weight gain and mortality among patients with BMI <16 kg/m2. Similar trends were observed with clinical treatment failure.
Conclusion
Weight gain after ART initiation is associated with improved survival and decreased risk for clinical failure, especially in the lower BMI strata. Prospective trials to promote weight gain after ART initiation among malnourished patients in resourced-constrained settings are warranted.
Keywords: HIV, Africa, antiretroviral therapy, body mass index, nutrition, epidemiology
Introduction
The provision of antiretroviral therapy (ART) for HIV infection has expanded rapidly throughout sub-Saharan Africa since 2003; 2.1 million of an estimated 7 million people in need now receive treatment 1. Clinical and programmatic success has been reported from several countries in the region 2–5, but malnutrition complicates the provision of care in areas burdened by the twin epidemics of high HIV prevalence and food scarcity 6.
The World Health Organization (WHO) grades malnutrition according to body mass index (BMI), calculated as weight in kilograms divided by height in meters squared, as mild (BMI = 17.00–18.49), moderate (16.00–16.99), and severe (<16.00 kg/m2) 7. The causes of low BMI are multi-factorial and may represent a number of conditions from normal anthropometric variation to chronic inadequate food intake to wasting associated with HIV and other infections. Several analyses of patient outcomes have identified a low BMI at ART initiation as an independent predictor of early mortality. In Zambia, for example, patients with a BMI <16.0 kg/m2 had higher mortality in the first 90 days on ART (adjusted hazard ratio [AHR]: 2.4, 95%CI: 1.8–3.2) when compared to those above this BMI threshold 3. In rural Malawi, a BMI <15.9 at ART initiation carried a six-fold increased hazard of death at three months compared to a BMI >18.5 kg/m2 (AHR: 6.0, 95%CI: 4.6–12.7) 8. In Tanzania, a BMI <16.0 was associated with two-fold higher mortality compared to a BMI >18.5 kg/m2 (AHR: 2.1, 95%CI: 1.1–4.2) over a median period of 10.9 months 9.
While some studies have identified at-risk groups based on baseline indicators of malnutrition, few have determined what impact improved nutrition may have on subsequent clinical outcomes while on ART. This is a notable gap in the medical literature since the proposed benefits of early weight gain serve as the theoretical foundation for food-by-prescription and food supplementation programs in resource-constrained settings 10, 11. In this analysis, we examine the association of six-month weight gain with subsequent clinical outcomes in a programmatic cohort in Lusaka, Zambia.
Methods
The Zambian national program for HIV care and treatment was implemented in Lusaka’s public health sector in April 2004 and has expanded rapidly 3, 12. Briefly, HIV-infected patients are enrolled in care and undergo a history and physical, WHO staging, and a CD4+ lymphocyte count. Weight and height (i.e. the components of BMI) are recorded at the initial visit, and weight is recorded at subsequent visits. Patients with WHO stage 4 disease; a CD4+ lymphocyte count <200 cells/mm3; or WHO stage 3 disease and a CD4+ lymphocyte count <350 mm3 are eligible to initiate ART. During the study period, the first line regimen was a non-nucleoside reverse transcriptase inhibitor (NNRTI), either efavirenz (EFV) or nevirapine (NVP), in combination with two nucleoside reverse transcriptase inhibitors (NRTIs): lamivudine (3TC) with either zidovudine (ZDV) or stavudine (d4T). In July 2007, tenofovir and emtricitabine (TDF/FTC) was introduced as the first line NRTI combination. Patients on treatment prior to July 2007 remained on the original regimen, except in cases of treatment failure or toxicity.
Toxicity and clinical disease progression are monitored closely after ART initiation through an intensive visit schedule. Once deemed stable by clinicians, patients return for pharmacy visits every one to three months and clinical visits every three months. A CD4+ lymphocyte count is performed every six months or at shorter intervals if there is evidence of disease progression. Determination of treatment failure is based on WHO staging (clinical failure) and/or CD4+ lymphocyte count (immunologic failure) criteria. These include a new or recurrent WHO stage 3 or 4 condition while on ART for greater than six months, a <50 cells/mm3 CD4+ lymphocyte increase after six months of ART, a CD4+ lymphocyte count <100 cells/mm3 after 12 months, a > 30% decline in CD4+ lymphocyte count from the peak post-ART value, or a decline in CD4+ lymphocyte count to value below that of treatment initiation. Plasma HIV RNA measurements, where available, are used sparingly. If adherence is determined to be adequate, patients failing treatment are changed to a second line regimen, typically a protease inhibitor (PI) with concomitant NRTI changes.
Nutrition counseling is provided at enrollment in the ART program, and clinicians have the option to refer patients at any visit for further information on healthy eating habits. Additionally, from 2004 to 2006 a World Food Programme (WFP) pilot study of food supplementation for HIV-infected persons was carried out at 10 clinics 13. This program provided individual and household rations for six to 12 months, based on an assessment of food insecurity in the home. Eligibility criteria did not include BMI or other anthropometric or biochemical measures.
We analyzed data from a cohort of HIV-infected adults (>15 years of age) who started ART between May 1, 2004 and April 31, 2008. To be included, patients were required to have a baseline BMI (i.e. at ART initiation), remain active in the program for at least six months, and have a follow-up weight measurement within the six-month window period. In the analyses of death or treatment failure, patients were censored at the time of voluntary withdrawal from the program or when classified as lost to follow-up (defined as 37 days after a scheduled pharmacy visit or, if no pharmacy visit was scheduled, 60 days after the last clinical visit). We compared the demographic and medical characteristics of those included and excluded from the analysis. For dichotomous or categorical variables, we calculated percentages; for continuous descriptors, we calculated means and standard deviations. We calculated mortality rates in person-years and generated corresponding exact Poisson confidence intervals for each estimate.
For our primary analysis, we categorized patients according to weight gain across two measures: absolute weight change from ART initiation to six months (≥10.00 kg, 5.00 – 9.99 kg, 2.50 – 4.99 kg, 0.00 – 2.49 kg, weight loss) and proportional weight change from ART initiation to six months (≥20.0%, 10.0 – 19.9%, 0.0 – 9.9%, weight loss). For each of these groups, we examined the risk of death and clinical treatment failure from six months onward, using Kaplan-Meier analysis and Cox Proportional Hazards models. For this analysis, we defined treatment failure as a worsening WHO stage after at least three months on therapy, a decline in the CD4+ lymphocyte count to a value <95% of the pre-treatment baseline, and/or a change to second-line treatment. Multivariable models were adjusted for age, gender, baseline hemoglobin, tuberculosis status, baseline WHO stage, initial ART regimen, and adherence. Adherence was based on the medication possession ratio defined as the number of days late for pharmacy refills divided by the total days on therapy, subtracted from 100%. The resulting figure represents the percentage of days a patient is known to have medication 14. Because entry BMI is a known effect modifier 3, 8, 9, we performed stratified analyses according to the above-mentioned WHO categories for malnutrition 7. We also included a weighted hazard ratio in our Cox Proportional Hazards model, to provide a summary measure based on the distribution of BMI in our population.
Available patient data through October 31, 2008 (the dataset freeze date) were considered in this report. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina), and were approved by the relevant ethical authorities of the University of Zambia (Lusaka, Zambia) and the University of Alabama at Birmingham (Birmingham, Alabama, USA).
Results
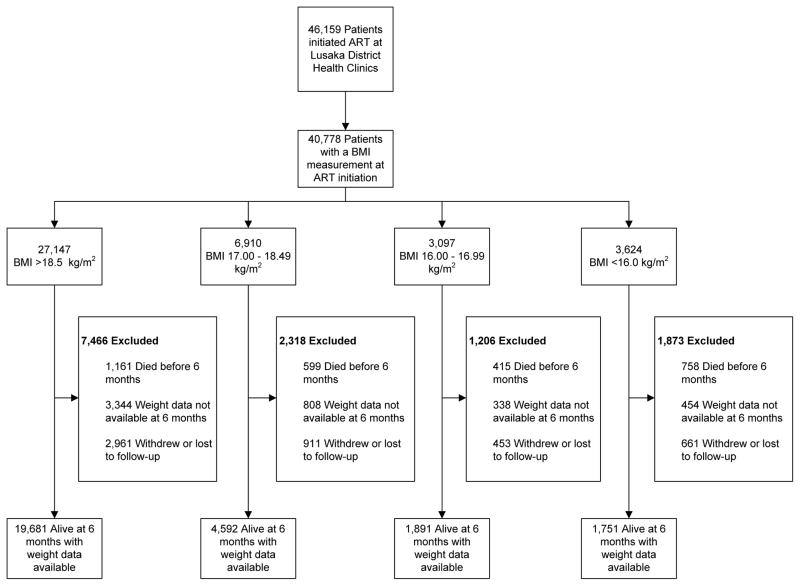
Between May 1, 2004 and April 31, 2008, 46,159 patients started ART at Lusaka district clinics, and 40,778 (88%) had a baseline BMI measurement. A broad range of BMI measurements were observed: 3,624 (9%) had a BMI <16.0; 3,097 (8%) had a BMI 16.00 – 16.99; 6,910 (17%) had a BMI 17.00 – 18.49; and 27,147 (67%) had a BMI >18.5 kg/m2.
Overall, 27,915 (68%) patients remained active in the program after six months and had a documented six-month weight measurement. Lower median CD4+ cell counts and hemoglobin, a higher prevalence of active tuberculosis (TB), and higher WHO clinical stages were observed in progressively lower BMI strata (TABLE 1). Among the 12,863 patients that were excluded, 4,986 (39%) were lost to follow-up or withdrew from the program, 2,933 (23%) had died prior to six months, and 4,944 (38%) did not have a weight measurement recorded at six months (FIGURE 1). Patients without a six-month weight measurement were more likely to be male (40% vs. 38%), have a baseline hemoglobin <10 mg/dL (9.1% vs. 7.4%), have a baseline CD4+ lymphocyte count <50 cells/mm3 (19% vs. 15%), or have a baseline WHO stage 3 or 4 (70% vs. 65%; p <0.01 for all comparisons).
Table 1.
Description of the patient cohort, stratified by baseline body mass index, Lusaka, Zambia (May 1, 2004 to April 30, 2008; N=27,915)
| Body mass index (kg/m2) | ||||
|---|---|---|---|---|
| ≥18.5 N=19,681 |
17.00 – 18.49 N=4,592 |
16.00 – 16.99 N=1,891 |
<16.0 N=1,751 |
|
| Age, mean (SD), y | 36 (9) | 36 (9) | 35 (9) | 34 (10) |
| Female, No. (%) | 12,818 (65) | 2,511 (55) | 1,026 (54) | 1,044 (60) |
| Weight, mean (SD), kg | 58.8 (9.1) | 49.0 (5.3) | 45.8 (5.1) | 41.0 (5.5) |
| CD4 cell count, mean (SD) | 162 (112) | 142 (102) | 132 (107) | 115 (99) |
| No. cells/mm3 (%) | ||||
| ≥ 350/mm3 | 792 (4.2) | 127 (2.9) | 48 (2.7) | 43 (2.5) |
| ≥ 200 – < 350/mm3 | 4,513 (24) | 907 (20) | 346 (19) | 250 (15) |
| ≥ 50 – < 200/mm3 | 11,248 (60) | 2,604 (59) | 976 (54) | 871 (51) |
| < 50/mm3 | 2,336 (12) | 798 (18) | 439 (24) | 529 (31) |
| Hemoglobin, mean (SD), g/dL | 11.2 (2.0) | 10.6 (2.1) | 10.3 (2.1) | 9.8 (2.0) |
| No. (%) | ||||
| ≥ 10 | 13,416 (74) | 2,645 (63) | 959 (56) | 744 (47) |
| ≥8 – < 10 | 3,659 (20) | 1,114 (27) | 545 (32) | 578 (36) |
| <8 | 971 (5.4) | 440 (11) | 211 (12) | 276 (17) |
| Tuberculosis (active) (%) | 2,394 (12) | 754 (17) | 340 (18) | 357 (20) |
| WHO stage at entry, No. (%) | ||||
| I or II | 7,963 (41) | 1,191 (26) | 352 (19) | 229 (13) |
| III | 10,253 (52) | 2,995 (65) | 1,353 (72) | 1,251 (72) |
| IV | 1,394 (7.1) | 394 (8.6) | 183 (9.7) | 266 (15) |
| Baseline Regimen Dispensed, No. (%) | ||||
| ZDV + 3TC + NVP | 8,711 (44) | 1,562 (34) | 560 (30) | 396 (23) |
| ZDV + 3TC + EFV | 683 (3.5) | 158 (3.4) | 73 (3.9) | 71 (4.1) |
| D4T + 3TC + NVP | 6,202 (32) | 1,670 (36) | 732 (39) | 746 (43) |
| D4T + 3TC + EFV | 704 (3.6) | 343 (7.5) | 182 (9.6) | 219 (13) |
| TDF + FTC + NVP | 1,443 (7.4) | 337 (7.4) | 123 (6.5) | 114 (6.5) |
| TDF + FTC + EFV | 1,884 (9.6) | 514 (11) | 217 (12) | 199 (11) |
Abbreviations: ART, antiretroviral therapy; WHO, World Health Organization; 3TC, lamivudine; D4T, stavudine; EFV, efavirenz; FTC, emtricitabine; NVP, nevirapine; TDF; tenofovir; ZDV, zidovudine.
The sum of each row or sub-column may not equal the total number of patients due to missing values in the ART service database.
Figure 1.
Description of the patient cohort, Lusaka, Zambia (May 1, 2004 to April 30, 2008)
Total patients alive at 6 months with weight data available: 27,915.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index.
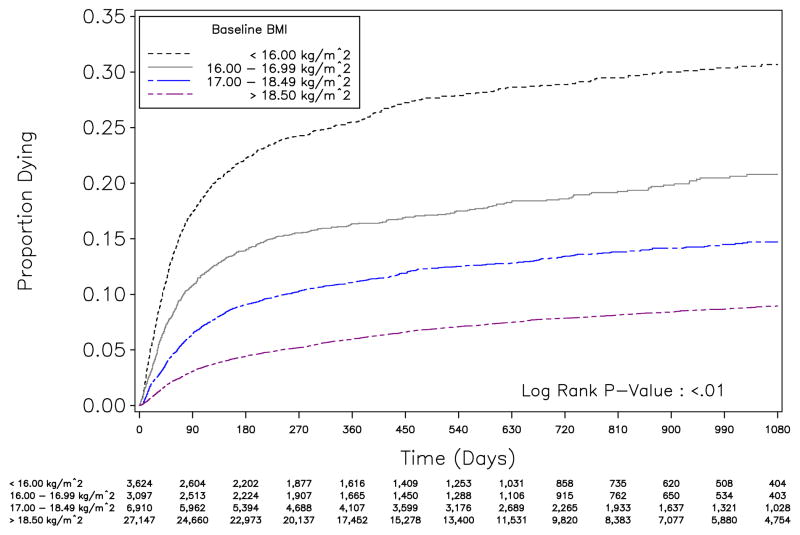
In Kaplan-Meier analysis, survival differed over time between different BMI strata (p <0.01; FIGURE 2). Mortality within the first 90 days of ART was elevated among those with BMI <16.0 (80/100 person-years [py]; 95% CI: 74, 86) compared to other BMI strata: 16.00 – 16.99 (47/100 py; 95% CI: 42, 53), 17.00 – 18.49 (27/100 py; 95% CI: 25, 14), and BMI >18.5 kg/m2 (13/100 py; 95% CI: 12, 14). After 90 days, the mortality rates appeared similar over time. These trends supported our decision to stratify subsequent analyses according to baseline BMI status.
Figure 2.
Time to death stratified by body mass index (BMI) at antiretroviral therapy initiation, Lusaka, Zambia (May 1, 2004 to April 30, 2008; N=40,778)
When we examined absolute weight gain according to baseline BMI strata, those in the <16.0 kg/m2 category had the largest proportion of patients gain ≥10 kg (39.2%) and the smallest proportion fail to gain any weight (7.3%). The proportion of patients who failed to gain weight was highest in the BMI >18.5 kg/m2 group. Similar trends were seen when we looked at categories of percentage weight gain (TABLE 2).
Table 2.
Weight change 6 months after antiretroviral therapy initiation, stratified by body mass index, Lusaka, Zambia (May 1, 2004 to April 30, 2008 N=27,915)
| Weight change at 6 months | Body mass index (kg/m2) | |||
|---|---|---|---|---|
| ≥18.5 | 17.00 – 18.49 | 16.00 – 16.99 | <16.0 | |
| ≥10 kg | 1,665 (8.5%) | 734 (16%) | 469 (25%) | 687 (39%) |
| 5 to <10 kg | 4,454 (23%) | 1,465 (32%) | 632 (33%) | 581 (33%) |
| 2.5 to <5 kg | 3,055 (16%) | 783 (17%) | 277 (15%) | 193 (11%) |
| 0 to <2.5 kg | 4,695 (24%) | 959 (21%) | 322 (17%) | 162 (9.3%) |
| <0 kg | 5,812 (30%) | 651 (14%) | 191 (10%) | 128 (7.3%) |
| ≥20% | 1,017 (5.2%) | 764 (17%) | 532 (28%) | 849 (49%) |
| 10% to <20% | 3,734 (19%) | 1,329 (29%) | 574 (30%) | 466 (27%) |
| 0% to <10% | 3,813 (19%) | 895 (20%) | 290 (15%) | 178 (10%) |
| <0% | 5,812 (30%) | 651 (14%) | 191 (10%) | 128 (7.3%) |
Abbreviation: ART, antiretroviral therapy.
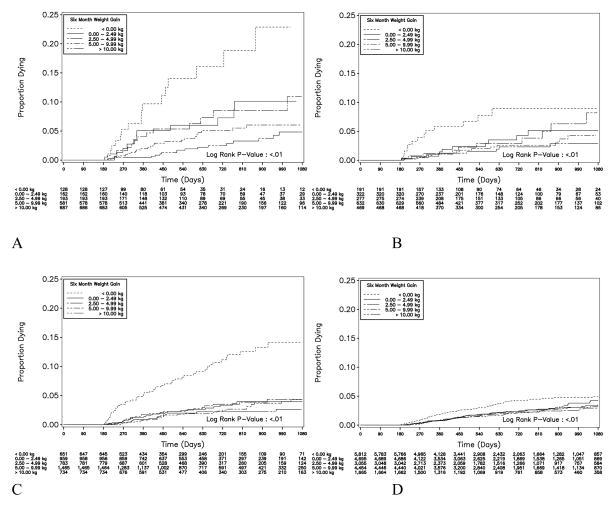
We performed Kaplan-Meier analysis to measure mortality after six months, based on weight gain in the initial six months on ART (FIGURE 3). Across every BMI strata, we observed elevated mortality among patients demonstrating weight losses from ART initiation to six months. At the lowest BMI strata (<16.0 kg/m2), the risk for mortality appeared inversely related to weight gain. Similar findings were demonstrated according to proportional weight gain (data not shown).
Figure 3.
Figure 3 (A – D): Time to death among patients surviving >6 months after antiretroviral therapy initiation in Lusaka, Zambia (May 1, 2004 to April 30, 2008; N=27,915), stratified according to 6-month weight gain: (A) BMI <16.0, (B) BMI 16.00–16.99, (C) BMI 17.00–18.49, and (D) BMI >18.5 kg/m2. The numbers shown at the bottom of the graphs represent the number of patients active in each six-month weight change category at 90 day intervals.
In multivariable analyses, we consistently observed an elevated hazard for death among individuals with demonstrated weight loss at six months. The risk was greatest among those with severe malnutrition (BMI <16.0 kg/m2, AHR 9.7; 95% CI 4.7 – 20.0). Similar trends were noted for clinical treatment failure, but the magnitude was lower (TABLE 3).
Table 3.
Risk of death or clinical treatment failure >6 months post-ART, stratified by weight change, Lusaka, Zambia (May 1, 2004 to April 30, 2008; N=27,915)
| Weight change at 6 months | Risk of death after 6 months, adjusted HR (95% CI) | ||||
|---|---|---|---|---|---|
| All patients | BMI >18.5 kg/m2 | 17.00 – 18.49 | 16.00 – 16.99 | <16.0 | |
| ≥10 kg | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 5 to <10 kg | 1.2 (0.9 – 1.6) | 0.9 (0.6 – 1.3) | 1.4 (0.7 – 2.6) | 1.8 (0.7 – 4.5) | 1.7 (0.9 – 3.4) |
| 2.5 to <5 kg | 1.6 (1.2 – 2.2) | 1.1 (0.7 – 1.7) | 2.1 (1.1 – 4.2) | 2.8 (1.1 – 7.5) | 3.0 (1.4 – 6.4) |
| 0 to <2.5 kg | 1.7 (1.3 – 2.3) | 1.3 (0.9 – 1.9) | 2.1 (1.0 – 4.1) | 2.5 (0.9 – 6.8) | 3.6 (1.6 – 8.2) |
| <0 kg | 3.2 (2.4 – 4.2) | 2.1 (1.4 – 3.1) | 8.2 (4.4 – 15.1) | 6.1 (2.4 – 15.5) | 9.7 (4.7 – 20.0) |
| ≥20% | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 10% to <20% | 1.3 (1.0 – 1.8) | 1.5 (0.9 – 2.5) | 1.4 (0.8 – 2.7) | 1.3 (0.6 – 3.0) | 1.4 (0.7 – 2.6) |
| 0% to <10% | 1.6 (1.2 – 2.1) | 1.6 (1.0 – 2.7) | 1.9 (1.1 – 3.5) | 2.1 (0.9 – 4.5) | 2.9 (1.5 – 5.3) |
| <0% | 3.3 (2.5 – 4.5) | 3.0 (1.8 – 5.0) | 8.0 (4.4 – 14.6) | 4.8 (2.1 – 11.0) | 8.1 (4.1 – 15.9) |
|
| |||||
| Risk of treatment failure after 6 months, adjusted HR (95% CI) | |||||
| All patients | >18.5 | 17.00 – 18.49 | 16.00 – 16.99 | <16.0 | |
|
| |||||
| ≥10 kg | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 5 to <10 kg | 1.0 (0.9 – 1.1) | 1.0 (0.8 – 1.2) | 1.1 (0.9 – 1.4) | 1.0 (0.6 – 1.4) | 0.8 (0.6 – 1.2) |
| 2.5 to <5 kg | 1.0 (0.9 – 1.2) | 1.0 (0.8 – 1.2) | 1.0 (0.8 – 1.4) | 1.3 (0.9 – 2.1) | 1.0 (0.6 – 1.7) |
| 0 to <2.5 kg | 1.2 (1.1 – 1.3) | 1.1 (1.0 – 1.3) | 1.3 (1.0 – 1.7) | 1.5 (1.0 – 2.3) | 1.9 (1.2 – 3.0) |
| <0 kg | 1.3 (1.2 – 1.5) | 1.3 (1.1 – 1.5) | 1.5 (1.1 – 2.1) | 2.2 (1.4 – 3.5) | 2.0 (1.2 – 3.4) |
| ≥20% | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 10% to <20% | 1.1 (0.9 – 1.2) | 1.0 (0.8 – 1.2) | 1.1 (0.8 – 1.4) | 0.9 (0.6 – 1.4) | 1.2 (0.8 – 1.7) |
| 0% to <10% | 1.1 (1.0 – 1.3) | 1.0 (0.9 – 1.2) | 1.2 (0.9 – 1.5) | 1.5 (1.0 – 2.1) | 1.5 (1.0 – 2.2) |
| <0% | 1.3 (1.2 – 1.5) | 1.3 (1.1 – 1.5) | 1.5 (1.2 – 2.1) | 2.2 (1.4 – 3.5) | 2.2 (1.3 – 3.8) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HR, hazard ratio.
Discussion
In our analyses, we observed an association between weight gain after ART initiation and improved clinical outcomes across all BMI strata. The improvements were most pronounced for BMI <16.0 kg/m2; failure to gain weight six months post-ART was associated with a nearly tenfold increased hazard of death compared to a ≥10 kg gain. In all BMI categories, individuals with a weight gain of at least 5 kg appeared to have improved outcomes over those who did not gain weight after six months. Our findings suggest that a minimum weight gain within the first six months may improve outcomes, but further study is clearly needed.
Although our programmatic database provided a large patient population, we recognize our primary limitation of missing data. We were unable to evaluate weight change for 12% of patients because they lacked a weight measurement within the six-month visit window. Those excluded because of missing data appeared to be statistically different from those who were included in the final analysis across a variety of characteristics, but it is unknown as to whether these would impact our results. Our cohort consisted primarily of individuals from an urban setting in a poor, sub-Saharan country, and the population may not be representative of rural areas or more developed countries, such as South Africa. In addition, although we were able to adjust for numerous potential confounders, we did not include in our model measures of food availability, food security, or socioeconomic status.
Weight loss has been recognized as a negative prognostic indicator since early in the HIV epidemic 15–17. Mortality correlates more closely with loss of lean body mass than total weight 18, and there is evidence for a disproportional increase in extracellular water and preferential repletion of fat stores with weight gain in HIV-infected persons 19. Our study did not examine body composition changes, but it is reasonable to assume that fat accumulation represented a significant component of the observed weight increases. Visceral fat accumulation, as a component of lipodistrophy syndrome, is associated with all drug classes (NRTI, NNRTI, and PI) commonly used in sub-Saharan Africa 20–22. Furthermore, maintenance of body cell mass is positively associated with protein intake in HIV-infected persons 23, but the regional dietary staples (e.g. cornmeal, green banana) are primarily carbohydrate 24. Further studies of body composition changes during post-ART weight gain are needed to better understand the association with survival and treatment success.
The proposed benefit of weight gain after ART initiation has served as the basis for randomized trials of supplementary feeding, but an analysis of trials among non-malnourished HIV-infected patients in the United States and Europe found inconsistent improvements in weight and no evidence of a survival benefit 25. In developing countries, but the data on macronutrient supplementation are primarily inferential 10, 11. Hunger is a frequently reported barrier to adherence 26, 27, and the side effects and toxicity of some antiretroviral medications can be potentiated if taken without food 26, 28, 29. However, there is some evidence that supplementary feeding programs may improve medication adherence and patient retention 13, 30, and multiple HIV programs have started providing this service to malnourished clients 31–33.
The association between weight gain and clinical outcomes among severely malnourished patients provides theoretical support to on-going food supplementation programs. We recognize, however, that our analysis was not designed to address causality. It is unclear from these results whether increased energy intake (and subsequent weight gain) contributed to survival of malnourished ART patients, or whether weight gain – like CD4 change – is simply a marker of an individual’s clinical response. By measuring the impact of food supplementation on clinical outcomes in prospective fashion, future studies could provide greater insight into these relationships. In focusing on the effectiveness of food supplementation programs in helping patients gain weight, issues such as ration size, number of dependents at home, and acceptability (e.g. taste) should be addressed 13, 34, 35.
In summary, in a large programmatic cohort, we found that weight loss was associated with risk for poor clinical outcomes across all BMI strata. Among those with severe malnutrition, weight gain was inversely related to death and clinical treatment failure in a dose-dependent fashion. Although they do not address causality, our results lend support to on-going programs for food supplementation among malnourished ART patients. However, given the expense, logistical barriers, and overlapping implications for health policy and economic development, further large, controlled studies of supplementary feeding at ART initiation are warranted to improve program success in resource-constrained settings.
Acknowledgments
Sources of funding: Investigator salary or trainee support is provided by the Fogarty International Center (R24-TW007988, K01-TW06670) and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061).
Footnotes
No conflicts of interest were reported by any author.
References
- 1.World Health Organisation Joint United Nations Programme on HIV/AIDS United Nations Children’s Fund. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector:progress report 2008. Geneva: 2008. [Google Scholar]
- 2.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004 Apr 9;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 3.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006 Aug 16;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 4.Bussmann H, Wester CW, Ndwapi N, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS. 2008 Nov 12;22(17):2303–2311. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash D, Katyal M, Brinkhof MW, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008 Nov 12;22(17):2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Agriculture Organization of the United Nations. The State of Food Insecurity in the World. Rome: [Accessed October 12, 2008; 2003.]. Available at: http://www.fao.org/docrep/006/j0083e/j0083e00.htm. [Google Scholar]
- 7.United Nations Administrative Committee on Coordination Sub-Committee on Nutrition. Fourth Report on the World Nutrition Situation. Geneva: 2000. [PubMed] [Google Scholar]
- 8.Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006 Nov 28;20(18):2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 9.Johannessen A, Naman E, Ngowi BJ, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marston B, De Cock KM. Multivitamins, nutrition, and antiretroviral therapy for HIV disease in Africa. N Engl J Med. 2004 Jul 1;351(1):78–80. doi: 10.1056/NEJMe048134. [DOI] [PubMed] [Google Scholar]
- 11.Piwoz E. Nutrition and HIV / AIDS: evidence, gaps, and priority actions. Washington, D.C: Academy for Educational Development [AED], Support for Analysis and Research in Africa [SARA]; 2004. [Google Scholar]
- 12.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007 Oct 24;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 13.Cantrell RA, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008 Oct 1;49(2):190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008 Aug;24(8):1031–1035. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Morb Mortal Wkly Rep. 1987 Aug 14;36( Suppl 1):1S–15S. [PubMed] [Google Scholar]
- 16.Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989 Sep;50(3):444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler DA, Gibert CL, Launer CA, et al. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 May 1;18(1):80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- 18.Kotler DP, Rosenbaum K, Wang J, Pierson RN. Studies of body composition and fat distribution in HIV-infected and control subjects. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Mar 1;20(3):228–237. doi: 10.1097/00042560-199903010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kotler DP, Wang J, Pierson RN. Body composition studies in patients with the acquired immunodeficiency syndrome. Am J Clin Nutr. 1985 Dec;42(6):1255–1265. doi: 10.1093/ajcn/42.6.1255. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen A, Calmy A, Schiffer V, et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med. 2008 Mar;9(3):142–150. doi: 10.1111/j.1468-1293.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 21.Kosmiski LA, Miller HL, Klemm DJ. In combination, nucleoside reverse transcriptase inhibitors have significant effects on 3T3-L1 adipocyte lipid accumulation and survival. Antivir Ther. 2006;11(2):187–195. [PubMed] [Google Scholar]
- 22.Seminari E, Tinelli C, Minoli L, et al. Evaluation of the risk factors associated with lipodystrophy development in a cohort of HIV-positive patients. Antivir Ther. 2002 Sep;7(3):175–180. [PubMed] [Google Scholar]
- 23.Williams SB, Bartsch G, Muurahainen N, Collins G, Raghavan SS, Wheeler D. Protein intake is positively associated with body cell mass in weight-stable HIV-infected men. J Nutr. 2003 Apr;133(4):1143–1146. doi: 10.1093/jn/133.4.1143. [DOI] [PubMed] [Google Scholar]
- 24.United States Agency for International Development. Food Commodity Fact Sheets, Updated January. 2006 Available at http://www.usaid.gov/our_work/humanitarian_assistance/ffp/crg/sec2.htm.
- 25.Mahlungulu S, Grobler LA, Visser ME, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev. 2007;(3):CD004536. doi: 10.1002/14651858.CD004536.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Hardon AP, Akurut D, Comoro C, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007 May;19(5):658–665. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 27.Au JT, Kayitenkore K, Shutes E, et al. Access to adequate nutrition is a major potential obstacle to antiretroviral adherence among HIV-infected individuals in Rwanda. AIDS. 2006 Oct 24;20(16):2116–2118. doi: 10.1097/01.aids.0000247580.16073.1b. [DOI] [PubMed] [Google Scholar]
- 28.Ammassari A, Murri R, Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001 Dec 15;28(5):445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organisation. Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach. Geneva: 2003. [Google Scholar]
- 30.Nash D. Characteristics of Facilities and Programs Delivering HIV Care and Treatment Services Are Associated with Loss to Follow-up Rates in Programs from 7 Sub-Saharan African Countries. 15th Conference on Retroviruses and Opportunistic Infections; Boston. 2008. [Google Scholar]
- 31.The United States President’s Emergency Plan for AIDS Relief. Emergency Plan Policy Change in Food and Nutrition Programming. 2007 Available at: http://www.pepfar.gov/documents/organization/98940.pdf.
- 32.The United States President’s Emergency Plan for AIDS Relief. Policy Guidance on the Use of Emergency Plan Funds to Address Food and Nutrition Needs. 2006 Available at: http://www.pepfar.gov/pepfar/guidance/77980.htm.
- 33.Mamlin J, Kimaiyo S, Lewis S, et al. Integrating nutrition support for food-insecure patients and their dependents into an HIV care and treatment program in Western Kenya. Am J Public Health. 2009 Feb;99(2):215–221. doi: 10.2105/AJPH.2008.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dibari F. The 2008 MSF Scientific Day. London: 2008. A Qualitative Investigation of Plumpynut® Consumption and Access in Adults enrolled in an MoH/MSF HIV Programme in Kenya. [Google Scholar]
- 35.Bowie C, Kalilani L, Marsh R, Misiri H, Cleary P. An assessment of food supplementation to chronically sick patients receiving home based care in Bangwe, Malawi: a descriptive study. Nutr J. 2005;4:12. doi: 10.1186/1475-2891-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]