SUMMARY
Zebrafish (Danio rerio) have become a valuable model for investigating the molecular genetics and development of the inner ear in vertebrates. In this study, we employed a prepulse inhibition (PPI) paradigm to assess hearing in larval wild-type (AB) zebrafish during early development at 5–6 days post-fertilization (d.p.f.). We measured the PPI of the acoustic startle response in zebrafish using a 1-dimensional shaker that simulated the particle motion component of sound along the fish's dorsoventral axis. The thresholds to startle-inducing stimuli were determined in 5–6 d.p.f. zebrafish, and their hearing sensitivity was then characterized using the thresholds of prepulse tone stimuli (90–1200 Hz) that inhibited the acoustic startle response to a reliable startle stimulus (820 Hz at 20 dB re. 1 m s−2). Hearing thresholds were defined as the minimum prepulse tone level required to significantly reduce the startle response probability compared with the baseline (no-prepulse) condition. Larval zebrafish showed greatest auditory sensitivity from 90 to 310 Hz with corresponding mean thresholds of −19 to −10 dB re. 1 m s−2, respectively. Hearing thresholds of prepulse tones were considerably lower than previously predicted by startle response assays. The PPI assay was also used to investigate the relative contribution of the lateral line to the detection of acoustic stimuli. After aminoglycoside-induced neuromast hair-cell ablation, we found no difference in PPI thresholds between treated and control fish. We propose that this PPI assay can be used to screen for novel zebrafish hearing mutants and to investigate the ontogeny of hearing in zebrafish and other fishes.
KEY WORDS: hearing, sensorimotor, startle response, lateral line
INTRODUCTION
Zebrafish, Danio rerio (Hamilton 1822), have become a valuable model for investigating the development and molecular genetics of the vertebrate inner ear (Whitfield, 2002; Nicolson, 2005). The early development of the zebrafish inner ear is similar to that of other vertebrates (Bang et al., 2001; Whitfield et al., 2002; Riley and Phillips, 2003) and its sensory hair cells are homologous to those found in mammals (Coffin et al., 2004). Over 50 genes are known to impact the zebrafish auditory inner ear and/or vestibular system (Granato et al., 1996; Whitfield et al., 1996; Whitfield et al., 2002; Riley and Phillips, 2003; Starr et al., 2004; Nicolson, 2005) and many of these genes are conserved and affect the inner ear development and function in other vertebrates, including humans (Nicolson et al., 1998; Moorman et al., 1999; Riley and Moorman, 2000; Busch-Nentwich et al., 2004; Kappler et al., 2004; Kozlowski et al., 2005). However, unlike mammals, zebrafish develop from eggs ex utero and are transparent during the first few weeks of life. These characteristics coupled with the animal's rapid generation time, ease of maintenance and accessibility of the inner ear make this animal an attractive genetic model to investigate inner ear development and hearing.
Despite the enormous potential of the zebrafish model to investigate the functional effects of genes on hearing, few behavioral hearing assays have been developed for zebrafish. The most commonly used behavioral measure of auditory function in larval zebrafish is the startle response (Bang et al., 2000; Bang et al., 2002). It is an innate, reliable and robust behavior elicited by fast, high intensity stimuli. The startle response is mediated by Mauthner cells (M-cells), which are large reticulospinal neurons that receive information from ipsilateral sensory afferents and synapse to contralateral spinal motor neurons (Eaton et al., 2001; Weiss et al., 2006). When activated, all of the motor neurons fire synchronously, causing the fish to bend into a characteristic ‘C’ shape away from the stimulus direction, which is easy to detect and differentiate from normal swimming motion. However, the use of the startle response only tests the grossest aspects of hearing and cannot be used to characterize differences in frequency selectivity or other auditory capabilities. Comparison of startle response thresholds with auditory-evoked potential (AEP) thresholds reveals a large difference in detection sensitivity between these two measures, which likely indicates that the startle response assay has a high rate of Type II error; i.e. the auditory stimulus is detected but is too weak to elicit a startle response. The development of acoustically evoked behavioral responses to pure tones in zebrafish has also been studied from 5 d.p.f. to adults (Zeddies and Fay, 2005) and a positive reinforcement conditioning assay has been developed recently for the assessment of hearing in adult zebrafish (Cervi et al., 2012).
The focus of this study was to develop a prepulse inhibition (PPI) paradigm to assess hearing in wild-type (AB) zebrafish during early larval development at 5–6 d.p.f. PPI is a well-studied phenomenon whereby a startle reflex elicited by a strong stimulus is inhibited by the prior presentation of a weaker stimulus (Hoffman and Ison, 1980). PPI and other behavioral suppression techniques have been used to investigate responses to acoustic stimuli since Yerkes (Yerkes, 1905), who showed that a pairing of tactile and acoustic stimuli elicited a greater response than a tactile stimulus alone; by systematically decreasing the intensity of acoustic stimuli and measuring response intensity, a behavioral hearing range could be constructed. Reflex inhibition and suppression methods have since been used to determine auditory sensitivity in rodents (Ison, 1982; Young and Fechter, 1983; Willott et al., 1994), chickens (Gray and Rubel, 1985) and humans (Ison and Pinckney, 1983). A PPI paradigm is advantageous over other behavioral techniques because it takes advantage of an innate response that does not need to be learned or conditioned, and the degree of inhibition has been shown to be proportional to the stimulus intensity (Young and Fechter, 1983; Neumeister et al., 2008).
Sound can be quantified in descriptive terms including pressure and particle motion. Most terrestrial ears respond to pressure, which is a scalar measure of sound that contains no directional information. In most cases, sound pressure can be readily measured using microphones or hydrophones. In contrast, particle motion is a vector measure of sound that includes directional cues and can be measured with accelerometers (or calculated from pressure gradient measurements). The inner ears of teleost fishes consist of one or more otolithic end organs that respond directly to particle motion and essentially function as accelerometers (Fay, 1984; Hawkins, 1993). Some fish, including adult zebrafish, have specialized adaptations that also allow them to sense the pressure component of sound; however, developmental studies have shown that 5 d.p.f. larval zebrafish lack these adaptations and would therefore only be sensitive to acoustic particle motion at this developmental stage (Higgs et al., 2003; Kimmel et al., 1995).
In this study, we assessed the acoustic (particle-motion) sensitivity of the inner ear in 5–6 d.p.f. larval, wild-type (AB) zebrafish using a PPI assay not previously used with fish. The M-cell-mediated startle response of zebrafish to an acoustic stimulus is modified by the prior presentation of a lower level acoustic stimulus. We show that the PPI assay is a more sensitive measure of the zebrafish auditory capability than the standard acoustic startle response assay. We also used the PPI assay to investigate the relative contribution of the lateral line to acoustic stimulus detection in 5–6 d.p.f. larval wild-type (AB) zebrafish and show that the lateral line is not involved in encoding the acoustic stimuli at the tested frequencies.
MATERIALS AND METHODS
Animals
Wild-type (AB) 5–6 d.p.f. zebrafish larvae (D. rerio) were obtained from an adult zebrafish colony housed at the University of Washington. Mating and egg collection were performed according to Westerfield (Westerfield, 2000). Fertilized eggs from mated adults were staged as detailed elsewhere (Kimmel et al., 1995) and raised in Petri dishes (density ≤50 larvae per dish) housed in incubators at 28.5°C. After 4 d.p.f., zebrafish larvae were fed live rotifers and then transferred to fresh embryo medium. At 4–6 d.p.f., larvae were transported to the testing facility in an insulated container, and were then tested the same day. All fish were transferred between containers and to the experimental apparatus using wide-bore pipettes in order to minimize damage of the lateral line neuromasts. Larvae were allowed to acclimate to the experimental lighting and temperature (27±1°C) conditions for 30 min before the experiments were conducted. Animal rearing and experimental procedures were approved by the University of Washington Animal Care and Use Committee.
Experimental setup
Sound produced by conventional speakers contains both acoustic pressure and particle motion. The use of a shaker allows for the fine stimulus control of acoustic particle motion in a single direction. The experimental apparatus consisted of a 96 square-well plate (containing 3.2 mm diameter wells) secured to a 0.635 cm thick acrylic platform that was mounted on to a vertically oriented Bruel-Kjaer Type 4810 shaker (Bruel and Kjaer, Naerum, Denmark). The apparatus was similar to that described elsewhere (Zeddies and Fay, 2005). Although the plate contained 96 wells, only a maximum of 36 central wells that formed a 6×6 array were used during the experiments because of the optical limitations of the high-speed camera. Individual fish with ~400 μl embryo medium were placed in each of the central test wells. The experimental apparatus was housed inside a sound attenuation chamber (Industrial Acoustics, New York, NY, USA) on a vibration-isolation air table, in order to minimize external vibratory noise. A TDT System III (Tucker Davis Technologies, Alachua, FL, USA) and a PC computer running a custom-written MATLAB stimulus generation program (The MathWorks, Natick, MA, USA) were used to relay the stimulus signal to a Bruel and Kjaer Type 2710 amplifier that drove the shaker and produced controlled vibratory stimuli along the dorsoventral axis of the fish within the well. An accelerometer (model 355B04, PCB Piezotronics., Depew, NY, USA) was mounted onto the acrylic platform in order to measure the acoustic particle acceleration of the fish in the plate wells. The output of the accelerometer was amplified (Model 482A PCB amplifier) and then relayed to the A–D input of the TDT System III. Stimulus generation, capture and TDT System III were controlled using Matlab and ActiveX software (Microsoft Corp., Redmond, WA, USA).
The zebrafish behavioral responses were recorded using a Photron Fastcam 1024PCI (Photron USA, San Diego, CA, USA) at 1000 frames s−1 (512×512 pixel resolution) synchronized to the vibratory stimulus via a transistor–transistor logic (TTL) pulse. TTL pulses from the camera were recorded at each frame capture using the System III and were later synchronized to the stimulus onset for analysis. All trials were illuminated from above using an LED array.
Acoustic stimuli
Acoustic stimuli were 24 ms cosine-squared gated 100 ms tones. Tonal stimuli of 90, 210, 310, 410, 540, 820, 1070 and 1200 Hz were created using MATLAB 2009b and sampled at 100 kHz. These frequencies were empirically determined during set up and initial testing to minimize distortion and motion in the non-vertical axes (i.e. x and y). The particle motion component of sound was measured using an accelerometer attached to the platform of the shaker system as a means to characterize acceleration in the experimental wells. During set up and initial testing, acceleration along the x-, y- and z-axes was measured using a PCB model 034K20 3-dimensional accelerometer, amplified using a Model 482A6 signal conditioner and then relayed to the System III. The accelerometer output was calibrated to the 355B04 accelerometer output prior to testing. Frequencies greater than 1200 Hz were not tested because of voltage and current input limitations of the shaker (Zeddies and Fay, 2005).
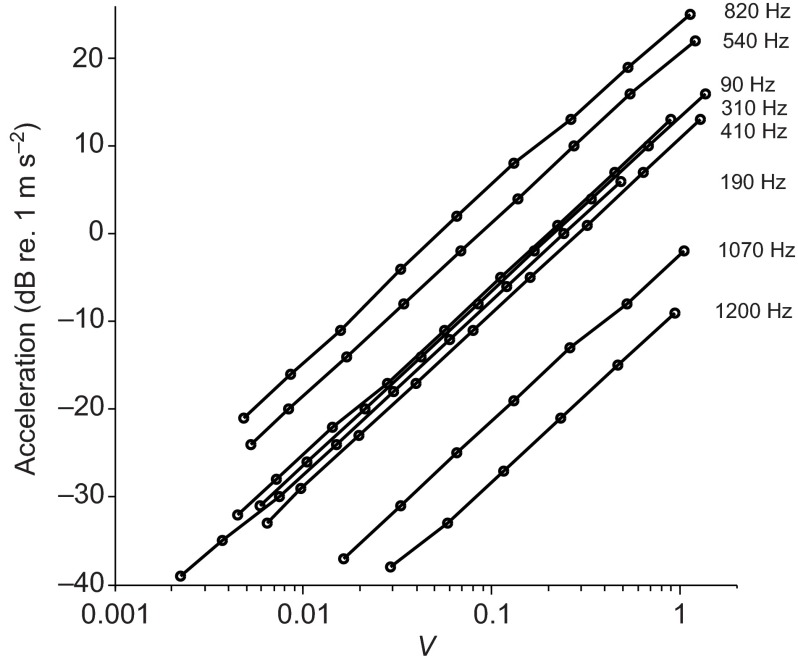
Before each experiment, acoustic stimuli were calibrated for frequency and amplitude. The 6×6 array of central wells were filled with ~400 μl embryo medium. The root mean square accelerometer voltage output was acquired for each input amplitude. These outputs were checked for linearity and, as expected, the doubling of the stimulus levels resulted in a doubling of the measured acceleration (i.e. the slopes of plots are 6 dB per stimulus level doubling) at all the frequencies tested (Fig. 1). The sensitivity of the accelerometer, calibrated by the manufacturer, was 1 V output 1 g−1 (9.8 m s−2) acceleration and the particle acceleration levels were determined using the formula:
 |
(1) |
Fig. 1.
Log-linear representation of the root mean square acceleration output of the shaker system (dB re. 1 m s −2) as a function of input voltage (V) by frequency with a 6×6 array of wells in a 96 well plate filled with 400 μl water. Each line indicates a separate frequency tested. Note that a doubling of the input voltage resulted in a doubling of the measured acceleration (6 dB increase).
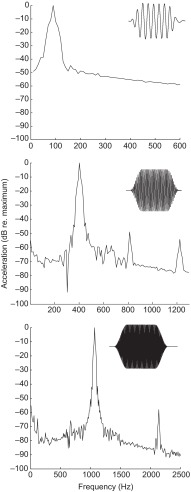
Samples of acoustic stimuli at the highest levels used to characterize the acoustic startle response were recorded. The rise–fall times of the acoustic stimuli were empirically determined and chosen as the shortest time that preserved the stimulus envelope. Fig. 2 shows the time waveform of the particle motion stimuli (see insets) and the fast-Fourier transform (FFT) of the stimuli for 90, 410 and 1070 Hz. The stimuli used contained little harmonic distortion. In all cases, the largest harmonic was attenuated at least 50 dB (re. 1 m s−2) relative to the fundamental frequency tested. At the highest levels used to characterize the acoustic startle response, significant particle motion was measured in the orthogonal (x- and y-) axes. However, this artifact of orthogonal motion in x and y during vertical (z-) axis stimulation was not observed at or near levels used to characterize the thresholds for the PPI of acoustic startle responses, as most of the x and y acceleration was at or below the measurable limit of the system (~−36 dB re. 1 m s−2).
Fig. 2.
Representative power spectrum of a subset (90, 410 and 1070 Hz) of sound stimuli used for pure-tone startle stimuli and prepulse stimuli measured at the highest level used (14 dB re. 1 m s−2). Data are normalized to a relative value of 0 dB assigned to the maximum sound level for the fundamental frequency tested. Insets, time course of pure-tone stimuli used for the startle response assay at 14 dB (re. 1 m s−2).
Acoustic startle response characterization
A characterization of the acoustic startle response was performed in order to differentiate the M-cell-mediated C-start response from other non-startle behaviors reported for zebrafish. There exist a large number of behaviorally interesting non-startle behaviors, such as the burst swim, J-bend turn, and routine locomotion [see table 1 in Wolman and Granato (Wolman and Granato, 2012)], but these behaviors are not associated with a positive C-start response. Previous studies have shown two different startle responses based on different latencies: a M-cell-mediated (short-latency) startle response occurs at a latency of approximately 5–7 ms while a long-latency startle occurs at >16 ms (Burgess and Granato, 2007; Kohashi and Oda, 2008). For all experiments, only the short-latency startle response was used to define a positive response.
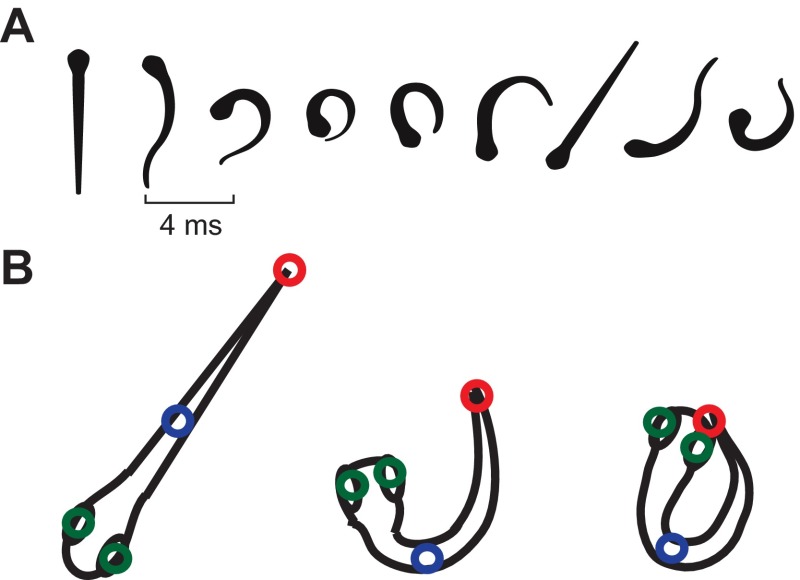
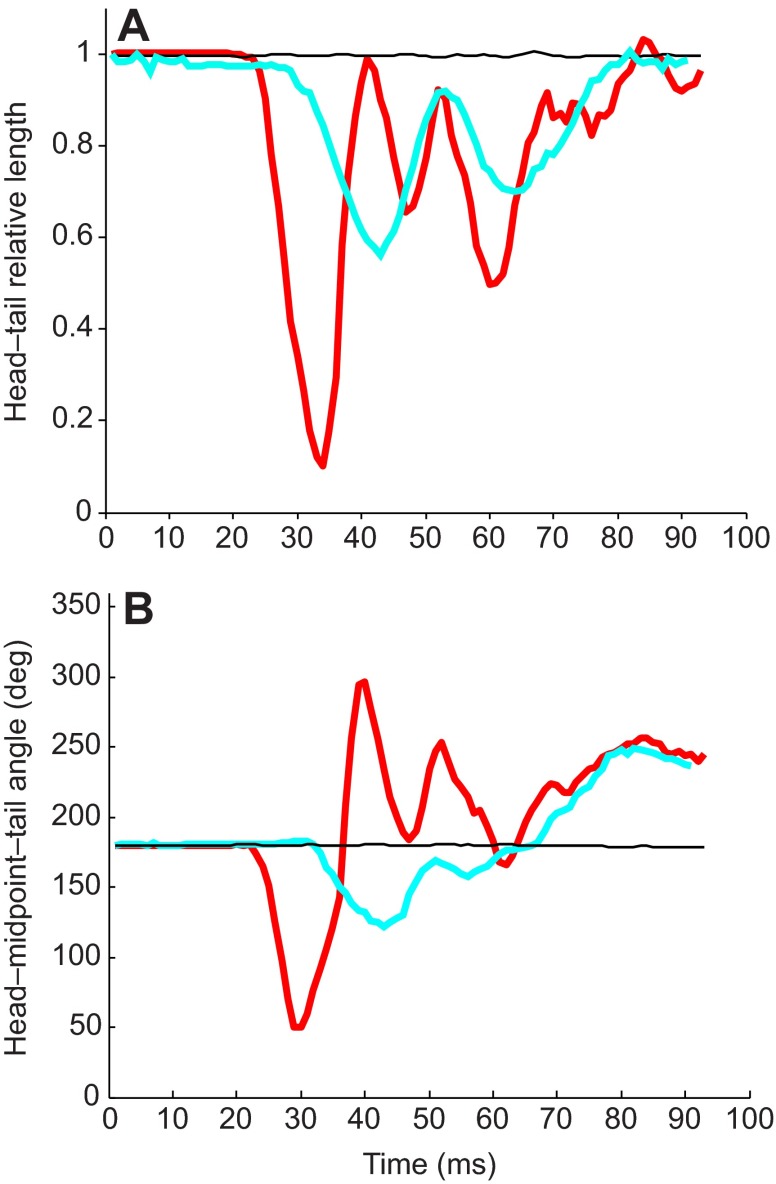
For acoustic startle characterization, individual larvae (N=9; 3×3 array) were presented with either 90 Hz at 14 dB (re. 1 m s−2) or 90 Hz at 8 dB pure-tone stimuli with a 15 ms cosine gated ramp and the behavioral responses were filmed for the first 100 ms after presentation of the stimulus. The video was then analyzed using a motion-tracking MATLAB script developed by Hedrick (Hedrick, 2008). Four points on the fish (the two eyes, the caudal edge of the swim bladder, and the caudal fin) were tracked for the duration of the response (Fig. 3B). These points were used to calculate two metrics: Euclidean length or distance between the head (defined as the midpoint between the two eyes) and tail, and the body angle (defined as the angle between the head, midpoint and tail). These two metrics were used to quantify C-start responses, non-startle responses and other behavioral responses. Positive startle responses were defined as responses that displayed a mean reduction in the Euclidean distance between the head and tail greater than 50% during the time period from the fish's initial movement to the apex of the C-bend of the fish's body, and reached maximum C-bend within 8 ms of onset of the startle response. The duration of the startle response was defined as the time of the initial movement of the fish to maximum flexion of the body C-bend. The latency of the startle response was defined as the time between the stimulus onset and initial movement of the fish; the stimulus onset was defined as the end point of the cosine gated ramp of the acoustic stimulus. Because latency was variable, only startle responses that occurred within <50 ms of stimulus onset were considered as part of the criteria for positive acoustic startle responses. These characterizations were tested on >20 previously untested responses to validate the accuracy and efficacy of the characterization parameters and then used to differentiate startle responses from non-startle responses in subsequent experiments.
Fig. 3.
(A) Diagram of time course of the acoustic startle response of 5–6 d.p.f. zebrafish, digitized from a representative positive response fish. The response is characterized by a fast, coordinated contraction on one side of the body, forming a distinctive C-shape (frame 4). Successive frames are 4 ms apart. (B) Diagrammatic representation of the four points marked throughout startle characterization: two eyes (green), caudal edge of the swimbladder (blue) and caudal fin (red). Point tracking was used to measure head–tail Euclidean length and head–midpoint–tail angle throughout responses.
Acoustic startle and PPI experiments
For the acoustic startle threshold experiments, each replicate (defined as one plate containing 24 fish arranged in a 6×4 array) consisted of stimuli at the frequencies mentioned above, and at intensities from −6 to −30 dB re. 1 m s−2 (varied in steps of 6 dB). That is, each replicate was presented with 45 stimuli presented in a repeated measures design. These trials were separated by a randomized inter-trial interval of 70±10 s based on preliminary data in order to reduce habituation. The behavioral responses were measured for the duration of the stimulus (100 ms). The C-start occurred at ~18 ms after stimulus onset while the long-latency startle was not characterized in this study. The videos were then analyzed using the criteria determined previously (above). For each trial, responses were coded binomially (1 for response, 0 for non-response). Plates that exhibited no responses were coded as having a threshold of 0 dB, one step (6 dB) above the highest presented stimulus level. After precise determination of the startle thresholds, prepulse experiments were conducted.
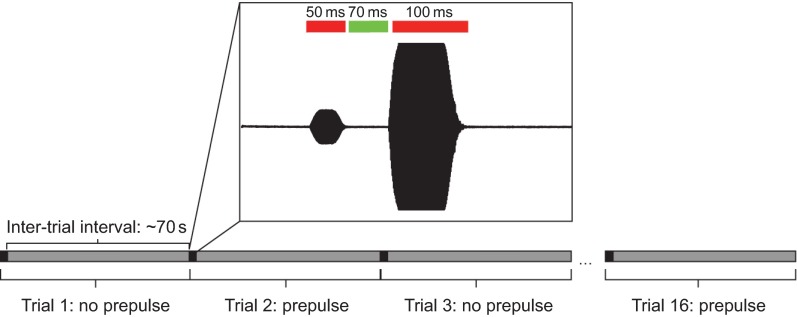
The experimental procedure for the PPI experiments was similar to that for the startle response experiments except that a frequency of 820 Hz at 20 dB re. 1 m s−2 was used as a universal startle stimulus. Each replicate consisted of 32 trials with four sound levels for each frequency presented in random order. These sound levels were empirically determined as the four largest sub-startle threshold levels. That is, these four levels were the highest levels presented that did not elicit a startle response. Additionally, three sound levels at or below the noise floor were tested to confirm the reliability of the response and to ensure that PPI did not extend to sound levels below the detection level of our system. A PPI trial consisted of a 50 ms randomized prepulse stimulus with a 24 ms ramp time followed by the startle stimulus. The inter-pulse interval, or the time between the end of the prepulse tone and the beginning of the startle tone, was 70 ms, which was empirically determined in preliminary experiments. Each PPI startle stimulus presentation (trial) was preceded by a no prepulse ‘catch’ trial in order to determine baseline startle response probability (Fig. 4). The catch trial also controlled for possible habituation to the stimuli. The PPI effect was calculated as the difference between the percentage response to the prepulse trial (Tn) and the mean response probability of the catch trials immediately preceding (Tn−1) and following the prepulse trial (Tn+1) using the formula:
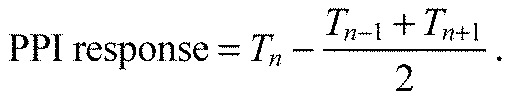 |
(2) |
Fig. 4.
Diagram of the prepulse inhibition (PPI) protocol. Prepulse trials and no prepulse catch trials are interleaved, with an inter-trial interval of ~70 s for a total of 16 trials per group. Prepulse frequency and level are randomized between trials, but all trials contain the same catch stimulus. Inset, example of a prepulse trial. A 50 ms prepulse stimulus is separated from the 100 ms catch stimulus by an empirically determined 70 ms inter-stimulus interval.
For all prepulse experiments, replicates were presented with no more than 16 total (prepulse and catch) stimuli in order to minimize habituation. After 16 presentations, fish were replaced with naive fish from the same cohort. Thus, for each dataset a total of 96 fish were used.
Lateral line ablation experiments
In order to assess the relative contribution of the lateral line in acoustic detection, the lateral line was ablated by aminoglycoside exposure. Larvae at 5 d.p.f. were exposed to 400 μmol l−1 neomycin in embryo medium for 1 h and then immediately rinsed four times in fresh embryo medium (Harris et al., 2003; Murakami et al., 2003; Owens et al., 2009; Namdaran et al., 2012). Neomycin exposure at concentrations greater than 100 μmol l−1 is known to significantly reduce swimming speed in larval zebrafish (Buck et al., 2012). Startle percentage to catch stimuli was measured after neomycin exposure and responses returned to baseline ~3–4 h post-exposure. To account for any additional effects, larvae were allowed to recover in fresh embryo medium for 6–12 hours before experimentation. This recovery period is not long enough for hair cell regeneration to occur (Ma et al., 2008). After recovery, the fish were tested as described above for the prepulse experiments. Five larvae from each cohort of aminoglycoside exposure were used to assess the efficiency of the aminoglycoside treatment.
To assess the efficiency of aminoglycoside ablation, lateral line superficial hair-cell neuromasts were labeled with the fluorescent vital dye DASPEI {2-[4-(dimethylamino)styryl]-N-ethylpyridinium iodide; 0.005% final concentration in embryo medium} for 15 min. Larvae were then rinsed twice in fresh embryo medium and anesthetized in 10 μg ml−1 MS222 (tricaine methanesulfonate, Sigma, St Louis, MO, USA). Larvae were visualized using an epifluorescence dissecting microscope with 450–490 nm laser. Ten neuromasts were evaluated per fish: supraorbital (SO1, SO2), infraorbital (IO1–4), mandibular (M2), middle (MI1, MI2) and otic (O2) (Raible and Kruse, 2000). Each neuromast was assigned a score of 0–2: 0 (little/no staining), 1 (reduced staining) and 2 (normal staining) for a combined score of between 0 and 20 per fish.
Data analysis
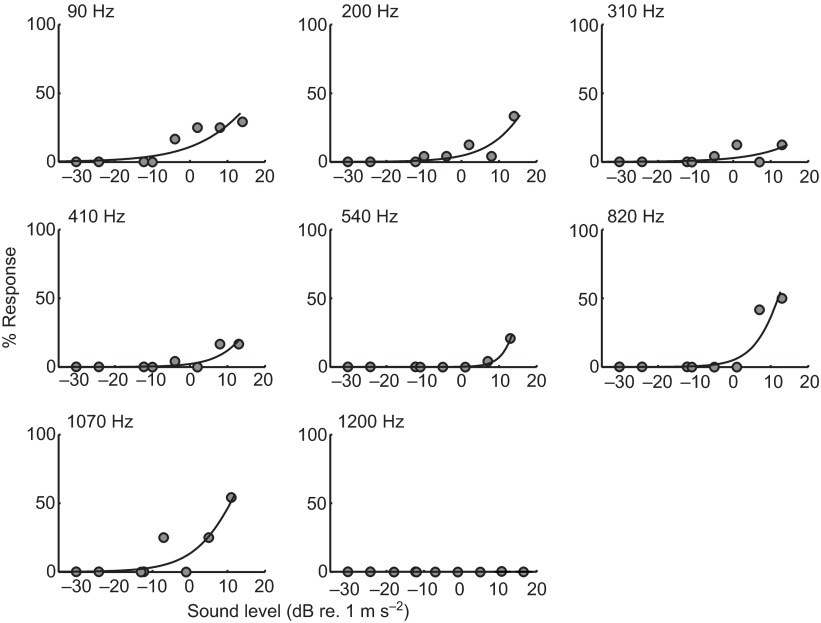
The binomial response data collected from each plate were analyzed using a curve-fitting procedure. For each frequency, responses at each stimulus level were converted to a response percentage. Assuming that the response percentage for a set of fish was a good measure of the probability of eliciting a response from any given fish, the thresholds for each frequency were determined by fitting the response percentages with a Weibull cumulative distribution curve using a maximum likelihood method (Wichmann and Hill, 2001; Treutwein, 1995). These curves show the best fit model to the data and are most accurate for the sound levels tested (Fig. 5); note that extrapolation of the curve's upper limits beyond the highest levels tested may not accurately reflect the expected startle response probabilities. Because we did not observe a response in the absence of stimuli, the startle response threshold was conservatively defined as the stimulus level at which the startle response could be reliably elicited >5% of the time. A startle response probability of 5% represents the 95% ‘confidence limit’ of the baseline no-response condition.
Fig. 5.
Representative examples of curve-fitting the acoustic startle percentage response at a given frequency at the various stimulus levels tested for 5–6 d.p.f. zebrafish. For each frequency, observed response percentages are shown as filled circles. The Weibull curve is fitted using an MLE criterion and threshold is characterized as the sound level (dB re. 1 m s−2; x-axis) at which the model predicts a response probability of 5% (y-axis).
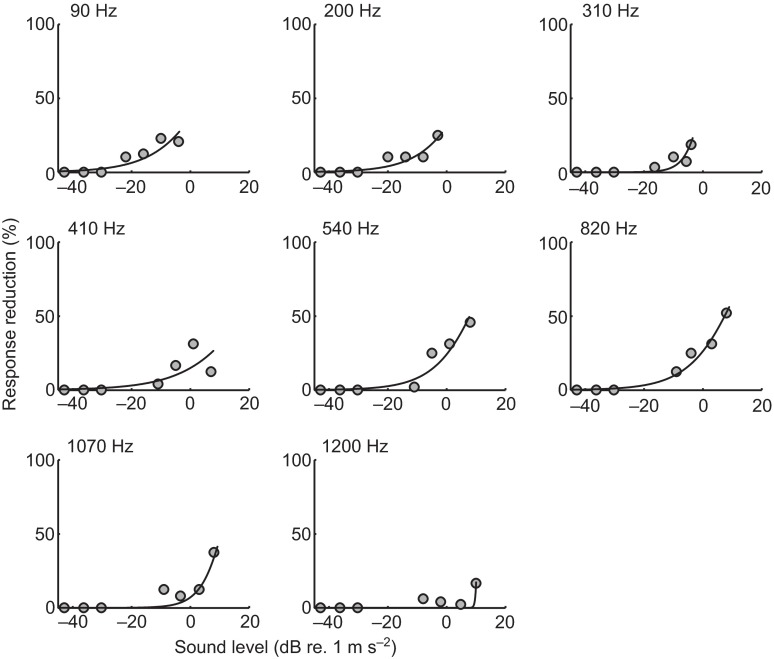
In the PPI experiments, a similar curve-fitting method was used to determine the threshold for the inhibition of the startle response. The binomial response data were converted into a response percentage, as in the case of the startle experiments. However, this response percentage was subtracted from the mean of the paired no-prepulse catch trials before and after stimulus presentation. This yielded a difference in startle probability from the expected value. This difference was then fitted to a cumulative Weibull distribution. The threshold for the inhibition of the startle response was determined for each prepulse frequency tested and was defined as the stimulus level that elicited a 5% reduction of the probability of startle response between PPI trials and the paired catch trials.
A one-way ANOVA was used to test for differences in startle duration between sound stimuli. Startle response data were heteroscedastic (Bartlett's test, P<0.01 for all frequencies), and therefore non-parametric methods were used to analyze all startle and PPI response data. Differences between thresholds as determined by the startle response paradigm and PPI paradigm were analyzed using a Friedman test (non-parametric equivalent of a repeated measures two-way ANOVA) (Zar, 1999), and frequency-specific differences between the two paradigms were analyzed using a post hoc pairwise Mann–Whitney U-test, as described by Siegel and Castellan (Siegel and Castellan, 1988). To assess differences in the lateral line ablation experiments, a Friedman test between treatment and frequency was conducted. All statistical analyses were conducted using MATLAB 2009b.
RESULTS
Acoustic startle response characterization
Acoustic startle responses to pure-tone stimuli were observed in 5 d.p.f. or older zebrafish and not in fish younger than 5 d.p.f. (data not shown) at the stimulus levels and frequencies tested in this study. Startle responses consisted of an initial quick C-bend of the body, followed by the refractory bends of the tail and head in alternating directions (Fig. 3A). Analysis of the high-speed kinematic data of the evoked startle behavior revealed a highly stereotyped and reliable acoustic startle response. Fig. 6 shows representative examples of the kinematic data for both startle and non-startle responses. Positive startle responses consisted of a mean (±s.d.) reduction in the Euclidean distance between the head and tail by 72±8% (N=18), which occurs during the time from the fish's initial movement to the apex of the C-bend of the fish's body. During this initial phase of the startle response, the head–midpoint–tail angle also decreased from 180 deg (initial) to a mean (±s.d.) angle of 45±5 deg. Changes in the Euclidean distance between head and tail and the head–midpoint–tail angle were highly correlated (r=0.83, N=18 responses) for the initial bend during stage I of startle (Foreman and Eaton, 1993). In contrast, changes in the Euclidean distance between head and tail (<40%) and the head–midpoint–tail angle (<60%) from the initial position for non-startle responses were much smaller.
Fig. 6.
Example of the acoustic startle response characterization. (A) Euclidean length from head–tail throughout the response, relative to initial length. Time 0 denotes the onset of the stimulus. A positive startle response (red) shows a marked decrease in head–tail distance at the apex of the initial C-bend, followed by smaller refractory bends. The non-startle response (cyan) shows some decrease, but is smaller in magnitude than the startle. No response (black) is also shown. (B) Head–midpoint–tail angle throughout the response. Initial position is 180 deg. Length and angle measurements are highly correlated (r=0.83, N=18 responses).
The mean latency of the short-latency startle response, defined as the time between the stimulus onset (end of the cosine gated ramp) and initial movement of the fish that met the startle response criteria, was 3.9±2.8 ms (mean ± s.d., N=15 responses). The mean (±s.d.) duration of the startle response from the initial movement of the fish to maximum flexion of the body C-bend was 7.1±0.74 ms (N=18 responses) and was not different at the sound levels tested (ANOVA, F1,35=0.05, P=0.82), indicating that the duration of the startle response was not dependent on stimulus level. The latency and duration of non-startle responses were much longer (>50 ms) and more variable in duration, and were not quantified.
Acoustic startle thresholds
The percentage of startle responses between the lowest and highest stimulus intensity increased at each frequency. Fig. 7 shows the startle response thresholds for acceleration as a function of frequency. Startle response thresholds were lowest at the lowest frequency tested, 90 Hz [median=−4 dB, interquartile range (IQR): −10 to 2 dB re. 1 m s−2] and 210 Hz (4 dB, IQR: −3 to 8 dB re. 1 m s−2). Startle thresholds above 210 Hz gradually increased, plateaued between 310 and 410 Hz (8 dB, IQR: 6 to 10 dB re. 1 m s−2), decreased slightly between 540 Hz (5 dB, IQR: 0 to 11 dB re. 1 m s−2) and 820 Hz (4 dB, IQR: −3 to 7 dB re. 1 m s−2), and then increased rapidly from 1070 Hz (15 dB, IQR: 13 to 17 dB re. 1 m s−2) to 1200 Hz (20 dB re. 1 m s−2). In general, startle sensitivity decreased with increasing frequency from 90 to 1200 Hz with the exception of a slight increase between 540 and 820 Hz. At the highest frequency tested, 1200 Hz, only five positive responses were observed at any stimulus level.
Fig. 7.
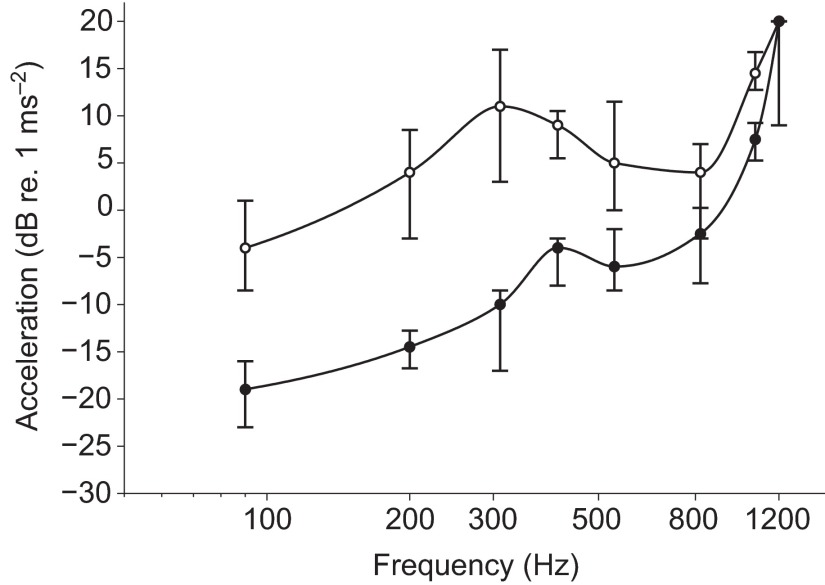
Behavioral audiogram of the acoustic startle response of 5–6 d.p.f. zebrafish (N=10 plates of 24 fish) to particle motion stimuli (open circles) and audiogram of hearing sensitivity using the PPI assay (N=10 plates, filled circles) to sub-threshold particle motion stimuli. Thresholds were defined as a 5% probability of startle for the startle response assay or a 5% inhibition of startle from the paired catch trials in the PPI assay. Data are presented as median ± 1 quartile. Lower numbers indicate higher sensitivities. Note that all prepulse thresholds are significantly lower than startle response thresholds.
PPI thresholds of the startle response
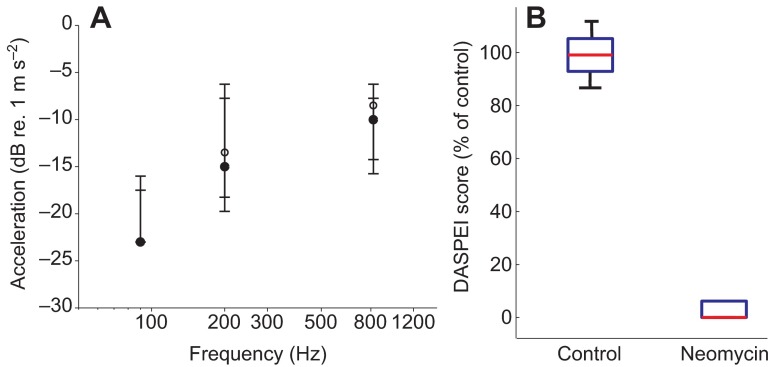
PPI thresholds were defined as the intensity of the prepulse stimulus at each frequency that resulted in a >5% reduction in the probability of a startle response to the standardized startle stimulus (820 Hz at 20 dB re. 1 m s−2). As with the startle response data, the PPI response data from 10 plates of 24 fish were fitted with a Weibull distribution. The resulting PPI response profiles at each prepulse frequency are shown in Fig. 8. Fig. 7 compares the median PPI thresholds and startle response thresholds in terms of acceleration as a function of prepulse frequency. In general, the PPI audiogram showed a steep increase in thresholds from 90 Hz (−20 dB, IQR: −23 to −16 dB re. 1 m s−2) to 210 Hz (−16 dB, IQR: −16 to −12 dB re. 1 m s−2), followed by a gradual threshold increase up to 820 Hz (−3 dB, IQR: −7 to 1 dB re. 1 m s−2), and then a rapid increase in thresholds up to 1200 Hz (20 dB, IQR: 9 to 20 dB re. 1 m s−2).
Fig. 8.
Example of curve-fitting to prepulse response data. Each point shown is the mean difference between the response percentage at that frequency and sound level (dB re. 1 m s−2; x-axis) and paired no-prepulse catch trials preceding and following the prepulse stimulus. Similar to the startle response data, a Weibull curve is fitted using an MLE criterion and threshold is characterized as the sound level at which the model predicts a response reduction of 5% (y-axis).
The PPI response is similar in shape to the startle response except that the PPI thresholds were significantly lower than the startle response thresholds (Friedman 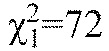 , P<0.001). Post hoc Mann–Whitney U-tests showed that these differences were frequency dependent (Fig. 7). PPI thresholds were significantly lower than startle thresholds at 90–1070 Hz (P<0.01 for all frequencies), but differences between prepulse and startle response thresholds at the highest frequencies tested (1200 Hz) were not significant (P=0.2). The greatest threshold difference was at 310 Hz for which the PPI threshold was ~21 dB lower than the startle threshold. PPI thresholds differed more from startle thresholds at lower frequencies (approximately 11 to 21 dB from 90 to 540 Hz, respectively) than at higher frequencies (approximately −7 to 0 dB from 820 to 1200 Hz, respectively).
, P<0.001). Post hoc Mann–Whitney U-tests showed that these differences were frequency dependent (Fig. 7). PPI thresholds were significantly lower than startle thresholds at 90–1070 Hz (P<0.01 for all frequencies), but differences between prepulse and startle response thresholds at the highest frequencies tested (1200 Hz) were not significant (P=0.2). The greatest threshold difference was at 310 Hz for which the PPI threshold was ~21 dB lower than the startle threshold. PPI thresholds differed more from startle thresholds at lower frequencies (approximately 11 to 21 dB from 90 to 540 Hz, respectively) than at higher frequencies (approximately −7 to 0 dB from 820 to 1200 Hz, respectively).
The degree of habituation was measured by 25 repeated presentations of a no-prepulse catch stimulus (820 Hz at 20 dB re. 1 m s−2) with a 70 s inter-stimulus interval. Response percentages followed a biphasic linear decrease, with the inflection point occurring around the 12th to 15th stimulus presentation. The mean (±s.e.m.) difference in response between the first presentation and the 15th presentation was 19±3% (N=5 plates), whereas the mean (±s.e.m.) difference in response between the first and 17th presentation was 33±4%. However, between the 17th presentation and the 25th presentation, the mean (±s.e.m.) difference in response percentage was only 3±4%.
Effect of lateral line ablation on PPI thresholds
In order to determine the relative contribution of the lateral line to acoustic stimulus detection, 5–6 d.p.f. zebrafish were exposed to 400 μmol l−1 neomycin for 30 min. Neomycin exposure resulted in a high incidence of hair cell death in the superficial neuromasts (DASPEI score: 1.2±0.19, mean ± s.e.m., N=8) compared with control fish, which showed DASPEI scores of 16±0.9. The PPI assay was conducted at a subset of frequencies (90, 210 and 820 Hz) used to construct the PPI audiogram. The PPI thresholds for neomycin-exposed fish did not differ from those of the control fish at any of the tested frequencies (Friedman test interaction,
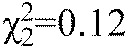 , P=0.25, N=10; Fig. 9). Thus, these results indicate that the mechanosensory superficial neuromasts do not contribute to auditory detection at the tested frequencies during this stage of development. Interestingly, the response probability for the no-prepulse catch trials was greatly decreased after neomycin exposure (81±3% for controls, 65±5% for neomycin-exposed fish, means ± s.d.), which suggests a potential negative effect of neomycin on the locomotor behavior of larval zebrafish (Buck et al., 2012) that persists 6–12 h after exposure.
, P=0.25, N=10; Fig. 9). Thus, these results indicate that the mechanosensory superficial neuromasts do not contribute to auditory detection at the tested frequencies during this stage of development. Interestingly, the response probability for the no-prepulse catch trials was greatly decreased after neomycin exposure (81±3% for controls, 65±5% for neomycin-exposed fish, means ± s.d.), which suggests a potential negative effect of neomycin on the locomotor behavior of larval zebrafish (Buck et al., 2012) that persists 6–12 h after exposure.
Fig. 9.
(A) PPI thresholds of 5–6 d.p.f. larval zebrafish (N=10 plates) treated with 400 μmol l−1 neomycin at 90, 210 and 820 Hz. There is no difference between neomycin-treated (open circles) and control (filled circles) fish. All data are presented as median ± 1 quartile. Note that at 90 Hz, medians for treated and control data overlap and lower quartile bars are slightly obscured. (B) Box plots of DASPEI scores for control and neomycin-treated fish, normalized to percentage relative to DASPEI scores for controls. The 400 μmol l−1 neomycin treatment had a high rate of superficial neuromast hair cell ablation. Note that the 95% confidence interval bars are obscured for the 400 μmol l−1 neomycin treatment.
DISCUSSION
To our knowledge, this study is the first to provide a behavioral audiogram for wild-type (AB) zebrafish during early larval development at 5–6 d.p.f. Our goal was to determine the acoustic sensitivity of larval wild-type zebrafish using the behavioral PPI assay, which quantifies the hearing thresholds of larval zebrafish to prepulse tones (90–1200 Hz) that inhibit the innate acoustic startle response to a reliable acoustic startle stimulus (820 Hz at 20 dB re. 1 m s−2). Our results demonstrate that larval zebrafish are most sensitive to low frequency acoustic stimuli from 90 Hz (lowest frequency tested) to 310 Hz and that the hearing thresholds established from the PPI audiograms were considerably lower than those previously obtained from startle audiograms. In addition, we provide evidence that the lateral line mechanosensory superficial neuromasts do not contribute to the detection of acoustic stimuli from 90 to 820 Hz during early development.
We found fast, C-start responses were reliably evoked by pure-tone acoustic stimuli in 5–6 d.p.f. wild-type (AB) zebrafish, whereas this behavior was absent in fish <5 d.p.f. (i.e. 4 d.p.f. zebrafish) over the range tested (up to 14 dB re. 1 m s−2 for frequencies of 90–1200 Hz). The variability in latency between the responses and the discrepancy between our measurement of latency and previously published accounts of startle latency (Burgess and Granato, 2007; Kohashi and Oda, 2008) were attributed to the ramped stimulus. In fact, some startle responses were observed to begin before the end of the ramp. Zeddies and Fay (Zeddies and Fay, 2005) found a similar timing of onset of the expression of acoustically evoked behavioral responses to pure tones in 5 d.p.f. zebrafish but could not characterize the startle response type because of equipment limitations. Earlier work by Eaton and colleagues (Eaton et al., 1977) suggests that the development of the startle response (M-cell-initiated C-start response) occurs very early in development. This fast startle behavior can be evoked by tactile stimulation as early as 44 h post-fertilization (h.p.f.) (Eaton et al., 1977) and by visual stimuli 68–79 h.p.f. (Easter and Nicola, 1996). Although Burgess and Granato (Burgess and Granato, 2007) reported occasional startle responses to uncalibrated broadband acoustic stimuli at 3 d.p.f., the reliable onset of acoustically evoked startle responses to pure-tone stimuli appears to occur at 5 d.p.f. This discrepancy in startle response onset may be due to the nature of the stimulus; the use of uncalibrated stimuli might contain low frequency elements that activate both the inner ear and the lateral line, leading to a higher activation of the M-cell at an earlier observed stage.
Acceleration thresholds from the pure-tone startle audiogram (Fig. 7) indicate that 5–6 d.p.f. zebrafish are most sensitive to low frequencies <310 Hz and that startle responses occur up to 1200 Hz (the highest frequency tested). The 820 Hz tone at 20 dB (re. 1 m s−2) had the highest response rate and was subsequently used as the startle-inducing stimulus for the PPI experiments. The gated acoustic stimuli used here contained little distortion arguing that the fish were responding specifically to the nominal stimulus frequencies.
Habituation to the startle-inducing catch stimulus was negligible in terms of its influence on the PPI response. Even though there was a small drop in the response percentage from the first presentation to the 16th presentation, as the PPI effect was measured relative to the response percentage of the catch trial, the effects of habituation were ameliorated. Habituation to the catch stimulus was also short lived and in agreement with previous studies (Roberts et al., 2011). Retesting fish greater than 6 h after initial habituation experiments showed no long-term effects of the stimulus presentations, and fish responded to catch stimuli at the same percentages as naive fish. In rodents, PPI assays for auditory sensitivity are conducted over multiple days to protect against habituation effects (Young and Fechter, 1983). A similar protocol may need to be developed for future comparative studies using the PPI assay with other fish species.
Response thresholds measured using the PPI experimental paradigm for 5–6 d.p.f. zebrafish were lower than startle response thresholds. These PPI thresholds represent the lowest sound levels for the prepulse test tones that are required to effectively inhibit or modify the M-cell-mediated startle response to a loud acoustic stimulus. Between 90 and 540 Hz the PPI thresholds were ~11–21 dB lower than the startle thresholds, and between 820 and 1200 Hz the PPI thresholds were <7 dB lower than startle thresholds (Fig. 7). The lowest PPI threshold was −20 dB re. 1 m s−1 at 90 Hz, which is similar to particle motion thresholds for single unit saccular afferent recordings in other fishes, such as toadfish [Opsanus tau; range: −90 to −37 dB re. 1 m s−2 at 100 Hz (Fay et al., 1994)], sturgeon [Acipenser fulvescens; −90 to −33 dB re. 1 m s−2 at 100 Hz (Meyer et al., 2010)] and goldfish [Carassius auratus; −90 to −8 dB re. 1 m s−2 at 140 Hz (Fay, 1984)].
Auditory thresholds derived from the PPI assay are known to be similar to those derived from electrophysiological methods. Young and Fechter (Young and Fechter, 1983) found PPI thresholds in rats to be similar to auditory brainstem-evoked potential (ABR) thresholds, while Walter and colleagues (Walter et al., 2012) found PPI thresholds were 10–15 dB SPL more sensitive than ABR thresholds in Mongolian gerbils. Our findings indicate that larval zebrafish have significant auditory capacity below levels that cause startle responses and the hearing threshold levels determined using the PPI paradigm are similar to AEP thresholds previously characterized for another otophysan fish, the goldfish (Radford et al., 2012). These findings suggest that the auditory system of 5 d.p.f. larval zebrafish is relatively sensitive and functional during early development and that the PPI procedure described here provides a good measure of hearing threshold levels in larval zebrafish. Because of the non-invasiveness of this technique, the auditory sensitivity of larval or juvenile fish (and their cohorts) can be tracked throughout their development, and in future studies should allow researchers to compare auditory thresholds as measured by PPI and other electrophysiological methods (e.g. AEPs or auditory single unit recordings) within a species.
Responses measured using the PPI assays were most likely mediated by the saccule. The fish inner ear consists of three otolithic end organs: the saccule, utricle and lagena (Popper and Fay, 1993). The upper frequency range (820–1200 Hz) suggests that PPI response is via the saccule because it is the only inner-ear end organ known to respond at these frequencies in otophysan fishes (Fay, 1981). The findings by Bang and colleagues (Bang et al., 2002) are also consistent with saccule-mediated sound detection in that most mutations found to affect the startle response (to a 400 Hz tone) had morphological defects associated with the saccular auditory pathway. The lagena of wild-type zebrafish does not develop until 12 d.p.f. (Riley and Moorman, 2000) and is thus non-functional in fish 5–6 d.p.f. While the utricle may serve an auditory role in adult sleeper gobies (Dormitator latifrons), its sensitivity is ~30 dB less than that of the saccule (Lu et al., 2004). However, studies on adult goldfish have shown that the utricular and saccular afferents are equally sensitive to particle motion stimuli (Fay, 1984; Fay and Olsho, 1979). Future studies that investigate the functional role of the three different end organs (saccule, lagena and utricle) in 5 d.p.f. zebrafish would be instrumental in determining whether each end organ differentially detects and encodes acoustic particle motion and pressure.
Indirect stimulation of the zebrafish inner ear by sound pressure can occur via gas-filled bladders in close proximity to the end organs and/or by means of skeletal adaptations such as the Weberian ossicles in zebrafish and other otophysan fishes that link the swim bladder to the inner ear (Higgs et al., 2003; Popper and Fay, 2011). However, in zebrafish the Weberian ossicles are not fully formed or ossified until ~36–37 mm total length (TL) or ~56 d.p.f. (Grande and Young, 2004). Higgs and colleagues (Higgs et al., 2003) showed, using AEP, no difference in sound pressure sensitivity of zebrafish during development from 10 to 45 mm TL. However, these authors showed that detectable frequencies >2000 Hz coincided with increases in body size (at ~17–20 mm TL), swim bladder size and connectivity of the Weberian elements, which is consistent with the hypothesis that the Weberian apparatus and swim bladder are responsible for transmitting higher frequency information to the inner ear (Von Frisch, 1938; Fay and Popper, 1974). Although the swim bladder is inflated and clearly visible in 5 d.p.f. zebrafish (~3.5 mm TL), the deflation of the swim bladder at this developmental stage does not affect the acoustically evoked behavioral response thresholds (Zeddies and Fay, 2005). These results suggest that 5–6 d.p.f. zebrafish do not respond to sound pressure, but instead respond exclusively to particle motion and direct acceleration of the inner ear otolithic organs. As a result, it is not surprising that at this early stage of development, the audiogram resembles that of fish that do not have specialized adaptations for detecting pressure.
Ablation of the mechanosensory superficial neuromasts using aminoglycosides had no effect on the hearing sensitivity of larval zebrafish at frequencies of 90, 210 and 820 Hz, which is consistent with AEP studies from adult goldfish (Higgs and Radford, 2013). At this stage of development, 5–6 d.p.f. zebrafish are only known to have superficial neuromasts capable of detecting vibrational stimuli up to 50 Hz (Liao et al., 2012). Lateral line canal neuromasts, which are capable of encoding frequencies up to 200 Hz (Kalmijn, 1988; Montgomery et al., 1995), do not develop until ~32 d.p.f. (~10 mm TL) (Webb and Shirey, 2003). Future studies are needed to determine whether there is multimodal overlap between the mechanosensory superficial neuromasts and the inner ear in 5–6 d.p.f. zebrafish at frequencies <50 Hz.
The PPI assay described here could be used as a valuable tool to screen for novel compounds that protect inner ear hair cells from noise-induced damage and investigate the molecular genetic basis of hearing in larval zebrafish. Molecular genetic studies on zebrafish hearing have thus far focused on unresponsive/deaf mutants with mutations affecting inner ear anatomical development (and/or its associated structures) or a loss of hair cell mechanotransduction (Nicolson, 2005). A PPI assay could be developed to screen for hearing phenotypes, such as those with reduced auditory sensitivity or frequency selectivity, and ultimately used to investigate the genetic basis of auditory processing during early zebrafish development.
ACKNOWLEDGEMENTS
We thank Dr Liz Whitchurch and Dr Kelly Owens for training assistance and help with experimental design, and Dr Sönke Johnsen for notes on the manuscript.
LIST OF ABBREVIATIONS
- ABR
auditory brainstem response
- AEP
auditory-evoked potential
- d.p.f.
days post-fertilization
- h.p.f.
hours post-fertilization
- M-cell
Mauthner cell
- PPI
prepulse inhibition
- TL
total length
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was supported by the National Institutes of Health [University of Washington Auditory Neuroscience Training Grant 2T32DC005361-11 to A.A.B., DC5987 to D.W.R.]. Deposited in PMC for release after 12 months.
REFERENCES
- Bang P. I., Sewell W. F., Malicki J. J. (2000). Behavioral screen for dominant mutations affecting zebrafish auditory system. Assoc. Res. Otolaryngol. Abs. 23, 177-187 [Google Scholar]
- Bang P. I., Sewell W. F., Malicki J. J. (2001). Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio). J. Comp. Neurol. 438, 173-190 [DOI] [PubMed] [Google Scholar]
- Bang P. I., Yelick P. C., Malicki J. J., Sewell W. F. (2002). High-throughput behavioral screening method for detecting auditory response defects in zebrafish. J. Neurosci. Methods 118, 177-187 [DOI] [PubMed] [Google Scholar]
- Buck L. M. J., Winter M. J., Redfern W. S., Whitfield T. T. (2012). Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear. Res. 284, 67-81 [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Granato M. (2007). Sensorimotor gating in larval zebrafish. J. Neurosci. 27, 4984-4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch-Nentwich E., Söllner C., Roehl H., Nicolson T. (2004). The deafness gene dfna5 is crucial for ugdh expression and HA production in the developing ear in zebrafish. Development 131, 943-951 [DOI] [PubMed] [Google Scholar]
- Cervi A. L., Poling K. R., Higgs D. M. (2012). Behavioral measure of frequency detection and discrimination in the zebrafish, Danio rerio. Zebrafish 9, 1-7 [DOI] [PubMed] [Google Scholar]
- Coffin A., Kelley M., Manley G. A., Popper A. N. (2004) Evolution of sensory hair cells. In Evolution of the Vertebrate Auditory System (ed. Manley G. A., Popper A. N., Fay R. R.), pp. 55-94 New York, NY: Springer-Verlag; [Google Scholar]
- Easter S. S., Jr, Nicola G. N. (1996). The development of vision in the zebrafish (Danio rerio). Dev. Biol. 180, 646-663 [DOI] [PubMed] [Google Scholar]
- Eaton R. C., Farley R. D., Kimmel C. B., Schabtach E. (1977). Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J. Neurobiol. 8, 151-172 [DOI] [PubMed] [Google Scholar]
- Eaton R. C., Lee R. K. K., Foreman M. B. (2001). The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog. Neurobiol. 63, 467-485 [DOI] [PubMed] [Google Scholar]
- Fay R. R. (1981) Coding of acoustic information in the eighth nerve. In Hearing and Sound Communication in Fishes (ed. Tavolga W. N., Popper A. N., Fay R. R.), pp. 189-221 New York, NY: Springer; [Google Scholar]
- Fay R. R. (1984). The goldfish ear codes the axis of acoustic particle motion in three dimensions. Science 225, 951-954 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Olsho L. W. (1979). Discharge patterns of lagenar and saccular neurones of the goldfish eighth nerve: displacement sensitivity and directional characteristics. Comp. Biochem. Physiol. 62, 377-386 [Google Scholar]
- Fay R. R., Popper A. N. (1974). Acoustic stimulation of the ear of the goldfish (Carassius auratus). J. Exp. Biol. 61, 243-260 [DOI] [PubMed] [Google Scholar]
- Fay R. R., Edds-Walton P. L., Highstein S. M. (1994). Directional sensitivity of saccular afferents of the toadfish to linear acceleration at audio frequencies. Biol. Bull. 187, 258-259 [DOI] [PubMed] [Google Scholar]
- Foreman M. B., Eaton R. C. (1993). The direction change concept for reticulospinal control of goldfish escape. J. Neurosci. 13, 4101-4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M., van Eeden F. J. M., Schach U., Trowe T., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., et al. (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399-413 [DOI] [PubMed] [Google Scholar]
- Grande T., Young B. (2004). The ontogeny and homology of the Weberian apparatus in the zebrafish Danio rerio (Ostariophysi: Cypriniformes). Zool. J. Linn. Soc. 140, 241-254 [Google Scholar]
- Gray L., Rubel E. W. (1985). Development of absolute thresholds in chickens. J. Acoust. Soc. Am. 77, 1162-1172 [DOI] [PubMed] [Google Scholar]
- Harris J. A., Cheng A. G., Cunningham L. L., MacDonald G., Raible D. W., Rubel E. W. (2003). Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 4, 219-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins A. D. (1993) Underwater sound and fish behaviour. In Behaviour of Teleost Fishes (ed. Pitcher T. J.), pp. 129-169 New York, NY: Springer; [Google Scholar]
- Hedrick T. L. (2008). Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 [DOI] [PubMed] [Google Scholar]
- Higgs D. M., Radford C. A. (2013). The contribution of the lateral line to ‘hearing’ in fish. J. Exp. Biol. 216, 1484-1490 [DOI] [PubMed] [Google Scholar]
- Higgs D. M., Rollo A. K., Souza M. J., Popper A. N. (2003). Development of form and function in peripheral auditory structures of the zebrafish (Danio rerio). J. Acoust. Soc. Am. 113, 1145-1154 [DOI] [PubMed] [Google Scholar]
- Hoffman H. S., Ison J. R. (1980). Principles of reflex modification in the domain of startle: I. Some empirical findings and their implications for the interpretation of how the nervous system processes sensory input. Psychol. Rev. 87, 175-189 [PubMed] [Google Scholar]
- Ison J. R. (1982). Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J. Comp. Physiol. Psychol. 96, 945-954 [PubMed] [Google Scholar]
- Ison J. R., Pinckney L. A. (1983). Reflex inhibition in humans: sensitivity to brief silent periods in white noise. Percept. Psychophys. 34, 84-88 [DOI] [PubMed] [Google Scholar]
- Kalmijn A. J. (1988) Hydrodynamic and acoustic field detection. In Sensory Biology of Aquatic Animals (ed. Atema J., Fay R. R., Popper A. N., Tavolga W. N.), pp. 83-130 New York, NY; London: Springer-Verlag; [Google Scholar]
- Kappler J. A., Starr C. J., Chan D. K., Kollmar R., Hudspeth A. J. (2004). A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc. Natl. Acad. Sci. USA 101, 13056-13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310 [DOI] [PubMed] [Google Scholar]
- Kohashi T., Oda Y. (2008). Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J. Neurosci. 28, 10641-10653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski D. J., Whitfield T. T., Hukriede N. A., Lam W. K., Weinberg E. S. (2005). The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev. Biol. 277, 27-41 [DOI] [PubMed] [Google Scholar]
- Liao J. C., Ballo O., Akanyeti O. (2012). Frequency response of afferent neurons in the posterior lateral line system of larval zebrafish (Danio rerio). 2012 Neuroscience Meeting Planner, Program No. 460.01. [Google Scholar]
- Lu Z., Xu Z., Buchser W. J. (2004). Coding of acoustic particle motion by utricular fibers in the sleeper goby, Dormitator latifrons. J. Comp. Physiol. A 190, 923-938 [DOI] [PubMed] [Google Scholar]
- Ma E. Y., Rubel E. W., Raible D. W. (2008). Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 28, 2261-2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Fay R. R., Popper A. N. (2010). Frequency tuning and intensity coding of sound in the auditory periphery of the lake sturgeon, Acipenser fulvescens. J. Exp. Biol. 213, 1567-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J. C., Coombs S., Halstead M. (1995). Biology of the mechanosensory lateral line in fishes. Rev. Fish Biol. Fish. 5, 399-416 [Google Scholar]
- Moorman S. J., Burress C., Cordova R., Slater J. (1999). Stimulus dependence of the development of the zebrafish (Danio rerio) vestibular system. J. Neurobiol. 38, 247-258 [PubMed] [Google Scholar]
- Murakami S. L., Cunningham L. L., Werner L. A., Bauer E., Pujol R., Raible D. W., Rubel E. W. (2003). Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio). Hear. Res. 186, 47-56 [DOI] [PubMed] [Google Scholar]
- Namdaran P., Reinhart K. E., Owens K. N., Raible D. W., Rubel E. W. (2012). Identification of modulators of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 32, 3516-3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister H., Szabo T. M., Preuss T. (2008). Behavioral and physiological characterization of sensorimotor gating in the goldfish startle response. J. Neurophysiol. 99, 1493-1502 [DOI] [PubMed] [Google Scholar]
- Nicolson T. (2005). The genetics of hearing and balance in zebrafish. Annu. Rev. Genet. 39, 9-22 [DOI] [PubMed] [Google Scholar]
- Nicolson T., Rüsch A., Friedrich R. W., Granato M., Ruppersberg J. P., Nüsslein-Volhard C. (1998). Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 20, 271-283 [DOI] [PubMed] [Google Scholar]
- Owens K. N., Coffin A. B., Hong L. S., Bennett K. O., Rubel E. W., Raible D. W. (2009). Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear. Res. 253, 32-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper A. N., Fay R. R. (1993). Sound detection and processing by fish: critical review and major research questions (Part 1 of 2). Brain Behav. Evol. 41, 14-25 [DOI] [PubMed] [Google Scholar]
- Popper A. N., Fay R. R. (2011). Rethinking sound detection by fishes. Hear. Res. 273, 25-36 [DOI] [PubMed] [Google Scholar]
- Radford C. A., Montgomery J. C., Caiger P., Higgs D. M. (2012). Pressure and particle motion detection thresholds in fish: a re-examination of salient auditory cues in teleosts. J. Exp. Biol. 215, 3429-3435 [DOI] [PubMed] [Google Scholar]
- Raible D. W., Kruse G. J. (2000). Organization of the lateral line system in embryonic zebrafish. J. Comp. Neurol. 421, 189-198 [PubMed] [Google Scholar]
- Riley B. B., Moorman S. J. (2000). Development of utricular otoliths, but not saccular otoliths, is necessary for vestibular function and survival in zebrafish. J. Neurobiol. 43, 329-337 [DOI] [PubMed] [Google Scholar]
- Riley B. B., Phillips B. T. (2003). Ringing in the new ear: resolution of cell interactions in otic development. Dev. Biol. 261, 289-312 [DOI] [PubMed] [Google Scholar]
- Roberts A. C., Reichl J., Song M. Y., Dearinger A. D., Moridzadeh N., Lu E. D., Pearce K., Esdin J., Glanzman D. L. (2011). Habituation of the C-start response in larval zebrafish exhibits several distinct phases and sensitivity to NMDA receptor blockade. PloS ONE 6, e29132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S., Castellan N. J. (1988) Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill; [Google Scholar]
- Starr C. J., Kappler J. A., Chan D. K., Kollmar R., Hudspeth A. J. (2004). Mutation of the zebrafish choroideremia gene encoding Rab escort protein 1 devastates hair cells. Proc. Natl. Acad. Sci. USA 101, 2572-2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B. (1995). Adaptive psychophysical procedures. Vision Res. 35, 2503-2522 [PubMed] [Google Scholar]
- Von Frisch K. (1938). The sense of hearing in fish. Nature 141, 8-11 [Google Scholar]
- Walter M., Tziridis K., Ahlf S., Schulze H. (2012). Context dependent auditory thresholds determined by brainstem audiometry and prepulse inhibition in Mongolian gerbils. Open J. Acoust. 2, 34-49 [Google Scholar]
- Webb J. F., Shirey J. E. (2003). Postembryonic development of the cranial lateral line canals and neuromasts in zebrafish. Dev. Dyn. 228, 370-385 [DOI] [PubMed] [Google Scholar]
- Weiss S. A., Zottoli S. J., Do S. C., Faber D. S., Preuss T. (2006). Correlation of C-start behaviors with neural activity recorded from the hindbrain in free-swimming goldfish (Carassius auratus). J. Exp. Biol. 209, 4788-4801 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th edn. Eugene, OR: University of Oregon Press; [Google Scholar]
- Whitfield T. T. (2002). Zebrafish as a model for hearing and deafness. J. Neurobiol. 53, 157-171 [DOI] [PubMed] [Google Scholar]
- Whitfield T. T., Granato M., van Eeden F. J., Schach U., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., et al. (1996). Mutations affecting development of the zebrafish inner ear and lateral line. Development 123, 241-254 [DOI] [PubMed] [Google Scholar]
- Whitfield T. T., Riley B. B., Chiang M. Y., Phillips B. (2002). Development of the zebrafish inner ear. Dev. Dyn. 223, 427-458 [DOI] [PubMed] [Google Scholar]
- Wichmann F. A., Hill N. J. (2001). The psychometric function: I. Fitting, sampling, and goodness of fit. Percept. Psychophys. 63, 1293-1313 [DOI] [PubMed] [Google Scholar]
- Willott J. F., Carlson S., Chen H. (1994). Prepulse inhibition of the startle response in mice: relationship to hearing loss and auditory system plasticity. Behav. Neurosci. 108, 703-713 [DOI] [PubMed] [Google Scholar]
- Wolman M., Granato M. (2012). Behavioral genetics in larval zebrafish: learning from the young. Dev. Neurobiol. 72, 366-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes R. M. (1905). The sense of hearing in frogs. J. Comp. Neurol. 15, 279-304 [Google Scholar]
- Young J. S., Fechter L. D. (1983). Reflex inhibition procedures for animal audiometry: a technique for assessing ototoxicity. J. Acoust. Soc. Am. 73, 1686-1693 [DOI] [PubMed] [Google Scholar]
- Zar J. H. (1999) Biostatistical Analysis, 4th edn. Upper Saddle River, NJ: Prentice Hall; [Google Scholar]
- Zeddies D. G., Fay R. R. (2005). Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 208, 1363-1372 [DOI] [PubMed] [Google Scholar]