Summary
The study of T cell memory and the target of vaccine design has focused on memory subsumed by T cells bearing the αβ T cell receptor. Alternatively, γδ T cells are thought to provide rapid immunity particularly at mucosal borders. Here we have shown that a distinct subset of mucosal γδ T cells mounts an immune response to oral Listeria monocytogenes (Lm) infection leading to the development of multifunctional memory T cells in the murine intestinal mucosa that is capable of simultaneously producing interferon-γ and interleukin-17A. Challenge infection with oral Lm, but not oral Salmonella or intravenous Lm, induced rapid expansion of memory γδ T cells suggesting contextual specificity to the priming pathogen. Importantly, memory γδ T cells were able to provide enhanced protection against infection. These findings illustrate a previously unrecognized role for γδ T cells with hallmarks of adaptive immunity in the intestinal mucosa.
Introduction
The ability of our body to generate long lasting immunity has become the foundation for strategies to vaccinate against life-threatening pathogens. The cellular and molecular basis for this protection has more recently been attributed to generating long-lived memory T and B cells with the capacity to rapidly respond to pathogen re-exposure. T cell memory has traditionally been studied in the context of CD8+ and CD4+ T cells expressing an αβ T cell receptor (TCR) (αβ T cells) but recent identification of nonconventional memory lymphocyte subsets (Sun et al., 2009; O'Leary et al., 2006; Paust et al., 2010) has provided incentive to reevaluate the ability of γδ T cells to generate memory populations. Overall, γδ T cell responses have been studied in the context of rapid, innate immunity and have rarely focused on γδ T cells as an arm of adaptive immunity. In primates, a few notable studies have demonstrated that a population of Vγ9Vδ2+ T cells respond to a challenge infection with recall-like expansion (Shen et al., 2002; Shao et al., 2009; Ryan-Payseur et al., 2012). However, in those studies, the role of the responding γδ T cell populations in the overall response is difficult to address and their contribution to protection is unclear. Given this, we decided to examine the γδ T cell response following oral Listeria monocytogenes (Lm) infection in mice.
Lm is a widely utilized pathogen for understanding immunity and γδ T cells appear to play both inflammatory and regulatory roles in response to Lm infection (Rhodes et al., 2008; Hamada et al., 2008b). However, immunity to oral Lm infection has not been widely studied since infection of mice with most available Lm strains does not mimic natural infection. In this study a bacterial mediator of invasion, internalin A (InlA), was modified to interact with murine E-cadherin and facilitate intestinal epithelial cell (IEC) invasion of mice (Mengaud et al., 1996; Lecuit et al., 1999; Wollert et al., 2007). This modified pathogen invades murine IECs when inoculated orally, recapitulating human Listeria infection (Wollert et al., 2007). In this manner, we could examine γδ T cell immunity in the intestinal mucosa following a true enteric infection.
γδ T cells are present in small numbers in most tissues of naïve mice. However, their presence is markedly pronounced at barrier surfaces. In particular, the intestine, lung, reproductive tracts, and skin maintain high proportions of γδ T cells. In the intestinal epithelium, a large percentage of intraepithelial lymphocytes are γδ T cells (Goodman and Lefrançois, 1988; Goodman and Lefrancois, 1989) and these express multiple V-regions with a high degree of junctional diversity (Asarnow et al., 1989). In contrast, γδ T cells in the skin, lung and reproductive tract express canonical TCRs with no or very limited junctional diversity (Allison and Havran, 1991). These cells are produced from the fetal and neonatal thymus, seed the epithelial surfaces in which they reside, and are maintained without further thymic input (Haas et al., 2012; Carding and Egan, 2002). On the other hand, γδ T cells expressing less restricted TCRs mainly reside in peripheral lymphoid tissues such as the lymph nodes (i.e., Vγ1 and Vγ2) and develop later in ontogeny (Carding and Egan, 2002; Korn and Petermann, 2012).
Distinct γδ T cell subsets are thought to be important for controlling Listeria infection and regulation of anti-listerial immunity (Hamada et al., 2008b; Hamada et al., 2008a; Rhodes et al., 2008). On one hand, γδ T cells responding to Listeria infection are an important source of the regulatory cytokine interleukin (IL)-10 (Rhodes et al., 2008; Hsieh et al., 1996) but γδ T cells are also as an important source of the proinflammatory cytokine IL-17A, which is a critical component of early anti-listerial immunity (Lockhart et al., 2006; Hamada et al., 2008b; Meeks et al., 2009). In naïve mice, IL-17 producing γδ T cells are typically found in peripheral lymph nodes (pLN) and are characterized as CD27− CD44hi (Ribot et al., 2009). Both Vγ2+ (Ribot et al., 2009; Roark et al., 2007; Hamada et al., 2008b) and Vγ4+ (Haas et al., 2012) γδ T cells produce IL-17 in adult or neonatal mice, respectively. Similarly to CD4+ helper T cells, γδ T cell fate is determined by the expression of transcription factors that function as master regulators of cytokine production. Thymic γδ T cells have high baseline expression of the transcription factor RORγt while signaling through CD27 and the TCR induce T-bet transcription factor expression and developmentally imprint γδ T cells for interferon-γ (IFN-γ) production (Ribot et al., 2009; Turchinovich and Hayday, 2011; Jensen et al., 2008). Thus, the production of IFNγ and IL-17A appears to be mutually exclusive as a result of specific developmental cues, although in vitro activation of human γδ T cells drives simultaneous production of IFN-γ and IL-17 (Haas et al., 2009; Caccamo et al., 2011).
One notable exception to the distribution of IL-17A producing γδ T cells appears to be their nearly complete absence from the mesenteric lymph nodes (MLN) (Do et al., 2011), suggesting tissue-specific migration or retention of this population. Given this and the role for γδ T cells in responses to bacterial infections, we examined the mucosal γδ T cell response to oral Lm infection. Surprisingly, our findings did not reveal an expected innate-like γδ T cell response but rather identified a mucosal γδ T cell response that shared numerous characteristics with an adaptive αβ T cell response. The responding mucosal γδ T cells were polyfunctional and were comprised of both IL-17A and IFN-γ producers and notably, IL-17A and IFN-γ double producers. Moreover, the mucosal γδ T cell subset was retained long-term and underwent extensive expansion upon oral challenge. Importantly, these Lm-elicited γδ T cells collaborated with αβ T cells to mediate protection against secondary infection. These findings illustrated a previously unrecognized role for γδ T cells with hallmarks of adaptive antimicrobial immunity in the intestinal mucosa. The preferential localization of memory γδ T cells to the intestinal mucosa may provide novel therapeutic opportunities for the treatment of intestinal diseases.
Results
A unique subset of γδ T cells is elicited by oral Lm infection and is maintained into memory
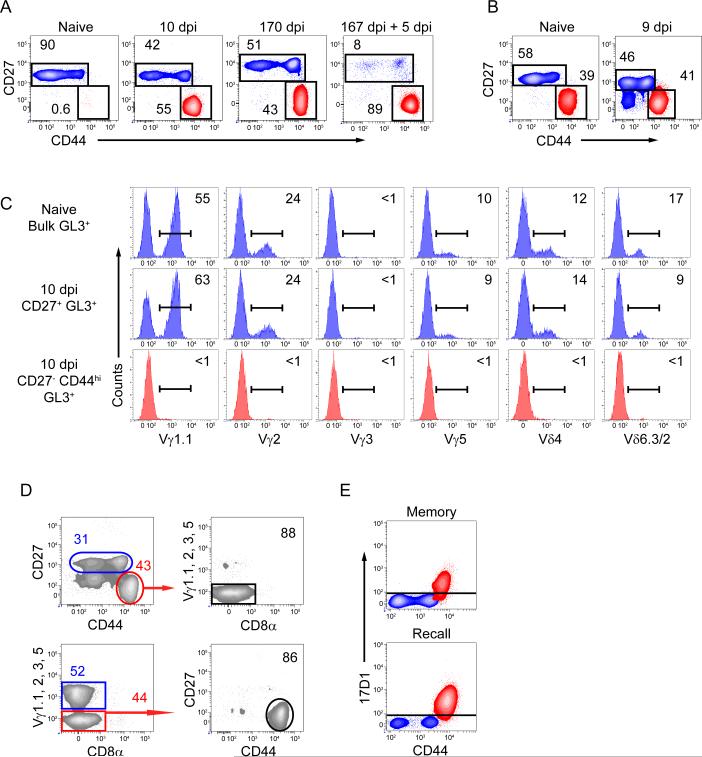
Following oral Lm infection, a large population of CD27− CD44hi γδ T cells which was not present in naive mice, appeared in the MLN and represented ~50% of the total γδ T cells (Figure 1A). Furthermore, only the CD27− CD44hi γδ T cells in the MLN incorporated bromodeoxyuridine (BrdU) following a 2-day pulse from 6-7 days post infection (dpi) demonstrating preferential reactivity of this subset to Lm infection (Figure S1A). This population was maintained for at least 5 months following infection and rapidly recalled to a secondary challenge (Figure 1A). While a population of CD27− CD44hi γδ T cells was consistently observed in pLN of naïve mice, a correspondingly similar increase in CD27− CD44hi γδ T cells was not observed in the pLN (Figure 1B) suggesting that the Lm-elicited γδ T cell response was limited to mucosal tissues. Moreover, MLN derived CD27− CD44hi γδ T cells were phenotypically distinct from innate-like (identified as CD27− CD44hi) IL-17 producing γδ T cells from the pLN as they lacked CD103, the chemokine receptor CCR6, and Vγ2 but expressed high T-bet and comparable RORγt (Haas et al., 2009; Gray et al., 2011) (Figure S1B). Further phenotypic analysis of Lm elicited MLN derived CD27− CD44hi γδ T cells demonstrated characteristics consistent with both innate and adaptive T cells since they expressed high common gamma chain (γc) cytokine receptors CD127 and CD25 and intermediate CD122 (Figure S1C). However, they did not express CD62L and were heterogeneous for Ly6C and CD43 (Figure S1C).
Figure 1. A distinct Lm-elicited γδ T cell subset is maintained into immunologic memory and recalls to secondary challenge.
(A and B) Representative contour plots are gated on γδ T cells (identified by staining with GL3, a Cδ TCR-specific mAb) from MLN (A) or pLN (B) on indicated dpi. Values represent the percentage of cells within the indicated gates. See also Figure S1. (C) Representative histograms gated on total naïve γδ T cells or indicated subsets from 10 dpi mice from MLN were analyzed for expression of the indicated Vγ or Vδ regions. Values corresponding to the percentage of cells within the gated population are shown. (D) Two distinct gating strategies can identify Lm-elicited γδ T cells. Representative contour plots of γδ T cells from 9 dpi MLN were analyzed for CD27, CD44, Vγ1.1, 2, 3, and 5, and CD8α. Values corresponding to the percentage of cells within the gated population are shown. (E) 17D1 identifies Vγ4Vδ1 expressing T cells following preincubation with GL3. γδ T cells from MLN of memory mice and memory mice given a secondary oral infection were characterized for CD27 and CD44 expression and 17D1 reactivity. Red contour represents CD27− CD44hi γδ T cells while blue contour represents CD27+ γδ T cells.
Since the Lm-elicited MLN γδ T cells appeared distinct from CD27− CD44hi pLN γδ T cells we sought to identify the TCR V region usage of Lm-responding cells. Using currently available antibodies, we determined that Lm-elicited CD27− CD44hi MLN γδ T cells did not express the Vγ1.1, 2, 3, or 5, or Vδ4 or Vδ6.3/2 TCRs (Garman et al., 1986) suggesting that these cells expressed the Vγ4 TCR chain (Figure 1C). Using a dump gate strategy employing all of the available Vγ TCR antibodies we demonstrated that the vast majority of Vγ4 γδ T cells were CD27− CD44hi indicating the reciprocal nature of this identification strategy (Figure 1D). These results were confirmed by reactivity with the 17D1 antibody in γδ T cells from Lm-immune mice at memory and after recall infection (Figure 1E). 17D1, an antibody which typically identifies Vγ3Vδ1 T cells, can instead identify Vγ4Vδ1 T cells if used following pre-incubation with the pan-γδ-specific mAb GL3 (Roark et al., 2004). Sequencing of the Vγ4 and Vδ1 TCRs from sort-purified cells further confirmed Vγ4Vδ1 TCR usage (Table 1). While the Lm-elicited population predominantly utilized canonical Vγ4 and Vδ1 TCRs, a subset of Vδ1 TCRs displayed diversity consistent with N additions surrounding the D region of the TCR.
Table 1.
Lm-elicited γδ T cell Vγ4 and Vδ1 TCRs sequences
| Vγ4 |
|||||||
|---|---|---|---|---|---|---|---|
| V | N | J | Frequency | In Frame | |||
| Canonical | EATYYCACW | DSSGF | 48/50 | Yes | |||
| EATYYCAW | DSSGF | 1/50 | No | ||||
| EATYYCACW | YSSGF | 1/50 | Yes | ||||
| Vδ1 |
|||||||
|---|---|---|---|---|---|---|---|
| V | N | D1 | N | J | Frequency | In Frame | |
| Canonical | GTYYCGSD | IGG | SSWDTR | 21/30 | Yes | ||
| Non-canonical | GTYYCGSD | IGG | IRA | TDKLVF | 3/30 | Yes | |
| GTYYCGSD | I | VGG | IRA | TDKLVF | 1/30 | Yes | |
| GTYYCGSD | IGG | T | TDKLVF | 1/30 | Yes | ||
| GTYYCGSD | RRD | T | TDKLVF | 1/30 | Yes | ||
| GTYYCGSD | M | G | YELVF | 1/30 | Yes | ||
| GTYYCGSD | I | SEG | Y | LPTNSSL | 1/30 | No | |
| GTYYCGSD | IGG | IPTNSSL | 1/30 | No | |||
Vγ4 and Vδ1 sequences were determined from sorted CD27neg CD44high γδ T cells from MLNs of mice infected 9 days previously. Canonical and non-canonical Vγ4 and Vδ1 TCR amino acid junctional sequences are displayed in reference to their frequency and in frame translation. Dashed line separates sequences that contain distinct J regions. Data in table is a compilation of sequences obtained from two independent experiments.
Lm-elicited γδ T cells rapidly mobilize into the blood and migrate to the lamina propria but not to the epithelium
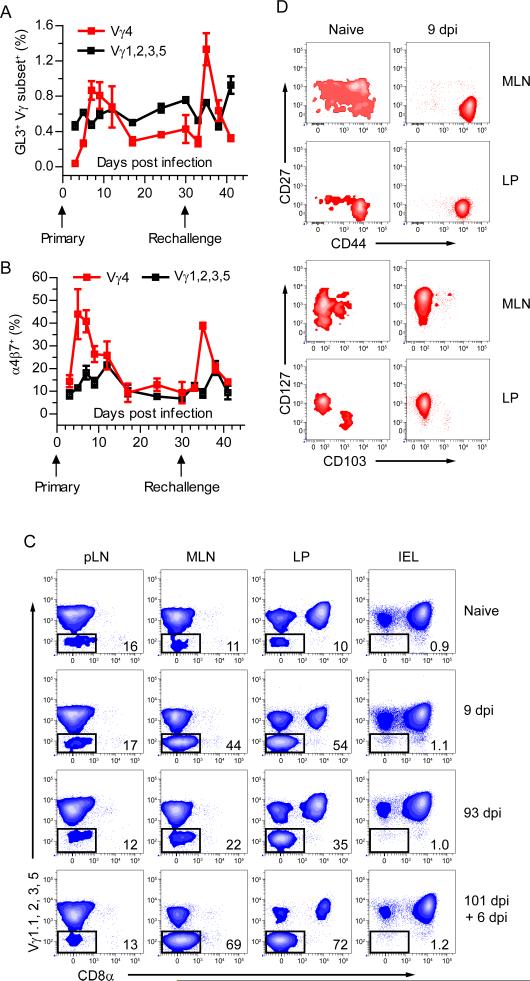
The memory characteristics of the Lm-elicited γδ T cell population were exemplified by the rapid appearance of Vγ4+ T cells in the blood after primary and secondary infection, demonstrating anamnesis (Figure 2A). Moreover, the secondary γδ T cell response was more rapid and robust than the primary response, reminiscent of a bona-fide memory response (Figures 2A and S2A). Although antigen presenting cells (APC) in the MLN imprint mucosal homing molecules on αβ T cells (Mora et al., 2005), this has not been demonstrated for γδ T cells. Transient upregulation of the mucosal addressin α4β7 on Lm-elicited Vγ4+ γδ T cells in the blood following primary and secondary infection implied instructional imprinting by APCs for intestinal migration (Figure 2B). Given this, we determined whether this population also accumulated within the intestinal effector sites, the lamina propria (LP) and the intestinal epithelium. Consistent with the expansion of CD27− CD44hi γδ T cells in the MLN, a large population of Vγ4+ γδ T cells emerged in the LP that contracted to form a stable memory population which rapidly reactivated following secondary exposure to Lm (Figure 2C). Interestingly, the Vγ4+ γδ T cell subset was not found in the intraepithelial lymphocyte (IEL) compartment at any point following infection suggesting distinct regulation of this effector cell subset (Figure 2C). While there are very few CD27− CD44hi γδ T cells in the MLN of a naïve mouse, we identified a small population of Vγ4+ γδ T cells in the MLN, pLN and LP of naïve mice representing ~10-15% of the total γδ T cells (Figure 2C). In naïve mice this population was phenotypically heterogeneous in the MLN being composed of three approximately equally sized subsets based on CD27 and CD44 expression (Figure 2D). However, 17D1-reactive γδ T cells in the MLN of naïve mice displayed a homogenous CD27− CD44hi phenotype suggesting that Lm-elicited γδ T cells may be derived from this population (Figure S2B). After infection the majority of Vγ4+ CD8α− T cells expanded in the MLN and LP were CD27− and CD44hi. Further, the population of γδ T cells that accumulated in the LP expressed high CD127 and CD44 and lacked expression of CD27 and CD103 similar to the Lm-induced MLN derived γδ T cells but distinct from pLN CD27− CD44hi γδ T cells (Figure 2D and S1B). In addition, in no case did the Vγ4+ cells in the pLN respond to infection (Figure 2C). Taken together, these data suggested that in response to oral infection a specific subset of Vγ4+ γδ T cells was activated in the MLN, mobilized into the bloodstream and subsequently homed to the LP where they were maintained long-term and underwent a robust response to secondary oral Lm infection.
Figure 2. Mobilization of Lm-elicited γδ T cells results in accumulation within intestinal LP but not within the epithelium.
(A and B) Blood was collected from Lm infected mice and analyzed for GL3, Vγ chain (A), and α4β7 (B) expression. Mice were given a secondary Lm infection at 30 dpi and monitored for an additional 11 days with 3 – 5 mice per group. Subsets labeled Vγ4 specifically refers to γδ T cells which do not express Vγ1.1, 2, 3, or 5. Data is represented as mean ± SEM. See also Figure S2A. (C) Representative contour plots are gated on γδ T cells from pLN, MLN, LP, and IEL at the indicated times following infection. Values represent the percentage of cells within the indicated gates. Representative contour plots are shown from multiple experiments with 3 – 5 mice per group. (D) Representative contour plots gated on Vγ4+ CD8α− γδ T cells (as indicated in (C)) from the MLN and LP were characterized for expression of CD44 and CD27 or CD127 and CD103. See also Figure S2B.
γδ T cells from Lm immune mice rapidly respond to secondary infection
We further examined the specificity and kinetics of the recall response by analyzing the response in orally-primed immune mice following a secondary oral or intravenous (iv) challenge. After oral rechallenge, an increase in the CD27− CD44hi γδ T cell subset in the MLN was evident by day 3, peaked at day 5 and began to decline at day 8 (Figure 3A). Consistent with the primary γδ T cell response, other subsets of γδ T cells failed to expand following a secondary oral Lm infection suggesting that the CD27− CD44hi γδ T cell subset selectively responded to the priming pathogen. Moreover, memory γδ T cells responded comparably to a bona-fide antigen-specific memory αβ CD8+ T cell recall response. Indeed, the γδ T cell response was greater in magnitude than the αβ CD8+ T cell recall response to the immunodominant LLO91-99 epitope (Figure S3A). Lm-elicited γδ T cells were also able to undergo further expansion consistent with traditional memory T cell prime-boosting following repeated challenges (Figure 3B).
Figure 3. Lm-elicited γδ T cells display contextual specificity to the priming pathogen.
(A) Mice infected 170 days previously were challenged either orally with 2×1010 CFU Lm or intravenously with 2×105 Lm. MLNs were harvested at the indicated times following secondary infection to enumerate γδ T cells based on CD27 and CD44 expression. Data is displayed as mean ± SEM and representative of at least two independent experiments of 4 – 5 mice per group. See also Figure S3A-D. (B) Mice which had received a secondary infection were rested an additional 124 days prior to a tertiary oral Lm infection with 2×1010 CFU and γδ T cells were analyzed for CD27 and CD44 expression 5 days later (red squares represent individual mice). Data is represented as mean ± SEM. (C and D) γδ T cells from the MLN of oral Lm immune mice or oral Lm immune mice rechallenged by oral infection with Lm, S. typhimurium strain BRD509, or S. typhimurium strain SL1344 were analyzed 5 days later for (C) CD27 and CD44 expression and (D) α4β7 expression. Representative data is displayed as mean ± SEM from two independent experiments with 3 – 4 mice per group. See also Figure S3E.
In contrast to the response to oral challenge, secondary intravenous (iv) infection of orally primed mice failed to induce expansion of any subset of γδ T cells in the MLN of an Lm immune mouse demonstrating that route of infection dictates recall of Lm elicited CD27− CD44hi γδ T cells (Figure 3A). To rule out sequestration of replicating Lm from the MLNs following an iv infection, we examined γδ T cell responses in the liver and spleen of Lm immune mice. First, both iv and oral infection with Lm resulted in high bacterial burdens in the liver and spleen (Figure S3B). Not surprisingly, the bacterial burdens were more elevated in the liver following an iv versus an oral infection. Despite the nearly 100-fold increase in bacterial burden in the liver, iv infection did not induce the expansion of CD27− CD44hi γδ T cells in the spleen or liver (Figure S3C). Moreover, iv challenge infection of orally primed mice failed to induce expansion of Lm-elicited γδ T cells residing in the liver (Figure S3D). However, expansion of γδ T cells in the liver occurred following oral infection of orally primed mice. It should be noted that both routes of infection generated a comparably robust antigen-specific CD8+ T cell response in the spleen and liver, suggesting distinct mechanisms regulate the expansion of Lm-elicited γδ and αβ T cells (Figure S3C). The inability of iv infection of orally primed mice to induce expansion of Lm-elicited CD27− CD44hi γδ T cells in the MLN and liver suggested that replicating bacteria and associated pathogen associated molecular patterns (PAMPs) alone were insufficient to induce expansion of Vγ4+ γδ T cells. Although the issue of γδ T cell specificity remains enigmatic, we tested whether infection with another intestinal bacterial pathogen could trigger a recall response in the Vγ4+ CD27− CD44hi T cell subset. Mice were primed by oral Lm infection and 64 days later were challenged by oral infection with Lm, S. typhimurium BRD509 (attenuated), or S. typhimurium SL1344 (pathogenic). Five days later, a substantial increase in the CD27− CD44hi γδ T cell subset was evident after Lm but not after S. typhimurium infection of either strain (Figure 3C) despite substantial bacterial (S. typhimurium) burden and immune activation in the MLN (Figure S3E). Moreover, only secondary Lm infection induced upregulation of α4β7 expression on γδ T cells (Figure 3D). Taken together, these data suggested that PAMPs or cytokines alone were unable to induce reactivation of Lm-elicited memory γδ T cells. Moreover, these results indicated a contextual specificity of the secondary γδ T cell response to the intestinal priming pathogen.
Oral Lm infection induces IL-17A and IFN-γ double-producing γδ T cells
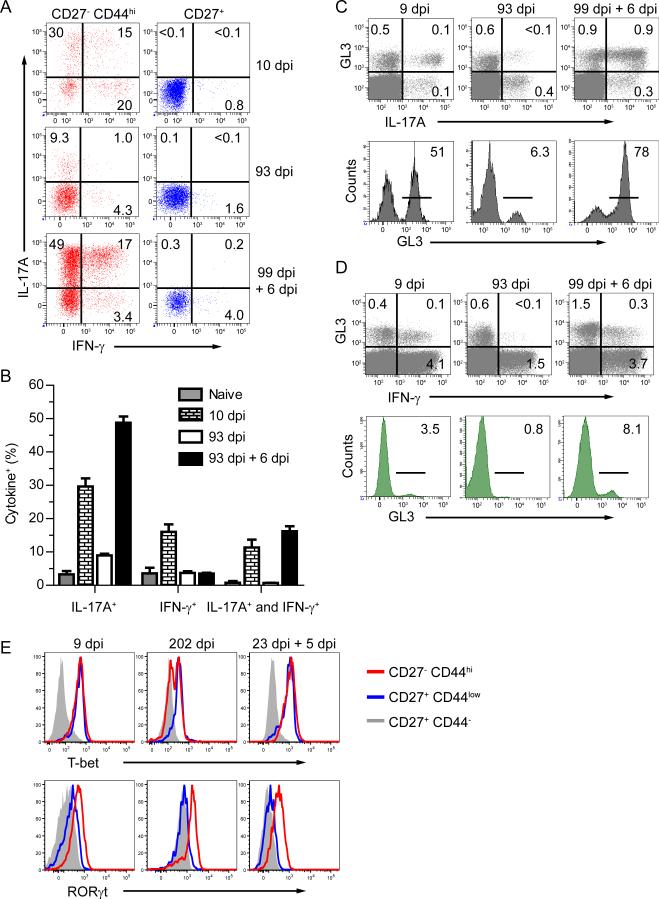
Cytokine production by naïve pLN derived γδ T cells is segregated based on expression of CD27 whereby CD27+ γδ T cells produce IFN-γ and CD27− γδ T cells produce IL-17A (Ribot et al., 2009). Contrary to this pattern of cytokine production, following oral Lm infection substantial amounts of IFN-γ and IL-17A were produced by CD27− CD44hi Lm elicited γδ T cells following CD3 crosslinking (Figure 4A and B). In addition, only a minor subset of CD27+ γδ T cells produced IFN-γ and this profile remained unchanged during distinct phases of the immune response to oral Lm infection (Figure 4A). Surprisingly, three distinct populations of cytokine-producing γδ T cells were induced in response to a primary oral Lm infection. Approximately 45% of the CD27− CD44hi γδ T cells produced IL-17A while ~35% produced IFN-γ. Most importantly, a substantial population of MLN γδ T cells were capable of simultaneously producing IFN-γ and IL-17A. However, >3 months after infection, small subsets of memory γδ T cells produced either IFN-γ or IL-17A, but not both. These findings indicate that the transition to memory included alterations in cytokine-producing abilities, just as occurs with memory αβ T cells. Upon secondary infection, a robust induction of cytokine production occurred with ~70% of the cells producing high amounts of IL-17A and ~25% of these also producing IFN-γ, while only a small subset solely produced IFN-γ (Figure 4A and B). Indeed, the Lm-elicited γδ T cell response became more pronounced relative to a polyclonally stimulated αβ T cell response between the primary and secondary infections. The γδ T cells and not the αβ T cells were the predominant source of IL-17A, but not IFN-γ, following both primary and secondary challenge (Figure 4C and D). Even though IFN-γ is predominately produced by αβ T cells, the contribution from γδ T cells increased relative to the primary response. Additionally, CD27− CD44hi MLN γδ T cells lacked granzyme B expression, suggesting an absence of lytic ability (Figure S4). Collectively these data supported the notion that responding γδ T cells functionally evolved through each phase of the immune response to infection from primary effector to memory to secondary effector. This process resulted in a specific mucosal γδ T cell subset becoming the predominant source of IL-17A while substantially increasing their contribution to IFN-γ production.
Figure 4. Evolution of the cytokine response from Lm-elicited γδ T cells.
(A and B) MLNs from naïve mice or mice infected with Lm were stimulated with αCD3 and αCD28-coated beads for 5 h in the presence of brefeldin A. γδ T cells were analyzed for CD27, CD44, IL-17A, and IFN-γ following stimulation. Representative dot plots from 3 – 5 mice per group and at least 2 experiments are displayed in (A) and represented as the mean ± SEM in (B). See also Figure S4. (C and D) MLNs from Lm infected mice were stimulated with beads as described for (A). γδ T cells were analyzed for IL-17A (C) and IFN-γ (D) following stimulation. Representative dot plots showing total CD3+ cells from 3 – 5 mice per group with values of the percentage of cells within gated quadrants displayed (upper panels). GL3 expression is determined from histograms gated on IL-17A (C) or IFN-γ (D) producing T cells from MLNs of infected mice. Numbers within histograms refer to the percentage of cells within the indicated gates. (E) MLNs were harvested from infected mice and γδ T cell subsets were analyzed for intracellular expression of T-bet and RORγt. Representative histograms are shown from 4 – 5 mice/group.
Considering the cytokine producing abilities just noted we examined expression of T-bet and RORγt directly ex vivo, the transcription factors that control IFN-γ and IL-17A production, respectively. Surprisingly, populations of CD27− CD44hi γδ T cells in the MLN expressed both T-bet and RORγt during all phases of the immune response (Figure 4E). Dual expression of T-bet and RORγt was especially prominent following primary and secondary infection but also occurred during memory homeostasis. At memory, CD27− CD44hi γδ T cells exhibited bimodal expression of T-bet which was rapidly upregulated following recall (Figure 4E). In contrast, memory CD27− CD44hi γδ T cells retained high expression of RORγt during memory which remained elevated following secondary challenge. These data pointed to distinct fates of the cytokine producing lineages during evolution of the response and indicated substantial potential for plasticity, particularly in the development of secondary γδ effector T cells from resting memory cells. Further, this data demonstrated long-term maintenance of simultaneous expression of T-bet and RORγt by γδ T cells responding to primary and secondary infections.
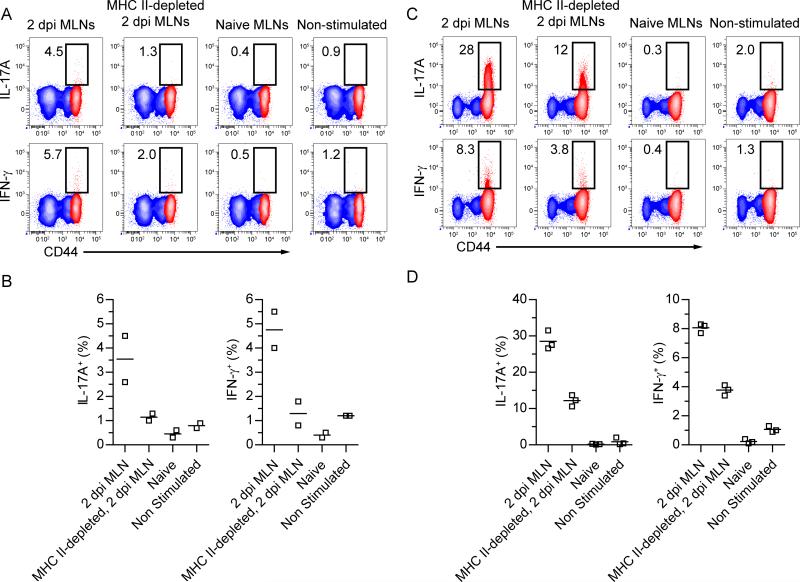
The antigen specificity of γδ T cells responding to pathogenic insult has been difficult to address over the past two decades. To begin to address the requirements for γδ T cell activation, cells from the MLN of day 2 Lm-infected mice were used to stimulate memory γδ T cells in culture. MLN cells from 2 dpi mice induced maximal expansion of naïve CD8+ T cells and contained high numbers of migratory APC populations indicating 2 dpi as an optimal time to examine the potential for induction of cytokine expression (data not shown). Activation of memory γδ T cells in these cultures resulted in induction of IFN-γ and IL-17A production to amounts that were similar to those induced by anti-CD3 stimulation (compare Figure 5A and B with Figure 4A). In addition, in vitro stimulation of effector γδ T cells with infected but not uninfected MLN cells induced substantial amounts of IL-17A and IFN-γ (Figure 5C). In both cases, depletion of major histocompatibility complex class II (MHC II) molecule expressing cells prior to stimulation substantially reduced cytokine induction. Taken together, these data suggest that an MHC II expressing cell is crucial for cytokine induction from mucosal γδ T cells.
Figure 5. MHC II expressing cells substantially contribute to Lm-elicited γδ T cell effector functions.
(A – D) 1×106 MLN cells from Lm-immune Thy1.2 mice (A and B) or Lm-immune Thy1.2 mice 5 days following a secondary oral Lm infection (C and D) were mixed with 1×106 MLN cells from Thy1.1 congenic mice as indicated above contour plots or left unstimulated. IL-17A and IFNγ production was determined by ICS of Thy1.2+ γδ T cells following 5 h in vitro in the presence of Brefeldin A. Red contour represents CD27− CD44hi Thy1.2+ γδ T cells while blue contour represents CD27+ Thy1.2+ γδ T cells. Values in contour plots represent the percentage of cytokine-producing cells from CD27− CD44hi γδ T cells. Data is representative of at least two independent experiments.
Lm-elicited γδ T cells provide protection following secondary infection
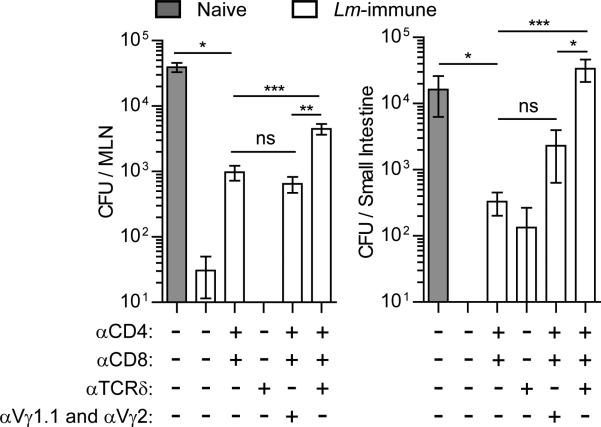
To determine whether the γδ T cell memory population was involved in anti-listerial immunity, Lm-immunized mice were depleted of CD4+ and CD8+ T cells (Figure S5A) with or without treatment with mAbs specific for Cδ (GL4) or Vγ1.1 and Vγ2 prior to secondary oral Lm infection. Consistent with other anti-TCRγδ mAb treatments which resulted in TCR internalization (Koenecke et al., 2009), GL4 mAb (Goodman and Lefrancois, 1989) treatment induced internalization of the γδ TCR and CD3 complex (Figure S5B), thereby hindering the ability of γδ T cells to respond following oral Lm infection (Figure S5C). Immune mice were nearly completely protected against infection in the MLN and intestine (Figure 6). Treatment with anti-Cδ alone did not affect protection, while CD4+ and CD8+ T cell depletion resulted in only a minimal loss of protection indicating that αβ T cell memory may not be as important following oral infection as it is thought to be following other routes of infection (Ladel et al., 1994; Harty et al., 1992; Sprent, 2002). However, anti-Cδ treatment along with CD4+ and CD8+ T cell depletion resulted in a substantially greater loss of protection in both the MLN and the intestine (Figure 6). Indeed, depletion of CD4+ and CD8+ T cells in conjunction with γδ T cell TCR internalization reduced protective capacity to that seen in naïve mice in the small intestine. This result suggested that the protective capacity of Lm-elicited γδ T cells was dependent on surface TCR expression. Memory Vγ4+ T cells were the likely γδ subset responsible for protection as treatment with mAb directed against Vγ1.1 and Vγ2 TCR in conjunction with CD4 and CD8 depletion did not lead to a greater loss of protection (Figure 6). Together, these data demonstrated that γδ and αβ T cells collaborate to provide protective immunity in the intestinal mucosa and that this protection appears dependent on TCR expression on γδ T cells.
Figure 6. γδ T cells collaborate with αβ T cells to protect against secondary infection.
Naïve mice or Lm-immune mice treated with the indicated combinations of GK1.5 (αCD4), 2.43 (αCD8), GL4 (αTCRδ), and 2.11 (αVγ1.1) and UC3-10A6 (αVγ2) were orally infected with 2×1010 CFU Lm. Bacterial burden was enumerated in the MLN and small intestine 4 days later. Data is presented as the mean ± SEM from 5 – 15 mice per group. *** p < 0.001, ** p < 0.01, * p < .05, ns p > 0.05. See also Figure S5.
Discussion
Here, we have identified a population of murine γδ T cells, distinct from previously described subsets, whose characteristics reflect the hallmarks of adaptive αβ T cells and resemble pathogen-specific IL-17-producing human γδ T cell subsets (Caccamo et al., 2011; Fenoglio et al., 2009; Dechanet et al., 1999). As noted in humans and primates, γδ T cells appear to have the capacity to form memory populations (Shen et al., 2002; Ryan-Payseur et al., 2012). However, their functional significance and contribution to protection in vivo has not been examined and there are currently no workable murine models to examine similar populations of γδ T cells. For the first time, we identified a murine model in which IL-17 producing pathogen-specific γδ T cells behave similarly to what was observed for primate γδ T cells. We have used this model to identify a protective subset of γδ T cells which is capable of simultaneous production of IL-17A and IFN-γ. In response to oral Lm infection a population of CD27− CD44hi γδ T cells was able to form a multifunctional memory population with the capacity to rapidly respond to a specific secondary infection and provide protection in collaboration with their αβ T cell memory counterparts. The γδ T cell response to Lm infection demonstrated a clear progression of primary effector cells to memory cells and subsequently to secondary effectors with unique cytokine and transcriptional profiles.
The memory γδ T cell population displayed contextual specificity to the priming pathogen highlighting their significance in mucosal immunity. Defining the specificity of γδ T cells has been a largely intractable process over the past two decades. To begin to address specificity in our system, we first examined the ability to recall orally primed Lm-immune mice with a secondary iv Lm infection. Interestingly, iv rechallenge failed to induce expansion of Lm-elicited γδ T cells. Since the majority of T cell priming following iv infection with Lm occurs in the spleen with little contribution from lymph node T cells (Klonowski et al., 2006), it may not be surprising that Lm-elicited MLN-derived γδ T cells did not proliferate in response to an iv infection. Pathogen products alone are capable of inducing IL-17 production from CCR6+ γδ T cells dependent on pattern recognition receptors (Martin et al., 2009). Thus, PAMPs induce expression of IL-1β and IL-23 which are capable of inducing IL-17A expression even in the absence of TCR signals (Sutton et al., 2009). Both oral and iv Lm infection resulted in elevated bacterial burden and therefore PAMPs in the liver. Yet, liver memory γδ T cells failed to expand following iv rechallenge. Together with the induction of α4β7 on responding γδ T cells, these data suggested a requirement for mucosal APCs in driving memory γδ T cell reactivation.
To further examine specificity, we challenged oral Lm-immune mice with S. typhimurium. Following oral infection with S. typhimurium, MLN derived APCs are capable of inducing expansion of memory CD8+ T cells (Jones-Carson et al., 2007). Importantly, both S. typhimurium and Lm infection induce expansion of human Vγ9+ T cells (Hara et al., 1992; Ryan-Payseur et al., 2012). Primate Vγ9+Vδ2+ T cells responding to Mycobacterium tuberculosis, Yersina enterolitica, Escherichia coli, and Lm recognize the pyrophosphate metabolite (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) (Puan et al., 2007; Begley et al., 2004; Ryan-Payseur et al., 2012) which is also produced by S. typhimurium (Cornish et al., 2006). However, challenge infection of Lm-immune mice with a high dose of an attenuated S. typhimurium strain (BRD509) or the highly virulent S. typhimurium parental strain (SL1344) failed to induce expansion of memory γδ T cells. Taken together, these results indicated, in the least, contextual specificity to the priming pathogen.
To date, murine IL-17-producing γδ T cells have not been reported to demonstrate the type of plasticity that is evident in CD4+ T cell subsets, such as the ability of IL-17 producing CD4+ T cells to produce IFN-γ or IL-10 (Bromson-Leeman et al., 2009; Zielinski et al., 2012). Identifying γδ T cells capable of producing both IL-17A and IFN-γ without in vitro manipulations has only convincingly been demonstrated for primate γδ T cells (Poggi et al., 2009; Fenoglio et al., 2009; Ryan-Payseur et al., 2012), and our results now extend this phenomenon to murine γδ T cells. Similar to IFN-γ+ IL-17+ γδ T cells from HIV-infected individuals, Lm-elicited memory γδ T cells co-expressed the canonical transcriptional regulators RORγt and T-bet. The precise lineage relationships between the cytokine producing Lm-elicited γδ T cell subsets transitioning from effectors to memory and subsequent secondary effectors remains unclear. Based on T helper-17 cell plasticity that leads to the acquisition of IFN-γ production (Nistala et al., 2010; Hirota et al., 2011; Huber et al., 2011), it may be likely that γδ T cells develop first into IL-17A producers and receive environmental queues to instruct IFN-γ production, but fate mapping experiments are needed.
γδ T cells have been reported to play dichotomous roles following Lm infection via non-oral routes. On one hand, they can produce IL-10 and regulate CD8+ T cell responses limiting immunopathology in the liver (Mombaerts et al., 1993; Rhodes et al., 2008; Fu et al., 1994). On the other hand, they can also protect against primary infection at least in part by production of IL-17A (Hamada et al., 2008b) and in the early response Vγ2+ cells produce IL-10 and IL-17A (Hamada et al., 2008b; Rhodes et al., 2008). However, as the response progresses Vγ4+Vδ1+ cells produce IL-17A and Vδ1−/− mice are more susceptible to infection (Hamada et al., 2008a). Moreover, following iv infection, γδ T cells are sufficient for protection in Tcra−/− or Tcrb−/− mice while protection following a secondary infection is predominately provided by αβ T cells but also involves γδ T cells (Mombaerts et al., 1993). In our case, depletion of CD4+ and CD8+ T cells resulted in only a modest loss of protection following secondary infection and treatment with only anti-Cδ antibody had little effect. However, following combined treatments a substantial loss of protection was observed in the intestine and MLN and this effect was attributable to Vγ4+ T cells. γδ T cells can provide effector functions without TCR engagement (Sutton et al., 2009) and treatment with anti-Cδ antibodies results in internalization of the TCR, rather than γδ T cell depletion (Koenecke et al., 2009). However, our results indicated the protective role of γδ T cells was likely TCR dependent. Both IL-17A or IFN-γ are important for protection from Lm infection (Harty and Bevan, 1995; Tripp et al., 1994; Hamada et al., 2008b; Meeks et al., 2009) with IL-17A inducing neutrophil recruitment (Meeks et al., 2009; Tam et al., 2012; Dejima et al., 2011) and IFN-γ orchestrating the early innate immune response (Kang et al., 2008). Whether similar events occur after oral infection is currently under study.
These studies have outlined an orchestrated mucosal γδ T cell immune response akin to the αβ response. While memory γδ T cells have been noted in primates, the murine equivalent was lacking hindering experimentation to provide insight into their roles in human diseases (Pang et al., 2012). Our findings suggested murine memory γδ T cells closely resembled human pathogen-specific Vγ9+Vδ2+ T cells. These Lm-elicited γδ T cells were maintained long-term and responded to secondary infection with robust proliferation and effector functions including simultaneous production of IFN-γ and IL-17A. Memory γδ T cells contributed extensively to protection from a secondary oral infection highlighting their importance at mucosal surfaces. Mucosal vaccine design may benefit from targeting such non-traditional memory lymphocyte populations.
Methods
Mice
Female Balb/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in specific-pathogen-free conditions. Six – 12 week old, age-matched mice were used for experiments. TCRδ H2B eGFP mice were obtained from I. Prinz (Hannover Medical School) and have been described previously (Koenecke et al., 2009). All animal experiments were conducted in accordance with the University of Connecticut Health Center Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
Bacteria and Infections
Listeria monocytogenes strain EGDe carrying a recombinant internalin A with a mutation in S192N and Y369S has been described in detail previously (Wollert et al., 2007) and was used for most infections. All mice were food and water deprived for ~4 hours prior to infection, housed individually with minimal bedding, and given an approximately 0.5 cm3 piece of bread inoculated with 2×109 (primary) or 2×1010 (secondary) colony-forming units (CFU) of Lm in PBS. Naïve mice are sham infected with bread inoculated with PBS. In experiments designed to quantify bacterial burden (Fig. 4), a recombinant Lm strain 10403s internalin A mutant which is naturally streptomycin resistant was used for primary (2×109 CFU) and secondary (2×1010 CFU) infections. Generation of an internalin A mutant on Lm strain 10403s was performed as described previously (Xayarath et al., 2009). Mice infected with Salmonella enterica serovar Typhimurium strain BRD509 (attenuated aroA−/aroD− mutant strain) and SL1344 (pathogenic strain from which BRD509 was derived) were infected via consumption of bread inoculated with 2×1010 or 5×107 CFU (respectively) as described above (kindly provided by S. J. McSorley). An oral infectious dose of 5×107 for SL1334 is 10-fold higher than the LD50 described for oral infections of Balb/c mice (Leung and Finlay, 1991).
Tissue Preparation
Single cell suspensions were prepared from MLN, pLN (consisting of the axillary, brachial, and inguinal lymph nodes), LP, and IEL as previously described (Sheridan and Lefrancois, 2012). Live cells were enumerated from single cell suspensions using a Vi-CELL Cell Viability Analyzer (Beckman Coulter).
Flow Cytometry
Cell suspensions were stained on ice for 30 minutes in the dark with various combinations of directly fluorochrome-conjugated antibodies purchased from Biolegend or eBiosciences. 17D1 was used to identify Vγ4Vδ1 expressing T cells as described previously (Roark et al., 2004). Following staining, cells were fixed for 20 minutes with 2% paraformaldehyde. In some cases, intracellular antigens were characterized following surface staining. For the identification of T-bet (Biolegend; 4B10) and RORγt (eBiosciences; AFKJS-9), cell staining and permeabilization was performed according to the permeabilization protocol of the BrdU Flow Kit (BD). For the identification of granzyme B, cells were permeabilized and fixed with Cytofix/Cytoperm buffer (BD) for 20 minutes on ice and then stained with an antibody specific for granzyme B (BD; GB11) in permeabilization buffer (BD) for 30 minutes on ice. MHC class I tetramer staining to identify LLO91-99-Kd specific T cells was performed at ambient temperature for 1 hour. For all samples, acquisition was performed on a LSR II flow cytometer (BD) and data were analyzed with either BD FACSDiva (BD) or FlowJo (TreeStar) software.
In vitro stimulations
Cell suspensions of MLNs were incubated with brefeldin A in RPMI 1640 supplemented with 10% fetal bovine serum, L-glutamine, gentamycin, penicillin, and streptomycin. Cells were stimulated with Dynabeads® Mouse T-activator CD3/CD28 (Life Technologies) according to manufacturer's instructions. Alternatively, cells from Lm-immune Thy1.2 mice or Lm-immune Thy1.2 mice 5 days following a secondary oral Lm infection were stimulated with an equal number of MLN cells from Thy1.1 congenic mice (either naïve, 2 dpi, or 2 dpi magnetically depleted of MHC II expressing cells). All stimulations were performed at 37°C and 5% CO2 for 5 h in the presence of brefeldin A. Cells were then surface stained as described above and treated with Cytofix/Cytoperm buffer (BD) for 30 minutes on ice. Cells were incubated with antibodies specific for IFN-γ (Biolegend; XMG1.2) and IL-17A (Biolegend; TC11-18H10.1) in permeabilization buffer (BD) for 30 minutes on ice.
In vivo antibody treatments
Mice were given i.p. injections with the indicated combinations of 500μg anti-CD8α (Bio X Cell; 2.43), 200μg anti-CD4 (Bio X Cell; GK1.5), 100μg anti-Cδ TCR (GL4), 100μg anti-Vγ1.1 (Biolegend; 2.11), and 100μg anti-Vγ2 (Biolegend; UC3-10A6) on day -3, -1, and +1, respective to recall infection.
BrDU incorporation
Mice were given 100μg bromodeoxyuridine (BrdU) i.p. on day 6 and 7 postinfection and MLN were harvested on day 8 postinfection. Incorporation of BrdU into dividing cells DNA was determined per manufacturer's guidelines (BD).
Bacterial Quantification
MLNs were mechanically dissociated through a 70μm filter. Small intestines were flushed with RPMI 1640 to remove luminal contents and mechanically dissociated using a gentleMACS™ Dissociater. All tissues were treated with 1% saponin (Calbiochem) for 1 hour at 4°C and serial dilutions were plated on Brain Heart Infusion agar plates supplemented with 50μg/mL streptomycin. Individual colonies were counted after 2 days at 37°C.
RT-PCR and sequencing
Nine dpi MLN were depleted of MHC II+ CD4+ CD8+ cells by MACS negative selection according to manufacturer's protocol (Miltenyi Biotec). CD27neg CD44high, and Vγ1.1, 2, 3, and 5neg γδ T cells were sorted from the enriched population on a FACS Aria II (BD). RNA from sorted cells was purified by Trizol (Life Technologies) and treated with DNA free DNAse (Life Technologies). cDNA was generated by RT-PCR using iSuperscript (Bio-Rad). PCR amplification of cDNA was carried out using previously reported TCR gamma and delta chain primers (Takagaki et al., 1989) with HIFI Platinum Taq polymerase (Life Technologies) and cloned into TOPO vectors (Life Technologies) for isolation. DNA from single colonies was extracted using QIAprep Miniprep (Qiagen). DNA sequences from cloned inserts were obtained from GeneWiz Inc. (South Plainfield, NJ) and sequence alignments were performed using Geneious software (Auckland, New Zealand). Canonical and non-canonical sequences were discriminated as reported before (Roark et al., 2004).
Statistical Analysis
A Mann–Whitney test was used for comparison of bacterial quantification data sets using Prism 5 (GraphPad).
Supplementary Material
Highlights.
Mucosal memory γδ T cells are generated following oral infection
Lm-elicited γδ T cells produce high amounts of both IL-17A and IFN-γ
Mucosal memory γδ T cells generate a bacteria-specific secondary response
Memory γδ T cells contribute to protection following reexposure
Acknowledgements
We thank the UCHC Flow Cytometry Core for expert assistance in cell sorting, the NIAID Tetramer Core Facility for providing LLO91 H-2Kd tetramers, S. J. McSorley (University of California, Davis) for providing Salmonella, and I. Prinz (Hannover Medical School) for providing TCRδ H2B eGFP reporter mice. Funding for this study was provided through support by the National Institutes of Health grants AI095544 (L.L.), AI076457 (L.L.), and AI041816 (N.E.F.) and by “Visualizing orally-induced T cell responses in the intestinal mucosa” reference number 2813 from the Crohn's and Colitis Foundation of America (B.S.S.). B.S.S, P.A.R., Q.M.P., and H.H.F. performed all experiments. F.A. and N.E.F. generated Lm InlAM in the 10403s strain. W.D.S. designed and provided Lm InlAM in the EGDe strain. W.D.S. and N.E.F. contributed to the overall study design. B.S.S. and L.L. designed the experiments, analyzed and interpreted the data, and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu. Rev. Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- Asarnow DM, Goodman T, Lefrancois L, Allison JP. Distinct antigen receptor repertoires of two classes of murine epithelium-associated T cells. Nature. 1989;341:60–62. doi: 10.1038/341060a0. [DOI] [PubMed] [Google Scholar]
- Begley M, Gahan CG, Kollas AK, Hintz M, Hill C, Jomaa H, Eberl M. The interplay between classical and alternative isoprenoid biosynthesis controls gammadelta T cell bioactivity of Listeria monocytogenes. FEBS Lett. 2004;561:99–104. doi: 10.1016/S0014-5793(04)00131-0. [DOI] [PubMed] [Google Scholar]
- Bromson-Leeman S, Bronson RT, Dorf ME. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J. Neuroimmunol. 2009;215:10–24. doi: 10.1016/j.jneuroim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, La MC, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, Fournie JJ, Dieli F. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- Cornish RM, Roth JR, Poulter CD. Lethal mutations in the isoprenoid pathway of Salmonella enterica. J. Bacteriol. 2006;188:1444–1450. doi: 10.1128/JB.188.4.1444-1450.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J. Clin. Invest. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejima T, Shibata K, Yamada H, Hara H, Iwakura Y, Naito S, Yoshikai Y. Protective role of naturally occurring interleukin-17A-producing gammadelta T cells in the lung at the early stage of systemic candidiasis in mice. Infect. Immun. 2011;79:4503–4510. doi: 10.1128/IAI.05799-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JS, Visperas A, O'Brien RL, Min B. CD4 T cells play important roles in maintaining IL-17-producing gammadelta T-cell subsets in naive animals. Immunol. Cell Biol. 2011 doi: 10.1038/icb.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, Murdaca G, Zocchi MR. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, Obrien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta t cells. J. Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrançois L. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J. Exp. Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur. J. Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, Krueger A, Forster R, Prinz I. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T, Hara H, Kishihara K, Tanaka K, Mayuzumi H, Ohta T, Matsuzaki G. Importance of murine Vdelta1gammadelta T cells expressing interferon-gamma and interleukin-17A in innate protection against Listeria monocytogenes infection. Immunology. 2008a;125:170–177. doi: 10.1111/j.1365-2567.2008.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 2008b;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J. Clin. Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh B, Schrenzel MD, Mulvania T, Lepper HD, Dimolfettolandon L, Ferrick DA. In vivo cytokine production in murine listeriosis - evidence for immunoregulation by gamma delta(+)3t cells. J. Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Connor W, Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Carson J, McCollister BD, Clambey ET, Vazquez-Torres A. Systemic CD8 T-cell memory response to a Salmonella pathogenicity island 2 effector is restricted to Salmonella enterica encountered in the gastrointestinal mucosa. Infect. Immun. 2007;75:2708–2716. doi: 10.1128/IAI.01905-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Marzo AL, Williams KJ, Lee SJ, Pham QM, Lefrancois L. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur. J. Immunol. 2009;39:372–379. doi: 10.1002/eji.200838741. [DOI] [PubMed] [Google Scholar]
- Korn T, Petermann F. Development and function of interleukin 17-producing gammadelta T cells. Ann. N. Y. Acad. Sci. 2012;1247:34–45. doi: 10.1111/j.1749-6632.2011.06355.x. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Flesch IE, Arnoldi J, Kaufmann SH. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KY, Finlay BB. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J. Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de JW, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunology. 2012;136:283–290. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol. Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Catellani S, Musso A, Zocchi MR. Gammadelta T lymphocytes producing IFNgamma and IL-17 in response to Candida albicans or mycobacterial antigens: possible implications for acute and chronic inflammation. Curr. Med. Chem. 2009;16:4743–4749. doi: 10.2174/092986709789878238. [DOI] [PubMed] [Google Scholar]
- Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Marker-Hermann E, Pasa-Tolic L, Nieves E, Giner JL, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int. Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur. J. Immunol. 2008;38:2274–2283. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O'Brien RL. Subset-specific, uniform activation among V gamma 6/V delta 1+ gamma delta T cells elicited by inflammation. J. Leukoc. Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J. Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan-Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector-functional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in nonhuman primates inoculated with Listeria monocytogenes DeltaactA prfA*. J. Immunol. 2012;189:1285–1293. doi: 10.4049/jimmunol.1200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Huang D, Wei H, Wang RC, Chen CY, Shen L, Zhang W, Jin J, Chen ZW. Expansion, reexpansion, and recall-like expansion of Vgamma2Vdelta2 T cells in smallpox vaccination and monkeypox virus infection. J. Virol. 2009;83:11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Isolation of mouse lymphocytes from small intestine tissues. Curr. Protoc. Immunol. 2012 doi: 10.1002/0471142735.im0319s99. Chapter 3, Unit3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. T memory cells: quality not quantity. Curr Biol. 2002;12:R174–R176. doi: 10.1016/s0960-9822(02)00735-2. [DOI] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, DeCloux A, Bonneville M, Tonegawa S. Diversity of τδ T-cell receptors on murine intestinal intraepithelial lymphocytes. Nature. 1989;339:712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- Tam S, Maksaereekul S, Hyde DM, Godinez I, Beaman BL. IL-17 and gammadelta T-lymphocytes play a critical role in innate immunity against Nocardia asteroides GUH-2. Microbes. Infect. 2012;14:1133–1143. doi: 10.1016/j.micinf.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J. Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Wollert T, Pasche B, Rochon M, Deppenmeier S, van den HJ, Gruber AD, Heinz DW, Lengeling A, Schubert WD. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 2007;129:891–902. doi: 10.1016/j.cell.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Xayarath B, Marquis H, Port GC, Freitag NE. Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis. Mol. Microbiol. 2009;74:956–973. doi: 10.1111/j.1365-2958.2009.06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.