Abstract
OBJECTIVES
To determine the prevalence of 25-hydroxyvitamin D (25[OH]D) deficiency and associations between 25(OH)D deficiency and cardiovascular risk factors in children and adolescents.
METHODS
With a nationally representative sample of children aged 1 to 21 years in the National Health and Nutrition Examination Survey 2001–2004 (n = 6275), we measured serum 25(OH)D deficiency and insufficiency (25[OH]D <15 ng/mL and 15–29 ng/mL, respectively) and cardiovascular risk factors.
RESULTS
Overall, 9% of the pediatric population, representing 7.6 million US children and adolescents, were 25(OH)D deficient and 61%, representing 50.8 million US children and adolescents, were 25(OH)D insufficient. Only 4% had taken 400 IU of vitamin D per day for the past 30 days. After multivariable adjustment, those who were older (odds ratio [OR]: 1.16 [95% confidence interval (CI): 1.12 to 1.20] per year of age), girls (OR: 1.9 [1.6 to 2.4]), non-Hispanic black (OR: 21.9 [13.4 to 35.7]) or Mexican-American (OR: 3.5 [1.9 to 6.4]) compared with non-Hispanic white, obese (OR: 1.9 [1.5 to 2.5]), and those who drank milk less than once a week (OR: 2.9 [2.1 to 3.9]) or used >4 hours of television, video, or computers per day (OR: 1.6 [1.1 to 2.3]) were more likely to be 25(OH)D deficient. Those who used vitamin D supplementation were less likely (OR: 0.4 [0.2 to 0.8]) to be 25(OH)D deficient. Also, after multivariable adjustment, 25(OH)D deficiency was associated with elevated parathyroid hormone levels (OR: 3.6; [1.8 to 7.1]), higher systolic blood pressure (OR: 2.24 mm Hg [0.98 to 3.50 mm Hg]), and lower serum calcium (OR: –0.10 mg/dL [–0.15 to –0.04 mg/dL]) and high-density lipoprotein cholesterol (OR: –3.03 mg/dL [–5.02 to –1.04]) levels compared with those with 25(OH)D levels ≥30 ng/mL.
CONCLUSIONS
25(OH)D deficiency is common in the general US pediatric population and is associated with adverse cardiovascular risks.
Keywords: rickets, vitamin D, cardiovascular risk factors, obesity, racial disparities
Vitamin D, known mainly for its role in calcium homeostasis, is now thought to be involved in various physiologic and pathologic processes in the human body.1,2 Rickets has reemerged in the United States in certain populations.3–9 25-Hydroxyvitamin D (25[OH]D) levels are the most commonly measured indicator of vitamin D status. Chronically low 25(OH)D levels, <15 ng/mL, considered by many to be 25(OH)D deficiency, may result in bone changes that are consistent with rickets.10 In adults, the optimal level of 25(OH)D has been suggested to be ≥30 ng/mL, a level associated with maximal suppression of parathyroid hormone (PTH) and reduced fracture rates.11
Few studies have evaluated the prevalence of 25(OH)D deficiency in children and adolescents in the United States. One study showed that 52% of 307 Hispanic and black adolescents in Boston, Mass, had 25(OH)D levels <15 ng/mL. Another found that 48% of 23 white preadolescent girls in Maine had 25(OH)D levels <20 ng/mL.12,13 More recently, a study of vitamin D levels in presumably healthy toddlers and infants attending a pediatrics clinic found that 12.1% (44 of 365) of the children had levels <20 ng/mL, and 40% (146 of 365) had levels <30 ng/mL.14
Given the limited available data on the prevalence of 25(OH)D deficiency among children and adolescents in the United States, we examined the prevalence of 25(OH)D deficiency (<15 ng/mL)12,15–18 and insufficiency (15–29 ng/mL) in US children and adolescents. In addition, we studied risk factors for 25(OH)D deficiency, and, because of evidence linking low 25(OH)D levels with cardiovascular risk factors in adults, we studied this association in children and adolescents as well. To do so, we analyzed data on children and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2001–2004.
PATIENTS AND METHODS
Study Population
NHANES 2001–2004 was a nationally representative cross-sectional survey of the civilian, noninstitutionalized US population performed by the National Center for Health Statistics. All of the participants underwent standardized interviews, physical examinations, and laboratory testing. The current analysis was limited to children and adolescents, 1 to 21 years of age, with 25(OH)D measurements and complete data on other variables of interest. Children and adolescents with missing data on 25(OH)D levels (n = 1571), poverty/income ratio (PIR; n = 607), country of birth (n = 8), obesity (n = 1205), milk intake (n = 354), and television and computer use (n = 72) were excluded from the multivariable analyses. 25(OH)D levels were measured in all of the children and adolescents aged ≥6 years from 2001–2002 and in all of the children and adolescents aged ≥1 year from 2003–2004. NHANES 2001–2004 was approved by the National Center for Health Statistics Institutional Review Board, and all of the participants ≥18 years provided informed consent, participants 12 to 17 years old and their parents provided informed consent, participants 7 to 11 years old provided assent and parental consent, and parents provided informed consent for those <7 years old.
Study Variables
NHANES 2001–2004 consisted of an in-home interview followed by a medical evaluation and blood sample collection at a mobile examination center. Demographic variables in the current analysis include age, gender, and race/ethnicity. Self-reported or, for those <12 years old, parent/guardian-reported race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other. Country of birth was categorized as those born within or outside of the United States. The PIR is a ratio of a family's income to their poverty threshold as defined by the US Census Bureau. NHANES defined PIR ≤1 as below the poverty threshold, and participants were divided into 3 categories of PIR: ≥5.00, 1.10 to 4.99, and ≤1.00. Diabetes mellitus was coded as present if participants reported that they had been told by a physician that they had diabetes mellitus or if they were taking insulin or oral hypoglycemics.
Milk consumption data were obtained from the diet, behavior, and nutrition section of the NHANES Sample Person Questionnaire and were categorized as daily, more than once per week but less than daily, and less than once per week. Fish intake was ascertained only in children 1 to 5 years old and women 16 to 49 years old by using a 24-hour dietary recall interview.
Participants were asked about hours spent watching television, playing video games, and using the computer. Combined television, video, and computer hours were categorized as none, ≤2 hours, 3 to 4 hours, and >4 hours per day. Vitamin D use as a supplement was assessed by using participant questionnaires and pill-bottle review to obtain the dose and duration of vitamin D intake. A participant was considered a regular vitamin D supplement user if he or she took 400 IU daily for ≥30 days.
During the examination, each participant's height and weight was measured. Obesity was categorized on the basis of weight in 1-year-olds and BMI in 2- to 21-year-olds. BMI was calculated as weight in kilograms divided by height in meters squared. In 1-year-olds, obesity was defined as levels exceeding the 95th percentile of the weight distribution on the basis of gender-specific weight curves. In 2- to 21-year-olds, obesity was defined by using age- and gender-specific centile curves on the basis of pooled international data and linked to the widely used adult obesity cutoff point of 30 kg/m2 proposed by Cole et al.19 Systolic and diastolic blood pressures were measured by trained personnel 3 times on all of the participants ≥8 years of age, and the mean value from these measurements was used for the current analysis. Hypertension was defined as a systolic or diastolic blood pressure exceeding the 95th percentile of levels for the median height of each participant's specific age and gender or >140/90 mm Hg in those ≥17 years of age.20
All of the blood measurements used in this study were drawn and performed as part of the NHANES 2001–2004 survey. High-sensitivity C-reactive protein (CRP) was measured by latex-enhanced nephelometry. Elevated CRP was defined as >0.15 mg/dL, the threshold of detection using this assay. Serum calcium, phosphate, and total and high-density lipoprotein (HDL) cholesterol levels were measured on the Beckman Synchron LX20 (Beckman Coulter, Brea, CA). Urinary albumin levels were measured by using a solid-phase fluorescent immuno-assay in all of the participants ≥6 years of age. Urinary creatinine levels were measured by using the Jaffe rate reaction with a CX3 analyzer (Beckman ASTRA, Brea, CA). The urinary albumin/ creatinine ratio (ACR) was calculated, and albuminuria was defined as an ACR >30 μg/mg. Serum PTH was measured at the University of Washington (Seattle, WA) on an Elecsys 1010 autoanalyzer (Roche Diagnostics, Mannheim, Germany) by using an electrochemiluminescent process, and levels >65 ng/mL, the upper limit of normal for this assay, were considered elevated.
25(OH)D was measured by using the Diasorin (formerly Incstar; Diasorin, Stillwater, MN) 25(OH)D assay. Three levels of control specimens were used to assess the quality of each 25(OH)D run. Coefficients of variation for serum 25(OH)D remained <11% throughout the 2001–2004 period. Vitamin D deficiency was defined as a level <15 ng/ mL, whereas levels 15 to 29 ng/mL and ≥30 ng/mL were considered insufficient and sufficient, respectively.
Statistical Analysis
The proportions of participants 1 to 21 years of age with 25(OH)D deficiency, insufficiency, and sufficiency were calculated by age group (1–6, 7–12, and 13–21 years). Participant characteristics were calculated by 25(OH)D categories (deficient, insufficient, and sufficient). Statistical significance of trends across these characteristics was determined by using linear and logistic regression for continuous and dichotomous variables, respectively. Next, by using logistic regression models, we calculated the odds ratios of 25(OH)D deficiency associated with risk factors, including demographic, socioeconomic, dietary, and lifestyle factors. In this analysis, we compared those with 25(OH)D deficiency to those with levels ≥15 ng/mL.
We then analyzed the association between 25(OH)D deficiency and cardiovascular risk factors using logistic regression for categorical variables, including hypertension, diabetes mellitus, albuminuria, and elevated CRP and PTH, as well as linear regression for continuous variables, including serum calcium, phosphate, systolic and diastolic blood pressures, and total and HDL cholesterol. Models included adjustment for age, gender, race/ethnicity, PIR, obesity, milk intake, television and computer use, and vitamin D supplement use. As sensitivity analyses, we also performed the above analysis only in participants who were not obese and, to test another cut point to define deficiency, by using <20 ng/mL. Data were analyzed by using appropriate NHANES sample weights in Stata SE 10.1 (Stata Corp, College Station, TX) to account for the complex NHANES sampling design, including unequal probabilities of selection, oversampling, and nonresponse. For all of the analyses, P values are 2-tailed with an α < .05 considered statistically significant.
RESULTS
Prevalence of 25(OH)D Deficiency and Insufficiency
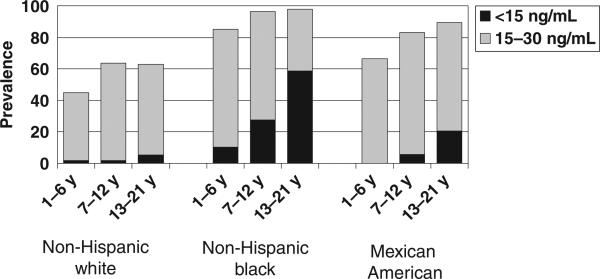
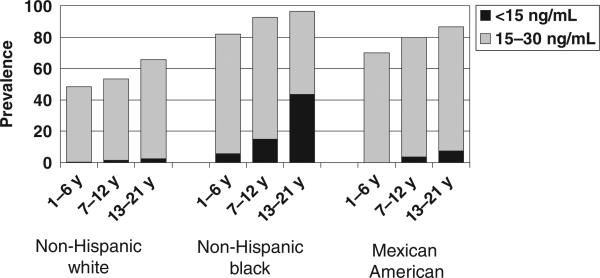
There were 9757 children in NHANES 2001–2004 included in the prevalence analyses. Nine percent of children had 25(OH)D levels <15 ng/mL, representing 7.6 million US children and adolescents with 25(OH)D deficiency. Sixty-one percent of the children had 25(OH)D levels between 15 and 29 ng/mL, representing 50.8 million US children and adolescents with 25(OH)D insufficiency (Figs 1 and 2 and Table 1).
FIGURE 1.
Prevalence of 25(OH)D insufficiency and deficiency by participant subgroup for 3012 girls in NHANES 2001–2004.
FIGURE 2.
Prevalence of 25(OH)D insufficiency and deficiency by participant subgroup for 3263 boys in NHANES 2001–2004.
TABLE 1.
Prevalence of 25 (OH)D Insufficiency and Deficiency Among 9757 Children and Adolescents in NHANES 2001-2004
| Population | 25 (OH)D Levels 15–29 ng/mL, % (95% CI) |
25 (OH)D Levels <15 ng/mL, % (95% CI) |
||
|---|---|---|---|---|
| Girls | Boys | Girls | Boys | |
| Non-Hispanic white | ||||
| 1–6 y old | 43 (29 to 57) | 48 (36 to 60) | 2 (0 to 5) | 0.4 (0 to 1) |
| 7–12 y old | 62 (52 to 72) | 52 (44 to 60) | 2 (0 to 3) | 1 (0 to 3) |
| 13–21 y old | 57 (50 to 66) | 63 (57 to 69) | 5 (3 to 8) | 3 (1 to 4) |
| Non-Hispanic black | ||||
| 1–6 y old | 75 (66 to 84) | 76 (70 to 83) | 10 (2 to 18) | 6 (2 to 10) |
| 7–12 y old | 69 (63 to 75) | 78 (73 to 83) | 28 (21 to 34) | 15 (11 to 19) |
| 13–21 y old | 39 (32 to 47) | 53 (46 to 60) | 59 (51 to 67) | 43 (36 to 51) |
| Mexican American | ||||
| 1–6 y old | 66 (51 to 81) | 70 (61 to 79) | — | — |
| 7–12 y old | 77 (71 to 84) | 76 (71 to 82) | 6 (2 to 9) | 4 (1 to 6) |
| 13–21 y old | 69 (62 to 76) | 79 (74 to 84) | 20 (13 to 28) | 7 (5 to 10) |
| Other race | ||||
| 1–6 y old | 58 (28 to 89) | 37 (13 to 61) | — | 2 (0 to 6) |
| 7–12 y old | 74 (63 to 85) | 81 (64 to 98) | 3 (0 to 7) | 2 (0 to 5) |
| 13–21 y old | 61 (47 to 76) | 57 (44 to 70) | 30 (14 to 46) | 28 (13 to 44) |
— represents a cell with no observations.
Factors Associated With 25(OH)D Deficiency (25[OH]D Levels <15 ng/mL)
Older children, girls, non-Hispanic blacks, Mexican Americans, other races, those born outside of the United States, those with a lower PIR, obese children, and those who spent more time watching television, playing video games, or using computers were more likely to have lower 25(OH)D levels (Table 2). In contrast, children who drank milk daily and those who took vitamin D supplements were less likely to have lower 25 (OH)D levels. These factors were associated with 25(OH)D deficiency after age, gender, and race/ethnicity adjustment and after multivariable adjustment (Table 3).
TABLE 2.
Participant Characteristics by 25 (OH)D Levels of 6275 Children Aged 1 to 21 Years From NHANES 2001–2004
| Characteristic | 25 (OH)D Levels, ng/mL |
P | ||
|---|---|---|---|---|
| ≥30 | 15–29 | <15 | ||
| Age, mean ± SE, y | 11.0 ± 0.3 | 13.0 ± 0.2 | 15.0 ± 0.3 | <.001 |
| Female gender, % ± SE | 47.0 ± 2.0 | 47.0 ± 1.0 | 60.0 ± 2.0 | .01 |
| Race/ethnicity, % ± SE | ||||
| Non-Hispanic white | 82.0 ± 2.0 | 57.0 ± 3.0 | 19.0 ± 3.0 | <.001 |
| Non-Hispanic black | 3.0 ± 0.4 | 14.0 ± 2.0 | 55.0 ± 3.0 | <.001 |
| Mexican American | 11.0 ± 2.0 | 22.0 ± 3.0 | 17.0 ± 3.0 | <.001 |
| Other | 4.0 ± 1.0 | 6.0 ± 0.7 | 10.0 ± 2.0 | .02 |
| Country of birth, % ± SE | ||||
| Born in United States | 97.0 ± 0.9 | 91.0 ± 0.8 | 89.0 ± 2.0 | <.001 |
| Born outside United States | 3.0 ± 0.8 | 9.0 ± 0.8 | 11.0 ± 2.0 | <.001 |
| PIR, % ± SE | ||||
| ≥5.00 | 19.0 ± 20 | 11.0 ± 0.9 | 7.0 ± 2.0 | <.001 |
| 1.10–4.99 | 64.0 ± 2.0 | 62.0 ± 20 | 52.0 ± 3.0 | .01 |
| 0.00–1.00 | 17.0 ± 2.0 | 27.0 ± 2.0 | 40.0 ± 3.0 | <.001 |
| Obese, % ± SE | ||||
| No | 91.0 ± 1.0 | 82.0 ± 1.0 | 72.0 ± 2.0 | <.001 |
| Yes | 9.0 ± 1.0 | 18.0 ± 1.0 | 28.0 ± 2.0 | <.001 |
| Milk intake, % ± SE | ||||
| Daily | 82.0 ± 1.0 | 76.0 ± 1.0 | 56.0 ± 2.0 | <.001 |
| More than once per week | 14.0 ± 1.0 | 18.0 ± 1.0 | 28.0 ± 2.0 | <.001 |
| Less than once per week | 4.0 ± 0.6 | 6.0 ± 0.6 | 17.0 ± 2.0 | <.001 |
| Fish eater (n = 3790), % ± SE | ||||
| Yes | 26.0 ± 2.0 | 21.0 ± 2.0 | 27.0 ± 3.0 | .47 |
| No | 74.0 ± 2.0 | 79.0 ± 2.0 | 73.0 ± 3.0 | |
| Television and computer use, hours per day, % ± SE | ||||
| None | 1.0 ± 0.5 | 1.0 ± 0.3 | 2.0 ± 0.7 | .54 |
| ≤2 h | 42.0 ± 2.0 | 33.0 ± 1.0 | 26.0 ± 2.0 | <.001 |
| 3–4 h | 39.0 ± 2.0 | 40.0 ± 1.0 | 34.0 ± 2.0 | .32 |
| >4 h | 18.0 ± 2.0 | 26.0 ± 1.0 | 38.0 ± 3.0 | <.001 |
| Vitamin D supplements, % ± SE | ||||
| No | 93.0 ± 0.9 | 96.0 ± 0.5 | 98.0 ± 0.5 | .001 |
| Yes | 7.0 ± 0.9 | 4.0 ± 0.5 | 2.0 ± 0.5 | .001 |
| PTH ±65 pg/mL (n = 2664), % ± SE | .001 | |||
| No | 97.0 ± 0.9 | 94.0 ± 1.0 | 87.0 ± 2.3 | |
| Yes | 3.0 ± 0.9 | 6.0 ± 1.0 | 13.0 ± 2.3 | |
| Phosphate, mg/dL (n = 3926), mean ± SE | 4.30 ± 0.03 | 4.30 ± 0.03 | 4.20 ± 0.03 | .08 |
| Calcium, mg/dL (n = 3926), mean ± SE | 9.74 ± 0.02 | 9.71 ± 0.02 | 9.60 ± 0.03 | <.001 |
| SBP, mm Hg (n = 4989), mean ± SE | 106.0 ± 0.5 | 107.0 ± 0.3 | 110.0 ± 0.6 | <.001 |
| DBP, mm Hg (n = 4989), mean ± SE | 58.0 ± 0.7 | 60.0 ± 0.4 | 61.0 ± 0.6 | .014 |
| Hypertension (n = 4989), % ± SE | .02 | |||
| No | 97.9 ± 0.6 | 97.6 ± 0.3 | 94.5 ± 1.1 | |
| Yes | 2.1 ± 0.6 | 2.4 ± 0.3 | 5.5 ± 1.1 | |
| Diabetes mellitus, % ± SE | .03 | |||
| No | 99.8 ± 0.1 | 99.3 ± 0.2 | 99.2 ± 0.3 | |
| Yes | 0.2 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.3 | |
| Albuminuria (ACR ±30 μg/mg), % ± SE | .6 | |||
| No | 96.0 ± 1.0 | 95.0 ± 0.5 | 96.0 ± 1.0 | |
| Yes | 4.0 ± 1.0 | 5.0 ± 0.5 | 4.0 ± 1.0 | |
| CRP, % ± SE, mg/dL | .02 | |||
| Low (≤0.15) | 79.0 ± 2.0 | 79.0 ± 1.0 | 67.0 ± 2.0 | |
| High (>0.15) | 21.0 ± 2.0 | 21.0 ± 1.0 | 33.0 ± 2.0 | |
| Total cholesterol, mean ± SE, mg/dL (n = 6036) | 165.0 ± 0.9 | 163.0 ± 1.0 | 167.0 ± 2.0 | .91 |
| HDL cholesterol, mean ± SE, mg/dL (n = 6036) | 53.0 ± 0.6 | 51.0 ± 0.3 | 52.0 ± 0.7 | .03 |
Data are presented as means ± SEs or percentages ± SEs of the population. SBP indicates systolic blood pressure; DBP, diastolic blood pressure. Percentages may not total 100% because of rounding.
TABLE 3.
Age-, Gender- and Race/Ethnicity-Adjusted and Multivariable-Adjusted Odds Ratios of 25 (OH)D Deficiency (<15 ng/mL) in 6275 Children in NHANES 2001–2004
| Characteristic | Age, Gender, and Race/Ethnicity Adjusted |
Multivariable Adjusteda |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age, y | 1.18b | 1.14 to 1.22 | <.001 | 1.16 | 1.12 to 1.20 | <.001 |
| Female gender | 1.90b | 1.60 to 2.40 | <.001 | 1.90 | 1.50 to 2.30 | <.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white (reference) | 1.00b | — | — | 1.00 | — | — |
| Non-Hispanic black | 24.20 | 15.90 to 36.90 | <.001 | 21.90 | 13.40 to 35.70 | <.001 |
| Mexican American | 3.70 | 2.10 to 6.50 | <.001 | 3.50 | 1.90 to 6.40 | <.001 |
| Other | 7.20 | 3.90 to 13.20 | <.001 | 7.30 | 3.70 to 14.30 | <.001 |
| Country of birth | ||||||
| Born in United States (reference) | 1.00 | — | — | 1.00 | — | — |
| Born outside United States | 1.00 | 0.70 to 1.50 | .97 | 1.10 | 0.70 to 1.60 | .66 |
| PIR | ||||||
| ≥5.00 (reference) | 1.00 | — | — | 1.00 | — | — |
| 1.10–4.99 | 1.00 | 0.60 to 1.90 | .88 | 1.00 | 0.50 to 1.90 | .98 |
| 0.00–1.00 | 1.30 | 0.60 to 2.50 | .50 | 1.20 | 0.60 to 2.40 | .62 |
| Obese | ||||||
| No (reference) | 1.00 | — | — | 1.00 | — | — |
| Yes | 2.00 | 1.50 to 2.60 | <.001 | 1.90 | 1.50 to 2.50 | <.001 |
| Milk intake | ||||||
| Daily (reference) | 1.00 | — | — | 1.00 | — | — |
| More than once per week | 1.40 | 1.10 to 1.80 | .01 | 1.40 | 1.10 to 1.80 | .01 |
| Less than once per week | 2.90 | 2.10 to 4.00 | <.001 | 2.90 | 2.10 to 3.90 | <.001 |
| Fish eater (n = 3790) | ||||||
| Yes (reference) | 1.00 | — | — | 1.00 | — | — |
| No | 1.20 | 0.80 to 1.70 | .46 | 1.10 | 0.70 to 1.60 | .67 |
| Television and computer use, h/d | ||||||
| Nonec | 2.20 | 0.70 to 6.40 | .16 | 2.20 | 0.70 to 6.60 | .17 |
| ≤2 h (reference) | 1.00 | — | — | 1.00 | — | — |
| 3–4 h | 1.20 | 0.90 to 1.50 | .26 | 1.10 | 0.90 to 1.50 | .37 |
| >4 h | 1.60 | 1.10 to 2.40 | .01 | 1.60 | 1.10 to 2.30 | .02 |
| Vitamin D supplements | ||||||
| No (reference) | 1.00 | — | — | 1.00 | — | — |
| Yes | 0.40 | 0.20 to 0.80 | .007 | 0.40 | 0.20 to 0.80 | .009 |
— indicates no data; OR, odds ratio.
Data show a multivariable adjusted for all other variables in the table except fish intake. The fish intake model was adjusted for all other variables in the table.
Data were adjusted for the other 2 variables: for age, data were adjusted for gender and race; for gender, data were adjusted for age and race; and for race, data were adjusted for age and gender.
Only 1.4% of the study population spent no time watching television, playing video games, or using the computer; therefore, this category was not used as the reference.
Association of 25(OH)D Deficiency With Cardiovascular Risk Factors
Children and adolescents with lower 25(OH)D levels were more likely to have lower serum calcium and HDL cholesterol levels, diabetes mellitus, and elevated CRP and PTH levels (Table 2). After multivariable adjustment, children and adolescents with 25(OH)D insufficiency had lower levels of total cholesterol and HDL cholesterol and higher diastolic blood pressures and were more likely to have elevated PTH levels than their counterparts with 25(OH)D ≥30 ng/mL (Table 4). Participants with 25(OH)D insufficiency were less likely to have elevated CRP levels, a finding that did not maintain statistical significance when evaluating those with 25(OH)D deficiency. Children with 25(OH)D deficiency had lower serum calcium and HDL cholesterol levels and higher systolic blood pressure than those with 25(OH)D levels ≥30 ng/mL after multivariable adjustment (Table 4). In addition, after multivariable adjustment, children and adolescents with vitamin D insufficiency and deficiency were more likely to have PTH levels >65 pg/mL and hypertension (Table 4).
TABLE 4.
Multivariable-Adjusted Differences in Cardiovascular Risk Factor Levels and OR of Risk Factors in Children and Adolescents With 25 (OH)D Deficiency (<15 ng/mL) and Insufficiency (15 to 29 ng/mL) Compared With Those With Levels ≥30 ng/mL Among 6275 children in NHANES 2001–2004
| Outcome | 25 (OH)D Levels 15–29 ng/mL |
25 (OH)D Levels <15 ng/mL |
||||
|---|---|---|---|---|---|---|
| Data | 95% CI | P | Data | 95% CI | P | |
| Serum calcium, mg/dL (n = 3926) | –0.03a | –0.09 to 0.02 | .21 | –0.09a | –0.15 to –0.04 | .002 |
| Serum phosphate, mg/dL (n = 3926) | –0.03a | –0.10 to 0.04 | .39 | –0.04a | –0.15 to 0.06 | .40 |
| Systolic blood pressure, mm Hg (n = 4989) | 0.78a | –0.08 to 1.64 | .08 | 2.24a | 0.98 to 3.50 | .001 |
| Diastolic blood pressure, mm Hg (n = 4989) | 1.68a | 0.20 to 3.16 | .03 | 1.60a | –0.54 to 3.75 | .14 |
| Total cholesterol, mg/dL (n = 6036) | –3.66a | –7.09 to –0.23 | .04 | –2.92a | –8.15 to 2.30 | .26 |
| HDL cholesterol, mg/dL (n = 6036) | –2.29a | –3.57 to –1.01 | .001 | –3.03a | –5.02 to –1.04 | .004 |
| PTH >65 pg/mL (n = 2664) | 2.0b | 1.10 to 3.80 | .04 | 3.6b | 1.80 to 7.10 | .001 |
| Hypertension (n = 4989) | 1.0b | 0.50 to 2.00 | .96 | 2.5b | 1.00 to 5.90 | .04 |
| Diabetes mellitus (n = 6275) | 2.8b | 0.80 to 10.40 | .12 | 1.9b | 0.40 to 9.70 | .41 |
| Elevated CRP (n = 6275) | 0.7b | 0.50 to 0.90 | .003 | 0.7b | 0.50 to 1.00 | .07 |
| Albuminuria (n = 6275) | 1.2b | 0.80 to 1.80 | .32 | 1.3b | 0.70 to 2.40 | .47 |
Data were multivariable adjusted for age, gender, race/ethnicity, obesity, PIR, television and computer use, milk intake, and vitamin D supplementation.
Data show Δ, which represents the difference between those with 25 (OH)D levels <15 ng/mL or 15 to 29 ng/mL compared with those with levels ≥30 ng/mL and OR is compared to those with 25 (OH)D ≥30 ng/mL.
Data show odds ratio, which represents the difference between those with 25 (OH)D levels <15 ng/mL or 15 to 29 ng/mL compared with those with 25 (OH)D ≥30 ng/mL.
Sensitivity Analyses
To avoid residual confounding by obesity, we analyzed the associations between 25(OH)D insufficiency and deficiency and cardiovascular risk factors only in nonobese children and adolescents. Results were similar to the associations reported above. After multivariable adjustment, those with 25(OH)D deficiency had a higher odds of having hypertension (OR: 3.81 [95% CI: 1.09 to 13.21]; P = .04), elevated PTH levels (OR: 3.79 [95% CI: 1.78 to 8.06]; P = .002), higher systolic blood pressure (OR: 2.36 mm Hg [95% CI: 1.04 to 3.69 mm Hg]; P = .001), and lower serum calcium levels (OR: –0.11 mg/dL [95% CI: –0.17 to –0.05]; P = .001) compared with those with 25(OH)D levels ≥30 ng/mL.
When analyzing the data by using <20 ng/mL as the cut point for deficiency, most of the associations did not change materially. In addition to previous associations, birth outside of the United States and television and computer use 3 to 4 hours per day became significant predictors of 25(OH)D deficiency (data not shown). In multivariable adjusted models, in addition to previous associations, 25(OH)D deficiency (<20 ng/mL) was also associated with higher diastolic blood pressure, lower total cholesterol, and a lower risk of elevated CRP but not associated with prevalent hypertension (data not shown).
DISCUSSION
The current study provides data on the prevalence of 25(OH)D deficiency and its correlates in a large, nationally representative sample of children and adolescents 1 to 21 years of age. In 2001– 2004, ~9% (7.6 million) of US children and adolescents had 25(OH)D deficiency, which predisposes them to the development of rickets. In addition, children and adolescents with 25(OH)D deficiency were more likely to have elevated PTH and hypertension and lower calcium and HDL cholesterol levels. Although the elevations in blood pressure and lipids associated with 25(OH)D deficiency are small and may not be clinically significant for an individual child, taken over the population and at such a young age, they may have long-term clinical consequences.21,22
Previous studies, albeit with smaller sample sizes, have also found a high prevalence of 25(OH)D deficiency among children and adolescents.6–8 Looker et al23 analyzed data from NHANES III to assess the prevalence of 25(OH)D deficiency in 12- to 19-year-old subjects. Of participants examined during the winter months, in southern latitudes, 1% of boys and 4% of girls had levels <10 ng/mL, whereas in those examined during the summer in the northern latitudes, <1% had levels <10 ng/mL. Although this study was nationally representative, it did not examine dietary or other lifestyle risk factors for 25(OH)D deficiency.
Consistent with other studies, non-white race/ethnicity was a very strong predictor of 25(OH)D deficiency. These associations were present even after adjustment for potentially modifiable risk factors for 25(OH)D deficiency, including obesity, milk intake, television watching, video game and computer use, and vitamin D supplement use, suggesting that there are other factors contributing to this disparity. Increased melanin in the skin has been shown to decrease production of precursors of vitamin D per the same dose of exposure to UV radiation.24,25 Our study found an association between 25(OH)D deficiency and hypertension. It is interesting to note that non-Hispanic blacks have a higher rate of hypertension than non-Hispanic whites, even in childhood.26 Differences in 25(OH)D levels may explain some of this disparity, and this is a subject that warrants further investigation.
In our analysis, girls had a higher prevalence of 25(OH)D deficiency compared with boys. Dietary intake of vitamin D from food was reported to be lower for female teenagers compared with male teenagers in NHANES III.27 Not all studies, however, report a gender difference in the prevalence of 25(OH)D deficiency.28
Less than daily and less than weekly milk intakes were associated with higher odds of 25(OH)D deficiency in our study. Our findings are consistent with smaller studies in infants, toddlers, and adolescents.12,14 Although fish is known to contain vitamin D, low fish intake was not associated with 25(OH)D deficiency in the current analysis. The American Academy of Pediatrics recommends 400 IU daily of vitamin D for children.29 Some experts suggest that, without adequate sun exposure, children and adults need ~800 to 1000 IU per day to achieve adequate levels.11,30,31 Vitamin D supplement intake was low in the current study (4% used 400 IU for ≥30 days). However, supplement use maintained a significant protective association against 25(OH)D deficiency.
Obese children were also more at risk of having 25(OH)D deficiency. This association is complex, because there is sequestration of vitamin D in fat tissue,32,33 and obese children may have a more sedentary, indoor lifestyle. Obese children may get less vitamin D from food sources than nonobese children34 or, most likely, a combination of all of the above and potentially other factors.
We found that participants with the lowest levels of 25(OH)D were more likely to have elevated PTH and low calcium levels, suggesting that there may be biochemical consequences to the low 25(OH)D levels. These biochemical abnormalities have been reported in other studies.6,14 A study of 193 girls 10 to 12 years old in Finland found that PTH levels were significantly higher and bone mineral density levels were lower in girls with lower 25(OH) D levels.35
There are few studies that have reported the association between 25(OH)D levels and cardiovascular risk factors in children. In a study of 217 obese children,36 lower HDL cholesterol and higher systolic blood pressure were associated with lower levels of 25(OH)D. Vitamin D is thought to be essential for maintaining adequate levels of apolipoprotein A-I, a major component of HDL cholesterol. Individuals with high 25(OH)D concentrations have the highest plasma apolipoprotein A-I concentrations, and there is a positive correlation between 25(OH)D and serum HDL cholesterol concentrations.37,38 Low HDL cholesterol levels are an established cardiovascular risk factor,39 suggesting potential long-term consequences for 25(OH)D deficiency in childhood. The unadjusted association observed between low 25(OH)D levels and elevated CRP disappeared after multivariable adjustment, suggesting that the relationship was confounded, possibly by obesity.
Our study has several important limitations. We are limited by a lack of information on several potential important confounders, including the season of measurement of 25(OH)D levels, the latitude of the participants’ home, and lack of information on sun exposure. In addition, we did not have measurements of fibroblast growth factor 23, which may mediate associations between phosphate and the vitamin D axis.40 However, our study did include a wide range of information, including demographics, dietary factors, and other laboratory factors, including PTH, which were all collected in a standardized fashion. Also, NHANES was designed such that participants in northern states are enrolled and screened during the summer and southern states during the winter. Therefore, the 25(OH)D levels measured may actually be higher than the average 25(OH)D levels present in the population. Lastly, our analysis of risk factors for and sequelae of 25(OH)D deficiency used a cross-sectional study design, and, therefore, caution should be taken when considering the direction of the associations, and, as in any observational study, causality cannot be established. Randomized, placebo-controlled clinical trials evaluating the beneficial effects of vitamin D supplementation on cardiovascular risk factors are needed to establish these as causal relations.
CONCLUSIONS
We found 25(OH)D deficiency to be common in children in the United States. Our analysis shows non-Hispanic blacks, Mexican Americans, girls, and older and obese children and adolescents to be at higher risk for 25(OH)D deficiency. In addition, 25(OH)D deficiency in children and adolescents was associated with cardiovascular risk factors, including hypertension, low HDL cholesterol levels, and elevated PTH levels. Although our study shows that a significant proportion of the US pediatric population has low 25(OH)D levels, only 4% of the population were taking the currently recommended supplement dose. It will be important to evaluate whether the recent increase in recommended vitamin D supplement dose29 decreases the percentage of the pediatric population that is 25(OH)D deficient.
WHAT'S KNOWN ON THIS SUBJECT: Small studies have evaluated the prevalence of vitamin D deficiency in the United States. Some of these have looked at risk factors for deficiency. A handful of reports looking at cardiovascular risk factors and associations with low vitamin D levels in children.
WHAT THIS STUDY ADDS: We describe the prevalence of vitamin D deficiency in the US pediatric population using a large nationally representative database. In addition we also showed an association between deficiency and cardiovascular risk factors such as blood pressure and HDL levels.
ACKNOWLEDGMENTS
Dr Kumar is supported by grant T32 DK007110-33; Dr Kaskel is supported by grants T32 DK007110-33, U01 DK63549, and U01 DK066174; and Dr Melamed is supported by grant K23 078774, all from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- PTH
parathyroid hormone
- NHANES
National Health and Nutrition Examination Survey
- PIR
poverty/income ratio
- CRP
C-reactive protein
- HDL
high-density lipoprotein
- ACR
albumin/creatinine ratio
Footnotes
This work was presented in abstract form at the meeting of the Pediatric Academic Societies; May 2-6, 2008; Honolulu, HI. www.pediatrics.org/cgi/doi/10.1542/peds.2009-0051
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Nagpal S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 3.Gessner BD, deSchweinitz E, Petersen KM, Lewandowski C. Nutritional rickets among breast-fed black and Alaska Native children. Alaska Med. 1997;39(3):72–74. 87. [PubMed] [Google Scholar]
- 4.McAllister JC, Lane AT, Buckingham BA. Vitamin D deficiency in the San Francisco Bay Area. J Pediatr Endocrinol Metab. 2006;19(3):205–208. doi: 10.1515/jpem.2006.19.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–337. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Kreiter SR, Schwartz RP, Kirkman HN, Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 7.Mylott BM, Kump T, Bolton ML, Greenbaum LA. Rickets in the Dairy State. WMJ. 2004;103(5):84–87. [PubMed] [Google Scholar]
- 8.Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80(6 suppl):1697S–1705S. doi: 10.1093/ajcn/80.6.1697S. [DOI] [PubMed] [Google Scholar]
- 9.Shah M, Salhab N, Patterson D, Seikaly MG. Nutritional rickets still afflict children in north Texas. Tex Med. 2000;96(6):64–68. [PubMed] [Google Scholar]
- 10.Pettifor JM. Vitamin D deficiency and nutritional rickets in children. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. 2nd ed. Elsevier Academic Press; Boston, MA: 2005. [Google Scholar]
- 11.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Gordon CM, Feldman HA, Sinclair L, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 16.Gloth FM, III, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274(21):1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 17.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 19.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. [PubMed] [Google Scholar]
- 21.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–709. [PubMed] [Google Scholar]
- 22.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8(7):657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 23.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 26.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- 27.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104(6):980–983. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86(1):150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 29.Wagner CL, Greer FR, Section on Breastfeeding and Committee on Nutrition. American Academy of Pediatrics Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 30.Glerup H, Mikkelsen K, Poulsen L, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247(2):260–268. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 32.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 33.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008;16(1):90–95. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 34.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Cheng S, Tylavsky F, Kroger H, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78(3):485–492. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 36.Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20(7):817–823. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 37.Auwerx J, Bouillon R, Kesteloot H. Relation between 25-hydroxyvitamin D3, apolipoprotein A-I, and high density lipoprotein cholesterol. Arterioscler Thromb. 1992;12(6):671–674. doi: 10.1161/01.atv.12.6.671. [DOI] [PubMed] [Google Scholar]
- 38.Carbone LD, Rosenberg EW, Tolley EA, et al. 25-Hydroxyvitamin D, cholesterol, and ultraviolet irradiation. Metabolism. 2008;57(6):741–748. doi: 10.1016/j.metabol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 40.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]