Abstract
Neuronal nitric oxide synthase (nNOS) membrane delocalization contributes to the pathogenesis of Duchenne muscular dystrophy (DMD) by promoting functional muscle ischemia and exacerbating muscle injury during exercise. We have previously shown that supra-physiological expression of nNOS-binding mini-dystrophin restores normal blood flow regulation and prevents functional ischemia in transgenic mdx mice, a DMD model. A critical next issue is whether systemic dual adeno-associated virus (AAV) gene therapy can restore nNOS-binding mini-dystrophin expression and mitigate muscle activity-related functional ischemia and injury. Here, we performed systemic gene transfer in mdx and mdx4cv mice using a pair of dual AAV vectors that expressed a 6 kb nNOS-binding mini-dystrophin gene. Vectors were packaged in tyrosine mutant AAV-9 and co-injected (5 × 1012 viral genome particles/vector/mouse) via the tail vein to 1-month-old dystrophin-null mice. Four months later, we observed 30–50% mini-dystrophin positive myofibers in limb muscles. Treatment ameliorated histopathology, increased muscle force and protected against eccentric contraction-induced injury. Importantly, dual AAV therapy successfully prevented chronic exercise-induced muscle force drop. Doppler hemodynamic assay further showed that therapy attenuated adrenergic vasoconstriction in contracting muscle. Our results suggest that partial transduction can still ameliorate nNOS delocalization-associated functional deficiency. Further evaluation of nNOS binding mini-dystrophin dual AAV vectors is warranted in dystrophic dogs and eventually in human patients.
INTRODUCTION
Dystrophin deficiency results in Duchenne muscular dystrophy (DMD), the most common lethal inherited muscle disease in boys (1). The 2.4 mb dystrophin gene contains 79 exons and it transcribes into a 14 kb cDNA. A highly promising approach to treat DMD is to restore dystrophin expression in all muscle cells in the body using gene replacement therapy. Currently, adeno-associated virus (AAV) is the only vector with proven evidence of whole body muscle transduction in small (such as mice) and large (such as dogs) animals (2,3). However, AAV is the smallest DNA virus with a viral particle size of only 20–25 nm. The maximal packaging capacity of an AAV vector is ∼5 kb (4). This is far below the size of the full-length dystrophin cDNA (∼12 kb).
To overcome this hurdle, investigators have tested a variety of smaller quasi-functional dystrophin genes. Among these, the naturally occurring Δ17-48 mini-dystrophin gene and the synthetic ΔH2-R19 mini-dystrophin gene are extremely promising. The Δ17-48 minigene was isolated from a 60-year-old mildly affected patient (5). This patient carried a large in-frame deletion which removed 46% of the dystrophin gene spanning from exon 17 to 48. The ΔH2-R19 minigene is an improved version of the Δ17-48 minigene (6). Compared with the Δ17-48 minigene, the ΔH2-R19 minigene is more effective in preventing muscle degeneration and recovering muscle force (6).
An essential function of dystrophin is to localize neuronal nitric oxide synthase (nNOS) to the sarcolemma (7). Membrane-associated nNOS is required to optimize muscle perfusion during contraction by protecting against excessive adrenergic vasoconstriction (8–10). Loss of sarcolemmal nNOS leads to muscle ischemia and accelerates the dystrophic process in DMD (8,10–12). Unfortunately, ΔH2-R19 mini-dystrophin cannot anchor nNOS to the muscle cell membrane (13,14). We recently found that dystrophin spectrin-like repeats 16 and 17 (R16/17) is the nNOS-binding domain (8,15). Importantly, the nNOS-binding domain R16/17 is absent in the ΔH2-R19 minigene (6). To increase the biological activity of the ΔH2-R19 minigene, we engineered R16/17 into mini-dystrophin and generated the nNOS-binding domain containing minigene (8,16). Transgenic over-expression of this minigene successfully attenuated functional ischemia and muscle injury in mdx mice, a commonly used mouse DMD model (8). Based on this encouraging finding, we developed a series of novel dual AAV vectors to express the nNOS-binding minigene (16). In a preliminary local muscle injection study, we found that one of our dual AAV sets (YZ27 and YZ22) resulted in robust mini-dystrophin expression in dystrophin-null mdx4cv mice and successfully established sarcolemmal nNOS expression (Fig. 1A) (16). In the current study, we examined whether systemic co-delivery of the YZ27 and YZ22 dual AAV vectors can restore normal blood flow regulation in contracting muscle and prevent exercise-induced muscle injury.
Figure 1.
Co-delivery of the mini-dystrophin dual AAV vectors through the vasculature results in efficient limb and abdominal muscle transduction. (A) The schematic outline of the ΔR2-15/ΔR18-19 mini-dystrophin dual AAV vectors. YZ27 carries the 5′ part of the minigene. YZ22 carries the 3′ part of the minigene. A GFP gene is fused in-frame to the N-terminal end of the mini-dystrophin gene. The nNOS-binding domain is located in dystrophin repeats 16 and 17. CMV, the cytomegalovirus promoter; pA, the polyadenylation signal; Hairpin-like structure, inverted terminal repeats of AAV virus; N, the N-terminal domain of dystrophin; H1, H3 and H4, hinges 1, 3 and 4 in the dystrophin rod domain; Numerical numbers, spectrin-like repeats in the dystrophin rod domain. (B) Representative direct fluorescence photomicrographs revealing GFP expression in different skeletal muscles. UL, upper limb muscle; EDL, extensor digitorum longus muscle; TA, tibialis anterior muscle; Quad, quadriceps muscle; Gas, gastrocnemius muscle; Abd, abdominal muscle. Scale bar: 100 μm. (C) Quantitative evaluation of GFP-positive myofibers in the indicated muscles. Results are from nine-infected mdx4cv mice (mean ± SEM). (D) Representative western blot results from indicated muscles. Top panel is probed with an anti-dystrophin C-terminal antibody. This antibody recognizes both full-length dystrophin in wild-type (WT) mice and mini-dystrophin expressed from the dual AAV vectors. Open arrowhead, full-length dystrophin; filled arrowhead, ΔR2-15/ΔR18-19 mini-dystrophin. Bottom panel is probed with the GFP antibody.
RESULTS
Systemic delivery of the mini-dystrophin dual AAV vectors results in broad skeletal muscle transduction in young adult dystrophic mice
We recently engineered a pair of dual AAV vectors (YZ27 and YZ22) to express the nNOS-binding ΔR2-15/ΔR18-19 mini-dystrophin gene (Fig. 1A) (16). This minigene carries the R16/17 nNOS-binding domain. For easy detection of the full-length protein, a GFP gene and a flag-tag were fused to the N-terminal and C-terminal end, respectively. Initial characterization by YZ27/YZ22 co-infection in the anterior tibialis muscle of dystrophin-deficient mice confirmed in-frame GFP expression and nNOS binding (16). Injection of either YZ22 alone or YZ27 alone did not yield GFP or dystrophin fragment expression (data not shown) (16). To determine whether intravenous delivery can lead to bodywide muscle transduction, we generated Y731F tyrosine mutant AAV-9 dual vectors. Dual vectors were co-delivered via the tail vein at the dose of 5 × 1012 vg particles/vector/mouse to 1-month-old mdx4cv mice, a strain of dystrophin-null mice that have been used in dual AAV mini-dystrophin gene therapy studies before (16,17). Four months after gene transfer, we evaluated transduction efficiency (Fig. 1). Consistent with our previous study (16), all GFP-positive myofibers were also positive for mini-dystrophin, Flag tag and nNOS (Fig. 2 and data not shown). Considerable expression was observed in the upper limb, extensor digitorum longus (EDL), tibialis anterior (TA), quadriceps, gastrocnemius and abdominal muscles (Fig. 1B). Quantification of the positive myofibers showed that ∼30–50% of myofibers expressed mini-dystrophin in these muscles (Fig. 1C). Limb muscle western blot revealed a single band corresponding to the predicted size of the GFP-fused mini-dystrophin protein (Fig. 1D). Consistent with a previous study, limited expression was observed in the heart and diaphragm (data not shown) (17).
Figure 2.
Dual AAV-mediated mini-dystrophin expression ameliorates histological lesions in dystrophic muscle. (A) Representative photomicrographs of the quadriceps muscle from BL6, mdx4cv and dual AAV-infected mdx4cv mice revealing dystrophin expression, general histology (HE) and nNOS expression. GFP, direct fluorescence photomicrographs; Hum Dys, indirect immunofluorescence staining with an antibody specific for human dystrophin; R4-6, indirect immunofluorescence staining with an antibody against dystrophin spectrin-like repeats 4 to 6. This region is deleted in the ΔR2-15/ΔR18-19 mini-dystrophin gene; nNOS activity, enzymatic staining for nNOS activity; nNOS IF, indirect immunofluorescence staining with an antibody against nNOS. Asterisk marks the same myofiber in serial sections. Black/white square, a myofiber not transduced by mini-dystrophin dual AAV vectors. (B) Quantification of centrally nucleated myofibers (CN) in the tibialis anterior muscle (TA) and the gastrocnemius muscle (Gastro). Sample size (number of muscles studied): N = 4 for BL6, N = 5 for mdx4cv, N = 9 muscles for AAV-infected mdx4cv. Approximately 100–250 myofibers were quantified for each indicated category. AAV neg myofiber, untransduced myofibers in dual AAV-infected mdx4cv mice; AAV pos myofiber, mini-dystrophin positive myofibers in dual AAV-infected mdx4cv mice. Asterisk, significantly different from that of BL6 and AAV pos myofiber. (C) Distribution of myofiber size in normal, mdx4cv and AAV-treated mdx4cv mice. Top panel, TA muscle; bottom panel, gastrocnemius muscle. Approximately 680 myofibers were measured for each muscle in each strain.
Dual AAV gene transfer restores sarcolemmal nNOS expression and improves muscle histopathology in mdx4cv muscle
To validate dual vector-mediated ΔR2-15/ΔR18-19 mini-dystrophin expression, serial muscle sections were examined for GFP, dystrophin and nNOS expression (Fig. 2A). As expected, GFP-positive myofibers were only observed in dual AAV-infected mdx4cv muscle. In adjacent sections, myofibers that were positive for GFP were recognized by the human dystrophin specific antibody (Hum Dys) but not by the R4-6 antibody, an antibody that reacts with an epitope absent in the synthetic mini-dystrophin gene (Fig. 2A). To evaluate membrane-bound nNOS expression, we performed nNOS immunofluorescence staining and in situ nNOS enzymatic activity assay. Both methods confirmed correct localization of nNOS on the muscle cell membrane in dual AAV-treated mdx4cv muscle (Fig. 2A).
Hematoxylin and eosin (HE) staining revealed histology improvement. In dystrophic muscles that received intravenous dual AAV injection, there appeared to be less inflammation, degeneration/regeneration and necrosis (Fig. 2A). Quantification of the percentage of centrally nucleated myofiber and the distribution of myofiber size further confirmed HE staining results (Fig. 2B and C). In both the TA and gastrocnemius muscles, central nucleation was significantly reduced in myofibers that were transduced by the dual vectors (AAV mini-dystrophin positive myofibers) but was not altered in myofibers that were not transduced (AAV mini-dystrophin negative myofibers) (Fig. 2B). Next, we examined myofiber size distribution in the TA and gastrocnemius muscles (Fig. 2C). Compared with normal C57Bl/6 (BL6) muscle, mdx4cv muscle showed a much broader range with more very small and very large myofibers. Dual AAV treatment shifted the pattern of myofiber size distribution. It was not completely normalized but was clearly improved compared with that of non-injected mdx4cv mice (Fig. 2C).
Intravenous administration of the mini-dystrophin dual AAV vectors increases muscle function in the DMD mouse model
To fully evaluate muscle physiology, we conducted ex vivo and in situ force measurement in the EDL and TA muscle, respectively (Fig. 3A–C). The physiological properties of the EDL muscle were studied in Ringer's buffer using freshly isolated muscle (Fig. 3). Compared with that of untreated mdx4cv mice, the dual AAV-treated mdx4cv EDL muscle had a significantly smaller muscle mass and cross-sectional area (CSA). Mini-dystrophin gene therapy significantly increased specific tetanic force of the EDL muscle. Upon repeated cycles of eccentric contraction stress, AAV-treated EDL muscle showed significantly less force loss (Fig. 3C). TA muscle force was measured in the intact animal in situ (Fig. 3B). Similar to what we saw in the EDL muscle, dual AAV therapy also significantly reduced muscle weight and CSA in the TA muscle (Fig. 3B). Consistently, treatment significantly enhanced tetanic forces of the TA muscle (Fig. 3B).
Figure 3.
Dual AAV minigene therapy corrects muscle hypertrophy, enhances contractility and preserves force generation when challenged with intensive chronic treadmill exercise. (A) Comparison of the EDL muscle weight, CSA, specific twitch and tetanic tension among BL6, untreated mdx4cv and dual AAV-treated mdx4cv mice. (B) Comparison of the TA muscle weight, CSA, specific twitch and tetanic tension among BL6, untreated mdx4cv and dual AAV-treated mdx4cv mice. (C) Eccentric contraction profiles of the EDL muscle in BL6, treated and untreated mdx4cv mice. (A–C) Asterisk indicates that the value in dual AAV-treated mdx4cv muscle is significantly different from those of untreated mdx4cv and BL6 muscles. (A and B) Cross indicates that the value in dual AAV-treated mdx4cv muscle is significantly different from that of untreated mdx4cv muscle only. (D) Quantitative comparison of specific muscle force of the EDL muscle in BL6 mice with or without chronic treadmill running. (E) Quantitative comparison of specific muscle force of the EDL muscle in mdx4cv mice with or without chronic treadmill running. Asterisk, significantly higher than treadmill challenged mice. (F) Quantitative comparison of specific muscle force of the EDL muscle in dual AAV-treated mdx4cv mice with or without chronic treadmill running.
Dual vector-mediated mini-dystrophin expression prevents exercise-induced loss of muscle force and ameliorates functional muscle ischemia in dystrophin-deficient mice
Two different approaches were used to determine whether dual AAV-mediated ΔR2-15/ΔR18-19 mini-dystrophin expression can treat the nNOS-dependent phenotypes of exercise-induced muscle force reduction and functional muscle ischemia. First, we examined muscle force change following chronic exercise challenge in mdx4cv mice. This method has been previously used to demonstrate focal muscle injury and compromised muscle force production in Fiona mice, a strain of full-length utrophin transgenic mdx mice (18). In this assay, mice were run on a treadmill twice a week for a total of eight weeks. At the end of the treadmill sessions, the EDL muscle force was measured and compared with that of non-exercised mice. In normal BL6 mice, repeated treadmill running did not compromise force production in the EDL muscle (Fig. 3D). In untreated mdx4cv mice, chronic treadmill challenge resulted in a significant reduction in the EDL muscle specific force at the stimulation frequency of 50, 100 and 150 Hz (a strong trend of reduction was seen at 80 Hz, but it did not reach statistical significance) (Fig. 3E). In sharp contrast, chronic treadmill exercise had a nominal effect on specific muscle force in dual AAV-treated mdx4cv mice (Fig. 3F). In other words, restoration of sarcolemmal nNOS by dual AAV-mediated mini-dystrophin therapy has successfully prevented muscle damage and the subsequent loss of force in mdx4cv mice.
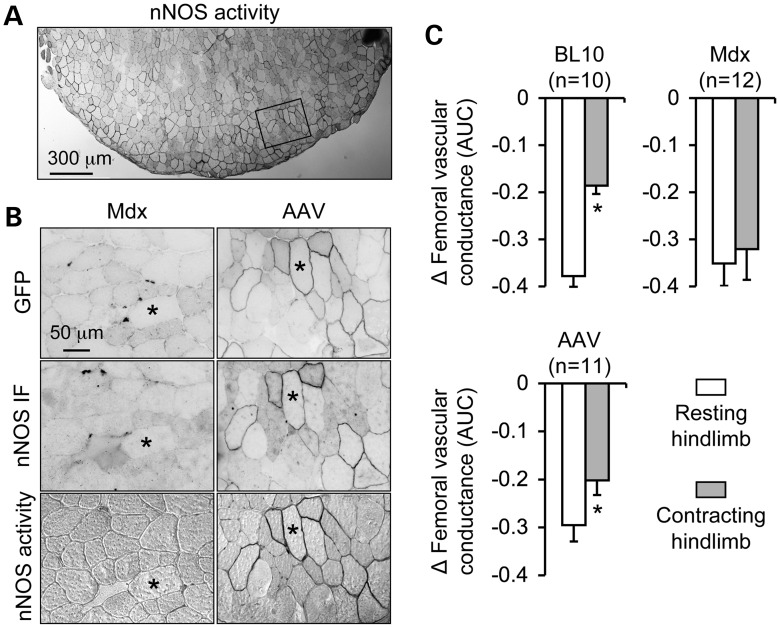
To determine whether we can achieve the same transduction efficiency in a different strain of dystrophin-null mice, we performed tail vein injection in 1-month-old mdx mice at the same dose as we have used for mdx4cv mice (5 × 1012 vg particles/vector/mouse) (Fig. 4 and Supplementary Material, Fig. S1). Consistent with our findings in mdx4cv mice (Fig. 1), intravenous dual AAV administration resulted in similar transduction efficiency in mdx mice (Fig. 4A and Supplementary Material, Fig. S1). As exemplified in the TA muscle, ∼30–50% of myofibers were transduced (Fig. 4A). All GFP-positive myofibers displayed sarcolemmal nNOS expression (Fig. 4B).
Figure 4.
Restoration of sarcolemmal nNOS expression by dual AAV minigene therapy ameliorates functional muscle ischemia. (A) Representative low magnification photomicrographs of nNOS activity staining of the TA muscle from a dual AAV-treated mdx mouse. High magnification images of the boxed areas are shown in the right column of (B). (B) Representative high power photomicrographs of the TA muscle from untreated (left column) and dual AAV-treated (right column) mdx mice. Top panel, GFP expression; middle panel, nNOS immunofluorescence staining; bottom panel, in situ nNOS enzymatic activity staining. Asterisk, the same myofiber in serial sections. (C) Quantitative comparison of the femoral vascular conductance response to norepinephrine in resting and contracting hindlimbs of BL10, untreated mdx and dual AAV-treated mdx mice. Asterisk, significantly different between resting and contracting muscle.
We have previously used an in vivo hemodynamic assay to show that mdx mice are susceptible to muscle ischemia during exercise due to loss of sarcolemmal nNOS (8,10). In this assay, we measured the transient decrease in hindlimb blood flow caused by femoral artery injection of the sympathetic vasoconstrictor norepinephrine when the hindlimb muscles were at rest. We then stimulated the sciatic nerve to evoke hindlimb muscle contractions and repeated the norepinephrine injection. Norepinephrine-mediated vasoconstriction normally is attenuated in the contracting hindlimbs in normal C57Bl/10 (BL10) mice, which optimizes blood flow to the working muscles (Fig. 4C) (8,10). Consistent with our previous publications (8,10), norepinephrine evoked similar decreases in femoral vascular conductance in the resting and contracting hindlimb muscles in mdx mice, directly demonstrating functional muscle ischemia (Fig. 4C). Following mini-dystrophin dual AAV treatment, adrenergic vasoconstriction was significantly attenuated during muscle contraction, showing a clear treatment effect in ameliorating functional muscle ischemia (Fig. 4C).
DISCUSSION
In this study, we evaluated therapeutic benefits of the nNOS-recruiting dual AAV vectors in mouse models of DMD by systemic gene transfer. We demonstrated that intravascular co-injection of two independent AAV vectors successfully reconstituted an intact mini-dystrophin expression cassette in the majority of muscles of young adult dystrophic mice (Figs 1, 4 and Supplementary Material, Fig. S1). With an encouraging transduction efficiency (∼30–50% of limb muscle myofiber were positive for mini-dystrophin), dual AAV therapy significantly ameliorated dystrophic pathology and improved muscle function (Figs 2 and 3). Specifically, treated mice showed less muscle inflammation, a significant reduction of myofiber degeneration/regeneration (as reflected by central nuclear quantification) and a partial correction of abnormal myofiber size distribution (Fig. 2). Dual AAV therapy also significantly alleviated muscle pseudohypertrophy, a unique disease-related feature in young adult dystrophic mice (Fig. 3) (6,19,20). Physiology assays demonstrated that treatment significantly enhanced tetanic muscle force and preserved muscle contractility under eccentric contraction stress (Fig. 3). Most importantly, dual AAV therapy reestablished sarcolemmal nNOS distribution in dystrophic muscle (Figs 2 and 4). As a consequence, norepinephrine-induced muscle ischemia during exercise was significantly blunted and muscle force loss caused by repeated exercise was prevented (Figs 3 and 4).
Functional muscle ischemia caused by nNOS delocalization has been recognized as a critical pathogenic mechanism in DMD for more than a decade (7,8,10,11). It may underlie a number of characteristic symptoms in patients such as muscle cramp, fatigue and force reduction. For this reason, treating muscle ischemia is an important goal in DMD gene therapy. Unfortunately, none of the gene therapy studies published so far has accomplished this mission (21). The molecular mechanism underlying dystrophic functional muscle ischemia has been delineated (10). In normal muscle, nitric oxide (NO) produced by sarcolemmal nNOS attenuates α-adrenergic vasoconstriction, thereby optimizing blood flow to meet the metabolic needs of the contracting muscles. Correct position of nNOS at the sarcolemma is vital in this process in order to facilitate ready diffusion of NO to the adjacent vasculature. In dystrophin-deficient muscle, nNOS is lost from the sarcolemma and the total nNOS content is reduced (7). As a result, NO-mediated vasodilation is compromised, resulting in functional muscle ischemia. Re-directing nNOS back to the membrane should restore NO-mediated blood flow regulation in contracting muscle and eliminate functional ischemia.
Early studies suggested that nNOS is connected to the sarcolemma by syntrophin (22). For this reason, it seems that restoration of syntrophin to the muscle cell membrane would be sufficient to localize nNOS to the sarcolemma. In support of this theory, Wang et al. (23) claimed that they achieved membrane-associated nNOS expression using a minimized dystrophin construct containing repeats R1, R2, R22, R23 and R24. Surprisingly, similar mini-/micro-dystrophin genes used by other laboratories have all failed to bring nNOS to the membrane, although syntrophin is recruited back to the sarcolemma by these minimized genes (8,13,14,24). We recently found that in addition to syntrophin, membrane targeting of nNOS also requires an nNOS-binding domain located in dystrophin R16/17 (8,15,18). Synthetic dystrophin genes that carry this domain can successfully restore nNOS to the membrane, while constructs that do not contain R16/17 cannot.
To translate our findings to DMD therapy, we decided to engineer the R16/17 nNOS recruiting domain to the ΔH2-R19 mini-dystrophin gene. We chose the ΔH2-R19 minigene because this minigene is originated from a truncated dystrophin gene in an extremely mild patient (5). Among dozens of abbreviated synthetic dystrophin constructs, the ΔH2-R19 minigene is the only one that we know works in human muscle (21). In a preliminary study, we compared two strains of transgenic mdx mice that either expressed the ΔH2-R19 minigene or our newly engineered nNOS-binding minigene (8). Encouragingly, supra-physiological expression of the nNOS-binding minigene, but not the ΔH2-R19 minigene, effectively normalized muscle blood flow regulation and prevented ischemic injury during exercise in transgenic mdx mice (8). In summary, our preliminary study has clearly established the therapeutic advantage of the nNOS-binding minigene and supports preclinical testing of the nNOS-binding minigene by AAV-mediated gene therapy.
The nNOS-binding minigene (ΔR2-15/ΔR18-19) is 6 kb. This exceeds the 5 kb packaging limit of a single AAV virion (4). In order to deliver a large gene with AAV, we have developed a number of dual vector strategies such as trans-activation, trans-splicing, overlapping and hybrid systems (13,16,25–28). The fundamental idea behind these approaches is to divide a large gene into two parts and package them in two different AAV vectors. The intact gene is reconstituted at the DNA level after co-infection and inter-molecular recombination of two different AAV genomes (29,30). Subsequent studies from several laboratories, including ours, demonstrate that dual AAV vectors not only result in saturated expression after direct muscle injection but also yield whole body muscle transduction following intravascular delivery (17,31–33). Collectively, these results reveal a high feasibility of treating muscular dystrophy with dual AAV vectors.
In light of abundant literature support, we developed the YZ27/YZ22 dual AAV vector set to express the 6 kb nNOS-binding mini-dystrophin gene (16). This set of vectors is designed using the principle of the overlapping strategy (27,34). Basically, the tail part of the 5′-end gene fragment is identical to that of the head part of the 3′-end gene fragment. Reconstitution is achieved through homologous recombination. Following systemic co-injection of the YZ27 and YZ22 vectors, mini-dystrophin expression was observed in 30–50% of the myofibers in all limb muscles and some body wall muscles (such as the abdominal muscle) (Figs 1 and 4). This level of mosaic expression is predicted to be therapeutically relevant in terms of reducing muscle pathology and increasing muscle strength (35). Indeed, histological examination and force measurement showed significant improvement in treated mice (Figs 2 and 3).
The primary goal of the current study is to test whether reconstitution of membrane-associated nNOS in a subset of myofibers can attenuate functional muscle ischemia in dystrophic mice. This is a highly relevant question because gene therapy is unlikely going to yield supra-physiological expression in every myofiber as we have seen in transgenic mice (8). To determine whether partial transduction (∼30–50% mini-dystrophin positive myofibers) can ameliorate functional muscle ischemia and reduce muscle injury, we have taken two independent approaches using two different strains of dystrophin-null mice (mdx and mdx4cv) (Figs 3 and 4). Mdx4cv mice are on the BL6 background and they carry a nonsense mutation in exon 50 (36). Mdx mice are naturally occurring dystrophin-null mice on the BL10 background and their mutation is located in exon 23 (37). For mdx4cv mice, we challenged them with chronic treadmill exercise. In this assay, the hindlimb EDL muscle generates much less force due to repeated muscle injury in the absence of sarcolemmal nNOS (18). Encouragingly, treadmill running did not result in EDL force loss in dual AAV-treated mdx4cv mice, suggesting that the restoration of nNOS has protected against exercise-induced muscle damage (Fig. 3F). We then used mdx mice to perform complementary experiments to directly quantify hindlimb blood flow regulation. The mdx mouse is the model originally used to establish the concept of functional ischemia in exercising dystrophic muscle (10). Consistent with the findings in mdx4cv mice (Fig. 3F), AAV treatment restored sarcolemmal nNOS and significantly reduced norepinephrine-mediated ischemia in contracting mdx muscle (Fig. 4C). Nevertheless, the protection offered by the nNOS-binding dual AAV treatment in this study (∼30% less hindlimb ischemia) was not as robust as the protection we previously observed in BL10 mice and transgenic mdx mice which had supra-physiologic expression of the nNOS-binding mini-dystrophin gene (∼80% less hindlimb ischemia) (Fig. 4C) (8,10). Taken together, the results from two complementary models/assays confirm that our dual AAV therapy has attenuated functional muscle ischemia during exercise and prevented muscle injury and the loss of muscle force induced by repeated exercise in the murine DMD model. This is an important milestone since it demonstrates for the first time that gene therapy can alleviate known functional consequences of nNOS membrane delocalization such as functional muscle ischemia, muscle injury and compromised force generation. On the other hand, our studies also suggest that additional optimization is needed to enhance transduction efficiency and achieve even better muscle protection.
MATERIALS AND METHODS
Animals
Animal experiments were approved by the Animal Care and Use Committees of the University of Missouri and Cedars-Sinai Medical Center and were in accordance with NIH guidelines. BL6, BL10, mdx4cv (B6Ros.Cg-Dmdmdx-4Cv/J) and mdx (C57BL/10ScSn-Dmdmdx/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Only young adult male mice were used in the study. All mice were housed in specific-pathogen free animal care facilities and kept under a 12 h light (25 lux)/12 h dark cycle with free access to food and water.
AAV production and gene delivery
The proviral cis plasmids have been published before (16). They include YZ27 and YZ22 (Fig. 1A). YZ27 contains the cytomegalovirus (CMV) promoter, a GFP gene fused to the N-terminal end of the dystrophin gene, and the 5′-end of the ΔR2-15/ΔR18-19 mini-dystrophin gene including the N-terminal domain, hinges 1 and 3, and spectrin-like repeats R1, R16, R17, R20 and a part of R21. YZ22 contains the 3′-end of the ΔR2-15/ΔR18-19 mini-dystrophin gene including a part of hinge 3, spectrin-like repeats R20 to R24, hinge 4, cysteine-rich domain and the C-terminal domain. YZ22 also contains a flag tag and the SV40 poly-adenylation signal. The Y731F tyrosine modified AAV-9 was purified and titrated according to our published protocol using a cap/rep helper plasmid generously provided by Dr Arun Srivastava (University of Florida, Gainesville, FL, USA) (38,39). Dual AAV vectors were delivered in pairs to 1-month-old mdx or mdx4cv mice via the tail vein. Each co-infection consisted of 5 × 1012 viral genome (vg) particles/vector/mouse. AAV transduction was evaluated at 4 months post-injection.
Morphology studies
General histology was examined by HE staining. GFP was visualized under the FITC channel using a Nikon E800 fluorescence microscope. Photomicrographs were taken with a Qimage REtiga 1300 camera. The ΔR2-15/ΔR18-19 mini-dystrophin was examined with Dys 3, a human dystrophin-specific mouse monoclonal antibody against an epitope located in dystrophin hinge 1 (1:20; Novocastra, Newcastle, UK) (8,13,40). Endogenous mouse dystrophin was detected with the H-300 rabbit polyclonal antibody against an epitope located between R4 to R6 (1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The nNOS protein was detected with a polyclonal antibody (1:2000; Santa Cruz Biotechnology). In situ NOS activity staining was performed according to our published protocol (8,12,18). The percentage of centrally nucleated myofibers was determined by manually counting the total number of myofibers and the total number of myofibers carrying centrally located nuclei in 8 μm HE stained sections. The percentage of central nucleation was calculated with the formula, % central nucleation = 100 × (total number of myofibers carrying centrally located nuclei)/(total number of myofibers). In mini-dystrophin dual AAV vector infected mdx4cv mice, the percentage of centrally nucleated myofibers was determined separately for mini-dystrophin positive and mini-dystrophin negative myofibers. In these cases, mini-dystrophin expression was confirmed in the adjacent section. The myofiber size was determined from the digitized images using the quantitative image analysis module of the extended version of the Photoshop CS5.5 software (Adobe Systems Incorporated, San Jose, CA, USA).
Western blot
Whole muscle lysate was obtained from frozen limb muscles according to our previous publications (12,16). Dystrophin was detected with a monoclonal antibody against the dystrophin C-terminal domain (Dys2, 1:100, clone Dy8/6C5, IgG1; Novocastra, Newcastle, UK). This antibody recognizes both endogenous full-length dystrophin and ΔR2-15/ΔR18-19 mini-dystrophin. GFP was determined with a monoclonal antibody against GFP (1:2000, clone 3E6, IgG2a; Invitrogen, Carlsbad, CA, USA).
Ex vivo evaluation of the EDL muscle function
Mice were anesthetized via intra-peritoneal injection of a cocktail containing 25 mg/ml ketamine, 2.5 mg/ml xylazine and 0.5 mg/ml acepromazine at 2.5 µl/g body weight. The EDL muscle was gently dissected and mounted to an intact muscle test system (Aurora Scientific, Inc., Aurora, ON, Canada) (41). Briefly, the proximal tendon of the EDL muscle was secured to a dual-mode servomotor transducer and the distal tendon was attached to a fixed post using a 4-0 suture (SofSilk USSC Sutures, Norwalk, CT, USA). Subsequently, the EDL muscle was submerged in a 30°C jacketed organ bath containing oxygenated (95% O2 and 5% CO2) Ringer's buffer. After 10 min equilibration, the optimal length (Lo) of the EDL muscle was measured with an electronic digital caliper (Fisher Scientific, Waltham, MA, USA). The maximum isometric tetanic force (Po) was measured at 150 Hz. The muscle CSA was calculated according to the following equation, CSA = (muscle mass, in gram)/[(optimal fiber length, in cm) × (muscle density, in g/cm3)]. A muscle density of 1.06 g/cm3 was used in calculation. Specific muscle force was determined by dividing the maximum isometric tetanic force with the muscle CSA. After tetanic force measurement, the muscle was rested for 10 min and then subjected to five rounds of eccentric contraction injury according to our previously published protocol (41). The percentage of force drop following each round of eccentric contraction was recorded. Data were recorded and analyzed using the Lab View-based DMC and DMA programs (Version 3.12, Aurora Scientific, Inc.).
In situ examination of the TA muscle function
The TA muscle force was measured according to our published protocol (12,41). Briefly, mice were anesthetized as described above. The TA muscle and the sciatic nerve were exposed. The mouse was transferred to a customer-designed thermo-controlled platform of the footplate apparatus (42). Subsequently, twitch and tetanic forces were measured in situ with a 305C-LR dual-mode servomotor transducer (Aurora Scientific, Inc.). Data recording and analysis were identical to methods described for the EDL muscle.
Evaluation of hindlimb muscle injury by chronic treadmill running
Mice were subject to chronic treadmill running as we described before using an Exer 3/6 treadmill system (Columbus Instruments, Columbus, OH, USA) (18,41). Briefly, mice were acclimated to the treadmill for 3 days and then challenged with treadmill running twice a week for a total of 8 weeks. Each treadmill session lasted 40 min including 10 min warm-up at the speed of 8 m/min followed by 30 min running at a speed of 12 m/min on a horizontal treadmill. At the end of 8-week treadmill exercise, mice were euthanized and tetanic force of the EDL muscle was measured as described above at the stimulation frequencies of 50, 80, 100 and 150 Hz. A separate set of mice (age and sex-matched) that did not undergo exercise running was used as controls for comparison. A statistically significant reduction of the EDL muscle specific force in exercised mice was considered a positive indication for the presence of muscle damage due to the loss of sarcolemmal nNOS (18).
Evaluation of functional muscle ischemia during hindlimb contraction
Femoral vascular conductance in mdx mice was evaluated using a pulsed Doppler velocimeter (Indus Instruments, Webster, TX, USA) based on our previously published protocol (8,10). Briefly, mice were anesthetized with isoflurane (1.5–2.0% in oxygen) and instrumented with a carotid artery catheter to measure blood pressure, a right femoral artery catheter to deliver norepinephrine and a left femoral artery Doppler ultrasound flow probe to measure hindlimb blood flow velocity. Core temperature was monitored using a rectal probe and was maintained at 37°C. Femoral vascular conductance (mean blood flow velocity/mean blood pressure) responses to intra-arterial injection of the α-adrenergic vasoconstrictor norepinephrine (12.5–25 ng in a volume of 5–20 µl) were measured with the left hindlimb at rest and during intermittent, tetanic contractions induced by sciatic nerve stimulation using 100 ms trains of pulses (100 Hz, 0.2 ms) at a rate of 30 trains per min (Model S88, Grass Instruments). Femoral vascular conductance responses to norepinephrine were calculated by integrating the area under the response curve.
Statistical analysis
Data are presented as mean ± standard error of mean. Statistical analysis was performed with the SPSS software (IBM Corporation, Armonk, NY, USA). For multiple group comparison, statistical significance was determined by one-way ANOVA followed by Bonferroni post hoc analysis. For two group comparison, statistical significance was determined by the Student t-test. Difference was considered significant when P < 0.05.
SUPPLEMENTARY MATERIAL
AUTHOR CONTRIBUTIONS
Conceived and designed experiments: D.D. and G.D.T. Performed experiments: Y.Z., Y.Y., L.L., C.H.H. and K.Z. Analyzed data: D.D., G.D.T. and Y.Z. Wrote the paper: D.D.
FUNDING
The study was supported by grants from the National Institutes of Health AR-49419 (D.D.), HL-91883 (D.D.) and AR-56221 (G.D.T.) and Muscular Dystrophy Association (D.D.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Thomas McDonald for excellent technical assistance. We thank Yi Lai for helpful discussion.
Conflict of Interest statement. Y.Y. and D.D. hold a patent on the nNOS binding mini-dystrophin gene.
REFERENCES
- 1.Kunkel L.M. 2004 William Allan award address. Cloning of the DMD gene. Am. J. Hum. Genet. 2005;76:205–214. doi: 10.1086/428143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yue Y., Ghosh A., Long C., Bostick B., Smith B.F., Kornegay J.N., Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostick B., Ghosh A., Yue Y., Long C., Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 4.Lai Y., Yue Y., Duan D. Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome > or =8.2 kb. Mol. Ther. 2010;18:75–79. doi: 10.1038/mt.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.England S.B., Nicholson L.V., Johnson M.A., Forrest S.M., Love D.R., Zubrzycka-Gaarn E.E., Bulman D.E., Harris J.B., Davies K.E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 6.Harper S.Q., Hauser M.A., DelloRusso C., Duan D., Crawford R.W., Phelps S.F., Harper H.A., Robinson A.S., Engelhardt J.F., Brooks S.V., et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 7.Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y., Thomas G.D., Yue Y., Yang H.T., Li D., Long C., Judge L., Bostick B., Chamberlain J.S., Terjung R.L., et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas G.D., Victor R.G. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J. Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas G.D., Sander M., Lau K.S., Huang P.L., Stull J.T., Victor R.G. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl Acad. Sci. USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sander M., Chavoshan B., Harris S.A., Iannaccone S.T., Stull J.T., Thomas G.D., Victor R.G. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D., Yue Y., Lai Y., Hakim C.H., Duan D. Nitrosative stress elicited by nNOSmu delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 2011;223:88–98. doi: 10.1002/path.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai Y., Yue Y., Liu M., Ghosh A., Engelhardt J.F., Chamberlain J.S., Duan D. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Judge L.M., Haraguchi M., Chamberlain J.S. Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex. J. Cell Sci. 2006;119:1537–1546. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- 15.Lai Y., Zhao J., Yue Y., Duan D. alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc. Natl Acad. Sci. USA. 2013;110:525–530. doi: 10.1073/pnas.1211431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum. Gene Ther. 2012;23:98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odom G.L., Gregorevic P., Allen J.M., Chamberlain J.S. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol. Ther. 2011;19:36–45. doi: 10.1038/mt.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D., Bareja A., Judge L., Yue Y., Lai Y., Fairclough R., Davies K.E., Chamberlain J.S., Duan D. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J. Cell Sci. 2010;123:2008–2013. doi: 10.1242/jcs.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastoret C., Sebille A. Mdx mice show progressive weakness and muscle deterioration with age. J. Neurol. Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- 20.Lynch G.S., Hinkle R.T., Chamberlain J.S., Brooks S.V., Faulkner J.A. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J. Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan D. Duchenne muscular dystrophy gene therapy: lost in translation? . Res. Rep. Biol. 2011;2:31–42. doi: 10.2147/RRB.S13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillier B.J., Christopherson K.S., Prehoda K.E., Bredt D.S., Lim W.A. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 23.Wang B., Li J., Qiao C., Chen C., Hu P., Zhu X., Zhou L., Bogan J., Kornegay J., Xiao X. A canine minidystrophin is functional and therapeutic in mdx mice. Gene Ther. 2008;15:1099–1106. doi: 10.1038/gt.2008.70. [DOI] [PubMed] [Google Scholar]
- 24.Yue Y., Liu M., Duan D. C-terminal truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double knock-out mice. Mol. Ther. 2006;14:79–87. doi: 10.1016/j.ymthe.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A., Yue Y., Duan D. Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequence. Hum. Gene Ther. 2011;22:77–83. doi: 10.1089/hum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh A., Yue Y., Lai Y., Duan D. A hybrid vector system expands aden-associated viral vector packaging capacity in a transgene independent manner. Mol. Ther. 2008;16:124–130. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- 27.Duan D., Yue Y., Engelhardt J.F. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol. Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- 28.Duan D., Yue Y., Yan Z., Engelhardt J.F. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat. Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 29.Lai Y., Yue Y., Bostick B., Duan D. In: Muscle Gene Therapy. Duan D., editor. New York: Springer Science+Business Media, LLC; 2010. pp. 205–218. [Google Scholar]
- 30.Ghosh A., Duan D. Expending adeno-associated viral vector capacity: a tale of two vectors. Biotechnology and Genetic Engineering Reviews. 2007;24:165–177. doi: 10.1080/02648725.2007.10648098. [DOI] [PubMed] [Google Scholar]
- 31.Lostal W., Bartoli M., Bourg N., Roudaut C., Bentaib A., Miyake K., Guerchet N., Fougerousse F., McNeil P., Richard I. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum. Mol. Genet. 2010;19:1897–1907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh A., Yue Y., Shin J.-H., Duan D. Systemic trans-splicing AAV delivery efficiently transduces the heart of adult mdx mouse, a model for Duchenne muscular dystrophy. Hum. Gene Ther. 2009;20:1319–1328. doi: 10.1089/hum.2009.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A., Yue Y., Long C., Bostick B., Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol. Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh A., Yue Y., Duan D. Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J. Gene Med. 2006;8:298–305. doi: 10.1002/jgm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamberlain J.S. Gene therapy of muscular dystrophy. Hum. Mol. Genet. 2002;11:2355–2362. doi: 10.1093/hmg/11.20.2355. [DOI] [PubMed] [Google Scholar]
- 36.Chapman V.M., Miller D.R., Armstrong D., Caskey C.T. Recovery of induced mutations for X chromosome-linked muscular dystrophy in mice. Proc. Natl Acad. Sci. USA. 1989;86:1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sicinski P., Geng Y., Ryder-Cook A.S., Barnard E.A., Darlison M.G., Barnard P.J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 38.Shin J.H., Yue Y., Duan D. Recombinant adeno-associated viral vector production and purification. Methods Mol. Biol. 2012;798:267–284. doi: 10.1007/978-1-61779-343-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong L., Li B., Mah C.S., Govindasamy L., Agbandje-McKenna M., Cooper M., Herzog R.W., Zolotukhin I., Warrington K.H., Jr., Weigel-Van Aken K.A., et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl Acad. Sci. USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue Y., Li Z., Harper S.Q., Davisson R.L., Chamberlain J.S., Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hakim C.H., Li D., Duan D. Monitoring murine skeletal muscle function for muscle gene therapy. Methods Mol. Biol. 2011;709:75–89. doi: 10.1007/978-1-61737-982-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakim C.H., Wasala N.B., Duan D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterio muscle in situ in mice. J. Vis. Exp. 2013;73:e50183. doi: 10.3791/50183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.