Introduction
Modern treatment with antiretroviral therapy (ART) has improved survival for patients with human immunodeficiency virus (HIV) infection. Cardiovascular disease and other non-AIDS conditions are increasingly becoming key health concerns as this patient population continues to age.1–3 HIV-infected patients are more likely than the general population to develop cardiovascular disease, probably because of a combination of traditional risk factors, HIV-related inflammation, and effects of antiretroviral drugs.4 Among the cardiovascular complications of HIV infection, HIV-associated pulmonary arterial hypertension (PAH) is especially severe and is associated with significant mortality.5
Pulmonary hypertension (PH) is a disease process associated with an increase in the mean pulmonary artery pressure (mPAP). PAH defines an increase in the mPAP specifically related to arteriopathy of the pulmonary vasculature. PAH can be idiopathic, familial, or secondary to a variety of conditions such as connective tissue disease, congenital systemic-to-pulmonary shunts, drugs and toxins, liver cirrhosis, or HIV infection.6 PAH leads to a progressive increase in mPAP and pulmonary vascular resistance (PVR), and a decrease in cardiac output (CO). Pulmonary artery pressure may normalize or decrease as progressive right heart failure occurs and CO decreases, ultimately leading to exercise limitation and death.
HIV could represent a major cause of PAH (HIV-PAH). There are 34 million individuals worldwide with HIV infection.7 Given that PAH occurs in 0.5% of patients with HIV, there may be as many as 200,000 patients with HIV infections affected by PAH worldwide.8 If the natural history of PAH is as ominous in HIV infection as it is in other patient populations, PAH could become a major health care concern in the future.
Epidemiology
Since the first case was identified in 1987,9 PAH has become a well-recognized complication of HIV infection. The initial prevalence estimate of 0.5% was derived from a large Swiss cohort of 1200 largely untreated HIV-infected individuals who used injection drugs.10 Prevalence estimates have varied considerably over time.8 The most recent estimates come from a prospective cohort study of 7648 HIV-infected individuals in France. Participants were screened for unexplained dyspnea using a questionnaire followed by echocardiography and pulmonary artery catheterization (PAC), yielding a prevalence of HIV-PAH of 0.46% (95% confidence interval 0.32–0.64),11 similar to that determined in the original Swiss cohort.
Prevalence estimates have varied depending on the population being studied, as seen in several echocardiographic studies that have evaluated the prevalence of elevated pulmonary artery systolic pressures (PASP) in the setting of HIV infection. Ina study performed at San Francisco General Hospital, tricuspid regurgitant jet velocity (TRV) and right atrial pressure were used to estimate PASP in 196 HIV-infected individuals and 52 age-matched uninfected controls.12 HIV-infected individuals had a higher PASP compared with controls (median 27.5 mm Hg, interquartile range 22–33 mm Hg, compared with 22 mm Hg, interquartile range 18–25 mm Hg; P<.001). A PASP of greater than 30 or 40 mm Hg was founding 35.2% of HIV patients compared with 6.6% of controls (P<.001), and 7.7% of HIV patients compared with 1.9% of controls (P = .005), respectively. After adjustment for age, gender, smoking, stimulant use, and intravenous drug use, HIV-infected individuals had a 5 mm Hg higher mean PASP and a 7-fold greater odds of having a pulmonary artery systolic pressure greater than 30 mm Hg (P<.001). A study of 656 HIV-infected individuals demonstrated that among individuals with a detectable tricuspid regurgitant (TR) jet, 57% had evidence of PH defined as right ventricular (RV) pressure greater than 30 mm Hg.13 Finally, a study of HIV-infected individuals in Spain reported that 9.9% of individuals had a TRV greater than 2.8 m/s.14 A retrospective study in patients attending the National Institutes of Health HIV clinic showed that 9.3% of patients had a TRV of 2.5 m/s or greater (PASP 30 mm Hg) and 0.4% had aTRV of at least 3.0 m/s (PASP 41 mm Hg).5
The cohorts included in these studies varied greatly, and the reason for significant differences seen in the prevalence could be related to demographics, intravenous drug use, or mode of transmission. Also notable is the large number of individuals who had echocardiographic abnormalities, but who did not meet criteria for a diagnosis of PAH. This finding raises the possibility that many more patients could have early or mild forms of PAH.5,13
Pathogenesis
Patients with HIV and PAH have plexogenic lesions, similar to patients with other diseases associated with PAH (Fig. 1), but whether the pathogenesis of disease is similar to PAH in these HIV-uninfected populations is unknown.15 Possible mechanisms that may be important in HIV-PAH include effects of HIV viral proteins, immune activation induced by HIV, or risk factors that are common in the HIV-infected population. Diastolic dysfunction, which is common in HIV, might also contribute to findings of elevated right-sided heart pressures.16
Fig. 1.
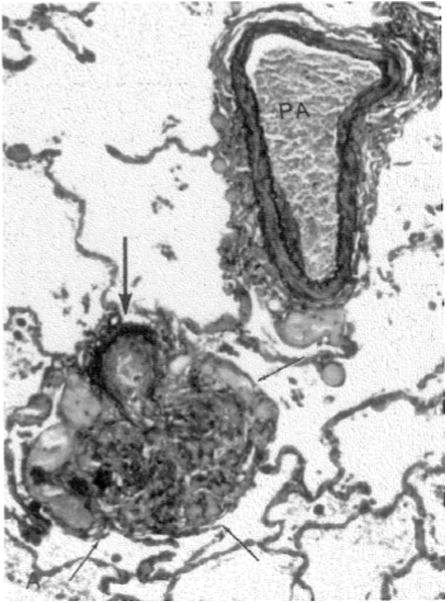
Elastic stain of lung reveals a predominantly unremarkable pulmonary artery (PA) with only a focal area of intimal sclerosis; immediately adjacent, there is a small muscular pulmonary artery (thick arrow) with severe intimal thickening and elastic tissue destruction that leads into an irregular mass of proliferating and focally dilated vascular channels (outlined by thin arrows), consistent with a plexiform lesion (Elastica van Gieson stain; original magnification 150×). (From Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Human Pathol 1987;18: 1295; with permission.)
HIV has never been shown to directly infect pulmonary vascular endothelial cells,17,18 but HIV viral antigens are present in pulmonary endothelium and may directly stimulate abnormal apoptosis, growth, and proliferation.19 Gp120, a viral protein necessary for the binding and entry of HIV into macrophages, has been shown to target human lung endothelial cells, increase markers of apoptosis, and stimulate the secretion of endothelin-1, a protein that is a potent vasoconstrictor.17 The negative factor (nef) antigen, critical for the maintenance of viral loads and for host-cell signaling interactions, has been localized to multiple pulmonary and vascular cells types.20 Primates infected with a simian immunodeficiency virus (SIV) expressing HIV nef protein develop lesions resembling plexiform lesions, and colocalization of HIV-1 nef has been demonstrated in pulmonary artery endothelial cells of HIV-infected individuals with PAH, but not in uninfected individuals or in individuals with idiopathic PAH.20,21 Specific nef signature sequences have been associated with PAH in 2 different HIV cohorts.22 Bone morphogenic protein receptor 2 (BMPR-2) mutations are associated with familial PAH and result in decreased signaling through BMPR-2.23 The HIV-1 tat (transcriptional transactivator) protein represses BMPR-2 gene expression in human macrophages in vitro, interfering with BMP–BMPR-2 transcriptional regulation.23 Exogenous tat protein has also been shown to activate endothelial cells, resulting in the release of growth factors,23 supporting the hypothesis that HIV viral proteins could induce aberrant endothelial function, leading to PAH.
There are other mechanisms by which HIV could cause PAH. HIV infection induces a chronic inflammatory state characterized by persistent immune activation and dysregulation,24 which could indirectly induce the release of proinflammatory cytokines and growth factors that could produce PAH.25 Even in the setting of effectively treated HIV infection, chronic inflammation persists and is independently associated with increased cardiovascular risk.26 Sputum inflammatory markers and activated CD8+ T cells are associated with elevated TRV and elevated PASP,27 demonstrating that HIV-associated inflammation may play a role in HIV-PAH. Increased expression of platelet-derived growth factor, a potent stimulus of smooth muscle cell and fibroblast growth and migration, has also been noted in lung tissue from patients with HIV-PAH.18 Similarly, vascular endothelial growth factor A induces vascular permeability and endothelial cell proliferation, and is produced by T cells infected by HIV in vivo.28
Coinfections associated with HIV have also been postulated to play a role. Human herpesvirus (HHV)-8 has been reported to be associated with PAH histologically.29 HHV-8 is associated with Kaposi sarcoma,30 and homosexual HIV-infected individuals have a high prevalence of HHV-8 infection ranging from 30% to 60%.31 However, HHV-8 infection has not been consistently associated with HIV and PAH in several studies.12,32,33
Other risk behaviors associated with HIV infection might also play a role in HIV-PAH. Stimulant drug use is common in HIV-infected populations, and HIV-infected individuals who use stimulant drugs are more likely to develop HIV-PAH.12 Injection drug use may act as a “second hit” in HIV and contribute to development of HIV-PAH.34 This hypothesis is supported by a recent study of rhesus macaques infected with SIV and treated with intramuscular morphine for 31 weeks. Animals developed significant pulmonary vascular remodeling including plexiform lesions, whereas animals either infected with SIV alone or treated with morphine alone did not.35
Survival
Survival reported for patients with HIV-PAH has consistently been worse than for either HIV infection or idiopathic PAH.36,37 In patients with HIV-PAH, mortality is most often secondary to PAH leading to right heart failure. Survival estimates for HIV-PAH come from a few large cohort studies and have varied over time, probably in part because of the availability of therapies for HIV and PAH.
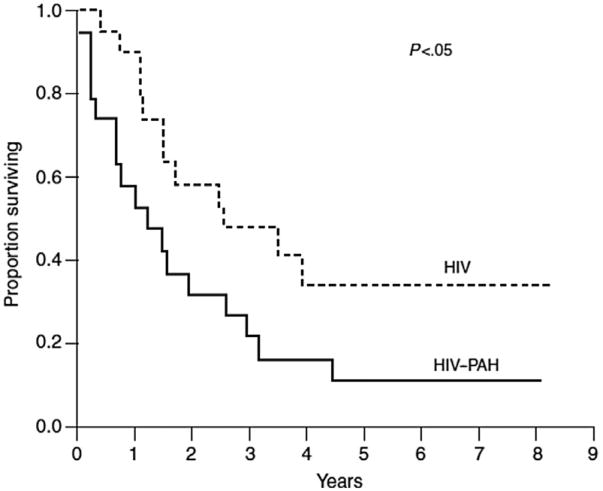
The first series to evaluate survival was a prospective cohort study of 19 HIV-PAH patients in a comparison with 19 HIV-infected controls performed before the wide availability of ART or PAH-specific therapy.36 Survival in HIV-PAH was 58%, 32%, and 21% at 1, 2, and 3 years, markedly worse than controls (Fig. 2). A lower CD4+ T-lymphocyte count and the diagnosis of PAH were associated with worse survival.
Fig. 2.
Kaplan-Meier plot demonstrating the probability of survival in patients with HIV infection and PAH compared with matched HIV-infected controls without PAH before the modern era of antiretroviral and PAH-specific therapy. (Reprinted with permission of the American Thoracic Society. Copyright (c) 2013 American Thoracic Society. From Opravil M, Pechere M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997;155:992. Official journal of the American Thoracic Society.)
The most recent data on survival of patients with HIV-PAH come from the French cohort. This series includes 77 patients with HIV-PAH evaluated between 2000 and 2008 and managed with modern therapy for HIV and PAH.38 In univariate analysis, a history of right-sided heart failure, baseline New York Heart Association Functional Class (NYHA FC) IV, cardiac index less than 2.8 L/min/m2, detectable HIV viral load, and CD4 count less than 200 cells/μL were associated with poor survival. In multivariate analysis, a low cardiac index and a low CD4 count remained associated with worse survival. Overall survival was 88%, 72%, and 63% at 1, 3, and 5 years, significantly better than prior series. In patients who received PAH-specific therapy, survival was 66% compared with 72% in those who did not.
Clinical Presentation and Diagnosis
Presenting complaints of HIV-PAH are the same as those for patients with idiopathic PAH. Symptoms are often nonspecific and insidious, so they are attributed to other complications of HIV or HIV itself. The time from presentation to the diagnosis is often long, from 6 months to 2 years. In a series of patients diagnosed with HIV-PAH before the year 2000, the most common presenting symptom was progressive shortness of breath (85%) followed by pedal edema (30%), nonproductive cough (19%), fatigue (13%), presyncope or syncope (12%), and chest pain (7%).15
Physical examination may be unremarkable, but often demonstrates typical findings of right-sided heart failure and volume overload. Auscultation may reveal a right-sided heave or S3, jugular veins may be distended, and there may be peripheral edema. The lung examination is frequently normal in patients with PAH, and abnormal lung findings may suggest an alternative diagnosis. The electrocardiogram may show signs of RV hypertrophy with right axis deviation and right atrial enlargement. A chest radiograph may reveal right heart enlargement and enlargement of the pulmonary arteries without lung findings.15 For most patients with HIV who have symptoms suggestive of PAH on initial evaluation, the next diagnostic test will usually be an echocardiogram, but routine screening with echocardiography for PAH in HIV-infected patients without a clinical suspicion of PAH may not be a useful or cost-effective approach.39
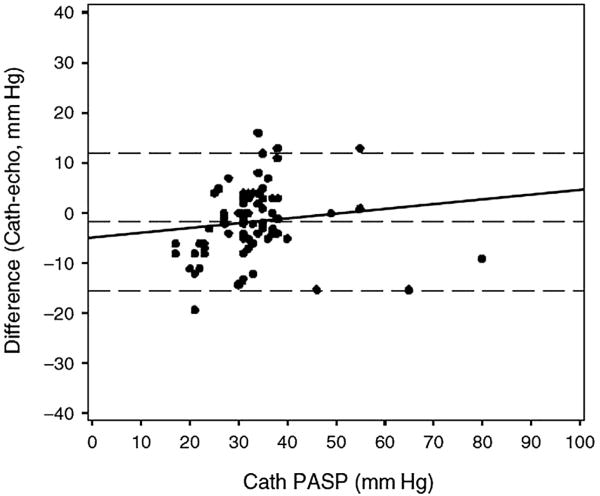
Echocardiography may not be sufficient to rule out PAH in individuals with a compatible clinical picture. Spectral Doppler is used to determine the peak velocity of the TR jet, which can be entered into modified Bernoulli equation to estimate the PASP (Fig. 3). This estimate may be unreliable if the peak TRV cannot be determined because there is minimal tricuspid regurgitation, an eccentric jet, or a very large jet. This lack of reliability may be particularly problematic in HIV-infected patients, and a low PASP on echo is not adequate enough to excluded the diagnosis of PAH (Fig. 4). In a study of Doppler echocardiography, estimates of PASP were inaccurate in 19.7% of cases, and 1 in 3 patients with HIV-PAH was missed.40
Fig. 3.
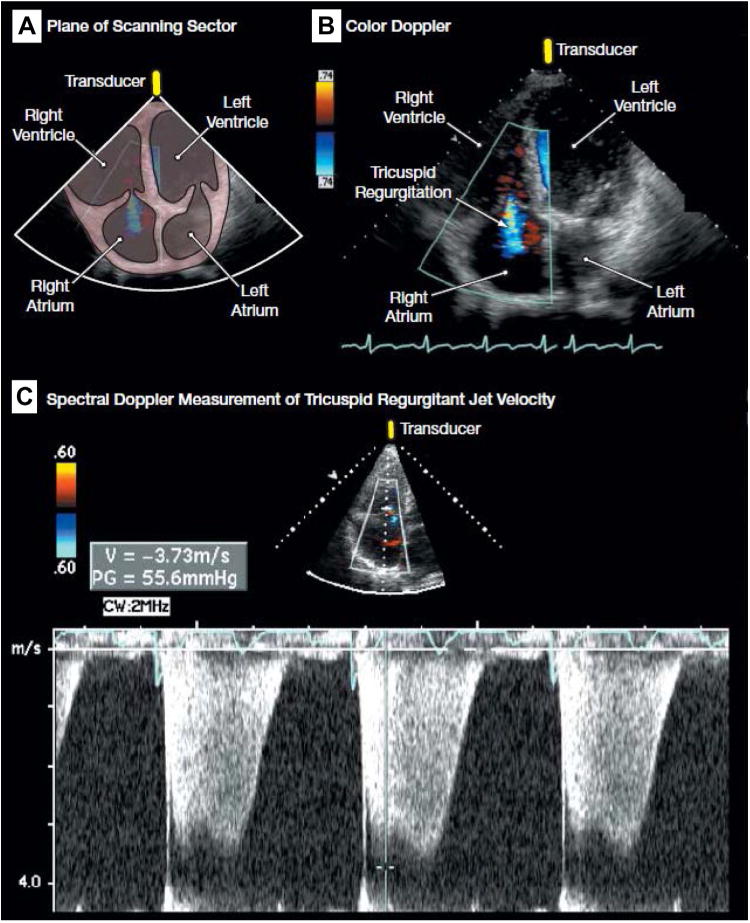
(A) Plane of scanning sector. (B) Color Doppler demonstrating tricuspid regurgitation (blue). (C) Spectral Doppler measurement of tricuspid regurgitant jet velocity for the estimation of pulmonary artery systolic pressure, demonstrating a peak tricuspid regurgitant jet velocity of approximately 3.7 m/s. Sampling of the peak tricuspid regurgitant jet velocity is used to estimate the RV to right atrial systolic pressure gradient (55.6 mm Hg in the figure) with the use of the modified Bernoulli equation (4 × [tricuspid regurgitant jet velocity]2). Pulmonary artery systolic pressure is quantified by adding the Bernoulli-derived pressure gradient to an estimate of mean right atrial pressure. (See video at http://jama.com/cgi/content/full/299/3/324/DC1.) (From Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension. JAMA 2008;299:326; with permission.)
Fig. 4.
Bland-Altman analysis demonstrating lack of agreement between the pulmonary artery systolic pressure estimated by Doppler echocardiogram and pulmonary artery systolic pressure (PASP) measured by right heart catheterization. (From Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS 2012;26:1968; with permission.)
Other echo findings such as RV enlargement, hypertrophy and systolic dysfunction, right atrial enlargement, characteristic pulmonic valve motion, and spectral Doppler characteristics should be considered when evaluating patients for possible PAH.41 Left ventricular systolic function and clinically relevant valvular disease should be excluded by echocardiography.
The gold standard for hemodynamic evaluation remains invasive assessment with PAC42; if echocardiography supports a possible diagnosis of PAH, PAC is mandatory before initiation of any PAH-specific therapy. To minimize complications and optimize data collection, PAC should be performed by a clinician with expertise in hemodynamic assessment and diagnostic evaluation of patients with PH. Maneuvers to exclude occult left-sided diastolic dysfunction, such as fluid challenge or exercise, may be performed in patients with risk factors (left atrial enlargement, left ventricular hypertrophy, diabetes, or hypertension). Acute vasodilator challenge may be performed during right heart catheterization; however, few patients with HIV-PAH who have a positive acute vasodilator response will have long-term responses to calcium-channel blockers.43
Before considering treatment for PAH, other causes of PH such as lung disease, valvular or left heart disease, chronic thromboembolic disease, and sleep apnea should be excluded as per guideline recommendations.44 Other diseases associated with PAH such as connective tissues disease, hemolytic disorders, portal hypertension, and congenital heart disease should be excluded because the approach to management and prognosis may be affected.
Treatment
Antiretroviral Treatment
The effects of ART in HIV-PAH are controversial. Two retrospective studies have shown a reduced incidence of HIV-PAH since ART became available, suggesting that HIV-targeted treatment is beneficial.8,45 A small French cohort reported that combination ART alone improved exercise tolerance as assessed by 6-minute walk (6MW) distance, but no change in hemodynamics was observed.38 In animal models, protease inhibitors have been shown to reverse hypoxia-induced PH.46 Data from the Swiss Cohort study showed that individuals with HIV-PAH diagnosed after 1995 had slightly improved survival compared with those diagnosed before this time, and a reciprocal relationship was noted between CD4 cell count and PAH incidence.38 There have been no large prospective studies of the effects of ART on HIV-PAH.
Current guidelines recommend that all HIV-infected patients be treated with antiretroviral agents regardless of CD4 T-cell count and viral load,47,48 so most patients diagnosed with HIV-PAH will already be on HIV therapy. In the authors' practice, antiretroviral therapy is started promptly in any individual newly diagnosed with HIV-PAH who was not previously on treatment. The choice of the initial antiretroviral regimen should be based on current guidelines; however, practitioners should take into consideration the likely need for PAH-specific therapies and relevant drug interactions.
Conventional Therapies
Initial treatment of patients with HIV-PAH will be determined by the severity of symptoms and hemodynamic compromise.44 Few studies have specifically addressed the treatment of HIV-PAH, so approach to treatment is based on studies performed in patients with idiopathic or associated PAH. Many patients will be diagnosed in the outpatient setting; however, presentation with acute on chronic decompensated right heart failure associated with severe hypoperfusion or hypotension is not uncommon. Patients who present with decompensated right heart failure should be triaged to an intensive care unit and managed with a PAC to titrate vasopressors and inotropic agents, to restore perfusion accordingly. Hypoxemia can worsen pulmonary vasoconstriction and should be corrected with supplemental oxygen. Diuretic therapy should be instituted in patients with volume overload, and adjusted to achieve normal right-sided filling pressures. Despite few supportive data, digoxin may be considered in patients with acute right heart failure and for chronic management to reduce symptoms of heart failure. Because of the risk of hemodynamic decompensation with dihydropyridine calcium-channel blockers and β-blockers, digoxin is also used as a first-line agent in patients with atrial arrhythmias and HIV-PAH.
Long-term oxygen therapy should be continued to treat hypoxemia. In observational studies, survival is improved in PAH patients treated with warfarin, and current guidelines recommend titration to an international normalized ratio of 1.5 to 2.5.44 Chronic digoxin therapy may be a useful adjunct to PAH-specific therapy.
Calcium-Channel Blockers
In several published reports, favorable long-term response to calcium-channel blockers has been limited to a minority of patients with idiopathic PAH.49 A favorable response to acute vasodilator challenge or oral calcium-channel blockers has been reported in only a few patients with HIV-PAH.36,38,43 As in all PAH patients, oral calcium-channel blockers should only be considered in those patients with a favorable response to acute vasodilator challenge, with careful long-term monitoring for signs of worsening PAH.
PAH-Specific Therapy
Retrospective cohort studies suggest that survival of patients treated with PAH-specific therapy in addition to ART is improved.38 Because there are few well-designed trials for HIV-PAH, choice and timing of therapy are based on data from other trials of PAH.50,51 Treatment of HIV-PAH is particularly challenging, owing to the potential for significant drug interactions and adverse effects. Safe initiation of PAH-specific therapy in a patient on ART requires planning and good communication with HIV specialists and pharmacists, as well as frequent patient follow-up to monitor for adverse effects and drug interactions.
Phosphodiesterase Inhibitors
Sildenafil and tadalafil function by inhibiting the metabolism of cyclic guanosine monophosphate, the second messenger that mediates the effects of nitric oxide, causing selective pulmonary vasodilation.52 Sildenafil treatment in PAH improves exercise tolerance, and tadalafil improves exercise tolerance and reduces time to clinical worsening.53 Data on the use of these agents in HIV-PAH is largely extrapolated from trials in PAH, but results from small series and case reports of HIV-PAH have been encouraging.54,55 Ritonavir and other protease inhibitors are inhibitors of cytochrome P450 CYP3A4 and CYP 2C9, which are important in the metabolism of sildenafil and tadalafil. Marked increases in sildenafil levels have been observed during coadministration with indinavir,56 saquinavir, and ritonavir,57 and some guidelines consider this combination to be contraindicated. The clinical relevance of this interaction is unclear because increased sildenafil levels have not been associated with hypotension or adverse effects in pharmacokinetic studies,57 and successful coadministration of ritonavir and sildenafil in HIV-PAH patients has been reported.58 Tadalafil levels are less affected by ritonavir, and guidelines suggest only close monitoring if tadalafil therapy is initiated.
Endothelin Receptor Antagonists
Blockade of the endothelin receptor with bosentan (nonselective) or ambrisentan (selective) improves hemodynamics, exercise tolerance and prevents clinical worsening in patients with PAH. Bosentan was studied prospectively in 16 HIV-PAH patients in the prospective BREATHE-4 trial. After 16 weeks, NYHA FC improved by at least 1 class in 14 patients, cardiac index improved by 39%, mPAP decreased by 21%, and 6MW distance improved by 91 ± 60 m.59 Another study examined longterm effects of bosentan in 59 patients after a median of 29 months of therapy, and showed short-term improvements in symptoms, exercise tolerance, and hemodynamic parameters.60 In both studies, bosentan was well tolerated.
Ambrisentan has not been specifically studied in patients with HIV-PAH, but appears to have efficacy similar to that of bosentan.61 Ambrisentan treatment is associated with a lower frequency of liver function test abnormalities. Monthly liver function testing is not mandatory, and pharmacokinetic studies suggest that there is no significant drug interaction with the protease inhibitor, ritonavir.62–64
Prostacyclin Analogues
Although no long-term trials in HIV-PAH patients have been performed, improvements in hemodynamics and exercise tolerance have been observed in small series of patients treated with catheter-based and inhaled prostanoid treatment. In one report, 6 patients with HIV-PAH were treated with intravenous epoprostenol for 12 to 47 months with improvements in NYHA FC. Acute improvements in mPAP, cardiac index, and PVR persisted at the time of repeat right heart catheterization.43 Another observational study reported on 3 patients with severe HIV-PAH treated with 1 year of subcutaneous treprostinil. All patients had improvement in NYHA FC, increased 6MW distance by at least 75 m (baseline 313–500 m), and had improvement in hemodynamics determined by echocardiography.65 Intravenous or subcutaneous treatment requires placement of an indwelling catheter, which may not be desirable in HIV-infected patients at risk for catheter-related infection and injection drug use.
Published experience in HIV-PAH with inhaled prostanoids is limited, but offers a desirable alternative delivery method. The effects of inhaled iloprost were reported in a study of 8 patients with severe HIV-PAH.66 Hemodynamic effects following initial iloprost treatment improved, with a 31% reduction in PVR and a 21% increase in cardiac index. Treprostinil is another inhaled prostanoid with the advantage of reduced dosing frequency. HIV-PAH patients were included in the TRIUMPH study of treprostinil, which demonstrated improved exercise tolerance when treprostinil was added to baseline oral therapy.67 The number of HIV-PAH patients, however, was too small for meaningful analysis.
Treat-to-Target Approach and Monitoring Effects of PAH-HIV Treatment
In the authors' practice, before initiation of PAH-specific therapy all patients undergo a thorough diagnostic evaluation, including right heart catheterization, to ensure that they meet criteria for a diagnosis of PAH and that other possible causes of PH or PAH are excluded. All patients undergo a baseline 6MW distance test to assess exercise tolerance, and this test is repeated at each subsequent clinic visit.50,68 Conventional therapy with diuretics and oxygen are initiated, as well as warfarin if patients can comply with necessary monitoring. Treatment with either a phosphodiesterase inhibitor or an endothelin receptor antagonist is generally the first line. The choice of initial therapy is often dictated by concomitant ART therapy and the willingness and ability of the patient to follow up with necessary monthly monitoring for liver function test abnormalities, contraception, and pregnancy testing. Patients are closely followed in cooperation with the HIV clinic, and the initial therapy is titrated based on the patients' objective exercise tolerance and 6MW distance. The authors frequently initiate inhaled prostanoid, usually treprostinil, in patients who remain symptomatic despite maximal oral therapy or as a first-line agent in patients with severe symptoms at the time of presentation. Catheter-based therapies are avoided in this population, given the challenges of managing the therapy, risk of infection, and risk of the catheter being used for illicit drug administration (which is common in the authors' patient population). Despite the paucity of trials of combination therapy, a treat-to-target approach for patients with PAH has been advocated, with additional oral and inhaled therapies until patients' subjective and objective exercise tolerance improves to an acceptable level.
Summary
The development of PAH in HIV-infected individuals is associated with high morbidity and mortality, and HIV-PAH may be an increasing problem as HIV-infected individuals survive longer. The pathogenesis of HIV-PAH is not completely understood, but HIV proteins, chronic immune activation, coinfections, or synergistic effects of other risk factors may be important. Physicians treating HIV-infected patients should monitor for symptoms of unexplained or new dyspnea, and patients should undergo an echocardiogram to estimate pulmonary artery pressures and to look for other signs of right heart dysfunction. A complete assessment including PAC is mandatory in confirming the diagnosis of HIV-PAH. PAH-specific therapy should be initiated by a physician with experience in managing PAH, in close collaboration with HIV specialists and pharmacists. An improved understanding of the pathogenesis of HIV-PAH is needed to inform the optimal approach to treatment.
Key Points.
As patients with human immunodeficiency virus (HIV) infection worldwide survive longer, the prevalence of HIV-associated pulmonary arterial hypertension (HIV-PAH) is likely to increase.
The development of PAH in HIV-infected individuals is associated with worse functional capacity and survival.
The underlying mechanism leading to HIV-PAH remains unclear and is an area of active investigation.
The optimal approach to therapy for individuals with HIV-PAH is uncertain, but should include aggressive management of HIV infection and careful use of PAH-specific therapies given possible significant drug interactions.
Acknowledgments
Grant Support: Dr Hsue is supported by grants from the NIH (R01HL095130, R01HL91526, and R01HL090480).
Footnotes
Conflicts of Interest: Dr Barnett has no conflicts of interest to declare. Dr Hsue has received honorarium from Gilead.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccara F. Cardiovascular complications and atherosclerotic manifestations in the HIV-infected population: type, incidence and associated risk factors. AIDS. 2008;22:S19–26. doi: 10.1097/01.aids.0000327512.76126.6e. http://dx.doi.org/10.1097/01.aids.0000327512.76126.6e. [DOI] [PubMed] [Google Scholar]
- 3.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–6. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009;23:2021–7. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension. JAMA. 2008;299:324–31. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 7.WHO/UNAIDS. Geneva (Switzerland): WHO/UNAIDS; 2012. Global report: UNAIDS report on the global AIDS epidemic 2012. Available at: http://www.aidsinfo.unaids.org. [Google Scholar]
- 8.Zuber JP, Calmy A, Evison JM, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38:1178–85. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 9.Kim KK, Factor SM. Membranoproliferative glomerulonephritis and plexogenic pulmonary arteriopathy in a homosexual man with acquired immunodeficiency syndrome. Hum Pathol. 1987;18:1293–6. doi: 10.1016/s0046-8177(87)80417-3. [DOI] [PubMed] [Google Scholar]
- 10.Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–71. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–13. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 12.Hsue PY, Deeks SG, Farah HH, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–33. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondy KE, Gottdiener J, Overton ET, et al. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–86. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 14.Quezada M, Martin-Carbonero L, Soriano V, et al. Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS. 2012;26:1387–92. doi: 10.1097/QAD.0b013e328354f5a1. [DOI] [PubMed] [Google Scholar]
- 15.Mehta NJ, Khan IA, Mehta RN, et al. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118:1133–41. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 16.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333:1107. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Monti G, Fartoukh M, et al. Plateletderived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J. 1998;11:554–9. [PubMed] [Google Scholar]
- 19.Mette SA, Palevsky HI, Pietra GG, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis. 1992;145:1196–200. doi: 10.1164/ajrccm/145.5.1196. [DOI] [PubMed] [Google Scholar]
- 20.Marecki JC, Cool CD, Parr JE, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174:437–45. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George MP, Champion HC, Simon M, et al. Physiologic changes in a non-human primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;48:374–81. doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almodovar S, Knight R, Allshouse AA, et al. Human immunodeficiency virus nef signature sequences are associated with pulmonary hypertension. AIDS Res Hum Retroviruses. 2012;28:607–18. doi: 10.1089/aid.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldwell RL, Gadipatti R, Lane KB, et al. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J Leukoc Biol. 2006;79:192–201. doi: 10.1189/jlb.0405194. [DOI] [PubMed] [Google Scholar]
- 24.Fauci AS, Pantaleo G, Stanley S, et al. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–63. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Morse JH, Barst RJ, Itescu S, et al. Primary pulmonary hypertension in HIV infection: an outcome determined by particular HLA class II alleles. Am J Respir Crit Care Med. 1996;153:1299–301. doi: 10.1164/ajrccm.153.4.8616557. [DOI] [PubMed] [Google Scholar]
- 26.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–40. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ascherl G, Hohenadl C, Schatz O, et al. Infection with human immunodeficiency virus-1 increases expression of vascular endothelial cell growth factor in T cells: implications for acquired immunodeficiency syndrome-associated vasculopathy. Blood. 1999;93:4232–41. [PubMed] [Google Scholar]
- 29.Cool CD, Rai PR, Yeager ME, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113–22. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 31.Martin JN, Ganem DE, Osmond DH, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 32.Montani D, Marcelin AG, Sitbon O, et al. Human herpes virus 8 in HIV and non-HIV infected patients with pulmonary arterial hypertension in France. AIDS. 2005;19:1239–40. doi: 10.1097/01.aids.0000176230.94226.06. [DOI] [PubMed] [Google Scholar]
- 33.Nicastri E, Vizza CD, Carletti F, et al. Human herpesvirus 8 and pulmonary hypertension. Emerg Infect Dis. 2005;11:1480–2. doi: 10.3201/eid1109.040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George MP, Champion HC, Gladwin MT, et al. Injection drug use as a “second hit” in the pathogenesis of HIV-associated pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:1144–6. doi: 10.1164/rccm.201204-0609ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spikes L, Dalvi P, Tawfik O, et al. Enhanced pulmonary arteriopathy in simian immunodeficiency virus-infected macaques exposed to morphine. Am J Respir Crit Care Med. 2012;185:1235–43. doi: 10.1164/rccm.201110-1909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opravil M, Pechere M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155:990–5. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 37.Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89:2722–7. doi: 10.1161/01.cir.89.6.2722. [DOI] [PubMed] [Google Scholar]
- 38.Degano B, Guillaume M, Savale L, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 39.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension. ACCP evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 40.Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26:1967–9. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond RJ, Hinderliter AL, Willis PW, IV, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–9. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 42.Champion HC, Michelakis ED, Hassoun PM, et al. Comprehensive invasive and noninvasive approach to the right ventricle–pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 43.Aguilar RV, Farber HW. Epoprostenol (prostacyclin) therapy in HIV-associated pulmonary hypertension. Am J Respir Crit Care Med. 2000;162:1846–50. doi: 10.1164/ajrccm.162.5.2004042. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association Developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Pugliese A, Isnardi D, Saini A, et al. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect. 2000;40:282–4. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 46.Gary-Bobo G, Houssaini A, Amsellem V, et al. Effects of HIV protease inhibitors on progression of monocrotaline- and hypoxia-induced pulmonary hypertension in rats. Circulation. 2010;122:1937–47. doi: 10.1161/CIRCULATIONAHA.110.973750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 48.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the international antiviral society—USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 49.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 50.Galié N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 51.Galié N, Rubin LJ, Hoeper MM, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 52.Barnett CF, Machado RF. Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag. 2006;2:411–22. doi: 10.2147/vhrm.2006.2.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galié N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 54.Ieong MH. Noninfectious pulmonary complications of HIV. Clin Pulm Med. 2006;13:194–202. [Google Scholar]
- 55.Schumacher YO, Zdebik A, Huonker M, et al. Sildenafil in HIV-related pulmonary hypertension. AIDS. 2001;15:1747–8. doi: 10.1097/00002030-200109070-00026. [DOI] [PubMed] [Google Scholar]
- 56.Merry C, Barry MG, Ryan M, et al. Interaction of sildenafil and indinavir when co-administered to HIV-positive patients. AIDS. 1999;13:101–7. doi: 10.1097/00002030-199910220-00001. [DOI] [PubMed] [Google Scholar]
- 57.Muirhead GJ, Wulff MB, Fielding A, et al. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50:99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinello P, Cicalini S, Pichini S, et al. Sildenafil plasma concentrations in two HIV patients with pulmonary hypertension treated with ritonavirboosted protease inhibitors. Curr HIV Res. 2012;10:162–4. doi: 10.2174/157016212799937263. [DOI] [PubMed] [Google Scholar]
- 59.Sitbon O, Gressin V, Speich R, et al. Bosentan for the treatment of human immunodeficiency virusassociated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:1212–7. doi: 10.1164/rccm.200404-445OC. [DOI] [PubMed] [Google Scholar]
- 60.Degano B, Yaici A, Le Pavec J, et al. Long-term effects of bosentan in patients with HIV-associated pulmonary arterial hypertension. Eur Respir J. 2009;33:92–8. doi: 10.1183/09031936.00094808. [DOI] [PubMed] [Google Scholar]
- 61.Galié N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008;117:3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 62.Venitz J, Zack J, Gillies H, et al. Clinical pharmacokinetics and drug-drug interactions of endothelin receptor antagonists in pulmonary arterial hypertension. J Clin Pharmacol. 2012;52:1784–805. doi: 10.1177/0091270011423662. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Yehuda O, Pizzuti D, Brown A, et al. Long-term hepatic safety of ambrisentan in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2012;60:80–1. doi: 10.1016/j.jacc.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Gillies H, Wang X, Staehr P, et al. PAH therapy in HIV: lack drug-drug interaction between ambrisentan and ritonavir. Am J Respir Crit Care Med. 2011;183:A5913. [Google Scholar]
- 65.Cea-Calvo L, Escribano Subias P, Tello de Menesses R, et al. Treatment of HIV-associated pulmonary hypertension with treprostinil. Rev Esp Cardiol. 2003;56:421–5. doi: 10.1016/s0300-8932(03)76889-4. in Spanish. [DOI] [PubMed] [Google Scholar]
- 66.Ghofrani HA, Friese G, Discher T, et al. Inhaled iloprost is a potent acute pulmonary vasodilator in HIV-related severe pulmonary hypertension. Eur Respir J. 2004;23:321–6. doi: 10.1183/09031936.03.00057703. [DOI] [PubMed] [Google Scholar]
- 67.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–22. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 68.Vachiéry JL, Yerly P, Huez S. How to detect disease progression in pulmonary arterial hypertension. Eur Respir Rev. 2012;21:40–7. doi: 10.1183/09059180.00009011. [DOI] [PMC free article] [PubMed] [Google Scholar]