Abstract
Objectives
Patients with ulcerative colitis (UC) who are in clinical remission may still have underlying endoscopic inflammation, which is associated with inferior clinical outcomes. The goal of this study was to determine the prevalence of, and factors associated with, active endoscopic disease in patients with UC who are in clinical remission.
Design
Prospective observational study in a single center. Patients with UC in clinical remission (by SCCAI) were enrolled prospectively at time of surveillance colonoscopy. Disease phenotype, endoscopic activity (Mayo sub-score) and histological score (Geboes) were recorded, and blood was drawn for peripheral blood biomarkers.
Results
149 patients in clinical remission were prospectively enrolled in this cohort; 81% had been in clinical remission for > 6 months, and 86% were currently prescribed maintenance medications. At endoscopy 45% of patients in clinical remission had any endoscopic inflammation (Mayo endoscopy sub-score >0) and 13% had scores >1. In a multivariate model, variables independently associated with a Mayo endoscopic score >1 were remission for < 6 months (p=.001), WBC (p=0.01) and CRP (p=0.009). A model combining these three variables had a sensitivity of 94% and a specificity of 73% for predicting moderate-severe endoscopic activity in patients in clinical remission (AUC 0.86). In an unselected sub-group of patients who had peripheral blood mononuclear cell mRNA profiling, GATA3 mRNA levels were significantly higher in patients with endoscopic activity.
Conclusions
Duration of clinical remission, WCC and CRP can predict the probability of on-going endoscopic activity despite clinical remission in patients with UC. These parameters could be used to identify patients who require intensification of treatment to achieve mucosal healing.
Keywords: Ulcerative colitis, disease activity, endoscopy, histology, CRP
Introduction
Ulcerative colitis is a chronic inflammatory condition, which can require clinic visits, hospitalizations and surgery due to complications from on-going intestinal inflammation (1;2). Historically, the primary goal of therapy was symptom resolution or control, and reduction of intestinal damage was a secondary aspiration. Agents such as prednisone have been very effective in improving patients’ symptoms, but less impressive in inducing healing of the intestinal mucosa (3). Routine evaluation to look for resolution of macroscopic inflammation for patients on maintenance therapy has not been part of standard clinical practice (4).
In recent years, the importance of achieving mucosal healing, rather than just symptom resolution, has been recognized. Longitudinal studies have reported that patients who achieve mucosal healing have lower rates of hospitalization, less need for immunosuppressive therapy, and a reduced risk of colectomy (3;5;6). In addition, the risk of colorectal neoplasia is higher in patients with UC and endoscopic evidence of colitis when compared to those who have a normal-appearing colon (7;8). Many of the maintenance agents used to treat UC have demonstrated an ability to induce mucosal healing, but rates of healing vary from 20-80% in clinical trials, often depending on the definition of “mucosal healing” used (9). In fact, persistent endoscopic inflammation is noted in many patients who are felt to be in “clinical remission” (10). These patients may be difficult to identify, as the correlation between clinical symptoms and endoscopic findings is variable (11). Although clinical scoring systems for UC may correlate with composite clinical / endoscopic scores in patients with moderate-severe symptoms, their performance in patients in clinical remission is unknown (12).
Given the importance of endoscopic healing in long-term outcomes with UC, and the limitations of using symptoms alone to screen for underlying macroscopic inflammation, identification of markers of intestinal inflammation are needed in patients in remission. Two studies have described the correlation between serum and fecal biomarkers in patients with endoscopically active and inactive UC, but both had few patients in clinical remission, or with mild endoscopic activity (13;14). The goal of this study was to enroll a cohort of patients with UC in clinical remission, to determine the prevalence of endoscopic colitis in these patients, and to identify surrogate markers that could predict this underlying endoscopic inflammation.
Methods
This was a prospective observational study performed at a single tertiary referral center. The study was approved for enrollment of human subjects by the local Institutional Review Board (protocol # 2009-P-000314/1). All patients with a confirmed history of ulcerative colitis who attended the endoscopy unit for a clinically-indicated surveillance colonoscopy were screened. All patients received the same bowel preparation (magnesium citrate). Clinical disease activity was determined using the Simple Clinical Colitis Activity Index (SCCAI), a validated score of colitis activity that has been shown to correlate well with endoscopic indices (15-17). In order to be considered “in remission” for enrollment in this study, participants had to have an SCCAI score <2.5 at the screening visit, and have had no changes in their UC medications or any steroid use in the prior month (15).
Each enrolled patient had baseline demographic and ulcerative colitis disease history recorded. This included disease location, duration, prior and current medication use, family history, extraintestinal disease, smoking status and NSAID use. During the index colonoscopy, endoscopic activity was classified using the sigmoidoscopy sub-score of the Mayo activity index, based on the most inflamed segment of the colon, if any (Suppl Table 1) (18). Histological activity in all segments was classified using the Geboes score, by a GI pathologist (JDG) blinded to endoscopic scores (19). A total Geboes score was assigned to biopsies from each colonic segment, and the highest score (most inflamed segment by histology) was used as the cumulative histology score for each patient.
A baseline blood sample was drawn for measurement of white blood count (WBC), hematocrit (Hct), erythrocyte sedimentation rate (ESR in mm/hr) and C-reactive protein (CRP in mg/L) in all patients. In an unselected sub-group of patients, peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll methods (Ficoll) for total RNA extraction (Qiagen RNAMini kit). 1 μg total RNA was reverse-transcribed to cDNA using the RT² First Strand Kit (Qiagen), and mixed with RT² SYBR Green Mastermix (Qiagen) for PCR reaction. PCR was performed on a RT² Profiler PCR Array (Th17 autoimmunity & inflammation array, SA Biosciences) and cycled on an Applied Biosystems cycler. This PCR array profiles the expression of 84 genes related to the Th17 regulatory network (http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-073A.html).
Dichotomous variables were analyzed for outcomes using χ2 test or Fisher’s exact test where appropriate, and continuous variables analyzed using t-test if normally distributed, or Wilcoxon test for non-normal data. Correction for multiple testing was included. The predictive model for endoscopic outcomes was build using forward stepwise logistic regression, with a p value of 0.1 required for entry. Data was analyzed with JMP 7.0 (SAS Institute Inc., North Carolina). Power calculations determined we had 80% power to detect a difference in CRP means of 2mg/L (STD 4mg/L) between groups, or a 3-fold difference in probability of endoscopic inflammation between groups compared by phenotypic features (e.g. gender). PS Power and sample size calculator was used (http://biostat.mc.vanderbilt.edu/PowerSampleSize).
For PCR array data analysis, an average ΔC(t) for each gene, and each gene within a group was calculated. ΔΔC(t) for each gene between groups determined, and 2(-ΔΔCt) used to measure fold-change.
Results
We prospectively recruited 149 patients who met our enrollment criteria (Figure 1). The baseline demographic, and phenotype characteristics of these patients are detailed in Table 1. As would be expected in a surveillance cohort, most patients (81%) had been in remission for greater than 6 months, and the majority (86%) was prescribed maintenance medication.
Figure 1.

Flow chart of patient numbers at enrollment and in each endoscopic group
UC; ulcerative colitis; WCC, white cell count; CRP, C-reactive protein; PBMC, peripheral blood mononuclear cells.
Table 1.
Baseline characteristics of enrolled cohort (N=149)
| Mean Age in yrs (SEM) | 37 (2) |
| Male (%) | 50 |
| Mean Disease Duration in yrs (SEM) | 21 (2) |
| Clinical Remission > 6 months (%) | 81 |
| Steroid use in last 12 months (%) | 13 |
| Disease geography | |
| Left-sided colitis (%) | 52 |
| Extensive colitis (%) | 48 |
| Current medications | |
| Mesalamine (%) | 76 |
| Azathioprine / mercaptopurine (%) | 18 |
| Infliximab (%) | 6 |
| Laboratory Markers (SEM) | |
| WCC (K/uL) | 6.3 (2) |
| Hematocrit (%) | 41 (0.4) |
| ESR (mm/hr) | 11 (1) |
| CRP (mg/L) | 3.6 (0.4) |
At the enrollment colonoscopy, endoscopic activity (a Mayo endoscopic sub-score of at least 1) was recorded in 67/149 (45%) of the cohort; 32% had a score of 1, 12% had a score of 2, and 1% had a score of 3 (Table 2). A sub-set of 15 patients had their endoscopy images re-scored by a second blinded endoscopist to determine the interobserver agreement for the endoscopic score. The kappa statistic was 0.7, suggesting substantial agreement between endoscopists (20).
Table 2.
Comparative characteristics of patients with evidence of any endoscopic inflammation (Mayo sub-score > 0, n=67), and those with no endoscopic inflammation (n=82)
| Variable | Endo. Active | Endo. Normal |
|---|---|---|
| Mean Age in yrs (SEM) | 48 (2) | 54 (2) |
| Male (%) | 49 | 50 |
| Mean Disease Duration in yrs (SEM) | 20 (3) | 22 (3) |
| Clinical Remission > 6 months (%) | 69 | 90 |
| Steroid use in last 12 months (%) | 21 | 6 |
| Disease geography | ||
| Left-sided colitis (%) | 51 | 54 |
| Extensive colitis (%) | 49 | 40 |
| Current medications | ||
| Mesalamine (%) | 82 | 72 |
| Azathioprine / mercaptopurine (%) | 21 | 16 |
| Infliximab (%) | 9 | 4 |
| Laboratory Markers (SEM) | ||
| WCC (K/uL) | 6.8 (0.2) | 5.9 (0.2) |
| Hematocrit (%) | 41 (0.4) | 41 (0.4) |
| ESR (mm/hr) | 11 (1) | 11 (1) |
| CRP (mg/L) | 4.8 (0.4) | 2.4 (0.4) |
| Mean Histological score | 1.7 (0.2) | 1.6 (0.2) |
Representative endoscopic images of patients with each Mayo endoscopy sub-score from this study are shown in Supplementary Figure 1. The mean CRP was 4.8mg/L (median 2.2mg/L) in those patients with any endoscopic inflammation (score > 0), and 7.3mg/L (median 3.2mg/L) in those with a Mayo score >1. In comparison, patients with a normal-appearing colon had a mean CRP of 2.4mg/L (median 1.3mg/L). The mean Geboes histological score was 1.7 (SEM 0.3) in those with endoscopic inflammation; histological scores ranged from 0 (no structural abnormalities) to 5.4 (ulcer or granulation tissue) in this patient group.
In order to determine which phenotype or treatment variables were associated with any underlying endoscopic inflammation, an initial univariate analysis was performed for associations (p<0.1) with endoscopic score > 0 (Suppl. Table 2). In this screening phase, patients in clinical remission for > 6 months had a reduced risk of current endoscopic activity (RR 0.6, 95% CI 0.4, 0.7, p=0.001). In contrast, patients who required steroids in the preceding 12 months had a higher risk of any current endoscopic inflammation (RR 1.8, 95% CI 1.3, 2.6, p=0.01). White blood count and CRP were also significantly different between those with or without any endoscopic inflammation (p=0.03, and 0.02 respectively by t-test).
In a multi-variate nominal logistic regression model that included these variables, clinical remission for < 6 months and CRP level remained independently associated with presence of any endoscopic activity (p=0.0002 for whole model). Combining remission status and CRP level in the model yielded a sensitivity of 50% and specificity of 85% to detect any underlying endoscopic activity in patients in clinical remission (AUC 0.68 for model, Figure 2). CRP alone only provided an optimal sensitivity of 66% and specificity of 58% at a cut-off of 1.6mg/L (AUC 0.62).
Figure 2.
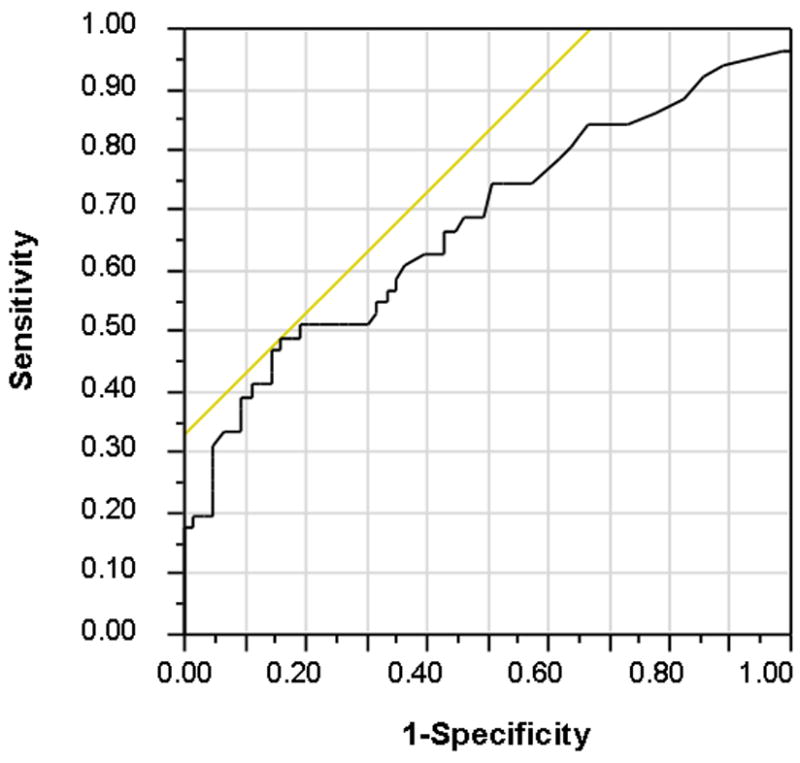
Receiver operating characteristics curve for combined model of remission duration and CRP in identifying patients with any endoscopic inflammation (Mayo sub-score > 0)
Since the criteria for mucosal healing in UC clinical trials usually only require improvement in endoscopic appearance to a Mayo sub-score of <2, we also analyzed how a combined model would perform in identifying patients with moderate-severe endoscopic activity (21). In a multivariate model, variables independently associated with a Mayo endoscopic score >1 were remission for < 6 months (p=.001), WBC (p=0.01) and CRP (p=0.009). A model combining these three variables had a sensitivity of 94% and a specificity of 73% for predicting moderate-severe endoscopic activity in these patients. The receiver operating characteristic curve of the combined model had an AUC of 0.86 (Figure 3).
Figure 3.
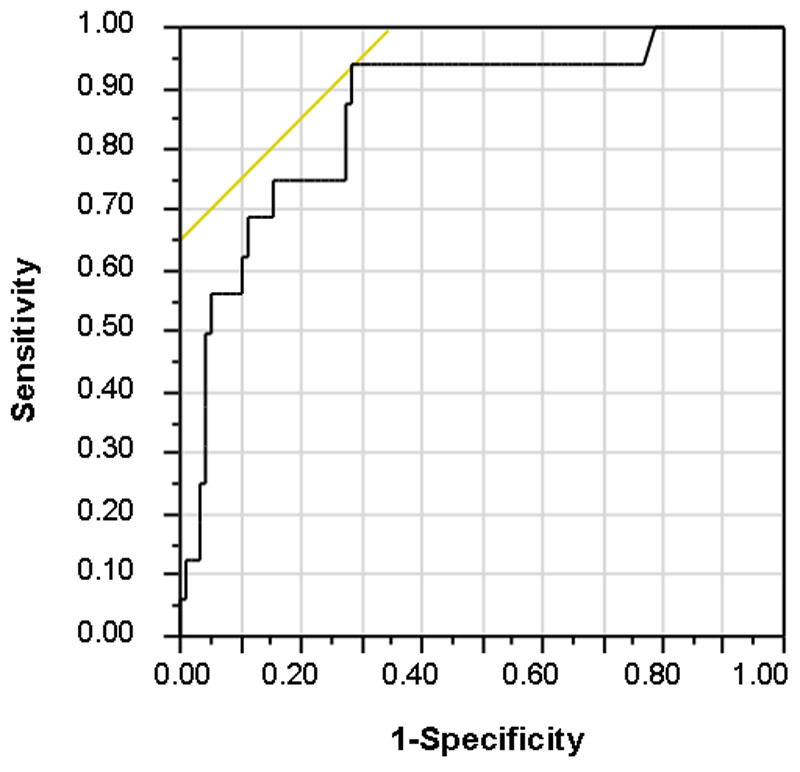
Receiver operating characteristics curve for combined model of remission duration, WCB and CRP in identifying patients with endoscopic inflammation (Mayo sub-score >1)
CRP alone, at a cut-off of 2.2mg/L, had a sensitivity of 82% and specificity of 65% for detection of moderate-severe endoscopic activity (AUC 0.76). Since screening for endoscopic inflammation is the primary goal of any peripheral biomarker, a lower CRP cut-off of 1.2mg/L would provide a sensitivity of 90% for endoscopic inflammation in this population, at the expense of lower specificity (40%). Conversely, 95% of patients with a normal colon had a CRP less than 6.7mg/L in this study.
Finally, we sought to determine whether the gene expression profile of peripheral blood mononuclear cells could identify patients with underlying endoscopic inflammation. A PCR array containing probes for 81 genes involved in systemic inflammatory pathways was utilized in 12 patients; 6 with endoscopic activity (Mayo score >1) and 6 with normal endoscopic appearance. As can be seen in Figure 4, a number of genes were differentially expressed between patients with endoscopic inflammation, compared to patients with mucosal healing. However, only GATA-3 expression was significantly different between both groups, with a 1.6-fold increase in expression of GATA-3 by peripheral blood mononuclear cells in patients with endoscopic inflammation (p=0.04).
Figure 4.

Heat map and fold change expression of RNA of named genes in PBMC samples from patients with normal-appearing colon (0), and those with endoscopic inflammation (Mayo score>1). Red cells indicate relatively up-regulated genes, green cells represent relatively down-regulated genes.
Discussion
In recent years there has been much focus on the importance of mucosal healing in patients with IBD, and the detrimental effects of chronic intestinal inflammation (9;22). Although much of the evidence to support mucosal healing is evolving, ensuring our patients achieve this outcome makes therapeutic sense. In patients who do not respond clinically to standard treatments for UC, re-evaluation with sigmoidoscopy or colonoscopy is common practice (12). However, in patients who achieve clinical remission, confirmation of endoscopic healing is not routinely performed beyond surveillance colonoscopy (4). Whether this approach misses patients with on-going endoscopic inflammation is unclear.
This study provides novel information on this issue, with evidence that almost half (45%) of patients who meet standard criteria for “clinical remission” have on-going endoscopic inflammation. Although only 13% exhibited moderate-severe endoscopic inflammation at colonoscopy, this still represents a significant cohort of patients who have not achieved the optimal clinical outcome, even though most (86%) were prescribed appropriate therapies. Recent data has reported a lack of mucosal healing in 62% of patients with UC after a course of steroids, and these patients had a higher risk of negative clinical outcomes, such as hospitalizations, immunosuppressant use, and colectomy (3). Indirect data from other groups has also associated the lack of mucosal healing with adverse clinical outcomes (6). Whether patients such as in our study, in clinical remission but with mild-moderate endoscopic activity, also have a higher risk of adverse outcomes is unknown, although further follow-up of this complete inception cohort over time will provide some data in this regard. The population of patients with persistent endoscopic activity also raises the question of whether we should be treating patients with UC primarily to achieve mucosal healing, irrespective of their lack of clinical symptoms. Prospective trials to address this topic are awaited, and likely to inform regulatory approval and third party cost coverage in the future.
Given the potential importance of identifying patients with underlying endoscopic inflammation despite clinical remission, we have identified clinical and biochemical features that confer a higher risk of on-going macroscopic colitis. Other groups have used serum and fecal biomarkers to correlate with endoscopic activity, but these studies contained few patients in clinical remission. Solem et al. demonstrated that CRP correlates with endoscopic scores, but this study contained only included 5 patients in clinical remission (14). Schoepfer et al. reported the correlation between fecal calprotectin and CRP and endoscopic scores in a group that included 26 patients with UC in clinical remission (13). In our large cohort of patients in clinical remission, the combination of duration of remission (< 6 months), WBC and CRP level demonstrated a sensitivity of 94% and a specificity of 73% to detect moderate-severe endoscopic activity. This predictive model is certainly a less costly approach than performing sigmoidoscopy / colonoscopy in all patients in clinical remission to assess for endoscopic activity, but would require validation in a separate (validation) cohort to confirm its test characteristics. Combining clinical history, WBC and CRP could allow clinicians to reserve follow-up sigmoidoscopy for patients in clinical remission at higher risk of endoscopic colitis.
The small differences in gene expression seen in PBMCs between patients with normal colons and those with endoscopic inflammation is not entirely surprising, given the small differences in gene expression, and levels of cytokines, seen peripherally even in patients with severe clinical and endoscopic colitis (23;24). The differential expression of GATA3 in the PBMCs of patients with endoscopic colitis is consistent with its role as a transcriptional regulator of type-2 helper T-cells (Th2), which are important in the pathogenesis of UC (25-27). It is likely that the underlying colitis in these patients activates Th2 pathways in circulating PBMCs via GATA3. Further validation of this finding in a larger cohort of patients will be required.
Limitations of this study are the small proportion of patients with moderate-severe endoscopic inflammation (13%), so variables that occur at small frequencies but differ between groups may have been subject to a type II error. Assuming similar rates of Mayo scores >1 in the general population of patients with UC who undergo surveillance, one would need to enroll a cohort of ~700 surveillance patients to detect difference between variables that occur at frequencies of less than 20%. The study is strengthened by the prospective enrollment and standardized scoring of all patients, and comprehensive clinical phenotypes. The definition of clinical remission used is based on a valid, reliable and responsive noninvasive measure to assess disease activity in adults with UC (15;16). We focused on blood-based peripheral biomarkers of endoscopic activity, but did not include validated stool-based markers, such as calprotectin. Finally, we did not measure medication adherence directly, but prior studies from our patient population have reported 70-80% of mesalamine prescriptions are refilled over 6 months (28).
In conclusion, endoscopic inflammation is frequent in patients with UC who are in clinical remission. Duration of remission and CRP level may allow clinicians to predict which patients may benefit from assessment of endoscopic activity to ensure mucosal healing.
Supplementary Material
Representative endoscopic images from this study of endoscopic appearance of patients graded as Mayo sub-score 0-3
Acknowledgments
ACM is supported by NIH grant K23DK084338 and the generosity of Doris Toby Axelrod & Lawrence J. Marks. The study design, implementation and analysis was independent of the funding sources.
We acknowledge the assistance of the endoscopy nurses in enrolling patients and obtaining samples for this study.
Footnotes
Contributions:
LR - study design, acquisition of data, drafting of manuscript
GOL, TZ, AG, JDG, KF, JW, SCR, AC – acquisition of data, critical review of manuscript
ACM - study design, analysis & interpretation of data, statistical analysis, drafting of manuscript, study supervision.
Disclosures:
No conflicts of interest relevant to this manuscript for any authors
Reference List
- 1.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135(6):1907–13. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9(6):483–9. doi: 10.1016/j.cgh.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2(3):219–25. doi: 10.1016/j.crohns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Froslie KF, Jahnsen J, Moum BA, et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133(2):412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 7.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126(2):451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53(12):1813–6. doi: 10.1136/gut.2003.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineton dC, Peyrin-Biroulet L, Lemann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7(1):15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 10.Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21925. [DOI] [PubMed] [Google Scholar]
- 11.Gomes P, du BC, Smith CL, et al. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986;27(1):92–5. doi: 10.1136/gut.27.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhanda AD, Creed TJ, Greenwood R, et al. Can endoscopy be avoided in the assessment of ulcerative colitis in clinical trials? Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22879. [DOI] [PubMed] [Google Scholar]
- 13.Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851–8. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 14.Solem CA, Loftus EV, Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54(6):782–8. doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner D, Seow CH, Greenberg GR, et al. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(10):1081–8. doi: 10.1016/j.cgh.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 19.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–9. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3. [PubMed] [Google Scholar]
- 21.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DT. We once were blind and now we see: is it time to treat ulcerative colitis to achieve mucosal healing? Clin Gastroenterol Hepatol. 2011;9(6):456–7. doi: 10.1016/j.cgh.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Kabakchiev B, Turner D, Hyams J, et al. Gene expression changes associated with resistance to intravenous corticosteroid therapy in children with severe ulcerative colitis. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Perlvarez ML, Garcia-Sanchez V, Villar-Pastor CM, et al. Role of serum cytokine profile in ulcerative colitis assessment. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22865. [DOI] [PubMed] [Google Scholar]
- 25.Hosoya T, Maillard I, Engel JD. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev. 2010;238(1):110–25. doi: 10.1111/j.1600-065X.2010.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101(3):428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtani K, Ohtsuka Y, Ikuse T, et al. Increased mucosal expression of GATA-3 and STAT-4 in pediatric ulcerative colitis. Pediatr Int. 2010;52(4):584–9. doi: 10.1111/j.1442-200X.2009.03019.x. [DOI] [PubMed] [Google Scholar]
- 28.Moss AC, Chaudhary N, Tukey M, et al. Impact of a patient-support program on mesalamine adherence in patients with ulcerative colitis--a prospective study. J Crohns Colitis. 2010;4(2):171–5. doi: 10.1016/j.crohns.2009.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative endoscopic images from this study of endoscopic appearance of patients graded as Mayo sub-score 0-3


