Abstract
Background
Persistent pulmonary hypertension of the newborn (PPHN) is associated with increased oxidative stress in pulmonary arteries (PA). Betamethasone decreases the oxidative stress and improves anti-oxidant balance in PPHN. We investigated whether antenatal betamethasone improves pulmonary vasodilation and postnatal oxygenation in late preterm lambs with PPHN.
Methods
PPHN was induced by constriction of fetal ductus arteriosus from 128 to 136d gestation. Ewes were given 2 i.m. doses of betamethasone or saline at 24h and 12h before Cesarian-section delivery at 136d gestation, simulating late preterm birth. Newborn lambs were mechanically ventilated for 8h with monitoring of blood gas and hemodynamic variables. Lungs were harvested post mortem to determine oxidative stress markers and in vitro responses of PA.
Results
Postnatal arterial partial pressure of oxygen and pH were higher and the oxygenation index and arterial partial pressure of carbon dioxide lower in betamethasone treated lambs. PA pressure was lower and systemic pressure higher in betamethasone lambs. Betamethasone decreased the oxidative stress markers and increased endothelial nitric oxide synthase expression in ventilated PPHN lungs.
Conclusion
Antenatal betamethasone decreases oxidative stress and improves postnatal transition in late preterm lambs with PPHN. This study suggests a potential benefit for antenatal betamethasone in late preterm births.
INTRODUCTION
Persistent pulmonary hypertension of the newborn (PPHN) occurs when the pulmonary vascular resistance fails to decrease at birth (1), resulting in a failure to establish oxygenation by the lung. Infants with PPHN develop hypoxemia and increased risk of death and long-term disabilities (1, 2, 3). Late preterm birth is an important cause of respiratory failure and PPHN in newborn infants (4–6). PPHN occurs in association with surfactant deficiency and ventilation/perfusion mismatch in late preterm gestation neonates (6). Although advances in neonatal care decreased the mortality for affected infants, survivors of PPHN continue to have increased long-term disability rates (3, 7). Current treatment strategies for PPHN target the infants that already have hypoxemia and cardio-pulmonary instability inherent to the course of these critically ill neonates. In addition, ventilation with high fractional inspired O2 concentration (FiO2) even for short duration leads to oxidative stress and sustained vascular dysfunction in the newborn (8). Therefore, improving outcomes in this population may require application of antenatal therapies that facilitate normal adaptation of the lung and decrease lung injury in infants at risk for PPHN.
Previous studies in a fetal lamb model of PPHN induced by prenatal ligation of ductus arteriosus demonstrated that an increase in oxidative stress (9, 10) underlies the vascular dysfunction (9–12) in pulmonary arteries. The vascular dysfunction evolves antenatally and interferes with the transition of pulmonary circulation at birth. Postnatal application of superoxide dismutase (SOD) as a rescue therapy improves pulmonary vasodilation and oxygenation in this model of PPHN (13, 14). However, a strategy to correct the vascular dysfunction prenatally in preparation for birth-related transition is not currently available.
Our previous studies demonstrated that the glucocorticoid, betamethasone decreases superoxide levels, increases the expression of endothelial nitric oxide synthase (eNOS) and manganese SOD (MnSOD) and the bioavailability of NO in the pulmonary artery endothelial cells (PAEC) in PPHN lambs (15). Antenatal administration of betamethasone improves the in vitro relaxation response of pulmonary arteries isolated from unventilated lungs of both normal and PPHN fetal lambs (15, 16). Corticosteroids decrease oxidative stress in the presence of lung inflammation in asthma (17). Previous studies in fetal rats and lambs demonstrated that prenatal steroids induce an increase in anti-oxidant enzyme activity and expression (18–20). We proposed the hypothesis that antenatal betamethasone administration would improve postnatal pulmonary vasodilation and oxygenation in PPHN by decreasing oxidative stress in the lung. We tested the effects of antenatal betamethasone following a clinically used dosing regimen, which was modified to minimize the incidence of preterm labor in fetal lambs. We conducted the studies in intact lambs delivered at late preterm gestation after the prenatal induction of PPHN.
RESULTS
A total of 20 fetal lambs, 10 in each group had PPHN induced; 6 control and 6 betamethasone treated lambs each completed the 8 h of ventilation. 3 animals in the control group and 1 in betamethasone group died prior to completion of 8 hours of ventilation. Three ewes in the betamethasone group and one control ewe had preterm labor prior to C-section delivery of the fetus. In addition, 3 unventilated fetal lambs that had exposure to either saline or betamethasone and 3 normal term lambs that were ventilated were included for immunoblotting or vascular ring studies.
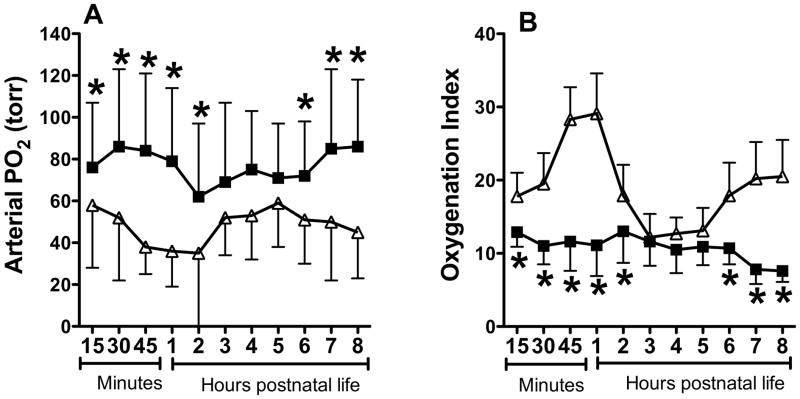
Betamethasone treated lambs had significantly better oxygenation during the first 2 hours, with a 2-fold difference by end of 1 hour (Figure 1A). The control some increase in the PO2 during hours 2-5 and the difference between the 2 groups was not significant during that time. However, betamethasone treated lambs had higher PaO2 at hours 6-8 compared to controls, with 40-90% differences in PaO2. The oxygenation index was also significantly lower in the betamethasone treated lambs, indicating lower severity of respiratory failure (Figure 1B).
Figure 1.
Effect of betamethasone on postnatal PaO2 (A) and oxygenation index (B). Data are mean±SD for 6 animals in each group. Filled squares indicate data for betamethasone treated PPHN group and open triangles for saline treated PPHN group (control). *P<0.05, from control. Betamethasone treated lambs had higher PaO2 and lower oxygenation index during postnatal ventilation.
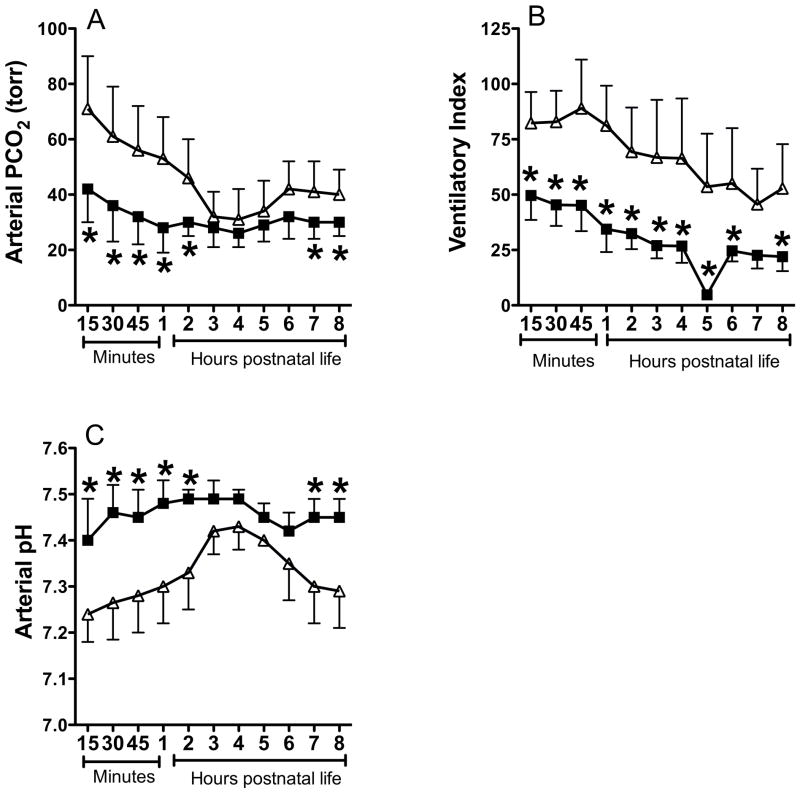
Betamethasone treated lambs had lower PaCO2 at hours 0–2, 7, and 8, indicating that lung compliance probably improved faster in this group compared to controls (Figure 2A). Since the lambs were delivered 7–8 days before term gestation, the improvement in compliance may be due to the beneficial effect of betamethasone on lung fluid clearance and surfactant release (21). The ventilatory index was consistently lower in betamethasone treated lambs (Figure 2B), as the ventilator pressure and rate were weaned for lower PaCO2. The arterial pH was higher in betamethasone treated lambs at hours 0–2 and 6–8 compared to controls (Figure 2C), consistent with lower PaCO2.
Figure 2.
Effect of betamethasone on postnatal PaCO2 (A), ventilatory Index (B), and arterial blood pH (C). Data are mean±SD for 6 animals in each group. Filled squares indicate data for betamethasone treated PPHN group and open triangles for saline treated PPHN control. *P<0.05, from control. Betamethasone (BMZ) treated lambs had lower PaCO2 and Ventilatory index and higher pH compared to controls.
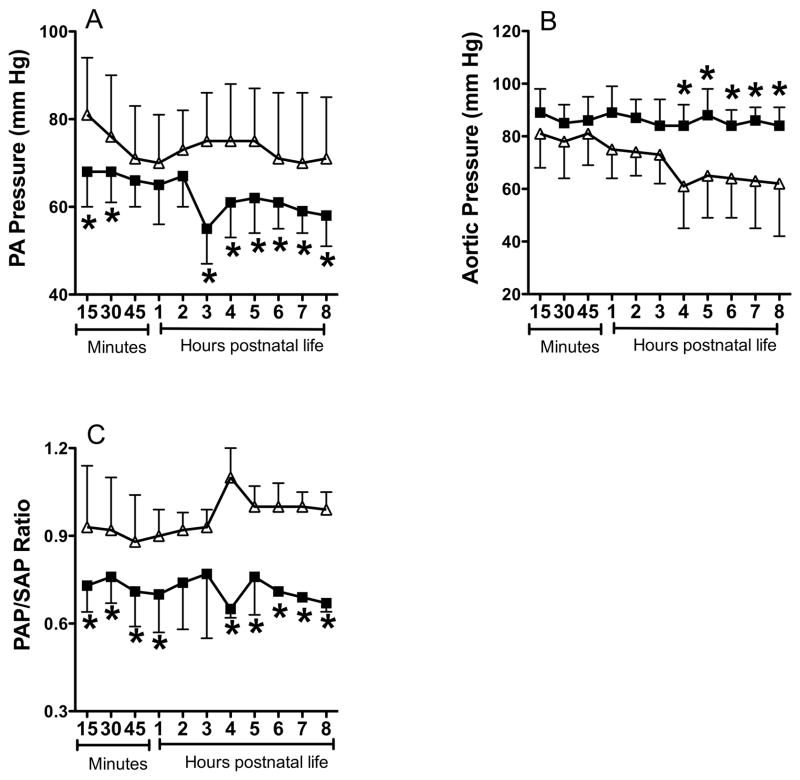
The PA pressure was 15% lower in the betamethasone treated group during the first hour and hours 3–8 of postnatal ventilation (Figure 3A). The aortic pressure was higher and more stable in the betamethasone treated lambs while it declined in the control lambs from hours 4–8 (Figure 3B). The PAP/SAP ratio was also lower in betamethasone treated lambs during the first hour and hours 4–8 of postnatal ventilation (Figure 3C).
Figure 3.
Effect of betamethasone on postnatal pulmonary artery (PA) pressure (A), aortic pressure (B), and ratio of PA pressure (PAP) to aortic pressure (SAP) during ventilation. Data are mean±SD for 6 animals in each group. Filled squares indicate data for betamethasone treated PPHN group and open triangles for saline treated PPHN control. *P<0.05, from controls. Betamethasone treated lambs had lower PA pressure, higher aortic pressure and lower PAP/SAP ratio compared to controls.
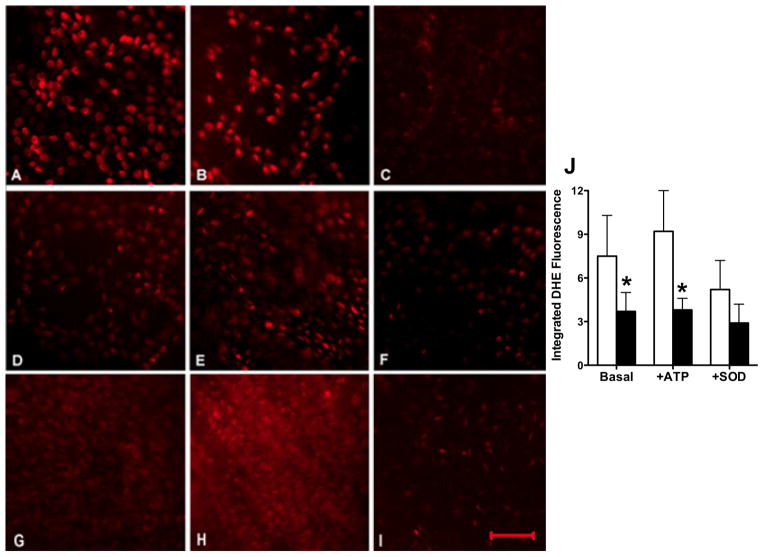
Pulmonary arteries from ventilated PPHN lambs had high DHE fluorescence at basal level (Figure 4A) and after stimulation with ATP (Figure 4B); the fluorescence was quenched by PEG-SOD (Figure 4C). Betamethasone treated animals had lower DHE fluorescence at basal level (Figure 4D and 4J) and after stimulation of pulmonary artery segments with ATP, which increases intra-cellular calcium levels (Figure 4E and 4J). Superoxide from xanthine+xanthine oxidase markedly increased the fluorescence in normal pulmonary artery (Figure 4H) as expected and the fluorescence was quenched by PEG-SOD (Figure 4I), indicating that changes in O2− levels were responsible for differences in DHE fluorescence in PPHN and after betamethasone.
Figure 4.
Dihydroethidine (DHE) fluorescence of pulmonary artery endothelium in ventilated PPHN lambs without (A–C) or with (D–F) prenatal betamethasone and a normal fetal lamb control (G–I). Representative fluorescence is shown for basal level (A, D and G), after ATP stimulation (B, E), after exposure to superoxide from xanthine+xanthine oxidase as positive control (H), and with superoxide scavenger, PEG-SOD, as negative control (C, F & I). Scale bar in panel I represents 50 μ and images are at 200x magnification. Summarized data are shown for 6 animals each for no betamethasone (open bars) and betamethasone treated pulmonary arteries (filled bars) in panel J as mean±SD. *P<0.05, from controls. Betamethasone treated lambs had lower DHE fluorescence at basal and stimulated conditions and the fluorescence was quenched by PEG-SOD indicating contribution of superoxide to DHE fluorescence.
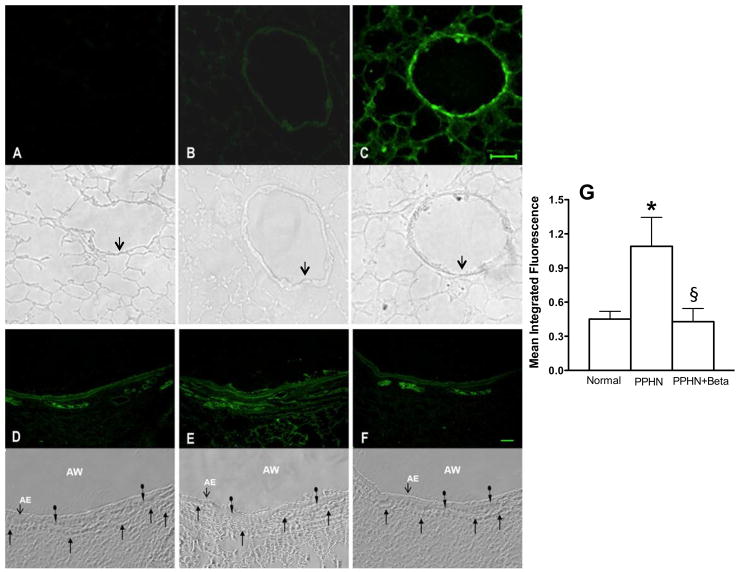
The 3-NT levels were low in unventilated lamb lungs (Figure 5B); exposure of lung section to peroxynitrite markedly increased the signal for 3-NT, as expected (Figure 5C). 3-NT levels were higher in the ventilated PPHN lambs (Figure 5E) compared to either normal ventilated lamb lungs (Figure 5D) or betamethasone treated ventilated lambs with PPHN (Figure 5F). The signal was higher in both airway and pulmonary arteries in PPHN lambs (Figure 5E). Betamethasone decreased the signal for 3-NT in both airway and pulmonary arteries in ventilated PPHN lambs (Figure 5F and 5G).
Figure 5.
Nitrotyrosine labeling of frozen lung section from unventilated (panels A–C) and ventilated lambs (D–F) with the corresponding phase contrast microscopy images shown directly below. For normal lung sections, panel A: Negative control, antibody preabsorbed with 3-nitro-L-tyrosine before applying to lung section; B: Basal nitrotyrosine immunolabeling; and C: Positive control, lung section pre-treated with 5μM peroxynitrite before nitrotyrosine immunolabeling. Scale bar in C is 50μ and magnification is 200x. Arrows in phase contrast images below point to airway epithelium. For ventilated lungs, panel D, ventilated normal lung; E, ventilated PPHN lung and F, PPHN lamb treated with betamethasone before ventilation. Magnification is 100x for panels D–F. AW=airway and AE=airway epithelium. The plain arrows indicate capillaries and circle arrows indicate airway smooth muscle. Summary data in panel G show increase in nitrotyrosine in PPHN lambs compared to ventilated normal lambs (normal) and decrease with betamethasone (PPHN+Beta). *P<0.05, from normal; §P<0.05, from PPHN lambs without betamethasone. Betamethasone treated PPHN lambs had lower nitrotyrosine levels in the lung sections.
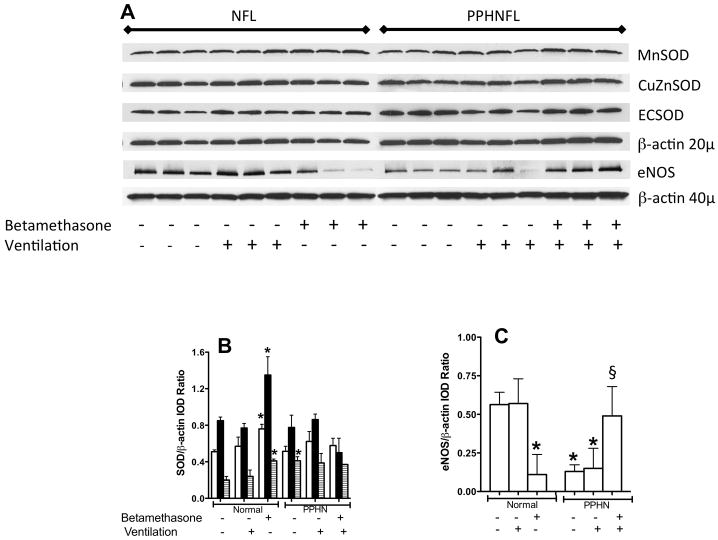
The levels of MnSOD, CuZnSOD and EC SOD in the unventilated normal lungs were increased by 50% with prenatal exposure to betamethasone (Figure 6, panels A & B). The protein levels of MnSOD and CuZnSOD did not change further with PPHN or with ventilation in either saline or betamethasone exposed lambs, while ECSOD expression was higher in PPHN unventilated lungs. Expression of eNOS was decreased in unventilated betamethasone exposed normal lungs (Figure 6A and 6C) and was also 4-fold lower in PPHN ventilated and unventilated lungs, as previously reported (9, 11). Prenatal exposure to betamethasone increased the eNOS expression by 3-fold in the ventilated PPHN lungs (Figure 6, panels A and C).
Figure 6.
A. Representative immunoblots showing protein levels of MnSOD, CuZnSOD, ECSOD and eNOS with beta-actin as internal control. Blots are shown for samples from unventilated and ventilated normal fetal lambs (NFL) and PPHN lambs (PPHNFL) with or without betamethasone as indicated on the figure labels. 20μg protein was loaded for SOD blots and corresponding β-actin and 40 μg loaded for eNOS and corresponding β-actin. Summarized data for integrated optical densities (IOD) of SOD/β-actin ratio (B) and eNOS/β-actin ratio (C) are shown as mean±SD for 3 lambs each. Panel B, open bars are for MnSOD, filled bars for CuZnSOD and hatched bars for ECSOD. *P<0.05, from normal unventilated lambs without betamethasone; §P<0.05, from PPHN ventilated lambs without betamethasone.
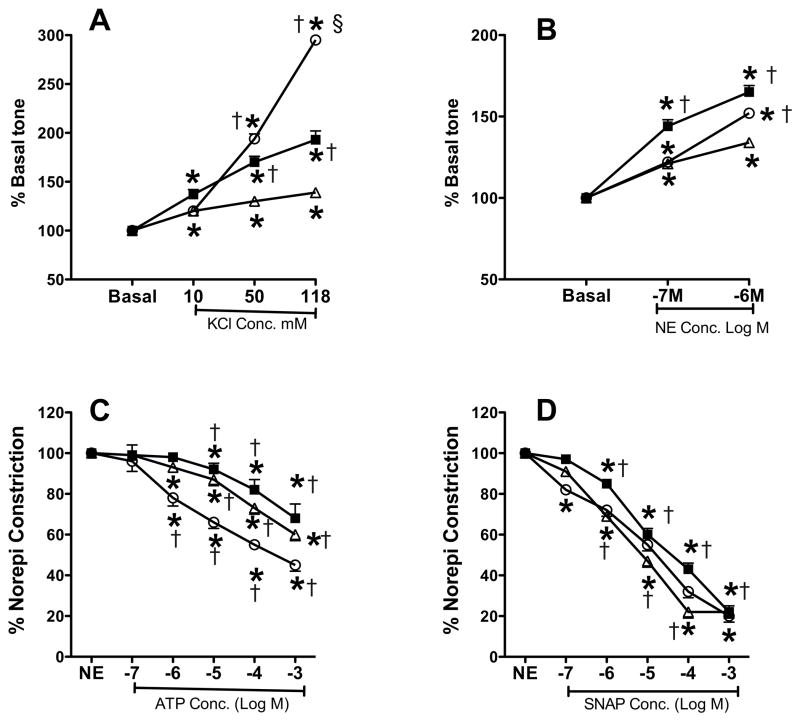
Pulmonary artery rings isolated from betamethasone treated lambs showed increased contractile responses to both norepinephrine and KCl (Figure 7, panels A & B) compared to saline treated PPHN lambs. Control rings from normal term ventilated lambs showed higher constrictor response at 118mM KCl compared to other groups, which may be related to their greater maturity and Smooth muscle mass (Figure 7A). The relaxation responses to NOS agonist, ATP and NO donor, SNAP were similar between the 2 PPHN groups (Figure 7, panels C and D).
Figure 7.
Contraction (A&B) and relaxation (C&D) responses of pulmonary artery rings from ventilated normal (open circle), PPHN (open triangle) and PPHN+betamethasone (filled square) lamb lungs. Data are mean±SEM for 12 rings for normal lambs and 24 rings for PPHN groups with or without betamethasone. For potassium chloride (KCl) and norepinephrine (NE) contraction responses, basal tone of each ring was normalized to 100%. For relaxation responses in C & D, tone achieved after norepinephrine (norepi) constriction was normalized to 100% for each ring. For panels A & B, *P<0.05 from basal tone; †P<0.05, from PPHN group without betamethasone; §P<0.05, from betamethasone treated group. Betamethasone enhanced the contractile response of rings to KCl and norepinephrine, whereas, rings from normal lambs achieved greater tone at maximal dose of KCl compared to both PPHN groups. For panels C&D, *P<0.05, from NE; †P<0.05, from preceding dose of ATP or S-nitroso-n-acetylpenicillamine (SNAP). Conc. = concentration. ATP and SNAP induced comparable relaxation response in all 3 groups.
DISCUSSION
We provide evidence that antenatal betamethasone improves oxygenation and facilitates pulmonary vasodilation in a model of PPHN in newborn lambs delivered at late preterm gestation. The effects of betamethasone were sustained for the 8-hour study period and were accompanied by decreased markers of oxidative stress in the airway and pulmonary arteries. Betamethasone also induced an increase in the levels of eNOS in the ventilated PPHN lungs. These data suggest that antenatal betamethasone can benefit postnatal transition in the presence of PPHN at late preterm gestation. We used both O2− and nitrotyrosine as markers of oxidative stress since previous studies demonstrated an upregulation of NADPH oxidase, a source of O2−, and uncoupling of eNOS (10, 12). Uncoupled eNOS generates both NO and O2− which combine to form peroxynitrite, a free radical that nitrates tyrosine residues. We observed an increase in nitrotyrosine with PPHN+ventilation and reduction in this marker with betamethasone.
Betamethasone has several physiological effects on the preterm lung including a decrease in lung fluid, an increase in anti-oxidant enzyme activity and expression (18–20) and surfactant release (21). Our previous studies demonstrated that betamethasone increases the expression of both eNOS and MnSOD in fetal PAEC when added to culture media for 48h (15). These changes were accompanied by a decrease in the superoxide and increase in NO levels (15). However, the potential benefit of betamethasone in facilitating postnatal transition in intact fetal lambs was not investigated in our previous in vitro studies.
Previous studies also reported that antenatal glucocorticoids facilitate birth related transition (16, 22–24). Antenatal steroids increased pulmonary vasodilation and expression of eNOS in lamb lungs at birth (22–24). We observed an increase in the expression of 3 SOD isoforms in the unventilated normal fetal lungs with betamethasone in the present study. However, we did not observe a change in SOD expression with betamethasone in ventilated PPHN lungs. It is likely that ventilation with high FiO2 necessary to sustain PaO2 in PPHN lungs disrupted this effect of betamethasone. Another possible explanation for the difference between our in vitro data and the present study is that we gave betamethasone 24 h before delivery, resulting in a shorter exposure time. We previously observed a decrease in MnSOD expression in PAEC from PPHN lambs (15). Farrow et al reported an increase in MnSOD expression and activity in vascular smooth muscle cells isolated from PPHN lambs (25). Our present studies demonstrate no change in the total lung expression of MnSOD in PPHN, probably from these reciprocal changes in different cells. These data also suggest that changes in SOD expression may be cell specific in PPHN. In addition, we did not measure the total or isoform specific SOD activity in our studies, which may be altered by PPHN or betamethasone. Betamethasone decreased basal eNOS expression in unventilated normal lungs, but increased the eNOS expression in ventilated PPHN lamb lungs, consistent with our in vitro data from PAEC (15). However, the increase in eNOS expression in the lung was not accompanied by improved relaxation response of pulmonary arteries to NOS agonist, ATP. These data suggest that function of eNOS is still impaired, probably from prolonged ventilation of PPHN lambs with high FiO2.
Our model of fetal ductal constriction is known to cause PPHN in lambs similar to findings observed in neonates with PPHN (26, 27). We also delivered the lambs in our study one week before term gestation. The lambs in our study were not given exogenous surfactant. This model therefore reproduces the respiratory failure from both lung immaturity and PPHN. Currently, late preterm births are an important cause of respiratory failure, leading to PPHN in neonates. Walsh-Sukys et al observed that 32% of babies diagnosed with PPHN were delivered by C-section before the onset of labor in a large cohort from the NICHD Neonatal Research Network (2). C-section delivery before the onset of labor was noted to increase the risk of PPHN significantly in other studies (4, 5). Late preterm gestation neonates with respiratory failure have increased morbidity and mortality compared to term infants with comparable degree of respiratory failure (6). Our study suggests that antenatal betamethasone decreases the oxidative stress and improves oxygenation at comparable gestation.
The improvement in PaO2 in our study was not significant at hours 3–6. This is due to some increase in PO2 in control lambs and weaning of FiO2 in betamethasone treated lambs. The oxygenation index remained consistently low and PO2 consistently above 50 in betamethasone treated lambs. The data also showed variability inherent to the ventilation studies and small sample size typical of these complex studies (28). The PA pressure decreased in betamethasone treated animals, matching the improvement in oxygenation. In addition, the PaCO2 was lower and pH higher in betamethasone treated lambs. These data indicate that improvement in lung mechanics may also contribute to improved oxygenation in this model. Since an increase in pH and decrease in PCO2 can reduce pulmonary artery pressure, the effect of betamethasone on these variables may have contributed to pulmonary vasodilation independent of its effects on the oxidative stress. The systemic pressure was consistently higher in betamethasone treated lambs. These results suggest that an increase in cardiac output may also contribute to the improved oxygenation in betamethasone treated lambs. The in vitro relaxation response of pulmonary arteries was not improved by betamethasone. The mechanism of improved oxygenation in betamethasone treated lambs remains unclear from the in vitro studies.
A recent study reported that administration of postnatal hydrocortisone to lambs with PPHN induced by prenatal ductal constriction improves oxygenation and decreases oxidative stress in the lung during mechanical ventilation (28). PA pressure changes were not reported and it is unclear whether improved oxygenation resulted from pulmonary vasodilation or improved lung mechanics. Our data are overall consistent with the reported effects of postnatal hydrocortisone and both approaches offer complementary strategies to decrease oxidative stress and improve oxygenation in PPHN.
The limitation of our study is that the period of postnatal ventilation was limited to 8 hours. We chose this time point because of high mortality in the control lambs with PPHN after 8 hours of ventilation. We also observed that betamethasone increased the contractile response of the pulmonary arteries to norepinephrine and KCl. This is a well-known effect of steroids in systemic vessels and involves calcium sensitization of smooth muscle cells. Whether a similar mechanism accounts for the observed pulmonary artery responses is unclear. Although increased contractility may appear to counter pulmonary vasodilation at birth, it may enhance oxygenation by preserving vasoconstriction in the unventilated segments of the lung and promoting V/Q matching.
In conclusion, we observed that antenatal betamethasone improves oxygenation and pulmonary vasodilation during postnatal life in newborn lambs with PPHN, delivered at late preterm gestation. Our study supports the potential clinical use of antenatal betamethasone for indicated deliveries at late preterm gestation.
METHODS
The Institutional Animal Care and Use Committee of Medical College of Wisconsin approved the use of animals for studies described in this article.
Creation of PPHN model
Pregnant ewes of Dorset Cross or Speckled Face breed were obtained from Purdue University (West Lafayette, IN) at 118±4 days of gestation; average full term gestation for sheep is 145 days. Ewes underwent midline laparotomy and hysterotomy under general anesthesia at 128±2 d gestation. Fetal chest was exteriorized and a left lateral thoracotomy was done for constriction of ductus arteriosus, as previously reported (11, 12, 29). A catheter was placed in the pulmonary artery by direct puncture and the tip was directed into the left pulmonary artery during same procedure. The ductal constriction was maintained for 8 days (128±2 to 136±2 days). Catheters were placed in the SVC and aortic arch via left jugular vein and common carotid artery at the time of C-section delivery.
Ewes were randomly assigned to receive either betamethasone or to a control group that received equal volume of saline by i.m. injection. Betamethasone (Celestone, Merck-Schering Plough, Whitehouse Station, NJ) was given in 2 doses of 12 mg each, 24 h and 12 h before C-section delivery of the fetal lamb at 136+2 days gestation. Administration of betamethasone more than 24 hours before planned delivery has lead to induction of preterm labor, as previously reported (30). Fetal lambs were delivered by C-section under general anesthesia with 1–3% isoflurane inhalation.
Three fetal lambs without PPHN and without betamethasone exposure and three twin fetal lambs without PPHN and exposed to antenatal betamethasone were also included as additional controls for in vitro studies. Three term fetal lambs were also ventilated for 8 hours as additional controls for in vitro studies.
Ventilation of fetal lambs
The fetal trachea was intubated with a 4.0-cuffed endotracheal tube and the cuff was inflated to prevent air leak. Fetal lung liquid was suctioned to improve ventilation. Ventilation was commenced after umbilical cord was tied and cut and the newborn lamb was placed on a warmer. Lamb was sedated with intermittent doses of pentobarbital (5 mg/kg IV) and buprenorphine (0.005 mg/kg IM) and paralyzed with vecuronium (0.1 mg/kg IV and repeated as indicated). Lambs were given 10% Dextrose i.v. at 4 ml/kg/h to keep blood glucose conc. in the normal range. Surfactant was not used in order to simulate the typical respiratory transition in late preterm newborn at birth and to avoid confounding the effects of betamethasone on respiratory failure. Ventilation was commenced with an Infant Star Time cycled, pressure limited ventilator (Infrasonics, Sorrento Valley, CA) with peak inspiratory pressures (PIP) of 25–35 cm of H2O, positive end expiratory pressure (PEEP) of 5–6 cm H2O, frequency of 30–40 breaths/min, inspiratory time of 0.4 to 0.5 sec and FiO2 of 50%, as reported previously (14). The ventilator settings were selected to keep arterial partial pressure of CO2 (PaCO2) in a target range of 40–55 mmHg, arterial partial pressure of O2 (PaO2) in a range of 50–70 mmHg and pH>7.20. The ventilator settings were not changed after the target blood gas tensions were reached. Arterial blood gas tensions and blood pH were measured at 15, 30, 45 and 60 minutes and every hour for the 8h of ventilation. Systemic and pulmonary artery pressures and heart rate were continuously monitored. Oxygenation index was calculated using the formula:mean airway pressure × FiO2 × 100×÷PaO2 and Ventilatory Index was calculated using the formula Respiratory rate × (PIP− PEEP) × PaCO2÷1000.
Pulmonary artery Ring studies (12)
After the ventilation studies were completed, lambs were euthanized with an overdose of pentobarbital. Lungs were harvested and third-fifth generation intrapulmonary arteries with an internal diameter of 300– 500 μM were dissected and isolated from the lung. The arteries were cut into rings 1-mm in length, suspended with stainless steel hooks in water-jacketed chambers and were studied using standard tissue bath techniques (12). They were allowed to equilibrate for 45 min and stretched to a passive tension of 0.8 Gm. Investigation of the effects of potassium chloride (KCl) and norepinephrine on basal tone was done without pre-constriction of the rings. KCl was added in concentrations of 10, 50 and 118 mM. In some rings, constriction response to 10−7–10−6M norepinephrine was tested. Relaxation responses to 10−7–10−3M conc. of ATP, a NOS agonist and to S-nitroso-n-acetyl penicillamine (SNAP), an NO donor were determined after pre-constriction of the rings with 10−7–10−6M norepinephrine. Percent relaxation was calculated by normalizing the ring tension observed with norepinephrine to 100%, as described before (12). A total of 12 rings from 3 normal term ventilated lambs and 24 rings each from 6 PPHN lambs were used for the in vitro studies.
Expression of eNOS and SOD isoforms in the lung
A part of the right upper lobe was flash frozen in liquid nitrogen immediately after harvest of the lungs. Samples from unventilated normal lambs with or without exposure to betamethasone, ventilated normal term lambs and ventilated PPHN lambs were included as controls. Samples were homogenized in modified RIPA buffer (11, 12), sonicated to break the cells and insoluble debris was removed by centrifugation. Protein concentration was measured and an aliquot (20μg) of the protein was used for immunoblotting with antibodies for cytosolic copper zinc SOD (CuZnSOD), extracellular SOD (ECSOD) and MnSOD and an aliquot of 40μg for eNOS. Each sample was also immunoblotted for β-actin, used as the internal loading control. Autoradiograms were imaged with Adobe PhotoShop v5.5 and the relative band densities were quantified using NIH Image 1.62. Integrated optical densities (IOD) for SOD isoforms, eNOS and β-actin were measured and the ratios of SOD/β-actin and eNOS/β-actin were calculated for each sample.
DHE fluorescence in PA segments with intact endothelium
Second generation branches of pulmonary arteries were dissected fresh from the lungs of saline and betamethasone treated PPHN lambs and an unventilated normal lamb. A 1-mm length of the artery was cut open, placed in 6-well plates with HBSS and endothelium was exposed. The PA segments were incubated with HBSS alone or containing 100U/ml of PEG-SOD for 30 min. Then DHE (10−5M) and HBSS with or without 10−5M ATP were added (12). In some studies, PA from a normal unventilated fetal lamb were incubated for 45 min with 1mM Xanthine plus 20mU/ml Xanthine oxidase (Sigma Chemical, St Louis, MO) to generate superoxide (O2− ) as a positive control for DHE staining. After 15 min, PA segments were washed, placed in micro-wells of 8-well chamber glass slides and cover-slipped. DHE fluorescence of endothelium was visualized and imaged with a fluorescent microscope (NIKON Eclipse 600), equipped with a SPOT RT Slide camera and software (Diagnostic Instruments, Inc.). Fluorescence was quantified using MetaVue software (Universal Imaging Corp., Downingtown, PA).
Immunofluorescence for 3-nitrotyrosine (3-NT)
The right middle lobe of the lung was gently inflated with optimum cutting temperature compound (OCT, Ted Pella Inc., Redding, CA) infused into the bronchus. The lobe was wrapped and placed on dry ice to firm up the OCT. Blocks were prepared by cutting 2–3 mm thick sections and were frozen in liquid nitrogen. For 3-NT labeling, 10μ sections of the frozen tissue were cut and mounted on slides. Tissue was fixed in 100% acetone, washed, blocked with 5% bovine serum albumin (BSA) and probed with 5μg/ml anti-nitrotyrosine antibody (Upstate-Millipore, Billerica, MA) overnight at 4°C. In some studies, sections from normal unventilated lung were incubated with 5μM peroxynitrite for 5 min to increase 3-NT formation in the lung as positive control for 3-NT. Sections were washed and incubated with 20μg/ml goat anti-mouse IgG (Alexa Fluor 488, Invitrogen-Life Technologies, Grand Island, NY) and fluorescence visualized using a Nikon Eclipse TE-300 fluorescent microscope with excitation at 485nm and emission at 530nm. Fluorescent images of similar-sized pulmonary arteries and airway were captured and quantified as described above.
Statistical analysis of data
All data are shown as mean±SD, except for ring tension studies where data are shown as mean±SEM. We compared changes in the hemodynamic and blood gas values over time between control and betamethasone treated groups by two-way ANOVA and Student-Newman Keuls Post-hoc test when a significant difference (p<0.05) was noted by ANOVA. Densitometry ratios for western blots and DHE/NT fluorescence in pulmonary arteries between the groups were compared by ANOVA and Newman-Keuls post-hoc test. Ring tension data were compared between different groups and at different doses by two-way ANOVA and Newman-Keuls post-hoc test.
Acknowledgments
Statement of financial support: Supported by grants 1RO3 HD059076 and RO1 HL057268 from National Institutes of Health, Bethesda, MD; a research grant from Ikaria Inc., Clinton, NJ; grant UL1RR031973 from Clinical and Translational Science Institute and Advancing Healthier Wisconsin Foundation of Medical College of Wisconsin, Milwaukee, WI; Children’s Research Institute of Children’s Hospital of Wisconsin, Milwaukee, WI; and Muma Endowed Chair in Neonatology at Children’s Hospital of Wisconsin, Milwaukee, WI.
We thank Dr. Satyan Lakshminrusimha at the University of Buffalo for his valuable suggestions on the ventilation study protocol.
References
- 1.Fox WW, Gewitz MH, Dinwiddie R, Drummond WH, Peckham GJ. Pulmonary hypertension in the perinatal aspiration syndromes. Pediatrics. 1977;59:205–11. [PubMed] [Google Scholar]
- 2.Walsh-Sukys MC, Tyson JE, Wright L, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Konduri GG, Vohr B, Robertson C, et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007;150:235–40. doi: 10.1016/j.jpeds.2006.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson KL, Zelig CM, Harvey JP, Cunningham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28:19–24. doi: 10.1055/s-0030-1262507. [DOI] [PubMed] [Google Scholar]
- 5.Winovitch KC, Padilla L, Ghamsary M, Lagrew DC, Wing DA. Persistent pulmonary hypertension of the newborn following elective cesarean delivery at term. J Matern Fetal Neonatal Med. 2011;24:1398–402. doi: 10.3109/14767058.2010.551681. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandrappa A, Rosenberg ES, Wagoner S, Jain L. Morbidity and mortality in late preterm infants with severe hypoxic respiratory failure on extra-corporeal membrane oxygenation. J Pediatr. 2011;159:192–198. e3. doi: 10.1016/j.jpeds.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berti A, Janes A, Furlan R, Macagno F. High prevalence of minor neurologic deficits in a long-term neurodevelopmental follow-up of children with severe persistent pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr. 2010;36:45–50. doi: 10.1186/1824-7288-36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62:313–8. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinhorn RH, Russell JA, Morin FC. Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol (Heart Circ Physiol) 1995;268:H1483–89. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 10.Brennan LA, Steinhorn RH, Wedgwood S, et al. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–91. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 11.Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H204–11. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 12.Konduri GG, Bakhutashvili I, Eis A, Pritchard KA. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1812–H1820. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- 13.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–9. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 14.Lakshminrusimha S, Russell JA, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–7. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res. 2008;63:67–72. doi: 10.1203/PDR.0b013e31815b43ee. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Tolsa JF, Shen H, Raj JU. A single dose of antenatal betamethasone enhances isoprenaline and prostaglandin E2-induced relaxation of preterm ovine pulmonary arteries. Biol Neonate. 1998;73:182–9. doi: 10.1159/000013976. [DOI] [PubMed] [Google Scholar]
- 17.Sadowska AM, Klebe B, Germonpre P, De Backer WA. Glucocorticosteroids as antioxidants in treatment of asthma and COPD. New application for an old medication? Steroids. 2007;72:1–6. doi: 10.1016/j.steroids.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Frank L, Lewis PL, Sosenko IR. Dexamethasone stimulation of fetal rat lung antioxidant enzyme activity in parallel with surfactant stimulation. Pediatrics. 1985;75:569–74. [PubMed] [Google Scholar]
- 19.Walther FJ, David-Cu R, Metha EI, Polk DH, Jobe AH, Ikegami M. Higher lung antioxidant enzyme activity persists after a single dose of corticosteroids in preterm lambs. Am J Physiol. 1996;271:L187–191. doi: 10.1152/ajplung.1996.271.2.L187. [DOI] [PubMed] [Google Scholar]
- 20.Walther FJ, Jobe AH, Ikegami M. Repetitive prenatal glucocorticoid therapy reduces oxidative stress in the lungs of preterm lambs. J Appl Physiol. 1998;85:273–8. doi: 10.1152/jappl.1998.85.1.273. [DOI] [PubMed] [Google Scholar]
- 21.DeLemos RA, Shermeta DW, Knelson JH, Kotas R, Avery ME. Acceleration of appearance of pulmonary surfactant in the fetal lamb by administration of corticosteroids. Am Rev Respir Dis. 1970;102:459. doi: 10.1164/arrd.1970.102.3.459. [DOI] [PubMed] [Google Scholar]
- 22.Houfflin-Debarge V, Deruelle P, Jaillard S, et al. Effects of antenatal glucocorticoids on circulatory adaptation at birth in the ovine fetus. Biol Neonate. 2005;88:73–78. doi: 10.1159/000084646. [DOI] [PubMed] [Google Scholar]
- 23.Grover TR, Ackerman KG, Le Cras TD, Jobe AH, Abman SH. Repetitive prenatal glucocorticoids increase lung endothelial nitric oxide synthase expression in ovine fetuses delivered at term. Pediatr Res. 2000;48:75–83. doi: 10.1203/00006450-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Hooper SB, Wallace MJ, Probyn ME, Harding R. Effects of antenatal corticosteroid treatment on pulmonary ventilation and circulation in neonatal lambs with hypoplastic lungs. Pediatr Pulmonol. 2006;41:844–54. doi: 10.1002/ppul.20453. [DOI] [PubMed] [Google Scholar]
- 25.Farrow KN, Wedgwood S, Lee KJ, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010;174:272–81. doi: 10.1016/j.resp.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin FC. Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in newborn lamb. Pediatr Res. 1989;25:245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest. 1989;83:1849–1858. doi: 10.1172/JCI114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez M, Lakshminrusimha S, Wedgwood S, et al. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;302:L595–603. doi: 10.1152/ajplung.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288:L648–54. doi: 10.1152/ajplung.00288.2004. [DOI] [PubMed] [Google Scholar]
- 30.Liggins GC. Premature delivery of fetal lambs infused with glucocorticoids. J Endocrin. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]