Abstract
Purpose
To determine if National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) scores decrease with worsening visual acuity (VA) in American Indian/Alaska Natives (AI/AN), as well as determine the other associated explanatory factors for vision-related quality of life
Methods
The study included 414 randomly selected AI/AN tribal members aged 40 years or older from the Pacific Northwest. We excluded candidates who were deceased, seriously ill, had dementia, or otherwise were unable to perform subjective testing such as visual field testing. The participants completed the NEI-VFQ-25, as well as a detailed eye examination. We defined visual impairment as presenting distance VA 20/40 or worse in the better-seeing eye. The main outcome measures were NEI-VFQ-25 composite and subscale scores. We compared median NEI-VFQ-25 composite and subscale scores in those with visual impairment to those without visual impairment.
Results
Visual impairment occurred in 53 (12.8%, CI: 9.6–16.0) participants. The NEI-VFQ-25 median composite score was significantly lower in those with visual impairment as compared to those without visual impairment (77.5 vs. 90.1, p=.001). A univariate analysis showed VA to be significantly (p ≤ .05) associated with all subscales except ocular pain. When controlling for age, gender, income level, education, percent AIAN heritage, and marital status, a multivariate proportional odds model analysis showed VA to be the best predictor of NEI-VFQ 25 composite scores.
Conclusion
Visual impairment is common in Northwest AI/AN. The NEI-VFQ-25 was sensitive to differences in VA, suggesting it is a valid measure of vision-related quality of life in AI/AN.
INTRODUCTION
Impaired vision is the second leading type of impairment in AIAN1, but little is known about the impact of visual impairment on quality of life (QOL) in AI/AN populations. The Northwest Tribal Vision Impairment Prevention study (NWTVP) recently reported that AI/AN populations in the Northwest have a high prevalence of visual impairment when compared to other ethnic groups2.
Several studies show that vision-specific QOL is related to visual acuity (VA), with QOL decreasing as VA worsens3–8. However, other studies have found that cultural and language differences can impact QOL measures and their subsequent scores9–11. Other studies of disease prevalence in AI/AN have concluded that researchers should be mindful of the occasional incongruence between AI/AN cultural beliefs and the tenets of Western medicine, and also of the cultural heterogeneity that exists even between AI/AN tribes. Indeed, according to an article by McCabe, this heterogeneity is “manifested in the epidemiology of disease and the cultural values and beliefs of each group”12. To our knowledge, researchers have not published studies concerning visual impairment and its effect on QOL in AI/AN populations, nor have any measures of vision-specific QOL been validated specifically in these populations.
We employed the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) to examine the association between VA and vision-specific QOL. The NEI-VFQ-25 is a 25-item interviewer administered questionnaire, used as a tool for measuring vision-specific QOL3, 4. Researchers have used the NEI-VFQ-25 in Caucasian, African-American, and Latino populations with good reliability3–8. The purpose of the current study was to determine whether NEI-VFQ-25 scores decrease with worsening VA in AI/AN while controlling for other potential confounders such as socio-economic and other demographic variables, and to compare the mean NEI-VFQ-25 scores with those from other studies.
MATERIALS AND METHODS
The Institutional Review Boards of Legacy Health System and the Northwest Area Indian Health Board approved this study. We obtained informed consent from all participants.
Participants
We randomly selected three tribes from the 43 federally recognized tribes in the Northwest region of the United States, one each from Washington, Oregon, and Idaho. Tribes were considered eligible for the study if they had a tribal enrollment of at least 400 members aged 40 years or older. Using a Community-Based Participatory Research13 philosophy, tribal members were integral in recruiting, scheduling, screening, and disseminating information. After selecting tribes, tribal community health representatives (CHR) used the tribal enrollment database to perform an age-stratified, random selection of tribal members 40 years of age or older. We excluded candidates who were deceased, had dementia or otherwise were unable to perform subjective testing such as visual field testing or to understand the questionnaire. We used the telephone, direct mail, and door-to-door visits, as needed, to schedule participants for the study.
National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25)
Prior to the study we asked tribal elders, community health representatives and tribal officials to evaluate whether the questions of the NEI-VFQ-25 were culturally appropriate. The members of this ad hoc community-based group recommended a change to the descriptors for one of the questions in the instrument; specifically, group members recommended adding the term “beadworking” to the descriptors of near-vision activities in question number 6, since this was a common activity of AI/AN requiring near vision.
The NEI-VFQ-25 contains an appendix of optional questions that can be added to the instrument's original 25 questions. We added Appendix question A2, which asks the participant to rate their current eyesight (with glasses or contacts, as applicable) and calculated these results into the General Vision subscale as directed in the published scoring algorithm for the instrument14.
Trained interviewers administered the modified NEI-VFQ-254 prior to the assessment of visual acuity. We created the NEI-VFQ-25 composite and subscale scores using the published method14. In short, we recoded the questions of the respective subscales into a 0–100 scale, and then averaged them to create a subscale score (excepting General Health, which includes only one question). A higher score indicates better quality of life. We averaged the subscale scores to create an overall composite score4.
Visual Acuity Testing
We recorded presenting distance vision with current correction, if any, using the Early Treatment of Diabetic Retinopathy Study (ETDRS) charts, and converted ETDRS scores into a logMAR scale of distance visual acuity15. A higher logMAR score indicates worsening vision. We defined visual impairment as presenting distance visual acuity 20/40 or worse in the better-seeing eye, which is similar to other studies5, 16. We also categorized visual impairment according to the recommendations of the World Health Organization as none (visual acuity better than 20/40), mild (visual acuity between 20/40 and 20/63), and moderate/severe (visual acuity 20/80 or worse)17.
Statistical Analysis
We report the median value of NEI-VFQ-25 subscale and composite scores because the scores were not normally distributed and were predominantly left-skewed. However, we also report mean scores to allow for comparisons between our sample and those of other studies. We used Chi-square analysis to compare the age distribution in categories between the participants and the tribes overall. We used the nonparametric Kruskal-Wallis analysis to determine significant differences between the visually normal and visually impaired groups.
For internal consistency reliability, we calculated Cronbach's α18, which estimates the correlation of subscale questions with the subscale score. For group comparisons, an acceptable minimum Cronbach's α is .703.
The NEI-VFQ-25 subscale and composite scores are ordinal in nature. Therefore, we used a proportional odds model, since it is robust with respect to any choices of cut-off values for categorization of scores and is efficient when the response variable is ordinal19. The linear regression model is not an appropriate model for these NEI-VFQ-25 ordinal response variables since linear regression models require a continuous response variable20. The proportional odds model is a variation of the logistic regression model to accommodate an ordinal polytomous response variable. For example, a model equation with one covariate is typically defined as
where j= number of ordinal response categories, θ= intercept, x= covariate value, and β= slope or beta coefficient. This model implies that the cumulative odds ratio of Y≤ j for x1 vs. x2 is
When x is an indicator variable for control and treatment groups, this equation creates an odds ratio for the treatment effect (for more details, see page 152 of the book referenced19).
We explored univariate and multivariate analyses. Our model included the NEI-VFQ-25 composite and subscale scores as dependent variables, and visual acuity and socio-demographic factors (gender, percent AI/AN, income level, marital status, education level, employment status, and age) as explanatory variables. We also used a sensitivity analysis to determine the amount of visual acuity change that results in a change in VFQ score.
Employment data was initially collected as a participant's response to one of the following options: employed, self employed, out of work more than one year, out of work less than one year, homemaker, student, retired, or unable to work. We determined that no statistically significant differences (p>0.05) existed in composite NEI-VFQ 25 scores between the sub-categories of “employed” (employed; self-employed). Similarly, we found no differences between the sub-categories of “unemployed” (out of work; homemaker; student; retired; unable to work). Therefore, we collapsed these subcategories into a dichotomous “employed/not employed” variable for analysis.
Income level was determined using the 2003 Federal Poverty Level (FPL) guidelines, in which income is reported as a percentage of the FPL based on the amount of total household income and the number of people in the household21. We used SPSS® (v13.0, SPSS Inc. Chicago, Illinois) and R (R project, v2.60)22 for all statistical analyses, with an alpha set at 0.05.
RESULTS
Participants and Socioeconomic data
Out of 632 tribal members aged 40 years or older whom we selected for the study, we report data for 414 (65%) participants; 118 (19%) tribal members agreed to participate in the study, but did not attend; 80 (13%) tribal members declined to participate in the study; and 20 (3%) attended but had incomplete screening or questionnaire data and were excluded from further analysis.
Table 1 shows the demographic data for the included participants. Percentage AI/AN heritage was based on self-reports from participants. We compared the categorical age of the participants to that of the tribes overall. Using a Chi-square analysis, we found no significant differences (p >.05) in age between those who attended the screening and those in the tribes overall (age categories: 40–49 years; 50–59 years; 60–69 years; 70 years or older). This suggests that no differential bias in age exists between those enrolled and not enrolled. Comparative statistics for the other demographic characteristics were not available.
Table 1.
Demographic characteristics for participants in the Northwest Tribal Vision Project (n=414).
| Gender (% female) | 64% |
| Age [mean (SD)] in years | 55 (11) |
| Percent AI/AN | |
| Unknown | 4% |
| Less than 25% | 13% |
| 25% to 50% | 4% |
| Greater than 50% | 79% |
| Employed | 63% |
| Education | |
| Less than High School (HS) | 15% |
| HS graduate (diploma/GED) | 29% |
| Some college | 42% |
| Bachelors degree or higher | 14% |
| Married | 44% |
| Income * | |
| <100% Federal Poverty Level | 19% |
| 101%–150% Federal Poverty Level | 19% |
| 151%–200% Federal Poverty Level | 14% |
| >200% Federal Poverty Level | 42% |
Six (6) percent of participants responded “don't know” or “don't want to answer”.
Visual Impairment
When defined as presenting distance vision 20/40 or worse in the better-seeing eye, 12.8% (n=53; CI: 9.6–16.0) of the participants had visual impairment. When separated into categories using the WHO scale, 10.6% (n=44; CI: 7.7–13.5) had mild impairment, and 2.2% (n=9; CI: 0.8–3.6) had moderate/severe impairment. Of those with moderate/severe impairment, 2 had VA 20/200 or worse (0.5%; CI: 0.10–1.54).
Internal Consistency of the NEI-VFQ-25
Table 2 shows the internal consistency for the composite and subscale scores. Most subscales showed acceptable internal consistency reliability with a Cronbach's α ≥ 0.7018, with Driving (α=.69) borderline, and Vision-Dependent Social Functioning (α=.61) below the acceptable minimum. Internal consistency was also acceptable in the visually impaired group, with Vision-Dependent Social Functioning (α=.69) borderline.
Table 2.
Reliability analysis using Cronbach's of the 25-ltem National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) subscale and composite scores from participants in the Northwest Tribal Vision project̃.
| All participants (n=414) | Visually impaired (n=53) | |
|---|---|---|
| Subset | α | α |
| General Health | * | * |
| General Vision | .71 | .78 |
| Ocular Pain | .75 | .85 |
| Near Vision Activities | .78 | .88 |
| Distance Vision Activities | .79 | .82 |
| Vision-Specific Social Functioning | .61** | .69** |
| Vision-Specific Mental Health | .79 | .86 |
| Vision-Specific Role Difficulties | .83 | .92 |
| Vision-Specific Dependency | .85 | .90 |
| Driving | .69** | .71 |
| Color Vision | * | * |
| Peripheral Vision | * | * |
Reliability analysis not performed as subset was comprised of fewer than 2 questions.
Does not meet acceptable minimum of 0.70.
Comparison of mean NEI-VFQ 25 scores with other studies
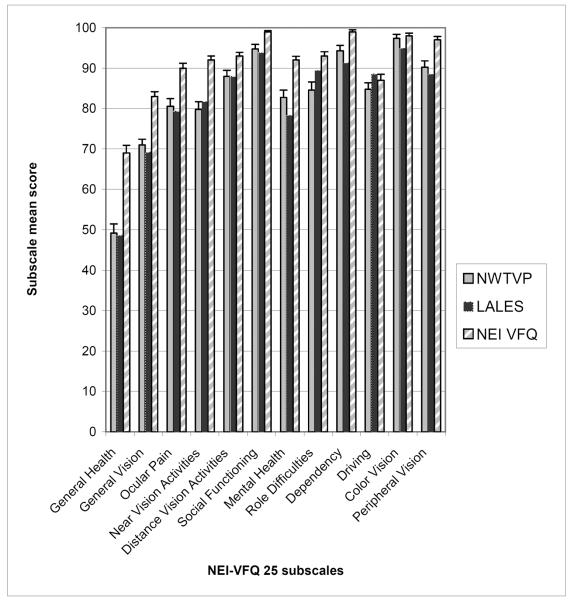
We compared the mean scores of those AI/AN with no visual impairment (Figure 1) to similar groups of visually normal participants in two previous studies; the NEI-VFQ reference group4 and the Los Angeles Latino Eye Study (LALES)5. The NEI-VFQ reference group included people of various ethnicities, with at least 30% coming from “underrepresented minority groups3,” and participants in the LALES study were self-identified as Latino5. Mean scores for all subscale categories were roughly similar between LALES and AI/AN participants from our sample, with AI/AN scoring equal to or greater than the LALES cohort in all subscales except Near Vision, Vision-Specific Role Difficulties, and Driving. Both the AI/AN and LALES cohorts scored lower than the NEI-VFQ reference groups in the majority of the NEI-VFQ-25 subscales; we found statistically significant differences (p<0.05) in scores between AI/AN and the reference group in all but 2 subscales (Driving and Color Vision).
Figure.
Comparison of mean NEI-VFQ 25 subscale and composite scores between the current study (Northwest Tribal Vision Project-NWTVP), Los Angeles Latino Eye Study (LALES*)5, and the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ) reference group4.
*Standard deviations were not reported in the LALES study reference; thus, confidence intervals could not be calculated.
Relationship Between Visual impairment and NEI-VFQ-25 Scores
Table 3 shows the median NEI-VFQ-25 scores. Scores for visually impaired participants were significantly lower (all p values < .05; Kruskal-Wallis) than those without visual impairment, except in the General Health and Ocular Pain subscales. A consistent decline in scores occurred with worsening WHO visual impairment- from no visual impairment, to mild visual impairment, and finally to moderate/severe visual impairment.
Table 3.
Comparison of NEI-VFQ-25 Subscale and Composite Scores in Northwest Tribal Vision Project participants with and without visual impairment** (Median/Mean).
| Entire sample (n=414) | No Impairment (n=361) | All impairment combined** (n=53) | Mild** impairment (n=44) | Moderate/severe** impairment (n=9) | Non-parametric p value* | |
|---|---|---|---|---|---|---|
| Composite score | 89 / 85 | 90 / 86 | 85 / 73 | 82 / 77 | 52 / 54 | <.001 |
| General Health | 50 / 49 | 50 / 50 | 50 / 43 | 50 / 44 | 50 / 39 | 0.087 |
| General Vision | 75 / 71 | 75 / 73 | 70 / 60 | 65 / 63 | 63 / 69 | <.001 |
| Ocular Pain | 88 / 80 | 88 / 81 | 88 / 77 | 88 / 79 | 63 / 69 | .742 |
| Near Vision Activities | 83 / 78 | 83 / 80 | 79 / 65 | 75 / 70 | 33 / 41 | <.001 |
| Distance Vision Activities | 92 / 86 | 92 / 88 | 79 / 69 | 79 / 75 | 25 / 39 | <.001 |
| Vision-Specific Social Functioning | 100 / 93 | 100 / 95 | 100 / 85 | 100 / 88 | 75 / 71 | <.001 |
| Vision-Specific Mental Functioning | 88 / 81 | 88 / 83 | 81 / 68 | 81 / 71 | 31 / 49 | <.001 |
| Vision-Specific Role Difficulties | 88 / 82 | 100 / 85 | 81 / 65 | 75 / 69 | 38 / 46 | <.001 |
| Vision-Specific Dependency | 100 / 96 | 100 / 94 | 100 / 79 | 100 / 85 | 42 / 48 | <.001 |
| Driving | 83 / 84 | 88 / 85 | 83 / 75 | 83 / 77 | 67 / 65 | 0.012 |
| Color Vision | 100 / 96 | 100 / 97 | 100 / 89 | 100 / 92 | 100 / 75 | 0.004 |
| Peripheral Vision | 100 / 88 | 100 / 90 | 75 / 74 | 75 / 79 | 50 / 50 | <.001 |
Kruskal-Wallis nonparametric p value comparing mean scores for those with and without visual impairment.
World Health Organization visual impairment categorization: none (visual acuity better than 20/40), mild (visual acuity between 20/40 and 20/63), and moderate/severe (visual acuity 20/80 or worse).
In Table 4, the mean NEI-VFQ-25 composite scores are shown for each level of visual acuity. Generally, the NEI-VFQ-25 composite scores tended to trend downward as visual acuity worsened; a notable exception occurred with one participant whose presenting visual acuity was 20/160 in both eyes, yet whose composite score was a 97. The instructions for the NEI-VFQ-25 ask that respondents “please answer all the questions as though (they) were wearing (their) glasses or contact lenses,” and this participant, whose manifest refraction was 20/20 in both eyes, did not bring their current eyeglasses to the screening, resulting in a worse presenting visual acuity than they might have received otherwise. Thus, this would appear to explain this anomalous composite score.
Table 4.
Mean NEI-VFQ 25 composite scores by degree of visual acuity (n=414).
| Snellen (20/x) | logMAR | Frequency | Mean VFQ25 Composite score (SD) |
|---|---|---|---|
| 10 | −0.3 | 1 | 86 |
| 12.5 | −0.2 | 9 | 93 (3.3) |
| 16 | −0.1 | 69 | 89 (9.6) |
| 20 | 0 | 180 | 86 (11) |
| 25 | 0.1 | 78 | 85 (13.3) |
| 32 | 0.2 | 24 | 84 (13.4) |
| 40 | 0.3 | 24 | 80 (19.4) |
| 50 | 0.4 | 13 | 79 (13.1) |
| 60 | 0.5 | 7 | 62 (25.2) |
| 80 | 0.6 | 2 | 51 (14.1) |
| 125 | 0.8 | 4 | 57 (24.8) |
| 160 | 0.9 | 1 | 97* |
| 200 | 1.0 | 1 | 31* |
| NLP | 1.7 | 1 | 12* |
Raw value; no mean or standard deviation could be calculated due to inadequate sample size.
Univariate Proportional Odds model
Table 5 shows univariate associations of composite NEI-VFQ-25 score with visual acuity and socioeconomic factors (age, sex, marital status, education, employment status, percent AI/AN heritage and income level), wherein higher NEI-VFQ-25 scores represent better QOL. Worsening visual acuity, higher age, and unemployed status were significantly associated (p values < .05) with a worsening NEI-VFQ-25 composite score. Highest income level (≥ 201% FPL) was associated with a better NEI-VFQ-25 composite score when compared to the lowest income level (< 100% FPL).
Table 5.
Univariate associations with NEI-VFQ 25 composite score* for participants in the Northwest Tribal Vision Project.
| β ** | OR† | Lower 95% confidence interval | Upper 95% confidence interval | p value | |
|---|---|---|---|---|---|
| Visual acuity | −0.43 | 1.54(1) | 1.37 | 1.72 | <0.001 |
| Age | −0.04 | 1.04(2) | 1.02 | 1.06 | <0.001 |
| Gender | −0.42 | 1.53(3) | 0.95 | 2.45 | 0.070 |
| Marital status | 0.06 | 0.94(4) | 0.61 | 1.47 | 0.197 |
| Education | 0.13 | 0.88(5) | 0.57 | 1.37 | 0.208 |
| Employment | −1.11 | 3.02(6) | 1.92 | 4.75 | <0.001 |
| % AI/AN heritage (baseline: 0 – 25%) | |||||
| 25 – 50% | 0.16 | 0.85(7) | 0.20 | 3.60 | 0.823 |
| 50 – 75% | 0.04 | 0.97(7) | 0.42 | 2.21 | 0.932 |
| 75 – 100% | −0.19 | 1.21(7) | 0.60 | 2.41 | 0.588 |
| Income level (baseline: < 100% FPL) | |||||
| 100–150% FPL | −0.01 | 1.01(8) | 0.52 | 2.00 | 0.966 |
| 151–200% FPL | −0.04 | 1.04(8) | 0.50 | 2.15 | 0.915 |
| ≥201% FPL | 0.95 | 0.39(8) | 0.21 | 0.73 | 0.003 |
NEI-VFQ-25 composite scores were categorized into five categories (from worsening to better quality of life): 0–20; 21–40; 41–60; 61–80; and 81–100.
Beta (β) coefficient from the Proportional Odds Model: a negative β indicates a higher likelihood of having a composite score worse than category 81–100.
OR: Odds ratio of NEI-VFQ-25 score worse than category 81–100:
VA: odds ratio with a 0.1 unit increase in logMAR visual acuity (worsening vision);
Age: odds ratio as 1 year older;
Gender: odds ratio of female vs. male;
Marital Status: odds ratio of not married vs. married;
Education: odds ratio of >high school or GED vs. less education;
Employment: odds ratio of unemployed vs. employed;
Percent American Indian/Alaskan Native heritage (% AI/AN): odds ratio as compared to category 0 –25% AI/AN; and
Income level: odds ratio as compared to category < 100% of FPL.
Multivariate Proportional Odds Model
Table 6 shows the results of a stepwise multivariate proportional odds model to determine the combination of variables associated with the NEI-VFQ-25 composite score. Candidate covariates included those covariates from the univariate model that were significant (visual acuity, age, employment status, and income level-see Table 5). We also included tribal affiliation to control for possible differences between tribes. The final model included only visual acuity, suggesting that the other covariates (age, employment, and income status) were not explanatory when visual acuity was in the model. In other words, the univariate associations between the NEI-VFQ-25 and these variables are explained by the variability in visual acuity.
Table 6.
Multivariate associations with NEI-VFQ 25 composite score for participants in the Northwest Tribal Vision Project.*
| β ** | OR† | Lower 95% confidence interval | Upper 95% confidence interval | p value | |
|---|---|---|---|---|---|
| Visual Acuity | −.37 | 1.44(1) | 1.27 | 1.64 | <0.001 |
| Age | −.01 | 1.01(2) | 0.99 | 1.04 | 0.244 |
| Employment | −.42 | 1.53(3) | 0.85 | 2.73 | 0.147 |
| Income level (baseline: < 100%) FPL) | |||||
| (101–150%FPL) | −.16 | 1.18(4) | 0.57 | 2.43 | 0.655 |
| (151–200% FPL) | −.46 | 1.58(4) | 0.71 | 3.49 | 0.252 |
| (≥ 201% FPL) | 0.46 | 0.63(4) | 0.31 | 1.28 | 0.193 |
| Tribal Affiliation (Baseline: Tribe 1) | |||||
| Tribe 2 | −.31 | 1.36(5) | 0.77 | 2.40 | 0.287 |
| Tribe 3 | 0.12 | 0.89(5) | 0.45 | 1.78 | 0.740 |
NEI-VFQ-25 composite scores were categorized into five categories (from worsening to better quality of life): 0–20; 21–40; 41–60; 61–80; and 81–100.
Beta (β coefficient from the Proportional Odds Model: a negative β indicates a higher likelihood of having a composite score worse than category 81–100.xs
OR: Odds ratio of NEI-VFQ-25 score worse than category 81–100:
VA: odds ratio with a 0.1 unit increase in logMAR visual acuity (worsening vision);
Age: odds ratio as 1 year older;
Employment: odds ratio of unemployed vs. employed;
Income level: odds ratio as compared to category < 100% of FPL; and
Tribal Affiliation: odds ratio as compared to Tribe 1.
Sensitivity Analysis
We found a strong, consistent relationship with VA, which was associated with the composite score (shown above) and with all subscales except General Health and Ocular Pain (data not shown). The beta coefficient for VA (−0.37) from Table 6 shows that an appropriate 3-unit change in logMAR VA (a clinically significant difference) resulted in one category of improvement in NEI-VFQ-25 composite scores. Overall, this suggests that the NEI-VFQ-25 is consistent and sensitive to changes in visual acuity in AI/AN.
DISCUSSION
The main objective for this study was to assess the utility of the NEI-VFQ-25 in detecting changes in vision-related QOL in AI/AN populations and to determine the association between VA and vision-related QOL scores in this population. Our results suggest that the NEI-VFQ-25 is a valid measurement of self-reported visual functioning in AI/AN. Better VA was associated with better self-reported vision-related functioning, with NEI-VFQ-25 composite scores increasing as acuity improved. NEI-VFQ-25 subscale and composite scores were significantly different between those with and without visual impairment. Furthermore, VA was shown to be the strongest explanatory covariate of NEIVFQ-25 scores.
Modifications of the NEI-VFQ-25
A review of the NEI-VFQ-25 by tribal members prior to the study suggested that most questions were applicable, but they suggested a slight modification. We made a minor change to the wording of question 6 “(How much difficulty do you have doing work or hobbies that require you to see well up close, such as cooking, sewing, fixing things around the house, or using hand tools?”) by adding “beadwork” to the list of examples in an effort to reflect a popular cultural endeavor that is also related to near-vision activities. We modified only the descriptors of this question, not the scale nor other wording. During the administration of the revised questionnaire, we further found that some of the questions on the NEI-VFQ-25 may make assumptions that were not necessarily applicable within the AI/AN populations. For example, question 14 asks, “Because of your eyesight, how much trouble do you have going out to see movies, plays, or sports events?” Given the rural locations of the tribes, these activities, as described, might not be available or relevant to the culture being sampled, and thus might result in a missing or inaccurate response. We suggest using community members to identify culturally appropriate examples (such as pow-wows, tribal meetings, dances, ceremonies, etc.) to better elicit responses to these questions.
Reliability of NEI-VQ-25 in AI/AN
Our results showed excellent reliability, which was comparable to the results of the LALES and other studies3–8, 10. In the overall sample, internal consistency reliability using Cronbach's α was found to be acceptable for 7 of the 9 subscales in which reliability estimates could be achieved. In the visually impaired group, Vision-Specific Social Functioning (α=0.69) failed to meet the acceptable minimum Cronbach's α of 0.70.
These results were similar to those reported in the LALES study5, which found that the subscales Ocular Pain, Vision-Specific Social Functioning and Driving subscales had an internal consistency reliability lower than the accepted minimum when the entire sample was used. This suggests that the psychometric performance of the NEI-VFQ-25 is reasonably consistent and a valid measure of vision-related functioning in AI/AN populations.
Comparisons With Other Studies
The similarity of mean NEI-VFQ-25 scores between the Latino participants of the LALES study and the AI/AN population in our sample, combined with the differences found between AI/AN and a predominantly white reference group suggests that some ethnic differences exist which influence perceptions of vision-related quality of life. It is interesting to note that AI/AN with no visual impairment scored relatively low on the General Health subscale (mean=49; wherein respondents rate their overall general health on a 5-item scale ranging between Excellent and Poor) and moderately on the General Vision subscale (mean=71; wherein respondents rate their current eyesight with present correction on a 6-item scale ranging between Excellent and Completely Blind). These scores seem to trend in a similar fashion with Latino respondents, but trail markedly behind the reference group scores. This may be related to unmeasured factors, such as depression, which are known to result in a diminished perception of general health and general vision.
Sensitivity Analysis
One of the more difficult aspects of QOL measures is the interpretation of the differences in scores: to wit, what do the scores actually mean in terms of clinical significance, and what degree of score change, either between groups or within groups over time, indicates a clinically meaningful difference? The LALES study used a 5-point difference in subscale scores5, 23. We had similar results showing that a 3-unit change in logMAR VA (a clinically significant difference) resulted in one category of improvement in NEI-VFQ-25 composite scores. This indicates that like the LALES study, we show that the NEI-VFQ-25 is a valid measure of vision related quality of life in AI/AN and that it is sensitive to differences in visual acuity.
However, the determination of a 5-point difference as a clinically significant change should be considered with caution. Changes in NEI-VFQ-25 subscale scores are derivative of the number of items within the subscale- the more items included, the smaller the amount of potential score change within the subscale when a single response is changed. Thus, a change in a single response option (eg. a 1 vs. a 2) on a single item in the instrument can, depending upon the subscale to which the item belongs, affect the subscale score by between 6.25 and 12.5 points. Therefore, we believe that further research is necessary to determine what level of NEI-VFQ-25 score change reflects a clinically meaningful change.
Linear regression vs. proportional odds model
Because the NEI-VFQ-25 uses ordinal response categories, the resulting subscale scores attained after recoding remain ordinal in nature, despite the recoding of each response into a 0–100 scale. This is demonstrated in that many integers between 0–100 are impossible to achieve after recoding. We found that a proportional odds model is more robust with smaller confidence intervals and a better fit to the data when compared to linear regression. Researchers may consider using a proportional odds model when analyzing NEI-VFQ-25 scores.
Limitations
We were only able to recruit 65% of those we contacted for the study despite offering after-hours and weekend appointment times. Reasons for the lower response rates may include socioeconomic issues such as disconnected or non-existent telephone service, cultural or regional issues such as transience (which may also be tied to low socio-economic status), “research fatigue,” in which a population previously amenable to research participation becomes exhausted, extensive travel distances to screenings, and even a pervasive mistrust of researchers24. Other studies have shown that recruitment rates are also generally lower in rural settings25, 26. We found no differences in age between our sample and the tribes overall; however, other differences may be present, such as employment status and income level. Nevertheless, the relationships of NEI-VFQ-25 composite scores with visual acuity were very consistent and significant (p <0.001, OR=1.44, Table 6) and it is unlikely that our results would be different even with a higher recruitment percentage.
Conclusions
Our study has indicated that the NEI-VFQ-25 is an appropriate instrument for measuring vision-related quality of life in AI/AN populations. We found significant associations between VA and vision-specific QOL in most of the NEI-VFQ-25 subscales as well as with the composite score. Furthermore, our results have shown significant differences in scores between AI/AN and the NEI-VFQ-25 reference group, but scores similar to those of Latinos. Future research is needed to determine whether these results can be generalized to all AI/AN tribes within the United States. Additional research is also warranted to study the effects of depression and other comorbidities on AI/AN QOL scores, as well as to determine whether the NEI-VFQ-25 is sensitive to interventions, such as the provision of eyeglasses, to improve QOL in AI/AN.
Acknowledgments
Financial Support: NEI 3 K23 EY0155501-01 (SLM) American Glaucoma Society (SLM) CDC U48 DP000024-01 (SLM) Good Samaritan Foundation
Footnotes
Conflict of Interest: None of the authors has a conflicting relationship with the material presented.
REFERENCES
- 1.Regional Differences In Disease. Indian Health Service; 2001. Available from: www.ihs.gov/ NonMedicalPrograms/ IHS_Stats/ files/ Regional_Differences_Charts2.pdf. [Google Scholar]
- 2.Mansberger SL, Romero FC, Smith NH, et al. Causes of visual impairment and common eye problems in Northwest American Indians and Alaska Natives. Am J Public Health. 2005;95:881–6. doi: 10.2105/AJPH.2004.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116:1496–504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 4.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 5.Globe DR, Wu J, Azen SP, Varma R. The impact of visual impairment on self-reported visual functioning in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1141–9. doi: 10.1016/j.ophtha.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Globe D, Varma R, Azen SP, Paz S, Yu E, Preston-Martin S. Psychometric performance of the NEI VFQ-25 in visually normal Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2003;44:1470–8. doi: 10.1167/iovs.02-0292. [DOI] [PubMed] [Google Scholar]
- 7.Broman AT, Munoz B, West SK, et al. Psychometric properties of the 25-item NEI-VFQ in a Hispanic population: Proyecto VER. Invest Ophthalmol Vis Sci. 2001;42:606–13. [PubMed] [Google Scholar]
- 8.Broman AT, Munoz B, Rodriguez J, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43:3393–8. [PubMed] [Google Scholar]
- 9.Berkanovic E. The effect of inadequate language translation on Hispanics' responses to health surveys. Am J Public Health. 1980;70:1273–6. doi: 10.2105/ajph.70.12.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley EA, Sloan JA, Novotny PJ, Garrity JA, Woog JJ, West SK. Evaluation of the National Eye Institute visual function questionnaire in Graves' ophthalmopathy. Ophthalmology. 2006;113:1450–4. doi: 10.1016/j.ophtha.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA. Pitfalls in measuring the health status of Mexican Americans: comparative validity of the English and Spanish Sickness Impact Profile. Am J Public Health. 1984;74:569–73. doi: 10.2105/ajph.74.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCabe M. Treating American Indians/Alaska Native elders. Geriatric Times. 2001;2 [Google Scholar]
- 13.Quality AfHRa . The Role of Community-Based Participatory Research: Creating Partnerships, Improving Health. Agency for Healthcare Research and Quality; Rockville, MD: 2003. [Google Scholar]
- 14.Mangione C. The National Eye Institute 25-Item Visual Function Questionnaire Scoring Algorithm. (Version) 2000;15 [Google Scholar]
- 15.Ricci F, Cedrone C, Cerulli L. Standardized measurement of visual acuity. Ophthalmic Epidemiol. 1998;5:41–53. doi: 10.1076/opep.5.1.41.1499. [DOI] [PubMed] [Google Scholar]
- 16.Wang JJ, Mitchell P, Smith W, Cumming RG, Attebo K. Impact of visual impairment on use of community support services by elderly persons: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:12–9. [PubMed] [Google Scholar]
- 17.Consultation on Development of Standards for Characterization of Vision Loss and Visual Functioning. World Health Organization; Geneva: 2003. [Google Scholar]
- 18.Cronbach JL. Coefficient alpha and the internal structure of tests. Psychometrika. 1951:297–334. [Google Scholar]
- 19.McCullagh P, Nelder JA. Generalized linear models. 2nd ed Chapman and Hall; London; New York: 1989. p. xix.p. 511. [Google Scholar]
- 20.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. 4th ed McGraw-Hill/Irwin; Boston; New York: 2004. p. 701. [Google Scholar]
- 21.Services HaH Annual Update of the HHS Poverty Guidelines. Federal Register. 2003:6456–6458.
- 22.Team RDC, Computing RFfS . R: A language and environment for statistical computing. 2.5.0 ed Vienna, Austria: 2007. [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed L. Erlbaum Associates; Hillsdale, N.J.: 1988. p. xxi.p. 567. [Google Scholar]
- 24.Daunt DJ. Ethnicity and recruitment rates in clinical research studies. Appl Nurs Res. 2003;16:189–95. doi: 10.1016/s0897-1897(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RB, McMahon SH, Hazey JA. Does practice location or academic connection affect recruitment of patients as research subjects? Fam Pract Res J. 1992;12:177–84. [PubMed] [Google Scholar]
- 26.Stark N, Paskett E, Bell R, et al. Increasing participation of minorities in cancer clinical trials: summary of the ”Moving Beyond the Barriers” Conference in North Carolina. J Natl Med Assoc. 2002;94:31–9. [PMC free article] [PubMed] [Google Scholar]