Abstract
Objective
We investigated the prevalence of congenital renal and urologic anomalies in children with congenital hypothyroidism to determine whether further renal and urologic investigations would be of benefit.
Study design
Prevalence of congenital hypothyroidism was obtained from the New York State Congenital Malformation Registry. The occurrence of urinary tract anomalies were calculated for children with congenital hypothyroidism and compared to children without congenital hypothyroidism. In addition we obtained congenital hypothyroidism data from New York State newborn screening, and the cases were matched to Congenital Malformation Registry.
Results
Analysis of Congenital Malformation Registry data showed 980 children with congenital hypothyroidism and 3 661 585 children without congenital hypothyroidism born in New York State (1992-2005). Children with congenital hypothyroidism have a significantly increased risk of congenital renal and urological anomalies with the odds ratio (OR) of 13.2 (10.6-16.5). The other significantly increased defects in congenital hypothyroidism were cardiac, gastrointestinal, and skeletal. Analysis of matched data confirmed an increase of congenital renal and urologic anomalies with OR of 4.8 (3.7-6.3).
Conclusions
Children with congenital hypothyroidism have an increased prevalence of congenital renal and urologic anomalies. We suggest that these children should be evaluated for the presence of congenital renal and urologic anomalies with renal ultrasonography, and that further studies of common genes involved in thyroid and kidney development are warranted.
Congenital malformations are the leading cause of infant mortality in the United States.1 Congenital hypothyroidism is the most common congenital endocrine disorder, affecting 1 in 3000 to 4000 newborns.2 Its incidence has increased 138% from 1978 to 2005 in New York State and 73% in the US from 1987 to 2002.3 Nearly all newborns are routinely screened for congenital hypothyroidism at birth in the United States.4 Thyroid dysgenesis is responsible for about 85% of cases of congenital hypothyroidism; dyshormonogenesis accounts for the remaining cases.5
Congenital hypothyroidism is associated with increased prevalence of congenital malformations.6-11 There are case reports of children with congenital hypothyroidism having defects in the development of the renal and urogenital systems.12-14 However, there are no studies specifically examining prevalence of congenital renal and urologic anomalies in the congenital hypothyroidism population. Congenital renal and urologic anomalies are the most common cause of end-stage kidney disease in children, accounting for almost 50% of the cases.15 Most children with congenital hypothyroidism are not routinely screened for the presence of congenital renal and urologic malformations.
Recently, mutations in PAX 8, TITF1, or FOXE1 genes have been associated with congenital hypothyroidism in patients with either isolated thyroid dysplasia or thyroid dysplasia with associated malformations involving kidney, lung, forebrain, and palate.16-20 PAX 8 is expressed in the developing central nervous system and kidney, including the ureteric bud, mesonephric ducts, and the main collecting ducts.17
PAX 2 and PAX 8 transcription factors are central regulators of kidney development. In mouse pronephros and mesonephros, they are found in both the mesenchymal primordium and the epithelial components. PAX 8 expression starts at the renal vesicle stage and is maintained until the end of nephron differentiation. PAX 2 and PAX 8 genes are necessary and sufficient to induce the nephric lineage. Embryos that are mutant for both genes fail to generate the nephric (Wolffian) duct and subsequently all 3 embryonic kidneys are defective.20,21
We hypothesized that children with congenital hypothyroidism have an increased prevalence of congenital renal and urologic anomalies. Our objective was to compare the prevalence of renal and urinary tract anomalies in children with and without congenital hypothyroidism and to determine whether renal ultrasound scanning would be beneficial in management of children with congenital hypothyroidism.
METHODS
Prevalence data for congenital hypothyroidism and congenital anomalies was obtained from the New York State Department of Health. The Congenital Malformations Registry of the New York State Department of Health is a repository of case reports on children who are born or reside in New York State and are diagnosed before the age of 2 years with any structural, functional, or biochemical abnormality determined genetically or induced during gestation and not due to birthing events.22
Since there are published reports of increased incidence of malformations of other major organ systems, we also examined prevalence of congenital malformations of heart, gastrointestinal, and skeletal systems in the data set. In addition we obtained congenital hypothyroidism data from the New York State newborn screening database and matched it to the Congenital Malformation Registry data.
Statistical Analysis
Prevalence, odds ratios (OR), and 95% confidence intervals (CI) were calculated for the risk of having congenital anomalies in children with hypothyroidism and in those without. Statistical analysis was done with SAS (Cary, North Carolina).
RESULTS
There were 980 children with congenital hypothyroidism and 3 661 585 children without congenital hypothyroidism born in New York State from 1992-2005 (Table I). Children with congenital hypothyroidism had a significantly increased risk of congenital anomalies, with all ORs achieving statistical significance with P < .0001 (Table II).
Table I.
Prevalence rates of congenital anomalies in congenital hypothyroidism (CHT) and in general population (Non-CHT)
| Congenital anomalies | CHT (RATE/10 000) | Non-CHT (RATE/10 000) |
|---|---|---|
| Renal | ||
| Dysplastic kidney | 30.6 | 1.7 |
| Renal agenesis | 102 | 4.3 |
| Ectopic kidney | 30.6 | 1.7 |
| Hydronephrosis | 346.9 | 21.1 |
| Hydroureter | 20.4 | 1.5 |
| UPJ obstruction | 30.6 | 1.9 |
| Reflux | 20.4 | 0.4 |
| Hypospadias | 275.5 | 39.6 |
| Obstruction meatus | 20.4 | 0.3 |
| Posterior urethral valves | 10.2 | 0.7 |
| Cardiovascular | ||
| Atrial septal defect | 622.4 | 29 |
| Ventricular septal defect | 602 | 36.6 |
| Coarctation of aorta | 81.6 | 4.1 |
| Tetralogy of Fallot | 183.7 | 4.6 |
| Endocardial cushion defect | 275.5 | 3.1 |
| Gastrointestinal | ||
| Duodenal atresia/stenosis | 51 | 1.6 |
| Gastroschisis | 10.2 | 1.4 |
| Omphalocele | 40.8 | 1.3 |
| Oral clefts | 91.3 | 12.9 |
| Pyloric stenosis | 40.8 | 17.1 |
| Tracheoesophageal fistula | 61.2 | 2.4 |
| Skeletal | ||
| Craniosynostosis | 51 | 4 |
| Congenital hip dysplasia | 30.6 | 1.7 |
| Limb reduction | 40.8 | 3.3 |
Table II.
Odds ratios of congenital anomalies with 95% confidence intervals in Congenital Malformation Registry data
| Congenital anomalies | CHT (n = 980) | Non-CHT (n = 3 661 585) | OR (95% CI) |
|---|---|---|---|
| Renal | |||
| Dysplastic kidney | 3 | 622 | 18.1 (5.8-56.3) |
| Renal agenesis | 10 | 1574 | 23.9 (12.8-44.8) |
| Ectopic kidney | 3 | 622 | 18.1 (5.8-56.3) |
| Hydronephrosis | 34 | 7726 | 16.9 (12.1-23.9) |
| Hydroureter | 2 | 549 | 13.6 (3.4-54.7) |
| UPJ obstruction | 3 | 696 | 16.2 (5.2-50.3) |
| Reflux | 2 | 146 | 51.1 (12.7-207.3) |
| Hypospadias | 27 | 14 499 | 7.1 (4.9-10.5) |
| Obstruction meatus | 2 | 110 | 68.1 (16.8-275.9) |
| Posterior urethral valves | 1 | 256 | 14.6 (2.0 to 104.2) |
| Composite | 87 | 26 800 | 13.2 (10.6-16.5) |
| Cardiovascular | |||
| Atrial septal defect | 61 | 10 619 | 22.8 (17.6-29.6) |
| Ventricular septal defect | 59 | 13 401 | 17.4 (13.4-22.7) |
| Coarctation of aorta | 8 | 1501 | 20.1 (9.9-40.3) |
| Tetralogy of Fallot | 18 | 1684 | 40.7 (25.4-64.9) |
| Endocardial cushion defect | 27 | 1135 | 91.4 (62.1-134.5) |
| Composite | 173 | 28 340 | 27.5 (23.3-32.4) |
| Gastrointestinal | |||
| Duodenal atresia/stenosis | 5 | 586 | 32.0 (13.3 to 77.4) |
| Gastroschisis | 1 | 513 | 7.3 (1.0-59.0) |
| Omphalocele | 4 | 476 | 31.5 (11.8-84.5) |
| Oral clefts | 9 | 4723 | 7.2 (3.7-13.8) |
| Pyloric stenosis | 4 | 6261 | 2.4 (0.9-6.4) |
| Tracheoesophageal fistula | 6 | 879 | 25.7 (11.5-57.4) |
| Composite | 29 | 13 438 | 8.3 (5.7-11.9) |
| Skeletal | |||
| Craniosynostosis | 5 | 1464 | 12.8 (5.3-30.9) |
| Congenital hip dysplasia | 3 | 622 | 18.1 (5.8-56.3) |
| Limb reduction | 4 | 1208 | 12.4 (4.6-33.2) |
| Composite | 12 | 3294 | 13.8 (7.8-24.4) |
Odds of having congenital renal and urologic anomalies were much higher in children with congenital hypothyroidism with OR of 13.2 (10.6-16.5). OR for hydronephrosis, hypospadias, and renal agenesis were especially significant as evidenced by the narrower confidence intervals. Composite ORs calculated for renal, cardiovascular, gastrointestinal, and skeletal anomalies were all highly significant, with P values < .0001 (Table II).
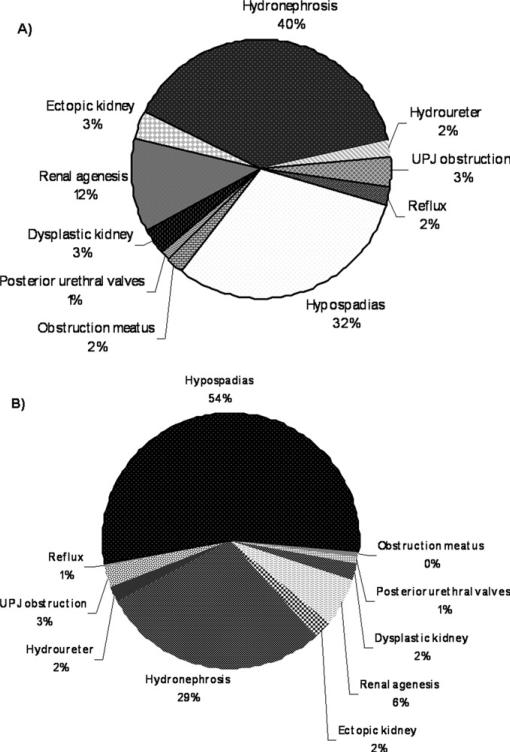
There were 1538 children with congenital hypothyroidism and 3 654 033 children without congenital hypothyroidism born in New York State from 1992 to 2005 registered in the New York State newborn screening database. A total of 418 of these (27%) matched to the Congenital Malformation Registry database. Data from the New York State newborn screening database was matched to the Congenital Malformation Registry data (Table III). Analysis of this data confirmed significantly increased prevalence of congenital renal and urological anomalies in congenital hypothyroidism with OR of 4.8(3.7-6.3). Hydronephrosis, UPJ obstruction, renal dysplasia and renal agenesis remained significantly increased in the matched data. There was, however, only 1 case of hypospadias and hydroureter in the matched data, resulting in nonsignificant OR. We observed differences in the distribution of congenital renal and urologic anomalies amongst congenital hypothyroidism and noncongenital hypothyroidism groups (Figure). Hydronephrosis was the major defect in congenital hypothyroidism population while hypospadias was most often seen in the general population. Of note, the renal and urologic anomalies seen in the congenital hypothyroid population are not discernible on a routine physical examination, but can easily be detected by a noninvasive renal ultra-sound. This is in contrast to hypospadias, which can be easily diagnosed on a routine physical examination.
Table III.
Odds ratios of congenital anomalies with 95% confidence intervals in data matched between Congenital Malformation Registry and New York State newborn screening database
| Congenital anomalies | CHT (n = 1538) | Non-CHT (n = 3 654 033) | OR (95% CI) |
|---|---|---|---|
| Renal | |||
| Dysplastic kidney | 10 | 629 | 38.0 (20.3-71.1) |
| Renal agenesis | 4 | 1631 | 5.8 (2.2-15.6) |
| Ectopic kidney | 2 | 632 | 7.5 (1.9-30.2) |
| Hydronephrosis | 30 | 8055 | 8.7 (6.0-12.6) |
| Hydroureter | 1 | 564 | 4.2 (0.6-29.9) |
| Atresia/Stenosis of ureter | 1 | 135 | 17.6 (2.5-126) |
| Hypospadias | 1 | 14 585 | 0.16 (0.02-1.2) |
| Anterior urethral obstruction | 1 | 31 | 76.7 (10.5-562.1) |
| UPJ obstruction | 4 | 691 | 13.8 (5.2-36.9) |
| Composite OR | 53 | 26 953 | 4.8 (3.7-6.3) |
| Cardiovascular | |||
| Atrial septal defect | 37 | 10 619 | 8.5 (6.1-11.7) |
| Ventricular septal defect | 44 | 13 401 | 8.0 (5.9-10.8) |
| Coarctation of aorta | 8 | 1501 | 15.9 (8.5-29.7) |
| Tetralogy of Fallot | 8 | 1684 | 11.3 (5.7-22.8) |
| Endocardial cushion defect | 10 | 1135 | 21.1 (11.3-39.3) |
| Composite OR | 109 | 28 340 | 9.8 (8.0-11.9) |
| Gastrointestinal | |||
| Duodenal atresia/stenosis | 4 | 586 | 16.1 (6.1-43.5) |
| Oral clefts | 4 | 4723 | 2.0 (0.8-5.4) |
| Pyloric stenosis | 3 | 6261 | 1.1 (0.4-3.5) |
| Composite OR | 11 | 11 570 | 2.3 (1.3-4.1) |
| Skeletal | |||
| Craniosynostosis | 1 | 1464 | 1.6 (0.2-11.5) |
Figure.
Congenital renal anomalies in A, CHT and B, non-CHT populations.
DISCUSSION
Our study shows significantly increased prevalence of specific congenital renal and urologic anomalies in congenital hypothyroidism. Although Cassio et al7 showed increased incidence of internal urogenital system malformations from 0.11% in noncongenital hypothyroidism to 0.43% in subjects with congenital hypothyroidism, these differences did not reach statistical significance, because they had only 1 child with congenital renal and urologic anomalies and the number of subjects with hypothyroidism were 235. Our findings of increased prevalence of gastrointestinal, cardiac, and skeletal anomalies among the congenital hypothyroidism group are consistent with other published reports.6-11
The strength of our study is that we used data from one of the largest population-based congenital malformation registries in the United States. About 80% of children in the Congenital Malformation Registry have only 1 malformation reported, but despite this we were able to demonstrate significant ORs. We further matched the data obtained from the newborn screening database to the Congenital Malformation Registry database, which confirmed the finding of increased odds of having congenital renal and urologic anomalies in children with congenital hypothyroidism.
This finding is biologically plausible, because recently there has been discovery of common genes involved in both thyroid and renal organogenesis. Pax 8 is one of these common genes expressed in the developing central nervous system and kidney, including the ureteric bud, mesonephric ducts, and the main collecting ducts. Mouse embryos that do not have Pax 8 and Pax 2 proteins fail to develop any pronephros. Pax 8 mutant mice show normal kidney development but die postnatally because of defective thyroid gland development. Bouchard et al20 used double mutant mice embryos to show that both Pax 2 and Pax 8 work cooperatively in the development of the kidney. Pax2+/–Pax 8+/–embryos had hypoplastic kidneys with decreased nephric tubules and glomeruli and increased stromal component. Pax2+/–Pax 8–/– embryos failed to develop a kidney, ureter, and genital tract.16
Another possible explanation for the multiple organ system malformations seen in children with congenital hypothyroidism could be that the thyroid hormones play an important role during early embryogenesis. However, to our knowledge there are no conclusive studies in human beings.
Limitations of our study include absence of any demographic data so we cannot make any observations about the prevalence on the basis of demographic variables. The Congenital Malformation Registry is compiled on the basis of hospital-generated data, so we may be underestimating the true prevalence of congenital renal and urologic anomalies. Another limitation of our study is that Congenital Malformation Registry is limited to children under 2 years of age, and, hence, we may be missing children who are diagnosed after that age. We do not know whether the cases of congenital hypothyroidism were transient or true hypothyroids. However, Oakley et al8 have reported an increased incidence of congenital malformations even with transient elevation in thyroid-stimulating hormone. Another potential limitation is that we do not have any information regarding the prenatal sonograms of the subjects in our study, and we do not know the timing and the reason for postnatal sonograms. Sensitivity of prenatal sonograms in detecting hydronephrosis is 80%, and because hydronephrosis can spontaneously resolve, we do not know whether cases reported to the Congenital Malformation Registry as having hydronephrosis were transient or persistent. On the other hand it is plausible that children who had abnormal renal ultrasounds were not admitted to the hospital and thus had not been reported to the Congenital Malformation Registry, in which case we may be underestimating the true prevalence of renal/urologic anomalies.
This is an observational study, and associations can be implied, but causality cannot be proven. Despite these limitations we show that children with congenital hypothyroidism are at increased risk of having associated renal and urologic anomalies. On the basis of our results, we recommend routine postnatal screening with renal ultrasound imaging to look for renal and urologic anomalies in children with congenital hypothyroidism. Since the most common types of congenital renal and urologic anomalies in congenital hypothyroidism, not identifiable on physical examination were hydronephrosis and renal dysplasia, early detection of these defects may prevent or delay the morbidity and mortality associated with end-stage kidney disease.
Acknowledgments
J.K. is supported by NIH/NIDDK-T32 DK007110-33. F.K. is supported by NIH/ NIDDK-T32 DK007110-33; U01 DK63549; U01 DK066174. R.W. is supported by NIH(K12).
Footnotes
The authors declare no potential conflicts of interest, real or perceived.
REFERENCES
- 1.Miniño AM, Heron MP, Murphy SL, Kochanek KD, Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004, Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Toublanc JE. Comparison of epidemiological data on congenital hypothyroidism in Europe with those of other parts of the world. Horm Res (Basel) 1992;38:230–5. doi: 10.1159/000182549. [DOI] [PubMed] [Google Scholar]
- 3.Harris KB, Pass KA. Increase in congenital hypothyroidism in New York State and in the United States. Molecular Genetics and Metabolism. 2007;91:268–77. doi: 10.1016/j.ymgme.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.National Newborn Screening & Genetics Resource Center ( genes-r-us.uthscsa.edu/)
- 5.Refetoff S, Dumont JE, Vassart G. Thyroid disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited diseases. McGraw Hill; New York: 2001. pp. 4029–76. [Google Scholar]
- 6.Roberts HE, Moore CA, Fernhoff PM, Brown AL, Khoury MJ. Population study of congenital hypothyroidism and associated birth defects, Atlanta, 1979-1992. Am J Med Genet. 1997;71:29–32. doi: 10.1002/(sici)1096-8628(19970711)71:1<29::aid-ajmg5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Cassio A, Tato L, Colli C, Spolletini E, Costantini E, Cacciari E. Incidence of congenital malformations in congenital hypothyroidism. Screening. 1994;3:125–30. [Google Scholar]
- 8.Oakley GA, Muir T, Ray M, Girdwood RWA, Kennedy R, Donaldson MDC. Increased incidence of congenital malformations in children with transient elevation of thyroid-stimulating hormone on neonatal screening. J Pediatr. 1998;132:726–30. doi: 10.1016/s0022-3476(98)70369-5. [DOI] [PubMed] [Google Scholar]
- 9.Fernhoff PM. Congenital hypothyroidism and associated birth defects: implications for investigators and clinicians. J Pediatr. 1998;132:573–4. doi: 10.1016/s0022-3476(98)70342-7. [DOI] [PubMed] [Google Scholar]
- 10.Kreisner E, Neto EC, Gross JL. High prevalence of extra thyroid malformations in a cohort of Brazilian patients with permanent primary congenital hypothyroidism. Thyroid. 2005;15:165–9. doi: 10.1089/thy.2005.15.165. [DOI] [PubMed] [Google Scholar]
- 11.Taji EA, Biebermann H, Límanová Z, Hníková O, Zikmund J, Dame C, et al. Screening for mutations in transcription factors in a Czech cohort of 170 patients with congenital and early-onset hypothyroidism: identification of a novel PAX8 mutation in dominantly inherited early-onset non-autoimmune hypothyroidism. Eur J Endocrinol. 2007;156:521–9. doi: 10.1530/EJE-06-0709. [DOI] [PubMed] [Google Scholar]
- 12.Satomura K, Michigami T, Yamamoto K, Hosokawa S. A case report of glomerulocystic kidney disease with hypothyroidism in a newborn infant. Nippon Jinzo Gakkai Shi. 1998;40:602–6. [Japanese] [PubMed] [Google Scholar]
- 13.Taha D, Barbar M, Kanaan H, Williamson BJ. Neonatal diabetes mellitus, congenital hypothyroidism, hepatic fibrosis, polycystic kidneys, and congenital glaucoma: a new autosomal recessive syndrome? Am J Med Genet A. 2003;22:269–73. doi: 10.1002/ajmg.a.20267. [DOI] [PubMed] [Google Scholar]
- 14.Jeha GS, Tatevian N, Heptulla RA. Congenital hypothyroidism in association with Caroli's disease and autosomal recessive polycystic kidney disease: patient report. J Pediatr Endocrinol Metab. 2005;18:315–8. doi: 10.1515/jpem.2005.18.3.315. [DOI] [PubMed] [Google Scholar]
- 15.United States Renal Data System [February 10, 2008];USRDS ADR Pediatrics. 2006 http://www.usrds.org/2006/pdf/08_peds_06.pdf.
- 16.Trueba SS, Auga J, Mattei G, Etchevers H, Martinovic J, Czernichow P, et al. PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. J Clin Endocrinol Metab. 2005;90:455–62. doi: 10.1210/jc.2004-1358. [DOI] [PubMed] [Google Scholar]
- 17.Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–51. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- 18.Krude H, Macchia PE, Di Lauro R, Grüters A. Familial hypothyroidism due to thyroid dysgenesis caused by dominant mutations of the PAX8 gene.. Proc of the 37th Annual Meeting of the European Society for Paediatric Endocrinology; Florence, Italy. 1998.p. 043. [Google Scholar]
- 19.Meeus L, Gilbert B, Rydlewski C, Parma J, Roussie AL, Abramowicz M, et al. Characterization of a novel loss of function mutation of PAX8 in a familial case of congenital hypothyroidism with in-place, normal-sized thyroid. J Clin Endocrinol Metab. 2000;89:4285–91. doi: 10.1210/jc.2004-0166. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–70. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and Pax8 Regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18:1121–9. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- 22.New York State Department of Health [May 15, 2007];Congenital Malformation Registry Annual Reports. http://www.health.state.ny.us/diseases/congenital_malformations.