Abstract
In animals circadian behavior can be analyzed as an integrated system - beginning with genes leading ultimately to behavioral outputs. In the last decade, the molecular mechanism of circadian clocks has been unraveled primarily by the use of phenotype-driven (forward) genetic analysis in a number of model systems. Circadian oscillations are generated by a set of genes forming a transcriptional autoregulatory feedback loop. In mammals, there is a “core” set of circadian genes that form the primary negative feedback loop of the clock mechanism (Clock/Npas2, Bmal1, Per1, Per2, Cry1, Cry2 and CK1ε). Another dozen candidate genes have been identified and play additional roles in the circadian gene network such as the feedback loop involving Rev-erbα. Despite this remarkable progress, it is clear that a significant number of genes that strongly influence and regulate circadian rhythms in mammals remain to be discovered and identified. As part of a large-scale N-ethyl-N-nitrosourea (ENU) mutagenesis screen using a wide range of nervous system and behavioral phenotypes, we have identified a number of new circadian mutants in mice. Here we describe a new short period circadian mutant, part-time (prtm), which is caused by a loss-of-function mutation in the Cryptochrome1 gene. We also describe a long period circadian mutant named Overtime (Ovtm). Positional cloning and genetic complementation reveal that Ovtm is encoded by the F-box protein FBXL3 a component of the SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligase complex. The Ovtm mutation causes an isoleucine to threonine (I364T) substitution leading to a loss-of-function in FBXL3 which interacts specifically with the CRYPTOCHROME (CRY) proteins. In Ovtm mice, expression of the PERIOD proteins PER1 and PER2 is reduced; however, the CRY proteins CRY1 and CRY2 are unchanged. The loss of FBXL3 function leads to a stabilization of the CRY proteins, which in turn leads to a global transcriptional repression of the Per and Cry genes. Thus, Fbxl3Ovtm defines a molecular link between CRY turnover and CLOCK/BMAL1-dependent circadian transcription to modulate circadian period.
The mechanism of circadian oscillators in mammals is generated by a cell autonomous autoregulatory transcription-translation feedback loop (Reppert and Weaver 2002; Lowrey and Takahashi 2004; Ko and Takahashi 2006). In the primary negative feedback loop, the bHLH-PAS transcription factors, CLOCK (and its paralog NPAS2) and BMAL1 (ARNTL) dimerize and activate transcription of the Period (Per1, Per2) and Cryptochrome (Cry1, Cry2) genes (Antoch et al. 1997; King et al. 1997; Gekakis et al. 1998; Kume et al. 1999; Bunger et al. 2000; DeBruyne et al. 2007). As the PER proteins accumulate, they form complexes with the CRY proteins, translocate into the nucleus, and interact with the CLOCK/BMAL1 complex to inhibit their own transcription (Lee et al. 2001). This leads to a fall in the inhibitory complex through turnover, and the cycle starts again with a new round of CLOCK/BMAL1-activated transcription. Additional pathways in the circadian gene network such as the second negative feedback loop (involving Rev-erbα) in the positive limb of the oscillator are thought to add robustness to the circadian mechanism (Preitner et al. 2002; Sato et al. 2004). Finally, post-translational modifications play critical roles in regulating the turnover, cellular localization and activity of circadian clock proteins (Lowrey et al. 2000; Eide et al. 2005; Gallego and Virshup 2007).
Despite this progress, it is clear that a significant number of genes that strongly influence and regulate circadian rhythms in mammals remain to be discovered and identified (Shimomura et al. 2001; Takahashi 2004). Forward genetic screens have been one of the most effective tools for circadian gene discovery (Takahashi et al. 1994; Vitaterna et al. 1994; Takahashi 2004), and we have used this approach to screen the mouse genome for circadian rhythm mutants generated in the Neurogenomics Project in the Center for Functional Genomics at Northwestern University (Vitaterna et al. 2006).
Mutagenesis, screening and identification of the part-time and Overtime genes
In an N-ethyl-N-nitrosourea (ENU) recessive screen using the BTBR T+ tf/J (BTBR/J) inbred mouse strain (Siepka and Takahashi 2005), we identified two mutants with short (21.4-h) and long (25.8-h) circadian periods in constant darkness (Figure 1). These two mutants were named part-time (prtm) and Overtime (Ovtm) (Siepka et al. 2007), respectively. Genetic mapping of prtm places this mutant on chromosome 10 in the region of Cry1 (Figure 2A). Complementation tests of prtm with a Cry1 null allele show that prtm is a new allele of Cry1 (Figure 2B). Sequencing of the Cry1 gene in prtm mutants reveals a T to C mutation in the second position of the splice donor site of exon 2 causing readthrough and premature termination in intron 2 (Figure 2C). Crosses of prtm with Cry2 null mutants to produce double homozygous mutants shows that these mice are arrhythmic similar to that seen in Cry1/Cry2 double mutant mice (Vitaterna et al. 1999). Thus, prtm is a loss-of-function allele of Cry1 and serves as a validation of the genetic screen.
Figure 1. ENU mutagenesis screen.
(A) Mutant mouse production. Male BTBR/J mice were treated with the chemical mutagen N-ethyl-Nnitrosourea (ENU) (1 × 250 mg/kg) and G1 offspring were used to breed 3-generation pedigrees to make ENU-induced mutations homozygous as described previously (Siepka and Takahashi 2005).
(B) Histogram distribution of freerunning period values for 3198 mice screened. The grey shaded area represents + 3 standard deviations (SD) from the mean. The original part-time and Overtime mutants are indicated by arrows.
(C) Representative actogram of a wild-type BTBR/J mouse. The actogram is double plotted where 48 hrs of activity are represented on each horizontal line. The mice were kept on a LD12:12 cycle (represented by the bar above) for the first 7 days and then released into constant darkness (DD) for 21 days (indicated by the arrowhead on the right).
(D) Actogram of the original prtm G3 mouse. The animal (a prtm homozygote) had a free running period of 21.4 h.
(E) Actogram of the original Ovtm G3 mouse. The animal (an Ovtm homozygote) had a free running period of 25.83 hr.
Figure 2. Genetic mapping and cloning of the part-time mutant.
(A) Genetic mapping of prtm to chromosome 10
(B) The prtm mutation occurs within the splice donor site at the 3′ end of Exon 2 of Cry1.
(C) Complementation test for prtm and Cry1. The left panel outlines the mating scheme for the complementation test. prtm mice were crossed to heterozygous Cry1 knock out (mCry1-KO) mice. Circadian behavior was recorded for 15 progeny. The actograms are representative of prtm/+ (top) and Cry-1KO/prtm (bottom) mice. In the period histogram distribution, black bars represent Cry1-KO/prtm mice (mean 22.15 hr; SD = 0.31; N = 8), while white bars represent prtm/+ mice (mean = 23.4 hr; SD = 0.23; N = 7). Student’s t-test (unequal variances, shows a significant difference between the two populations (DF = 13; T = −9.12; p = 5.2 × 10−7).
(D) Representative actogram of a prtm/prtm, Cry2 −/− double mutant mouse showing an arrhythmic phenotype similar to that seen with Cry1 −/−, Cry2 −/− double mutant mice.
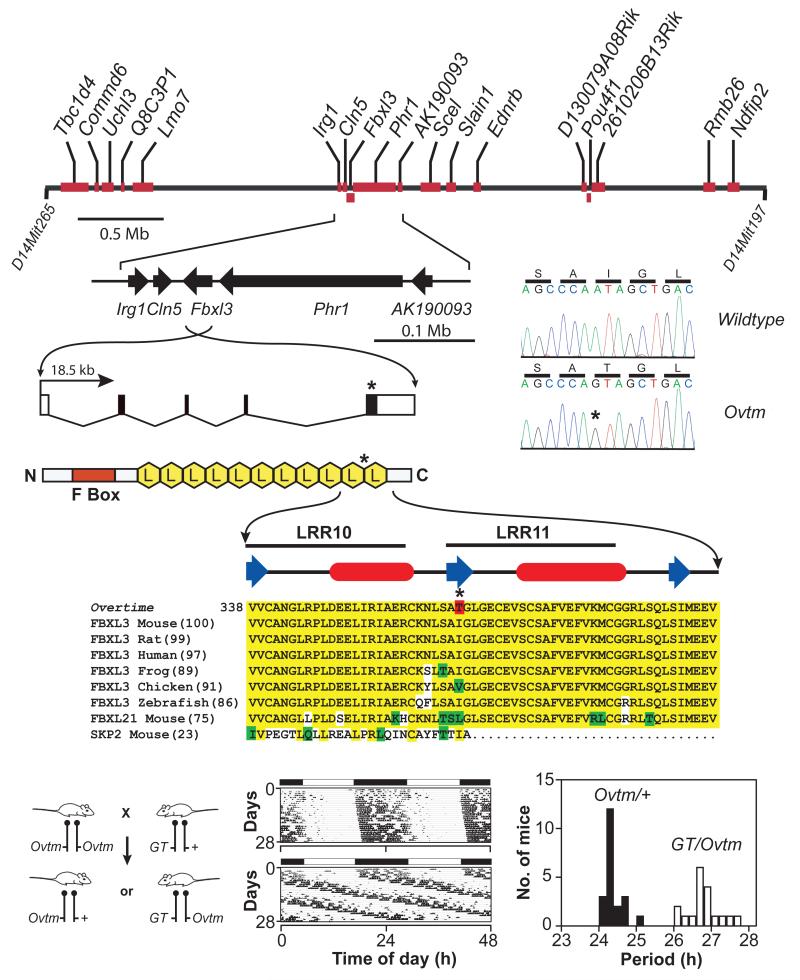
The second mutant, Ovtm maps to a 1.7 cM interval on chromosome 14 (Figure 3) (Siepka et al. 2007). This region corresponds to a 4 Mb interval and contains 18 open reading frames, none of which corresponds to previously known circadian clock genes (Lowrey and Takahashi 2004) (Figure 4A). We sequenced all annotated exons for the 18 candidate genes in the Ovtm interval and found only a single nonsynonymous point mutation within the coding region of Fbxl3. There is a single base transition from A to G in exon 5 of Fbxl3 in Ovtm mice as compared to wildtype BTBR/J mice. This mutation co-segregated perfectly with the long-period phenotype of Ovtm/Ovtm mice. The point mutation converts amino acid residue 364 from isoleucine to threonine in FBXL3 (Figure 4A, B). This isoleucine residue is highly conserved in FBXL3 from vertebrates and in the mouse paralog FBXL21 (Figure 4B). FBXL3 is a member of the F-box protein family with leucine rich repeats (LRR) which is defined by its founding member, SKP2 (S-phase kinase-associated protein-2) (FBXL1) (Jin et al. 2004). SKP2 is the F-box protein moiety in the SKP1-CUL1-F-box-protein (SCF)SKP2 E3 ubiquitin ligase complex which mediates the recognition and ubiquitination of the CDK2 inhibitor, p27Kip1, to target it for proteasomal degradation (Cardozo and Pagano 2004). FBXL3 which has 11 LRRs can be aligned with SKP2 which has 10 LLRs based on its protein structure; however the C-termini of SKP2 and FBXL3 are not conserved likely due to the recognition of different substrates (Hao et al. 2005). The Ovtm I364T mutation occurs in the C-terminus of FBXL3 between LRR10 and LRR11 where the alignment with SKP2 becomes divergent (Figure 4B). Because the LRR domains of F-box proteins are involved with substrate recognition with the SCF complex, we hypothesized that the I364T mutation could alter the interaction of FBXL3 with its substrates.
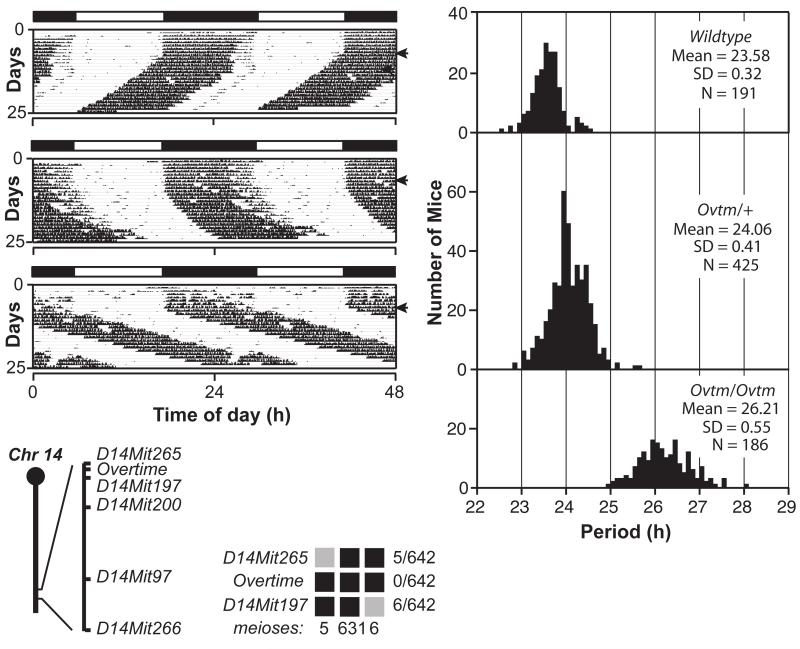
Figure 3. Semidominant phenotype of Overtime and genetic mapping.
(A) Representative actograms of wildtype, (B) Ovtm/+ and (C) Ovtm/Ovtm [BTBR/J × C57BL/6J] F2 mice. The actograms are plotted as described in Figure 1.
(D) Period distribution of F2 intercross progeny. The three panels from top to bottom represent wildtype, Ovtm/+ and Ovtm/Ovtm mice, respectively.
(E) Ovtm maps between D14Mit265 and D14Mit197 on chromosome 14. Haplotypes of the 321 Ovtm/Ovtm F2 intercross progeny (642 meioses) are on the right. Black boxes represent BTBR/J alleles, while grey boxes represent heterozygous alleles (BTBR/J and C57BL/6J). The number of recombinants per total meioses is indicated to the right of the haplotype map. From Siepka et al. (Siepka et al. 2007).
Figure 4. Positional cloning of Overtime and identification of Fbxl3 mutation.
(A) Physical map of the Ovtm interval. Ovtm maps to a 4 Mb region of chromosome 14. Red blocks represent the eighteen candidate genes within the interval. The asterisks indicate the location of the Ovtm mutation.
(B) FBXL3 is an F-box protein. FBXL3 contains one F-box domain (red box) and 11 leucine rich regions (LRR) (yellow hexagons) (Jin et al. 2004). b-strand (blue arrow) and a-helical (red ovals) regions (based on analysis using PROF in the PredictProtein server (Rost et al. 2004)) of LRR10 and LRR11 are indicated above the sequence. In the protein alignment, yellow indicates amino acid identity, green indicates conservative substitutions, white indicates non-conservative substitutions, and red indicates the I364T Ovtm mutation.
(C) Complementation test for Ovtm and Fbxl3. The left panel outlines the mating scheme for the complementation assay. Ovtm mice were crossed to heterozygous Fbxl3 gene trap (GT) mice. The actograms are representative of Ovtm/+ (top) and GT/Ovtm (bottom) mice. In the period histogram distribution black bars represent Ovtm/+ mice, while white bars represent GT/Ovtm mice. From Siepka et al. (Siepka et al. 2007).
Because we isolated only one mutant allele of Fbxl3 and either a second independent allele, rescue, or functional evidence is required for proof in positional cloning (Takahashi et al. 1994), we used genetic complementation tests to confirm that Ovtm was allelic with Fbxl3. The crosses show that Ovtm and an Fbxl3 gene trap (GT) fail to complement each other, thus providing independent and definitive evidence that Ovtm is an allele of Fbxl3 (Figure 4C). Interestingly, the period length of GT/Ovtm mice is indistinguishable from Ovtm homozygotes suggesting that the Ovtm mutant allele is likely a hypomorphic or loss-of-function allele.
Effects of the Overtime mutation on circadian clock gene expression
Because FBXL3 is likely a component of an SCF E3 ubiquitin ligase complex, we examined the in vivo expression patterns of circadian clock proteins in mouse tissues to explore whether Ovtm might alter their abundance by affecting degradation. Figure 5A shows the expression patterns of the clock proteins, CRY1, CRY2, PER1, PER2, CLOCK and BMAL1 in liver and cerebellum. In WT mice, there were low amplitude rhythms of CRY1 and CRY2 and high amplitude rhythms of PER1 and PER2 as reported previously (Lee et al. 2001). In Ovtm liver tissue, CRY1 and CRY2 protein patterns were not significantly altered; however, PER1 and PER2 levels were significantly reduced (Figure 5B). In the cerebellum, the effects of Ovtm were more striking. Although CRY1 levels were not different, CRY2 levels were significantly elevated in Ovtm mice consistent with the hypothesis that CRY degradation is impaired. In addition, there were very clear reductions in the levels of PER1 and PER2. The reduction of PER1 and PER2 levels in Ovtm mice is unexpected and counterintuitive. We would have expected to see an increase rather than a decrease in protein abundance if the PER proteins were targets of FBXL3 because the Ovtm mutation is a loss-of-function mutation. This suggests that it is unlikely that the PER proteins are targets of FBXL3 and that the reduction in PER levels could occur as a consequence of the negative feedback on CLOCK/BMAL1-dependent transcription.
Figure 5. Altered circadian clock gene expression in Overtime mice.
(A) Protein oscillation profiles of clock genes from liver and cerebellum. Wildtype and Ovtm mutant tissue was collected at indicated circadian times. Western blotting was performed on total protein extracts with indicated antibodies.
(B) Quantification of proteins from liver and cerebellum. Filled circles with solid line represent normalized values from wildtype, and open circles with dotted line represent normalized values from Ovtm mice.
(C) Real time RT-PCR analysis for clock gene expression in wildtype and Ovtm mice. Filled circles with solid line represent wildtype, and open circles with dotted line represent values from Ovtm mice. All cycling genes show a significant reduction of mRNA level from Ovtm mice compared to WT mice in liver and cerebellum except for Per2 in liver. From Siepka et al. (Siepka et al. 2007).
To explore the reasons for the reduction in PER1 and PER2 protein abundance, we profiled the in vivo circadian mRNA expression patterns for Cry1, Cry2, Per1, Per2 and Dbp in the liver and cerebellum of mice maintained in DD. As shown in Figure 5C, the Ovtm mutation caused significant reductions in the mRNA abundance of all of these cycling transcripts with the strongest effects being seen with Cry1 and Per2 in the cerebellum. At the mRNA level, both a delay in the peak time and a reduction in abundance can be seen. Importantly, although CRY1 and CRY2 protein levels were not lower in Ovtm mice, the corresponding mRNA levels for Cry1 and Cry2 are significantly reduced in both tissues. In addition, mRNA levels for the cycling CLOCK target gene, Dbp (Ripperger and Schibler 2006), were very strongly reduced in Ovtm mice. Thus, the mRNA profiling experiments point to an interesting and unexpected consequence of the Ovtm mutation: a reduction in steady-state mRNA expression of Cry1, Cry2, Per1, Per2 and Dbp which are all transcriptional targets of the CLOCK/BMAL1 complex (Gekakis et al. 1998; Kume et al. 1999; Yoo et al. 2005; Ripperger and Schibler 2006).
Comparison of the effects of Ovtm on protein vs. mRNA abundance suggests that there are two different effects on the expression of the CRY and PER proteins. The PER protein levels appear to be reduced as a consequence of reduced transcript levels. By contrast, the CRY protein levels are not reduced even in the face of reduced transcript levels. This suggests that potential reductions in CRY protein levels caused by reduced Cry transcript levels could be compensated by a reduction in protein degradation.
Interaction of OVTM with circadian clock proteins
The Pagano laboratory has found that FBXL3 targets CRY proteins for ubiquitination and degradation (Busino et al. 2007). To confirm these results and determine whether the Ovtm mutation affects interactions with CRY, we examined the interaction of FBXL3 or OVTM with circadian clock proteins by immunoprecipitation assays. Both FBXL3 and OVTM interacted strongly with native CRY1 and CRY2 proteins. Very weak or no interaction of FBXL3 was seen with PER1 and PER2, especially in comparison to that seen between the PERs and βTrCP1, an F-box protein known to interact with the PERs (Eide et al. 2005; Shirogane et al. 2005) (Figure 6A). In all experiments, there was a discernibly stronger interaction of the CRY proteins with FBXL3 relative to OVTM, but the difference was subtle.
Figure 6. Interaction of FBXL3 and OVTM with circadian clock proteins.
(A) NIH 3T3 cells were transfected with FLAG-Fbxl3, FLAG-Ovtm and V5-βTrcp1. Immunoprecipitation was performed with anti-Flag or anti-V5 antibody. Native immunoprecipitated proteins were further analyzed by Western blotting with anti-PER or anti-CRY antibodies.
(B) Confirmation of interaction between FBXL3 with CRY and PER2. Top panel, 293A cells were transfected with Cry-HA and FLAG-Fbxl3, FLAG-Ovtm. F+O is co-transfection of FLAG-Fbxl3, FLAG-Ovtm. Open arrowheads indicate CRY, filled arrow heads indicate FBXL3 or OVTM. Lower panel, Per-V5 were co-transfected with FLAG-Fbxl3, FLAG-Ovtm. Open arrowheads indicate immunoprecipitated FBXL3 or OVTM, filled arrowheads indicate FBXL3 or OVTM from input.
(C) Effects of Fbxl3 and Ovtm on CRY1 and FBXL3 protein stability. Cry1-HA was co-transfected with FLAG-Fbxl3 or FLAG-Ovtm with HA-Ubiquitin (Ub). Abundance of CRY1, FBXL3 and OVTM was measured by Western blotting. Upper panel: Inhibition of CRY degradation by MG132 treatment plus CHX treatment. Lower panel: Ovtm mutation cause accelerated degradation of FBXL3 through the proteasomal degradation pathway. β-actin was used as a loading control.
(D) Quantitation of the effects of Fbxl3 and Ovtm on CRY1 and FBXL3 protein stability. Upper panel: Effects of FBXL3 on CRY1 stability in 293A cells. Open squares: Cry1 only; filled circles: Cry1 co-transfected with Fbxl3; open circles: Cry1 co-transfected with Ovtm mutant. The half life of CRY1 is reduced by either FBXL3 (half life: 1.7 h) or OVTM (half-life: 2.5 h). OVTM co-expression was less effective in CRY1 degradation compared to FBXL3. Lower panel: effect of the Ovtm mutation on the half life of FBXL3. Closed circles and open circles represent FBXL3 and OVTM, respectively. In the presence of CRY1, the Ovtm mutation caused a reduction in the protein stability of OVTM.
(E) Stability of endogenous CRY1 and PER2 in Ovtm ear fibroblast cells. Protein extracts from harvested cells were analyzed with Western blotting using anti-CRY1 and anti-PER2 antibody. α-tubulin was used as a loading control.
(F) Half-life measurements of endogenous CRY1 and PER2 proteins in WT and Ovtm fibroblasts. Upper panel: Time course of CRY1 levels following addition of CHX. Closed circle are from WT fibroblasts and open circles are from Ovtm cells. CRY1 degradation is much more rapid in WT fibroblasts (half life: 5.2 h) than in Ovtm fibroblasts (>>9 h). Lower panel: Half-life measurements of PER2 proteins in WT and Ovtm fibroblasts. Closed circles are from WT fibroblasts and open circles are from Ovtm fibroblasts. From Siepka et al. (Siepka et al. 2007).
We also used tagged proteins in co-immunoprecipitation assays in 293A cells which are easily transfected and express relatively low levels of clock proteins. Both FBXL3 and OVTM interacted strongly with CRY1 and CRY2 (Figure 6B). To explore the weak interaction of FBXL3 with PER proteins, tagged Per constructs were also tested. All three PER proteins showed interactions with FBXL3 and OVTM, however, the strongest interactions were seen with PER2. Because PER2 interacts very strongly with CRY1 (Griffin et al. 1999; Kume et al. 1999; Lee et al. 2001), it is likely that the interactions seen here with FBXL3 may be indirect via CRY1.
Effects of OVTM on CRY degradation
To determine whether OVTM is less efficient than FBXL3 in inducing the degradation of CRY1, we compared the effects of FBXL3 and OVTM on the stability of CRY1 following cycloheximide treatment to prevent de novo protein synthesis in transfected cells (Figure 6C). In 293A cells, FBXL3 expression leads to the degradation of CRY1, and this degradation is blocked by the 26S proteasomal inhibitor MG132 (Figure 6C). OVTM is less effective than FBXL3 in causing CRY1 degradation under these conditions. Interestingly, the turnover of FBXL3 is also affected by the OVTM mutation (Figure 6C, bottom). FBXL3 is relatively stable with a half-life of greater than 7 h; whereas, OVTM has a much shorter half-life of 2.7 h (Figure 6D). Therefore, there are two effects of the OVTM mutation: a reduction in proteasome-mediated CRY1 degradation and a decreased stability of the OVTM protein itself, both of which could contribute to a loss-of-function phenotype.
To determine whether these changes in CRY1 stability seen in transfected cells are physiological, we used fibroblasts prepared from either WT or Ovtm mice and determined the half-lives of native CRY1 and PER2 proteins in these cells. As shown in Figure 6E and F, the half-life of CRY1 in Ovtm fibroblasts is extremely long (>>9 h) as compared to WT cells (half-life = 5.2 h). The overall levels of PER2 in Ovtm fibroblasts were very low similar to that seen in the cerebellum. When the half-life of PER2 was determined, however, there was no detectable difference in the half-life of PER2 in WT and Ovtm cells (Figure 6F). Thus, these experiments in fibroblasts from WT and Ovtm mice show that native CRY1, but not PER2, turnover is specifically altered by the Ovtm mutation.
Conclusions
We have shown that the ENU-induced Overtime mutant is caused by an I364T mutation in the mouse FBXL3 protein (Siepka et al. 2007), a member of the F-box protein with leucine rich repeats family (Jin et al. 2004). The OVTM protein is less efficient than FBXL3 in degrading CRY1 thus providing genetic evidence that FBXL3 appears likely to be a primary F-box protein within an SCF E3 ubiquitin ligase complex (Cardozo and Pagano 2004) that targets the CRY proteins for degradation in the proteasome (Figure 7). The I364T OVTM mutation lengthens circadian periodicity ~2.5 h in mice. We propose that the phenotypic effects of the Ovtm mutation occur primarily through two mechanisms: (1) loss of FBXL3 function leading to stability of CRY1 protein; and (2) repression of CLOCK/BMAL1-dependent transcriptional activation. These two processes lead to a striking reduction in the expression of the PER proteins which is caused by a reduction in transcription of the Per genes. By contrast, the levels of CRY are not reduced by the Ovtm mutation despite lower rates of Cry transcription. Because the transcription of the Cry1 gene is strongly attenuated by the Ovtm mutation, it is surprising that CRY1 protein levels are not lower. The low rate of CRY1 protein degradation in Ovtm tissues must offset the lower synthesis of CRY1 so that the steady state abundance of CRY1 protein is similar in Ovtm and WT mice. Importantly however, because the turnover rate of CRY1 is reduced, the clearance of CRY1 will be prolonged even if the initial steady-state abundance levels are comparable. This would then lead to a prolongation of the CRY-dependent repression phase of the circadian cycle (Godinho et al. 2007). If such a prolongation extended CRY repression for 2-3 hours, the period lengthening phenotype seen in Ovtm mice would follow as a consequence.
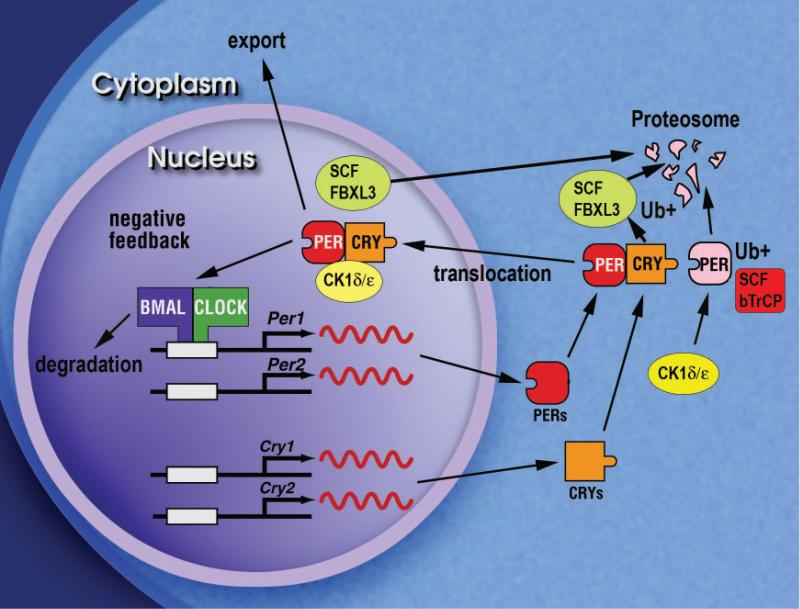
Figure 7. Model of the primary negative feedback loop within the circadian clock of mammals.
The diagram shows the primary negative autoregulatory feedback loop that constitutes the circadian oscillator mechanism in mammals. The BMAL1-CLOCK heterodimeric complex activates transcription of the Per1, Per2, Cry1 and Cry2 genes. The PER and CRY proteins accumulate in the cytoplasm and interact with each other, and then translocate into the nucleus with CK1δ/ε where the complex interacts with BMAL1-CLOCK to inhibit the transcription of the Per and Cry genes. The turnover of the PER and CRY proteins are selectively regulated by interaction with the F-box proteins, β-TrCP and FBXL3, respectively, to target them for ubiquitination and subsequent degradation by the proteasome.
These results highlight the significance of an SCFFBXL3 E3 ubiquitin ligase complex in regulating the stability and kinetics of CRY degradation. The specificity of the FBXL3 interaction with the CRY proteins is striking and suggests that additional F-box proteins may regulate other circadian clock components. The first example of an F-box protein playing a role in circadian rhythmicity was the Arabidopsis gene ZEITLUPE (ZTL) which encodes an F-box protein with an N-terminal LOV domain and C-terminal kelch repeats (Somers et al. 2000). ZTL targets the Arabidopsis clock protein, TOC1, for degradation by the proteasome and is thought to regulate circadian period by controlling TOC1 stability (Mas et al. 2003). In addition, the F-box protein, FKF1, mediates the cyclic degradation of CDF1, a repressor of the photoperiodic gene CONSTANS (Imaizumi et al. 2005), and the F-box protein, AFR, is a positive regulator of phytochrome A-mediated light signaling in Arabidopsis (Harmon and Kay 2003). FBXL3 is the second example of a mammalian F-box protein regulating the circadian clock proteins. The first example is βTrCP which has been shown to interact directly with the PER proteins (Eide et al. 2005; Shirogane et al. 2005). Evidence for βTrCP in the circadian pathway first emerged from Drosophila in which it was shown that Slimb, the ortholog of βTrCP, regulated circadian expression of PER and TIM (Grima et al. 2002; Ko et al. 2002). Interestingly, in Neurospora, the ortholog of βTrCP, FWD1, regulates the degradation of the clock protein, FREQUENCY (He et al. 2003). More recently, JETLAG, the Drosophila ortholog of Fbxl15, has been shown to play a critical role in light-induced TIM degradation by the proteasome (Koh et al. 2006). In Drosophila two different SCF complexes appear to control TIM levels: a circadian pathway involving Slimb and a light-dependent pathway involving JET (Koh et al. 2006). It will be interesting see whether similar types of mechanisms are conserved in mammals. Because of the differences in the roles of the PER and CRY proteins in Drosophila and in mammals (Allada et al. 2001; Young and Kay 2001), where CRY is primarily a circadian repressor (not a photoreceptor), FBXL3 appears to function in a circadian SCF complex-mediated pathway. Unlike Drosophila PER, the PER1 and PER2 proteins in mammals are transcriptionally induced by light in the SCN. It will be interesting to see whether βTrCP functions in a circadian or in a light-dependent SCF pathway for PER degradation (analogous to the TIM protein in Drosophila).
Using a forward genetics approach in mice, we have identified, FBXL3, as a new molecular component of the negative feedback loop that generates circadian rhythmicity. Ironically, in the same screen we also found a mutation in Cry1, the substrate for FBXL3. These experiments highlight the important role of CRY1 in the regulation of circadian period in which the loss-of-function leads to shortened periodicity whereas its over-expression by virtue of stabilization leads to lengthened periodicity.
ACKNOWLEDGMENTS
This chapter reports previously unpublished work describing the identification and candidate gene cloning of the part-time mutant and includes a shortened version of the identification and positional cloning of the Overtime mutant originally published by Siepka et al. (2007) in Cell. Research supported by NIH grants U01 MH61915, P50 MH074924 and R01 MH078024 to J.S.T. and R01 NS053616 to C.L. J.S.T. is an Investigator in the Howard Hughes Medical Institute.
REFERENCES
- Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O’Neill J, Chesham JE, Brooker D, Lalanne Z, Hastings MH, Nolan PM. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- Griffin EA, Jr., Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature. 2002;420:178. doi: 10.1038/nature01122. [DOI] [PubMed] [Google Scholar]
- Hao B, Zheng N, Schulman BA, Wu G, Miller JJ, Pagano M, Pavletich N P. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell. 2005;20:9. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Harmon FG, Kay SA. The F box protein AFR is a positive regulator of phytochrome A-mediated light signaling. Curr Biol. 2003;13:2091. doi: 10.1016/j.cub.2003.11.019. [DOI] [PubMed] [Google Scholar]
- He Q, Cheng P, Yang Y, Yu H, Liu Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. Embo J. 2003;22:4421. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian Clock gene. Cell. 1997;89:641. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Suppl 2):R271. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Ko HW, Jiang J, Edery I. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature. 2002;420:673. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas P, Kim WY, Somers DE, Kay SA. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature. 2003;426:567. doi: 10.1038/nature02163. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- Shirogane T, Jin J, Ang XL, Harper JW. SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem. 2005;280:26863. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Takahashi JS. Forward genetic screens to identify circadian rhythm mutants in mice. Methods Enzymol. 2005;393:219. doi: 10.1016/S0076-6879(05)93007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA. ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell. 2000;101:319. doi: 10.1016/s0092-8674(00)80841-7. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Pinto LH, Vitaterna MH. Forward and reverse genetic approaches to behavior in the mouse. Science. 1994;264:1724. doi: 10.1126/science.8209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS. Finding new clock components: past and future. J Biol Rhythms. 2004;19:339. doi: 10.1177/0748730404269151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Pinto LH, Takahashi JS. Large-scale mutagenesis and phenotypic screens for the nervous system and behavior in mice. Trends Neurosci. 2006;29:233. doi: 10.1016/j.tins.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102:2608. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]