Abstract
The SRY-related HMG-box 5 (SOX5) gene encodes a member of the SOX family of transcription factors. Recently, genome-wide association studies have implicated SOX5 as a candidate gene for susceptibility to four cardiac-related endophenotypes: higher resting heart rate (HR), the electrocardiographic PR interval, atrial fibrillation and left ventricular mass. We have determined that human SOX5 has a highly conserved Drosophila ortholog, Sox102F, and have employed transgenic Drosophila models to quantitatively measure cardiac function in adult flies. For this purpose, we have developed a high-speed and ultrahigh-resolution optical coherence tomography imaging system, which enables rapid cross-sectional imaging of the heart tube over various cardiac cycles for the measurement of cardiac structural and dynamical parameters such as HR, dimensions and areas of heart chambers, cardiac wall thickness and wall velocities. We have found that the silencing of Sox102F resulted in a significant decrease in HR, heart chamber size and cardiac wall velocities, and a significant increase in cardiac wall thickness that was accompanied by disrupted myofibril structure in adult flies. In addition, the silencing of Sox102F in the wing led to increased L2, L3 and wing marginal veins and increased and disorganized expression of wingless, the central component of the Wnt signaling pathway. Collectively, the silencing of Sox102F resulted in severe cardiac dysfunction and structural defects with disrupted Wnt signaling transduction in flies. This implicates an important functional role for SOX5 in heart and suggests that the alterations in SOX5 levels may contribute to the pathogenesis of multiple cardiac diseases or traits.
INTRODUCTION
The SRY-related HMG-box 5 (SOX5) gene is localized on chromosome 12p12 (OMIN 604975) and encodes a member of the SOX family of transcription factors that bind to DNA (1). SOX5 is expressed in multiple human tissues, including heart, liver, lung, kidney, spleen, fetal brain and testis (2). Studies have shown that SOX5 can modulate cell fate, control cell proliferation and regulate cartilage formation and neuron development (3–5). Recently, genome-wide association studies (GWASs) have implicated SOX5 as a candidate gene for susceptibility to four cardiac-related endophenotypes: higher resting heart rate (RHR) (6), the electrocardiographic PR interval (7), atrial fibrillation (AF) (8) and left ventricular mass (LVM) (9). These findings suggest an important functional role for SOX5 in the heart. Mice with Sox5 gene single null mutation were early lethal and had mild skeletal abnormalities (3). Sox5-deficient mice died at birth with respiratory distress and abnormal lung development, indicating that Sox5 is critical for proper in utero lung morphogenesis (10). To date, early lethality in loss of function Sox5 mouse models has prohibited analysis of adult cardiac function; a role for SOX5 in cardiac function has not been reported previously.
Drosophila melanogaster or fruit fly has been successfully used to characterize multiple genes associated with cardiac diseases (11–14). The basic mechanisms of heart development and control of cardiac function are highly conserved between human and Drosophila. Thus, discoveries made in the fly can be applied to higher species including human. In addition, Drosophila have a short life span and an oxygen transport system independent from its heart, which makes the organism more viable to genetic alterations that effect cardiac function (15). Anatomically, the fly heart lies close to the dorsal surface of the abdomen (14). Therefore, the morphological and rhythm changes can be readily analyzed in the relatively simple organization of the fly heart with the emerging biomedical imaging technology optical coherence tomography (OCT) (16).
Non-invasive OCT enables real-time, in vivo, micron-scale and three-dimensional (3D) imaging of biological tissues without the need to sacrifice and process specimens. OCT has been used for a wide range of clinical applications in human, including ophthalmology (16–19), endoscopy (20–26) and cardiovascular imaging (27–31). Drosophila's heart beats at a rate of several hundred beats per minute (BPM), making high imaging speed critical in order to capture the dynamics of its heart during various cardiac cycles. The utility of OCT for studying Drosophila cardiac functions has been reported by several studies (32–37). With a custom-built OCT imaging platform, we previously demonstrated acquiring cross-sectional OCT images at ∼120 frames/s, fast enough to capture the dynamics of the beating Drosophila heart, in order to assess the effect of Alzheimer's disease and dilated cardiomyopathy-associated presenilin gene. We found that either the overexpression or the silencing of the Drosophila ortholog of presenilin in the heart leads to cardiac dysfunction (36). To date, OCT has been used by our group and others to evaluate cardiac function using M-mode imaging at a single location of the Drosophila heart tube over time and concentrated on inferring structural information, such as heart dimensions during systole and diastole phase, as well as functional information, such as HR and arrhythmia prevalence, and to detect significant differences in these cardiac parameters in response to genetic alterations related to cardiac diseases (32–34,36). Previous studies, however, lacked the resolution and speed to resolve fine details such as cardiac wall dimensions, as well as the ability to measure wall dynamics using information from the entire cross-sectional heart chamber (32–34,36). With the advancements in the imaging speed as well as Doppler and phase sensitive detection, the use of OCT for Drosophila studies was further extended to study the dynamics of cardiac wall movement during various cardiac cycles (35,37).
In this study, we have developed a high-speed and ultrahigh-resolution OCT imaging system to non-invasively quantify Drosophila cardiac function more precisely and more comprehensively than previously possible. The new OCT system enables the 3D volumetric imaging of the Drosophila and rapidly captures the cross-sectional images of the heart tube over various cardiac cycles. By searching the Drosophila genome database, we determined that human SOX5 is highly conserved to a Drosophila ortholog Sox102F. SOX5 and Sox102F share 71% identity and 82% similarity in amino acid sequences. To circumvent the lethality of mouse models null for Sox5, we have employed transgenic Drosophila models and high-performance OCT imaging to quantitatively measure cardiac function in adult flies to assess the functional role of Sox102F in the heart. Our studies have demonstrated that the silencing of Sox102F in the heart results in cardiac dysfunction.
RESULTS
Drosophila cardiac function and heart structural analysis
We employed UAS-Sox102F-RNAi transgenic flies and an established 24B-GAL4 driver (38) to silence Sox102F specifically in the fly heart (38,39). The 24B-GAL4 line allows targeted expression in mesoderm and all cardiac and muscular cells with a uniform and high level of expression in the most anterior heart cells (40). Flies in which Sox102F was silenced by 24B-GAL4 (UAS-Sox102F-RNAi; 24B-GAL4) appeared normal at eclosion. The silencing of Sox102F led to a decrease in Sox102F expression to 55% compared with control flies that expressed a heterozygous 24B-GAL4 alone (24B-GAL4/+), as assessed by real-time reverse transcriptase (RT)–polymerase chain reaction (PCR) of cDNA from the hearts of the UAS-Sox102F-RNAi; 24B-GAL4 and 24B-GAL4/+ flies.
We quantitatively measured cardiac function in 30-day-old UAS-Sox102F-RNAi; 24B-GAL4 flies (n = 35) and age-matched control flies (n = 36) using the newly developed OCT system. Since the imaging resolution and speed were dramatically improved compared with our previous system (36), this analysis allows us to have a more precise measurement of the chamber dimensions, as well as enables us to use Doppler OCT to measure cardiac wall velocity versus time. As can be seen in Figure 1, where an example of volumetric and cross-sectional OCT images of an adult Drosophila are shown, OCT can readily identify at least the first three heart segments (ostia) of the fly (Fig. 1B).
Figure 1.
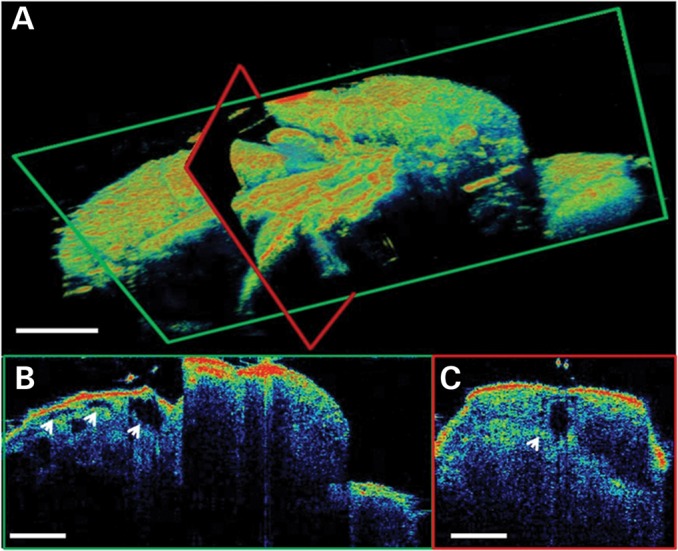
OCT imaging of adult Drosophila. (A) 3D reconstruction from a volumetric data set of an adult Drosophila. (B) Cross-sectional OCT view along the fly body showing detailed structures in the sagittal plane. Arrows point to the first three ostia, identified as empty, elongated tubes. (C) Cross-sectional OCT view across the fly's abdomen showing a detailed structure of the main heart chamber (conical heart chamber). As indicated by the arrow, the heart is observed as an empty space in the images. Images are false color coded and the higher intensity signal is represented by a color closer to red. Scale bars: 250 µm.
Analyses of OCT imaging data for assessing cardiac function were categorized as structural parameters, including the end systolic and diastolic vertical and transverse dimensions (ESDv, ESDt, EDDv and EDDt), end systolic and diastolic areas (ESA and EDA) and cardiac wall thickness and dynamical parameters, including HR, systolic and diastolic wall velocities and the prevalence of arrhythmia (Table 1). Compared with the age-matched controls, structurally, the silencing of Sox102F in flies (UAS-Sox102F-RNAi; 24B-GAL4) resulted in a significant decrease in each of the structural parameters, including ESDv, ESDt, EDDv, EDDt, ESA and EDA, and a significant increase in cardiac wall thickness from 10.80 ± 0.28 µm in control to 12.23 ± 0.30 µm in UAS-Sox102F-RNAi; 24B-GAL4 flies (Table 1, Fig. 2). Dynamically, the silencing of Sox102F caused a significant decrease in HR from 239 ± 8 to 211 ± 10 BPM in UAS-Sox102F-RNAi flies and both systolic and diastolic wall velocities (Table 1, Figs 2 and 3) and an increase in the cardiac arrhythmia prevalence, although arrhythmia prevalence did not reach statistical significance (Table 1).
Table 1.
Comparison of the cardiac structural and dynamical parameters in control flies and UAS-Sox102F-RNAi; 24B-GAL4 flies derived from the OCT images
| Parameter (mean ± SE) | 24B-GAL4/+ (n = 36) | UAS-Sox102F-RNAi; 24B-GAL4 (n = 35) | P-value | |
|---|---|---|---|---|
| Structural parameters | ESDt (µm) | 32 ± 4 | 19 ± 3 | 0.001* |
| EDDt (µm) | 64 ± 4 | 54 ± 3 | 0.02* | |
| ESDv (µm) | 37 ± 4 | 19 ± 3 | <0.0001* | |
| EDDv (µm) | 71 ± 4 | 53 ± 4 | 0.0006* | |
| ESA (µm2) | 1233 ± 160 | 562 ± 149 | 0.002* | |
| EDA (µm2) | 3473 ± 300 | 2241 ± 265 | 0.002* | |
| Cardiac wall thickness (µm) | 10.80 ± 0.28 | 12.23 ± 0.30 | 0.0006* | |
| Dynamical parameters | HR (BPM) | 239 ± 8 | 211 ± 10 | 0.02* |
| Systolic wall velocity (µm/s) | 877 ± 64 | 724 ± 33 | 0.02* | |
| Diastolic wall velocity (µm/s) | 762 ± 41 | 575 ± 29 | 0.0002* | |
| Arrhythmia prevalence | 15/35 (42%) | 21/36 (60%) | 0.1 |
*Statistically significant difference, P < 0.05.
Figure 2.
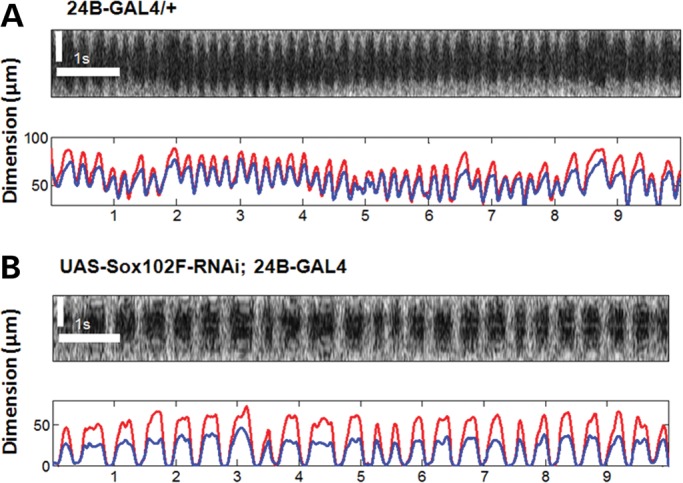
Obtaining functional information about cardiac function from structural OCT images. (A) A pseudo M-mode OCT image from the center of the main heart chamber (top) and the automatically generated plots for the heart dimensions (below) of a fly from the control group. Rapidly repeated cross-sectional OCT images over the clearest and largest heart chamber were performed for each fly where the single A-scan over the center of the heart chamber in each cross-sectional image is extracted to create a pseudo M-mode OCT image. The regularity of the heart beats can be visualized from the M-mode image as well as the resulting dimension plot. This particular fly has been characterized with a HR of 258 BPM, EDDt and EDDv of 80 and 108 µm, respectively, ESDt and ESDv of 49 and 71 µm, respectively, ESA and EDA of 2605 and 5644 µm2, respectively. (B) A pseudo M-mode OCT image (top) and the automatically generated plots for the heart dimensions (bottom) of a fly from the UAS-Sox102F-RNAi; 24B-GAL4 group. The M-mode image shows irregular beating patterns. This particular fly has been characterized with a HR of 138 BPM, EDDt and EDDv of 54 and 32 µm, respectively, ESDt and ESDv of 1 and 1.4 µm, respectively, ESA and EDA of 6 and 1036 µm2, respectively. Red lines in the dimension plots correspond to axial dimensions, whereas the blue lines correspond to transverse dimensions. Scale bars: 50 µm in the vertical dimension; 1 s in the horizontal dimension.
Figure 3.
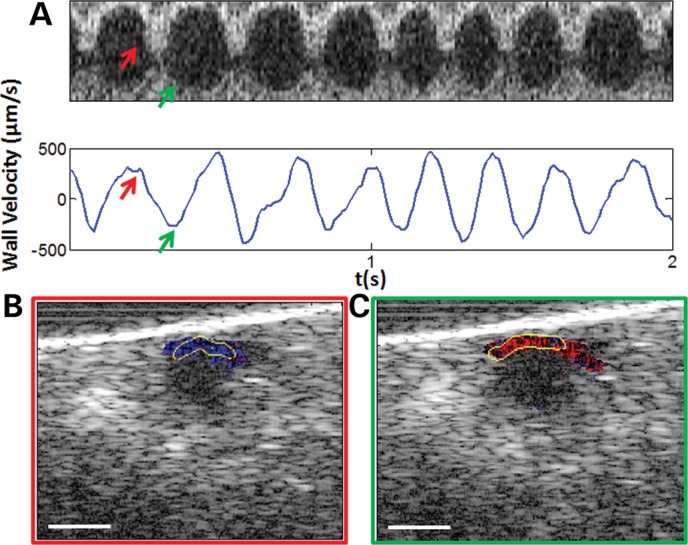
Extracting systolic and diastolic wall velocities using Doppler OCT. (A) A pseudo M-mode OCT image from the center of the main heart chamber (top) and the corresponding wall velocity plot extracted by Doppler analysis (bottom). A positive peak velocity is observed during the systole (an example is indicated by the red arrows), and a negative peak velocity is observed during the diastole (an example is indicated by the green arrows). A smoothing filter has been applied to the Doppler plot in order to highlight the velocity profile more clearly. (B and C) Cross-sectional color Doppler OCT image showing the localization of the Doppler signal at the upper portion of the heart wall during the systolic and diastolic phases. The outer boundary of the automatically segmented heart wall is shown by the yellow curve. Scale bars: 50 µm.
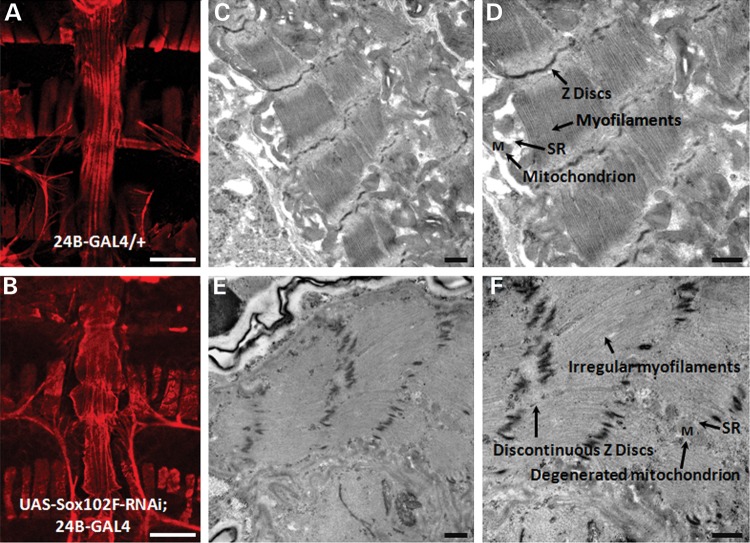
We analyzed 30-day-old flies' whole heart by F-actin immunostaining and heart ultrastructure by transmission electron microscopy (TEM). Fluorescent labeling of the whole adult heart with F-actin demonstrated that the silencing of Sox102F resulted in an enlarged and irregular cardiac tube and loss of myofibril structure (Fig. 4B) compared with that of control (Fig. 4A). TEM analysis of the adult hearts of control flies showed normal myofibril structure (Fig. 4C and D). In contrast, hearts from flies in which Sox102F was silenced revealed irregular and broken myofilament arrays with larger gaps between the individual filaments, discontinuous Z discs, irregular and smaller sarcoplasmic reticulum (SR) and degenerative mitochondria (Fig. 4E and F) consistent with whole heart and cardiac functional alterations. Collectively, these data demonstrate that the silencing of Sox102F led to cardiac hypertrophy, a decrease in the size of the heart chamber and a decrease in HR and wall velocities, accompanied by disrupted myofibril structure in adult flies.
Figure 4.
Whole heart and cardiac ultrastructure analysis. (A and B) Micrographs of the whole adult heart by F-actin immuno-fluorescent staining. (A) Control fly (24B-GAL4/+) showed a normal cardiac tube. (B) Silencing of Sox102F in the heart (UAS-Sox102F-RNAi; 24B-GAL4) resulted in an enlarged and irregular cardiac tube and loss of myofibril structure. (C–F) Ultrastructure of adult heart longitudinal sections between A1 and A3 segments by TEM. (C and D) Control fly showed normal myofibril structure. (E and F) Silencing of Sox102F in the heart led to irregular myofilament arrays, discontinuous Z discs, irregular and smaller SR and degenerative mitochondria. Myofilaments, Z discs, SR and mitochondrion were labeled and pointed with arrows. Magnification: (A and B) 10×, scale bars: 100 µm; (C and E) 20 000×; (D and F) 30 000×, scale bars: 500 nm.
Wing phenotype and wingless expression
Wing development is regulated by multiple signaling pathways, including Notch, Wnt, epidermal growth factor receptor, hedgehog and decapentaplegic. The wing pattern has provided an important tool in isolating and characterizing genes affecting these signaling pathways (41). Wnts are a family of secreted signaling proteins including wingless (wg), the central component of Wnt signaling pathway (42). To identify the functional pathway of SOX5, we silenced Sox102F specifically in the wing. SD-GAL4 drives the silencing of Sox102F in the wing disc in the pattern of the scalloped (sd) gene, which regulates the expression of a number of targeted genes including wg (42). Over 200 flies were analyzed for the wing phenotype. Heterozygous SD-GAL4 driver alone (SD-GAL4/+) showed normal wing (Fig. 5A–C). The silencing of Sox102F in the wing (UAS-Sox102F-RNAi; SD-GAL4) resulted in a significant increase in the L2, L3 and wing marginal veins; in particular, extra veins were formed in the distal part of L3 (Fig. 5D–F). We next examined the expression of the wg protein by immunostaining wing imaginal discs dissected from the third instar larvae with an anti-wg antibody. In control flies with heterozygous SD-GAL4 driver alone (SD-GAL4/+), wg was expressed as a broad strip in the notum, a thinner strip in the prospective wing margin-dorsal/ventral (D/V) boundary and a strip encircling the prospective wing blade (Fig. 5G). Consistent with the phenotype observed in adult flies, we observed remarkably increased wg expression levels and disorganized wg expression pattern in the third instar wing discs in which Sox102F was silenced, leading to extra strips in the prospective wing blade region (Fig. 5H). These data strongly support Sox102F as a regulator of wg expression.
Figure 5.
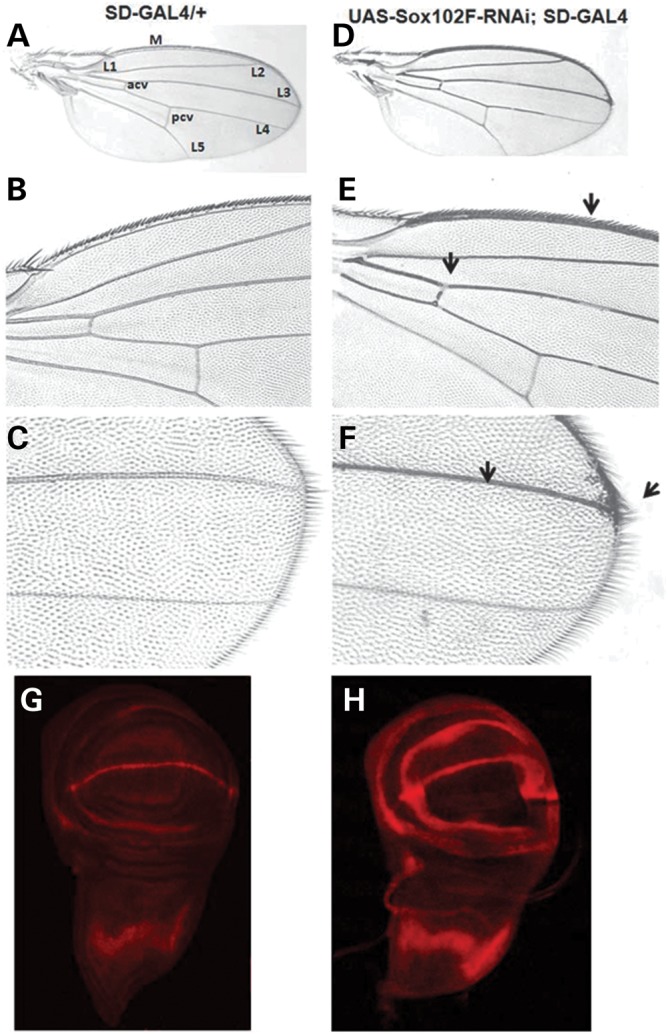
Silencing of Sox102F in the wing resulted in wing vein phenotypes and altered wg expression in wing disc. (A–C) Control fly with heterozygous SD-GAL4 driver alone (SD-GAL4/+) showed normal wing structure. (D–F) Silencing of Sox102F in the wing (UAS-Sox102F-RNAi; SD-GAL4) led to a significant increase in the L2, L3 and wing marginal veins; in particular, extra veins were formed in the distal part of L3 vein (arrows). (G) In control flies, wg was expressed as a broad strip in the notum, a thinner strip in the prospective wing margin-D/V boundary and a strip encircling the prospective wing blade. (H) In flies in which Sox102F was silenced, there were increased wg expression level and disorganized wg expression pattern, forming extra strips in the prospective wing blade region.
DISCUSSION
In this study, we have provided clear evidence indicating that the silencing of Sox102F leads to severe cardiac dysfunction and structural defects in adult flies, including cardiac hypertrophy, reduced heart chamber size and decrease HR and wall velocities, accompanied by disrupted myofibril structure, and that the expression of Sox102F can regulate wg expression. Variants in SOX5 have been significantly associated with multiple cardiac diseases or traits, including the higher RHR (6), electrocardiographic PR interval (7), AF (8) and LVM (9). This is the first report of a systematic assessment of the functional role of the Drosophila ortholog of SOX5 in a whole animal in vivo. These findings implicate that SOX5 may play an important role in regulating cardiac function.
We have developed a high-speed and ultrahigh-resolution OCT imaging system that enables successful characterization of structural and dynamical parameters of cardiac function in adult flies. Notably, the cardiac wall thickness is ∼10 µm, and the differences between the two groups included in this study were <2 µm. This highlights the necessity of using a high-resolution imaging system in order to be able to distinguish these subtle differences. Moreover, as the velocity of the heart wall is relatively low, as opposed to the velocity of blood flow traditionally measured with Doppler OCT methods, imaging and analysis protocols need to be carefully designed in order to overcome the deleterious effects of phase noise and bulk sample motion. A high-speed imaging system is imperative in this respect, as extracting Doppler information from rapidly scanned cross-sectional images is more reliable and robust compared with using an M-mode image from a single transverse position. This is due to the additional structural information gained from cross-sections. Higher speed imaging systems will provide 4D data sets (3D + time) in high frame rates, which will allow characterizing the velocity and other functional parameters along the entire heart tube of the fly. Important parameters such as cardiac output (Q) can then be extracted from the 4D (3D + time) data sets for the comprehensive assessment of cardiac function.
The Wnt signal transduction has been implicated as an important event that regulates cardiac development and function (43). The Wnt signaling pathway controls β-catenin, which enters the nucleus, binds to DNA and triggers expression of genes (44). SOX5 acts synergistically with a stable form of β-catenin and increases the expression of axin2, a negative regulator of the Wnt pathway in neural tube cells (5). Consistent with previous findings, we have demonstrated that the silencing of Sox102F promoted the formation of wing marginal veins and a remarkably increased and disorganized level of wg expression in flies. These data support the contention that SOX5 negatively regulates Wnt signaling via reducing wg expression in addition to increasing axin2 expression. The finding that the silencing of Sox102F resulted in cardiac hypertrophy accompanied with remarkably increased wg expression is consistent with the known role of Wnt signaling in heart formation. Down-regulation of the Wnt pathway leads to a thinner cardiac tube by gene expression profiling in Drosophila heart formation (45). The inhibition of Sox102F on the Wnt signaling may contribute to the pathogenesis that led to cardiac dysfunction in flies in which Sox102F was silenced.
A single-nucleotide polymorphism (SNP) rs17287293 which is located 3′ to SOX5 was significantly associated with the higher RHR in a meta-analysis of 15 GWASs (6). Epidemiologic studies have demonstrated that the higher RHR is significantly associated with increased cardiovascular disease and mortality risk independent of traditional risk factors in diverse subgroups, including healthy people in the general population (46,47), hypertensives (48) and those with established ischemic heart disease (47,49,50). A higher RHR also has been associated with poorer prognosis within subgroups of patients with cardiovascular disease (51–53). Compared with those whose RHR was consistent at <70 BPM, individuals whose rates increased from <70 to >85 BPM had a 90% higher risk of death from heart disease after 12 years of follow-up of 29 325 healthy men and women who had no known heart disease at the start of the study (50). In a 5-year follow-up, 5139 healthy middle-aged men who showed that an increase of >3 BPM in the RHR was associated with a 19% increase in all-cause mortality (54).
In other GWASs, the SNP rs11047543 located 3′ to SOX5 was significantly associated with both the electrocardiographic PR interval (7) and AF (8). The disturbances of the PR interval is considered an intermediate phenotype for AF and an increase risk of AF, which is independently associated with an increased risk of stroke, heart failure, dementia and death. The minor allele of SNP rs11047543 was significantly associated with a decrease in AF risk (7). Furthermore, the SNP rs10743465 3′ to SOX5 was the most significantly associated with LVM in a follow-up study for LVM on chromosome 12p11 (9). An increase in LVM is one of the most important cardiac risk factors for stroke, myocardial infarction and chronic heart failure, independent of age, sex and race-ethnicity (55,56). In a landmark study, each 50 g/m increase in LVM was associated with an adjusted relative risk of cardiovascular disease of 1.49 and 1.57 in men and women, respectively (57). Moreover, diastolic dysfunction is linked to increased mortality in a range of conditions, including post-acute myocardial infarction, hypertension and chronic renal failure (58–62). Closely linked to diastolic function, alterations in cardiac wall velocities are associated with several cardiovascular disorders, mostly pronounced with hypertrophic cardiomyopathy (63–68).
An action potential is the first step in the chain of events leading to contraction in cardiac cells. The action potential stimulates Ca++ entry into cardiomyocytes through voltage-gated L-type Ca++ channels (LTCCs). Antisense oligonucleotides against SOX5 have been shown to significantly decrease the maximum charge movement in mouse myoblast cells (69). Charge movement is regulated by LTCCs (69), and the amplitude of LTCCs was increased in hypertrophied and failing myocytes, contributing to cardiac hypertrophy (70). Moreover, the nitric oxide (NO) level is significantly correlated with the occurrence of LVM (71). SOX5 regulated shear stress-modulated gene expression in an NO-dependent manner in endothelial cells (72), which may contribute to LVM pathogenesis. Collectively, these findings suggest that Sox5 may regulate LTCCs and that genetic variants in SOX5 may alter atrial action potential and atrioventricular conduction influencing risk for the higher RHR, disturbed PR interval, AF and LVM (73).
In addition to associations with cardiac diseases or traits, the SNP rs11046966 3′ to SOX5 has been associated with chronic obstructive pulmonary disease (10); the intronic SNP rs1522232 in SOX5 was associated with the rapid progression of AIDS (74); the other intronic SNP rs1464500 in SOX5 was associated with metabolic side effects of antipsychotic drugs, such as high-density lipoprotein levels in patients taking perphenazine (75) and triglyceride levels in subjects taking statins (76). Moreover, two missense mutations in SOX5, Gln362Pro and Glu367Gln, five amino acids apart, were identified in 2 of the 190 amyotrophic lateral sclerosis patients and not detected in 190 normal controls (77). Further studies are warranted to elucidate the functional role(s) of SOX5 in multiple human diseases or traits.
In summary, we have demonstrated that the silencing of Sox102F in the Drosophila heart results in cardiac dysfunction. Together, these data provide the first in vivo evidence for an important functional role for SOX5 in the heart and suggest that the alterations in SOX5 levels may contribute to the pathogenesis of multiple cardiac diseases or traits.
MATERIALS AND METHODS
Transgenic flies and fly culture
Fly culture and crosses were carried out according to the standard procedures. The following fly strains were used: UAS-Sox102F-RNAi from the TRiP Project at Harvard Medical School (http://www.flyrnai.org/TRiP-HOME.html), 24B-GAL4 (38) and SD-GAL4 (42).
Animal preparation
Flies were anesthetized in a closed container using FlyNap® (Carolina Biological Supply Company) for 2 min. FlyNap® is an established anesthetizing agent used in Drosophila studies, and its main constituent, triethylamine, is shown to have minimal effect on influencing fly cardiac functions (13). After anesthesia, flies were taped on a microscope cover slide by their wings with the dorsal side facing up. OCT imaging was conducted within 30 min, before the effects of anesthesia wore off in order to minimize motion artifacts.
High-speed and ultrahigh-resolution OCT imaging system and OCT imaging for the Drosophila cardiac function assessment
A custom-built spectral domain OCT system was used for the study. The OCT system was optimized for high-speed and ultrahigh-resolution imaging, where a broadband superluminescent diode (Superlum, Dublin, Ireland) centered at ∼850 nm was employed to provide ∼3 µm axial resolutions in tissue. A long working distance near infrared objective (Thorlabs, Newton, NJ, USA) was used to achieve 8 µm resolution in the transverse dimensions. A 25 kHz line rate line-scan camera (e2v, Chelmsford, UK) was used to generate cross-sectional images at 110 frames/s with 226 axial scans per frame, which is sufficient to capture the chamber morphology at various cardiac cycles of a beating fly heart. The imaging speed and the transverse imaging resolution were improved by a factor of ∼2, and the axial resolution is improved by more than a factor of 3 compared with the OCT system used in our previous studies on Drosophila (36). Dynamical and structural parameters including HR, ESDv, ESDt, EDDv, EDDt, ESA, EDA, wall thickness and systolic and diastolic wall velocities of the heart wall were extracted from cross-sectional images obtained from the heart chamber ∼50 µm away from the chest. This cross-sectional imaging plane was selected for analysis as it consistently presents the clearest and largest heart chamber in all the flies. Each cross-sectional imaging session was acquired continuously for 10 s and repeated three times in order to capture multiple cardiac cycles for accurate analysis. Post-processing of the image data was performed with the custom-written MATLAB (Mathworks, Natick, MA, USA) code. The prevalence of arrhythmia in each fly group (%) was quantitatively analyzed via identifying the irregular heartbeat rhythm in the dimension plots.
Since the Drosophila hemolymph is essentially transparent due to lack of red blood cells, obtaining flow information from the hemolymph is not possible without using extrinsic contrast agents. However, it is possible to extract Doppler OCT information from the heart wall as demonstrated in previous studies (35,37). Doppler analysis of the cardiac wall relied on an established method in the OCT literature, which requires oversampling in the spatial dimension and extracting motion information by finding the phase difference between two successive A-scans and correcting the calculated phase for bulk motion effects (78,79). We were able to extract systolic and diastolic cardiac wall velocity profiles from the flies using Doppler OCT. The upper portion of the heart wall was automatically segmented in order to calculate velocity from the region of the wall that has movement in the vertical direction, as the Doppler analysis method employed in this study can only measure velocity in parallel to the scanning beam. From the resulting M-mode Doppler velocity plots, systolic and diastolic wall velocities were calculated by finding the mean of positive and negative peak velocity values, respectively.
Drosophila heart structural analysis by F-actin immunostaining microscopy and TEM
Hearts from 30-day-old control or silencing of Sox102F flies were dissected for whole heart and cardiac ultrastructure analyses. Fluorescent labeling of the whole adult heart tube with F-actin and imaging were done as described previously (80). In brief, 15 hearts from control or silencing of Sox102F flies were dissected in oxygenated hemolymph and fixed with 4% formaldehyde. The sarcomeric F-actin was stained with AlexaFluor® 594 phalloidin (red, Life Technologies, Grand Island, NY, USA) and imaged with a Zeiss confocal microscope. For TEM, 15 hearts from control or silencing of Sox102F flies were fixed, embedded, sectioned, examined and imaged as described previously (81). In brief, tissues were fixed in 2.0% glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA, USA) overnight at 4°C. They were rinsed in buffer, post-fixed in 1.0% osmium tetroxide in cacodylate buffer for 1 h at room temperature, rinsed in buffer and dehydrated through a graded series of ethanol to 100%. They were then infiltrated with Epon resin (Ted Pella, Redding, CA, USA) in a 1:1 solution of Epon:ethanol. The following day they were placed in fresh Epon for several hours and then embedded in Epon overnight at 60°C. Thin sections were cut on a Leica EM UC7 ultramicrotome, collected on formvar-coated grids, stained with uranyl acetate and lead citrate and examined in a JEOL JEM 1011 transmission electron microscope at 80 kV. Images were collected using an AMT digital imaging system (Advanced Microscopy Techniques, Danvers, MA, USA).
Analysis of the wing phenotype and immunostaining for wg expression
Wings from adult flies were dissected in isopropanol and mounted in Canada Balsam mounting medium. A total of 30 third instar wing discs were dissected, fixed, blocked and probed with primary and secondary antibodies accordingly. The following antibodies were used: mouse anti-wg antibody (1:1000, Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and the secondary anti-mouse Alexa 546 (1:200, Life Technologies).
cDNA synthesis and real-time RT–PCR
The cDNAs used for real-time RT–PCR were synthesized from total RNA isolated from the heart muscle of 30-day-old adult Drosophila. The cDNA was synthesized using SUPERSCRIPT Preamplification System for first-strand cDNA synthesis (Life Technologies). Real-time RT–PCR quantification with Sox102F or dActin-specific sense and antisense primers was done on an iCycler (BIO-RAD, Hercules, CA, USA) using SYBR Green PCR Core Reagents (Qiagen, Germantown, MD, USA) according to the manufacturer's instructions. The sequences of primers are: Sox102F 5′-TGGTGGCTCAGGAAGTCTCT, 3′-AACTGCTGAGGGGGTGTATG; RT–PCR product size 171 bp; Actin5C 5′-TACCCCATTGAGCACGGTAT, 3′-GGTCATCTTCTCACGGTTGG; RT–PCR product size 156 bp. The house-keeping gene dActin was used as an internal control and was co-amplified under the same PCR conditions. All standards and unknown samples were run in triplicates per reaction. The fluorescence intensity was calculated using iCycler software version 3.1. The expressions of Sox102F were given as a relative number of copies (%) of mRNA molecules, as calibrated by co-amplification of dActin.
Statistical analysis of cardiac function
A custom MATLAB (Mathworks) script was used to automatically segment the fly heart chamber from cross-sectional OCT images and extract functional and structural parameters described previously. Average values for these parameters were calculated from the measurement of multiple cardiac cycles and repeated imaging sessions. The mean ± SE of each parameter was reported for each fly group.
The Student's t-test was performed to statistically compare the difference between the UAS-Sox102F-RNAi; 24B-GAL4 group and the aged matched 24B-GAL4/+ control group and P < 0.05 was defined as statistically significant. Irregular heartbeat rhythm was identified if it occurred during imaging and the prevalence of cardiac arrhythmia was statistically compared between the two groups using a two-proportion z-test.
FUNDING
This work was supported by the Cure Alzheimer's Fund to R.E.T., the National Institute of Health (R01AG014713 and R01MH60009 to R.E.T.; R01CA75289 and R01HL095717 to J.G.F.; R00EB010071 to C.Z.; R03AR063271 to A.L.), the Air Force Office of Scientific Research (FA9550-07-1-0014 to J.G.F.) and a Massachusetts General Hospital ECOR Award to A.L. The Microscopy Core Facility at the MGH Program in Membrane Biology receives support from the Boston Area Diabetes and Endocrinology Research Center (DK 57521) and the Center for the Study of Inflammatory Bowel Disease (DK 43351).
Acknowledgments
Conflict of Interest statement. None declared.
REFERENCES
- 1.Remenyi A., Lins K., Nissen L.J., Reinbold R., Scholer H.R., Wilmanns M. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 2003;17:2048–2059. doi: 10.1101/gad.269303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wunderle V.M., Critcher R., Ashworth A., Goodfellow P.N. Cloning and characterization of SOX5, a new member of the human SOX gene family. Genomics. 1996;36:354–358. doi: 10.1006/geno.1996.0474. [DOI] [PubMed] [Google Scholar]
- 3.Smits P., Li P., Mandel J., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B., Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 4.Shim S., Kwan K.Y., Li M., Lefebvre V., Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Morales P.L., Quiroga A.C., Barbas J.A., Morales A.V. SOX5 controls cell cycle progression in neural progenitors by interfering with the WNT-beta-catenin pathway. EMBO Rep. 2010;11:466–472. doi: 10.1038/embor.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eijgelsheim M., Newton-Cheh C., Sotoodehnia N., de Bakker P.I., Muller M., Morrison A.C., Smith A.V., Isaacs A., Sanna S., Dorr M., et al. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum. Mol. Genet. 2010;19:3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeufer A., van Noord C., Marciante K.D., Arking D.E., Larson M.G., Smith A.V., Tarasov K.V., Muller M., Sotoodehnia N., Sinner M.F., et al. Genome-wide association study of PR interval. Nat. Genet. 2010;42:153–159. doi: 10.1038/ng.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olesen M.S., Holst A.G., Jabbari J., Nielsen J.B., Christophersen I.E., Sajadieh A., Haunso S., Svendsen J.H. Genetic loci on chromosomes 4q25, 7p31, and 12p12 are associated with onset of lone atrial fibrillation before the age of 40 years. Can. J. Cardiol. 2012;28:191–195. doi: 10.1016/j.cjca.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Della-Morte D., Beecham A., Rundek T., Wang L., McClendon M.S., Slifer S., Blanton S.H., Di Tullio M.R., Sacco R.L. A follow-up study for left ventricular mass on chromosome 12p11 identifies potential candidate genes. BMC Med. Genet. 2011;12:100. doi: 10.1186/1471-2350-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersh C.P., Silverman E.K., Gascon J., Bhattacharya S., Klanderman B.J., Litonjua A.A., Lefebvre V., Sparrow D., Reilly J.J., Anderson W.H., et al. SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am. J. Respir. Crit. Care Med. 2011;183:1482–1489. doi: 10.1164/rccm.201010-1751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocorr K., Reeves N.L., Wessells R.J., Fink M., Chen H.S., Akasaka T., Yasuda S., Metzger J.M., Giles W., Posakony J.W., et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc. Natl Acad. Sci. USA. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigoreaux J.O. Genetics of the Drosophila flight muscle myofibril: a window into the biology of complex systems. Bioessays. 2001;23:1047–1063. doi: 10.1002/bies.1150. [DOI] [PubMed] [Google Scholar]
- 13.Paternostro G., Vignola C., Bartsch D.U., Omens J.H., McCulloch A.D., Reed J.C. Age-associated cardiac dysfunction in Drosophila melanogaster. Circ. Res. 2001;88:1053–1058. doi: 10.1161/hh1001.090857. [DOI] [PubMed] [Google Scholar]
- 14.Curtis N.J., Ringo J.M., Dowse H.B. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, Drosophila melanogaster. J. Morphol. 1999;240:225–235. doi: 10.1002/(SICI)1097-4687(199906)240:3<225::AID-JMOR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura M., Ocorr K., Bodmer R., Cartry J. Drosophila as a model to study cardiac aging. Exp. Gerontol. 2011;46:326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W., Hee M.R., Flotte T., Gregory K., Puliafito C.A., et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drexler W., Morgner U., Ghanta R.K., Kärtner F.X., Schuman J.S., Fujimoto J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001;7:502–507. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakata L.M., DeLeon-Ortega J., Sakata V., Girkin C.A. Optical coherence tomography of the retina and optic nerve - a review. Clin. Exp. Ophthalmol. 2009;37:90–99. doi: 10.1111/j.1442-9071.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 19.Geitzenauer W., Hitzenberger C.K., Schmidt-Erfurth U.M. Retinal optical coherence tomography: past, present and future perspectives. Br. J. Ophthalmol. 2011;95:171–177. doi: 10.1136/bjo.2010.182170. [DOI] [PubMed] [Google Scholar]
- 20.Tearney G.J., Brezinski M.E., Bouma B.E., Boppart S.A., Pitvis C., Southern J.F., Fujimoto J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276:2037–2039. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 21.Bouma B.E., Tearney G.J., Compton C.C., Nishioka N.S. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest. Endosc. 2000;51:467–474. doi: 10.1016/s0016-5107(00)70449-4. [DOI] [PubMed] [Google Scholar]
- 22.Sivak M.V., Kobayashi K., Izatt J.A., Rollins A.M., Ung-runyawee R., Chak A., Wong R.C.K., Isenberg G.A., Willis J. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest. Endosc. 2000;51:474–479. doi: 10.1016/s0016-5107(00)70450-0. [DOI] [PubMed] [Google Scholar]
- 23.Suter M.J., Jillella P.A., Vakoc B.J., Halpern E.F., Mino-Kenudson M., Lauwers G.Y., Bouma B.E., Nishioka N.S., Tearney G.J. Image-guided biopsy in the esophagus through comprehensive optical frequency domain imaging and laser marking: a study in living swine. Gastrointest. Endosc. 2010;71:346–353. doi: 10.1016/j.gie.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X.D., Boppart S.A., Van Dam J., Mashimo H., Mutinga M., Drexler W., Klein M., Pitris C., Krinsky M.L., Brezinski M.E., et al. Optical coherence tomography: advanced technology for the endoscopic imaging of Barrett's esophagus. Endoscopy. 2000;32:921–930. doi: 10.1055/s-2000-9626. [DOI] [PubMed] [Google Scholar]
- 25.Hatta W., Uno K., Koike T., Yokosawa S., Iijima K., Imatani A., Shimosegawa T. Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma. Gastrointest. Endosc. 2010;71:899–906. doi: 10.1016/j.gie.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C., Tsai T.H., Lee H.C., Kirtane T., Figueiredo M., Tao Y.K.K., Ahsen O.O., Adler D.C., Schmitt J.M., Huang Q., et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos) Gastrointest. Endosc. 2012;76:32–40. doi: 10.1016/j.gie.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tearney G.J., Brezinski M.E., Southern J.F., Bouma B.E., Boppart S.A., Fujimoto J.G. Optical biopsy in human gastrointestinal tissue using optical coherence tomography. Am. J. Gastroenterol. 1997;92:1800–1804. [PubMed] [Google Scholar]
- 28.Barlis P., van Soest G., Serruys P.W., Regar E. Intracoronary optical coherence tomography and the evaluation of stents. Exp. Rev. Med. Devices. 2009;6:157–167. doi: 10.1586/17434440.6.2.157. [DOI] [PubMed] [Google Scholar]
- 29.Bezerra H.G., Costa M.A., Guagliumi G., Rollins A.M., Simon D.I. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc. Interv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang I.K., Bouma B.E., Kang D.H., Park S.J., Park S.W., Seung K.B., Choi K.B., Shishkov M., Schlendorf K., Pomerantsev E., et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez-Chico J.L., Alegria-Barrero E., Teijeiro-Mestre R., Chan P.H., Tsujioka H., de Silva R., Viceconte N., Lindsay A., Patterson T., Foin N., et al. Optical coherence tomography: from research to practice. Eur. Heart J. Cardiovasc. Imaging. 2012;13:370–384. doi: 10.1093/ehjci/jes025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf M.J., Amrein H., Izatt J.A., Choma M.A., Reedy M.C., Rockman H.A. Drosophila as a model for the identification of genes causing adult human heart disease. Proc. Natl Acad. Sci. USA. 2006;103:1394–1399. doi: 10.1073/pnas.0507359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choma M.A., Izatt S.D., Wessells R.J., Bodmer R., Izatt J.A. Images in cardiovascular medicine: in vivo imaging of the adult Drosophila melanogaster heart with real-time optical coherence tomography. Circulation. 2006;114:e35–e36. doi: 10.1161/CIRCULATIONAHA.105.593541. [DOI] [PubMed] [Google Scholar]
- 34.Allikian M.J., Bhabha G., Dospoy P., Heydemann A., Ryder P., Earley J.U., Wolf M.J., Rockman H.A., McNally E.M. Reduced life span with heart and muscle dysfunction in Drosophila sarcoglycan mutants. Hum. Mol. Genet. 2007;16:2933–2943. doi: 10.1093/hmg/ddm254. [DOI] [PubMed] [Google Scholar]
- 35.Choma M.A., Suter M.J., Vakoc B.J., Bouma B.E., Tearney G.J. Heart wall velocimetry and exogenous contrast-based cardiac flow imaging in Drosophila melanogaster using Doppler optical coherence tomography. J. Biomed. Optics. 2010;15:056020. doi: 10.1117/1.3503418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A., Zhou C., Moore J., Zhang P., Tsai T.H., Lee H.C., Romano D.M., McKee M.L., Schoenfeld D.A., Serra M.J., et al. Changes in the expression of the Alzheimer's disease-associated presenilin gene in Drosophila heart leads to cardiac dysfunction. Curr. Alzheimer Res. 2011;8:313–322. doi: 10.2174/156720511795563746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choma M.A., Suter M.J., Vakoc B.J., Bouma B.E., Tearney G.J. Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis. Models Mech. 2011;4:411–420. doi: 10.1242/dmm.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 39.Fischer J.A., Giniger E., Maniatis T., Ptashne M. Gal4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- 40.Zikova M., Da Ponte J.P., Dastugue B., Jagla K. Patterning of the cardiac outflow region in Drosophila. Proc. Natl Acad. Sci. USA. 2003;100:12189–12194. doi: 10.1073/pnas.2133156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturtevant M.A., Bier E. Analysis of the genetic hierarchy guiding wing vein development in Drosophila. Development. 1995;121:785–801. doi: 10.1242/dev.121.3.785. [DOI] [PubMed] [Google Scholar]
- 42.Paumard-Rigal S., Zider A., Vaudin P., Silber J. Specific interactions between vestigial and scalloped are required to promote wing tissue proliferation in Drosophila melanogaster. Dev. Genes. Evol. 1998;208:440–446. doi: 10.1007/s004270050201. [DOI] [PubMed] [Google Scholar]
- 43.Eisenberg L.M., Eisenberg C.A. Wnt signal transduction and the formation of the myocardium. Dev. Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Liebner S., Cattelino A., Gallini R., Rudini N., Iurlaro M., Piccolo S., Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell. Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeitouni B., Senatore S., Severac D., Aknin C., Semeriva M., Perrin L. Signalling pathways involved in adult heart formation revealed by gene expression profiling in Drosophila. PLoS Genet. 2007;3:1907–1921. doi: 10.1371/journal.pgen.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooney M.T., Vartiainen E., Laatikainen T., Juolevi A., Dudina A., Graham I.M. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2009;159:612–619. doi: 10.1016/j.ahj.2009.12.029. e613. [DOI] [PubMed] [Google Scholar]
- 47.Kannel W.B., Kannel C., Paffenbarger R.S., Jr, Cupples L.A. Heart rate and cardiovascular mortality: the Framingham study. Am. Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 48.Benetos A., Rudnichi A., Thomas F., Safar M., Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- 49.Woodward M., Webster R., Murakami Y., Barzi F., Lam T.H., Fang X., Suh I., Batty G.D., Huxley R., Rodgers A. The association between resting heart rate, cardiovascular disease and mortality: evidence from 112,680 men and women in 12 cohorts. Eur. J. Prev. Cardiol. 2012 doi: 10.1177/2047487312452501. doi:10.1177/2047487312452501. [DOI] [PubMed] [Google Scholar]
- 50.Nauman J., Janszky I., Vatten L.J., Wisloff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–2587. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 51.Fox K., Ford I., Steg P.G., Tendera M., Robertson M., Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 52.Fox K., Steg P.G. Elevated heart rate proven to increase coronary events. Cardiovasc. J. Afr. 2008;19:276–278. [PubMed] [Google Scholar]
- 53.Diaz A., Bourassa M.G., Guertin M.C., Tardif J.C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur. Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 54.Jouven X., Empana J.P., Escolano S., Buyck J.F., Tafflet M., Desnos M., Ducimetiere P. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am. J. Cardiol. 2009;103:279–283. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 55.Gosse P., Dallocchio M. Left ventricular hypertrophy: epidemiological prognosis and associated critical factors. Eur. Heart J. 1993;14(Suppl. D):16–21. doi: 10.1093/eurheartj/14.suppl_d.16. [DOI] [PubMed] [Google Scholar]
- 56.Haider A.W., Larson M.G., Benjamin E.J., Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J. Am. Coll. Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 57.Levy D., Garrison R.J., Savage D.D., Kannel W.B., Castelli W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 58.Moller J.E., Egstrup K., Kober L., Poulsen S.H., Nyvad O., Torp-Pedersen C. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am. Heart J. 2003;145:147–153. doi: 10.1067/mhj.2003.46. [DOI] [PubMed] [Google Scholar]
- 59.Hillis G.S., Moller J.E., Pellikka P.A., Gersh B.J., Wright R.S., Ommen S.R., Reeder G.S., Oh J.K. Noninvasive estimation of left ventricular filling pressure by E/e’ is a powerful predictor of survival after acute myocardial infarction. J. Am. Coll. Cardiol. 2004;43:360–367. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Wang M., Yip G.W., Wang A.Y., Zhang Y., Ho P.Y., Tse M.K., Yu C.M., Sanderson J.E. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J. Hypertens. 2005;23:183–191. doi: 10.1097/00004872-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 61.Sharma R., Pellerin D., Gaze D.C., Mehta R.L., Gregson H., Streather C.P., Collinson P.O., Brecker S.J. Mitral peak Doppler E-wave to peak mitral annulus velocity ratio is an accurate estimate of left ventricular filling pressure and predicts mortality in end-stage renal disease. J. Am. Soc. Echocardiogr. 2006;19:266–273. doi: 10.1016/j.echo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Nagueh S.F., Appleton C.P., Gillebert T.C., Marino P.N., Oh J.K., Smiseth O.A., Waggoner A.D., Flachskampf F.A., Pellikka P.A., Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 63.Matsumura Y., Elliott P.M., Virdee M.S., Sorajja P., Doi Y., McKenna W.J. Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart. 2002;87:247–251. doi: 10.1136/heart.87.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckberg D.L., Gault J.H., Bouchard R.L., Karliner J.S., Ross J. Mechanics of left ventricular contraction in chronic severe mitral regurgitation. Circulation. 1973;47:1252–1259. doi: 10.1161/01.cir.47.6.1252. [DOI] [PubMed] [Google Scholar]
- 65.Kovick R.B., Fogelman A.M., Abbasi A.S., Peter J.B., Pearce M.L. Echocardiographic evaluation of posterior left-ventricular wall motion in muscular-dystrophy. Circulation. 1975;52:447–454. doi: 10.1161/01.cir.52.3.447. [DOI] [PubMed] [Google Scholar]
- 66.Chikamori T., Dickie S., Poloniecki J.D., Myers M.J., Lavender J.P., Mckenna W.J. Prognostic-significance of radionuclide-assessed diastolic function in hypertrophic cardiomyopathy. Am. J. Cardiol. 1990;65:478–482. doi: 10.1016/0002-9149(90)90814-h. [DOI] [PubMed] [Google Scholar]
- 67.Pak P.H., Maughan W.L., Baughman K.L., Kass D.A. Marked discordance between dynamic and passive diastolic pressure-volume relations in idiopathic hypertrophic cardiomyopathy. Circulation. 1996;94:52–60. doi: 10.1161/01.cir.94.1.52. [DOI] [PubMed] [Google Scholar]
- 68.Galetta F., Franzoni F., Santoro G. Left ventricular diastolic function assessed using tissue Doppler imaging in elderly athletes. Int. J. Cardiol. 2004;94:339–340. doi: 10.1016/j.ijcard.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Zheng Z., Wang Z.M., Delbono O. Charge movement and transcription regulation of L-type calcium channel alpha(1S) in skeletal muscle cells. J. Physiol. 2002;540:397–409. doi: 10.1113/jphysiol.2001.013464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benitah J.P., Alvarez J.L., Gomez A.M. L-type Ca(2+) current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 71.Hua L., Li C., Xia D., Qu P., Li Z., Zhang W., Feng X. Relationship between hypertensive left ventricular hypertrophy and levels of endothelin and nitric oxide. Hypertens. Res. 2000;23:377–380. doi: 10.1291/hypres.23.377. [DOI] [PubMed] [Google Scholar]
- 72.Braam B., de Roos R., Bluyssen H., Kemmeren P., Holstege F., Joles J.A., Koomans H. Nitric oxide-dependent and nitric oxide-independent transcriptional responses to high shear stress in endothelial cells. Hypertension. 2005;45:672–680. doi: 10.1161/01.HYP.0000154683.33414.94. [DOI] [PubMed] [Google Scholar]
- 73.Olsson S.B., Cotoi S., Varnauskas E. Monophasic action potential and sinus rhythm stability after conversion of atrial fibrillation. Acta Med. Scand. 1971;190:381–387. doi: 10.1111/j.0954-6820.1971.tb07446.x. [DOI] [PubMed] [Google Scholar]
- 74.Le Clerc S., Limou S., Coulonges C., Carpentier W., Dina C., Taing L., Delaneau O., Labib T., Sladek R., Deveau C., et al. Genomewide association study of a rapid progression cohort identifies new susceptibility alleles for AIDS (ANRS Genomewide Association Study 03) J. Infect. Dis. 2009;200:1194–1201. doi: 10.1086/605892. [DOI] [PubMed] [Google Scholar]
- 75.Adkins D.E., Aberg K., McClay J.L., Bukszar J., Zhao Z., Jia P., Stroup T.S., Perkins D., McEvoy J.P., Lieberman J.A., et al. Genomewide pharmacogenomic study of metabolic side effects to antipsychotic drugs. Mol. Psychiatry. 2011;16:321–332. doi: 10.1038/mp.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barber M.J., Mangravite L.M., Hyde C.L., Chasman D.I., Smith J.D., McCarty C.A., Li X., Wilke R.A., Rieder M.J., Williams P.T., et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS One. 2010;5:e9763. doi: 10.1371/journal.pone.0009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daoud H., Valdmanis P.N., Gros-Louis F., Belzil V., Spiegelman D., Henrion E., Diallo O., Desjarlais A., Gauthier J., Camu W., et al. Resequencing of 29 candidate genes in patients with familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011;68:587–593. doi: 10.1001/archneurol.2010.351. [DOI] [PubMed] [Google Scholar]
- 78.White B., Pierce M., Nassif N., Cense B., Park B., Tearney G., Bouma B., Chen T., de Boer J. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography. Optics Express. 2003;11:3490–3497. doi: 10.1364/oe.11.003490. [DOI] [PubMed] [Google Scholar]
- 79.Leitgeb R., Schmetterer L., Drexler W., Fercher A., Zawadzki R., Bajraszewski T. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Optics Express. 2003;11:3116–3121. doi: 10.1364/oe.11.003116. [DOI] [PubMed] [Google Scholar]
- 80.Alayari N.N., Vogler G., Taghli-Lamallem O., Ocorr K., Bodmer R., Cammarato A. Fluorescent labeling of Drosophila heart structures. J. Vis. Exp. 2009;32:e1423. doi: 10.3791/1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sullivan William M.A., Scott Hawley R. Drosophila Protocols. Woodbury, NY: Cold Spring Harbor Labroratory Press; 2000. p. 245 pp. [Google Scholar]