Abstract
We investigated the hypothesis that oxidative stress in persistent pulmonary hypertension of the newborn (PPHN) impairs voltage gated potassium (Kv) channel function. We induced PPHN in fetal lambs by prenatal ligation of ductus arteriosus; controls had sham ligation. We studied changes in the tone of pulmonary artery rings and Kv channel current of freshly isolated pulmonary artery smooth muscle cells (PASMC) using standard techniques. 4-Aminopyridine (4-AP), a Kv channel antagonist, induced dose dependent constriction of control PA rings; this response was attenuated in PPHN pulmonary arteries. Exogenous superoxide and peroxynitrite inhibited the response to 4-AP in control rings. Tiron, a superoxide scavenger, improved the response to 4-AP in PPHN rings. 4-AP inhibited the NOS- independent relaxation response to ATP in control PA rings. Relaxation response to ATP was blunted in PPHN rings and was improved by NOS antagonist, n-nitro-l- arginine methyl ester (L-NAME). 4-AP attenuated this response in L-NAME treated PPHN rings. Exogenous superoxide suppressed 4-AP sensitive Kv current in control PASMC. Kv channel current was attenuated in cells from PPHN lambs and was restored by tiron. Oxidative stress impairs Kv channel function in PPHN. Superoxide scavengers may improve pulmonary vasodilation in PPHN in part by restoring Kv channel function.
Adenosine 5’triphosphate (ATP) is a purine nucleotide that contributes to the birth related pulmonary vasodilation in fetal lambs (1-3). ATP causes vasodilation by both stimulation of nitric oxide (NO) release (4-5) and by NO-independent mechanisms (4-6). Persistent pulmonary hypertension of the newborn (PPHN) occurs when pulmonary vascular resistance fails to decrease at birth. Studies in fetal lambs with PPHN induced by prenatal constriction of ductus arteriosus demonstrated impaired nitric oxide – cGMP mediated vasodilation and an increase in oxidative stress in the pulmonary arteries (7-9). Increased superoxide (O2•−) formation comes from a number of sources including NADPH oxidase (9) and uncoupled nitric oxide synthase (10) in this model of PPHN. Superoxide impairs vasodilation in part by reducing the availability of NO. The reaction of O2•− with NO results in the formation of peroxynitrite (11), which also contributes to impaired vasodilator responses. Scavenging O2•− with superoxide dismutase (SOD) or SOD mimic, tiron improves vasodilator response in PPHN (12,10).
Vascular smooth muscle cell (VSMC) K+ channels mediate both NO-dependent and NO independent vasodilator responses in a number of vascular beds including pulmonary arteries. Potential NO- independent agonists for smooth muscle K+ channels include endothelium derived hyperpolarizing factors (EDHF) - either hydrogen peroxide (H2O2) or metabolites of cytochrome P450 pathway (13,14) and ATP (6). Among the K+ channels, voltage gated (Kv) and high conductance Ca2+ activated channels (BKCa) contribute the majority of K+ current (15). Developmental studies identified a maturational increase in Kv channel expression and activity during fetal to neonatal transition (16). Previous studies in VSMC from the ductal ligation model of PPHN demonstrated that a decrease in KCa channel activity and expression occur in PPHN (17). In contrast, the role of altered Kv channel responses in PPHN and specifically the role of oxidative stress in impairing the Kv channel responses are unknown. Oxidative stress from exposure to high glucose or pulmonary hypertension was shown to impair vasodilation by decrease in Kv channel function in adult animal models and adult patients (18-20). We investigated the hypothesis that oxidative stress impairs Kv channel function and NO independent vasodilator responses to ATP in PPHN induced by prenatal ductal constriction. We used isolated pulmonary artery rings and whole cell patch clamp of pulmonary VSMC from fetal lambs that underwent prenatal ductal constriction and control fetal lambs that had sham ligation of ductus arteriosus. The objectives of our studies are to investigate the functional responses of Kv channels in pulmonary arteries and Kv channel current of VSMC in control and PPHN lambs to identify the specific contribution of O2•− in the impaired vasodilation.
Materials and methods
Creation of PPHN model
Pregnant ewes were obtained at 118±2 days of gestation. After a period of acclimation, ewes underwent midline laparotomy and hysterotomy under general anesthesia at 128±2 d gestation. Fetal chest was exteriorized and a left lateral thoracotomy was done for ligation of ductus arteriosus (10). In control lambs, ductus arteriosus was exposed but not ligated. The ductal constriction was maintained for 8 days (128±2 to 136±2 days). Fetal lambs were delivered by C-section, euthanized with an overdose of pentobarbital, and then lungs were harvested. Third-5th generation pulmonary arteries were dissected for vascular ring studies (10) and 5-7th generation arteries for isolation of VSMC. The use of animals in the research protocol was approved by the Institutional Animal Care and Use Committee of Zablocki VA Medical Center and Medical College of Wisconsin.
Pulmonary artery Ring studies (10)
Third-fifth generation intrapulmonary arteries with an internal diameter of 300-500 μM were dissected and isolated from the lung. The arteries were cut into rings 1-mm in length, suspended with stainless steel hooks in water-jacketed chambers and were connected to force displacement transducers (FTO3, Grass Instruments). The artery rings were bathed in 2 ml of physiological salt solution (PSS) kept at 37°C and aerated to maintain normal acid-base status and oxygenation of tissue. They were allowed to equilibrate for 45 min and stretched to a passive tension of 0.8 Gm. Investigation of the effects of 4-AP on basal tone was done without pre-constriction of the rings. After equilibration and observing stable ring tension, 4-AP was added in concentrations of 10-5-10-2M. Ring tension was measured 10 min after the addition of each dose. In some experiments, PA rings from control lambs were pre-incubated with xanthine (10-4M)+xanthine oxidase (10mU/ml) or 5×10-5M menadione (21,22) to increase O2•− levels in the vessels or 10-4M peroxynitrite to provide nitrosative stress or 10-4M tiron to scavenge O2•− prior to the addition of 4-AP. In other experiments, PA rings from PPHN lambs were incubated with the same agents prior to the addition of 4-AP. Evaluation of the relaxation response to ATP was done in rings pre-constricted with 10-6-10-7M norepinephrine. This dose of norpepinephrine gave stable constriction that reached 50% of maximal tension observed with 100 mM KCl. The tension reached with norepinephrine constriction for each ring was normalized to 100% and the percent change from this tension with each dose of ATP was calculated. Relaxation responses to 10-8-10-3 M doses of ATP were determined. Separate rings were pre-treated with 10-4 M concentrations of N-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, alone or with 10-3M 4-AP followed by incremental exposure to ATP. In some studies, control rings were treated with a combination of L-NAME and KCa channel antagonist, Iberiotoxin (10-7M) or KATP channel antagonist, glybenclamide (10-5M) followed by determination of the relaxation response to ATP.
Expression of Kv 1.5 channel protein in pulmonary arteries
Fifth to seventh generation pulmonary arteries were dissected clear of surrounding parenchyma, flash frozen in liquid nitrogen, pulverized and placed in modified RIPA buffer (10). The mixture was homogenized, sonicated to break the cells and insoluble debris was removed by centrifugation. Protein concentration was measured and an aliquot (15μg) of the protein was used for immunoblotting with Kv 1.5 channel protein antibody and with antibody for glyceraldehyde 3 phosphate dehydrogenase (GAPDH), used as internal loading control. Autoradiograms were imaged with Adobe PhotoShop v5.5 software and the relative band densities were quantified using NIH Image 1.62. Integrated optical density for Kv 1.5 channel protein and GAPDH were measured and the ratios of Kv 1.5/GAPDH were calculated for each sample.
Whole cell patch clamp of vascular smooth muscle for Kv channels
Fifth to seventh generation pulmonary arteries were dissected clear of surrounding parenchyma and placed in ice-cold HBSS. VSMC were enzymatically dispersed from these arteries using published methods (23). Whole cell recordings of K+ currents were obtained in freshly isolated pulmonary artery smooth muscle cells by amphotericin-perforated patch clamp method using an amplifier (Axopatch 200B, Axon instruments) and pclamp 8 software (Axon instruments) as previously described (24). Macroscopic K+ currents were generated by progressive 10- mv depolarizing steps (500 ms duration, 5-s intervals) from a constant holding potential of -60 to +60 mV. Currents were sampled at 3 kHz and filtered at 1 kHz. After control currents were recorded, the Kv channel blocker 4-AP was applied at 3 mM concentration. In a single cell, Kv current was defined as the difference between outward current recorded in drug-free bath solution and after superfusion with 3 mM 4-AP (18). Trials were performed in triplicate and averaged to estimate peak current amplitudes (picoamperes per picofarad) to normalize for cellular membrane area. The membrane capacitance of each cell was estimated by integrating the capacitative current generated by a 10-mV hyperpolarizing pulse after electronic cancellation of pipette-patch capacitance. In some experiments, smooth muscle cells from control PA were treated with xanthine (10-4M)+xanthine oxidase (10mU/ml) to generate O2•− and catalase (500U/ml) to scavenge H2O2, a metabolite of O2•− (18). Control studies were done with addition of xanthine and catalase. Tiron (10-4M), an O2•− scavenger was added to cells from PPHN pulmonary arteries to evaluate the effect of oxidative stress on Kv current.
Drug preparation
All the chemicals used were obtained from Sigma Chemical Co (St Louis, MO). Antibody for Kv 1.5 channel was obtained from Alomone laboratories and the GAPDH antibody from Abcam, Cambridge, MA.
Statistical analysis
Data are shown as mean±1 SD. Changes in vascular ring tension with incremental doses of 4-AP or ATP ± different blockers were analyzed by two-way ANOVA. When a significant difference (P < 0.05) was found, a Duncan’s multiple range test was done to determine which means were different. Comparison of densitometric data for Kv 1.5 channel protein from control and pulmonary hypertension groups was done by unpaired t test.
Results
Response of pulmonary artery rings to 4-AP
PA rings from control animals had a vigorous constrictor response to incremental doses of 4-AP (Figure 1 A). Addition of Xanthine+xanthine oxidase or menadione to generate O2•− resulted in attenuation of the constrictor response to 4-AP in control rings. Similarly, addition of peroxynitrite caused attenuation of the constrictor response to 4-AP in control rings (Figure 1 A). Addition of tiron, an O2•− scavenger did not alter the response of control rings to 4-AP (Figure 1A). These results together suggest that Kv channels are active and contribute to the resting tone in the fetal pulmonary arteries and that oxidative stress impairs this basal Kv channel activity. The response to 4-AP is blunted in vascular rings from PPHN lambs, suggesting decreased basal Kv channel activity in PPHN (Figure 1 B). Addition of xanthine+xanthine oxidase or menadione to generate superoxide or peroxynitrite to increase nitrosative stress did not cause additional attenuation of the response to 4-AP. The O2•− scavenger, tiron restored the constrictor response to 4-AP in PPHN rings (Fig 1 B).
Figure 1.
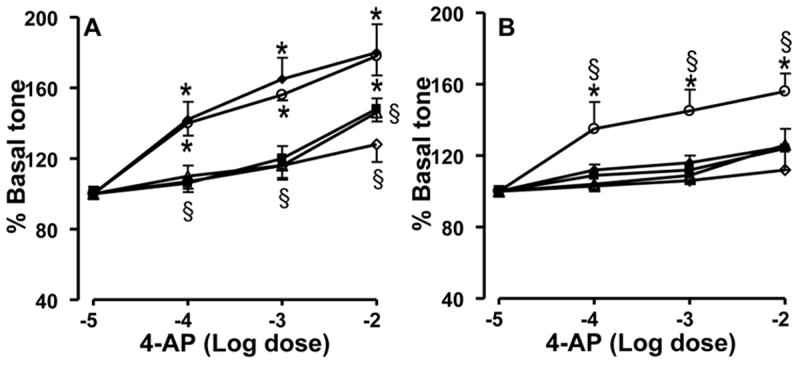
Effect of 4-AP on the basal tone of pulmonary artery rings from control (A) and PPHN lambs (B). Data are mean±SD for 15 rings from 5 animals each for 4-AP alone (-◆-), tiron+4AP (-○-), xanthine+xanthine oxidase+4-AP (-■-), peroxynitrite+4AP (-△-) and menadione+4-AP (-◇-). * Indicates P<0.05 from -5M conc. of 4-AP and § from 4-AP alone. The increase in basal tone in response to 4-AP in control rings was attenuated by xanthine+xanthine oxidase, menadione, and peroxynitrite (A). The attenuated response to 4-AP in PPHN rings was improved by superoxide scavenger, tiron and not altered further by xanthine+xanthine oxidase, menadione, or peroxynitrite (B).
Response of PA rings to ATP
Control PA rings showed a dose dependent relaxation response to ATP (Figure 2 A). Response to ATP was partly attenuated by NOS inhibitor, L-NAME. The NO independent response observed in L-NAME treated control PA rings was attenuated by 4-AP (Figure 2A). PA rings from PPHN lambs showed no relaxation response to ATP (Figure 2B), as we reported previously (10). The NOS inhibitor, L-NAME improved the relaxation response to ATP in PPHN rings, as reported previously (10). 4-AP inhibited the relaxation response observed in L-NAME treated PPHN rings with ATP (Figure 2B). The KCa channel antagonist Iberiotoxin(10-7M) and KATP channel blocker, glybenclamide (10-5M) did not alter the relaxation response of L-NAME treated control PA rings to ATP (Figure 2C & 2D).
Figure 2.
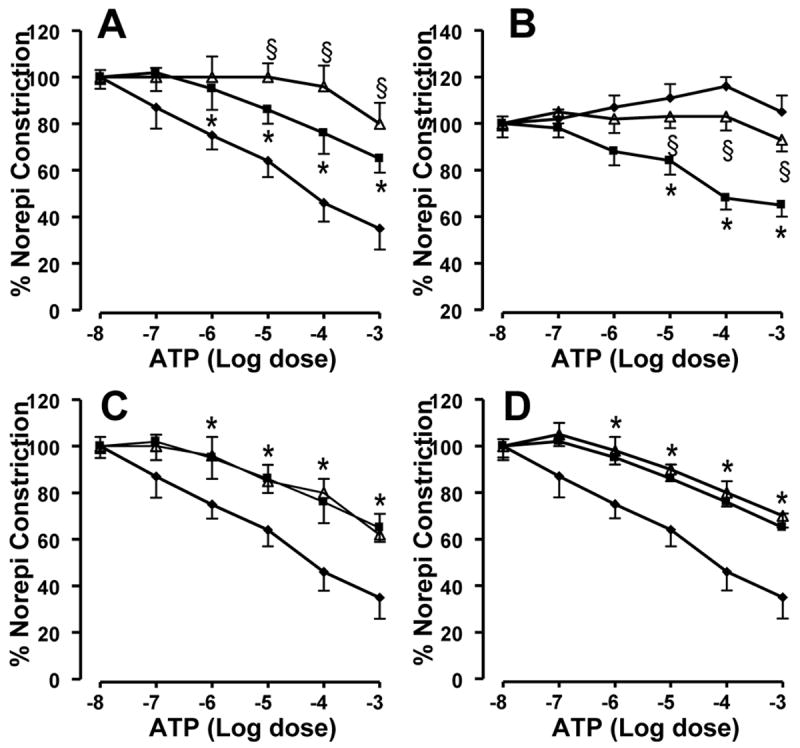
Effect of 4-AP on the NO independent response to ATP in control (A) and PPHN (B) pulmonary artery rings. Data are mean±SD for 15 rings from 5 animals each for ATP alone (-◆-), L-NAME+ ATP (-■-) and L-NAME+4-AP+ATP (-△-). * indicates P<0.05 from ATP alone and § from L-NAME+ATP. The relaxation response to ATP in L-NAME treated PA rings was attenuated by 4-AP in control and PPHN lambs (A & B). Effect of KCa channel blocker Iberiotoxin (C) and KATP channel blocker, glybenclamide (D) on the relaxation response to ATP in L-NAME treated control PA rings. Data are mean±SD for 12 rings from 4 animals each for ATP alone (-◆-), L-NAME+ ATP (-■-) and L-NAME+iberiotoxin+ATP (-△-) in panel C or L-NAME+glybenclamide+ATP (-△-) in panel D. * Indicates P < 0.05 from ATP alone. Both Iberiotoxin (10-7M) and glybenclamide (10-5M) failed to attenuate the NOS independent relaxation response to ATP.
Expression of Kv 1.5 channel protein in PPHN
The protein levels of Kv 1.5 channel were not different between control and PPHN pulmonary arteries (Figure 3). Although the ratio of Kv 1.5 to GAPDH appeared to be lower in PPHN group, the difference was not significant (P=0.07, Figure 3). These data are consistent with the report by Linden et al that Kv 1.5 mRNA levels are not altered in PPHN (25). These data also support the functional studies in vascular rings and isolated smooth muscle cells that scavenging O2•− was effective in improving the Kv channel responses.
Figure 3.
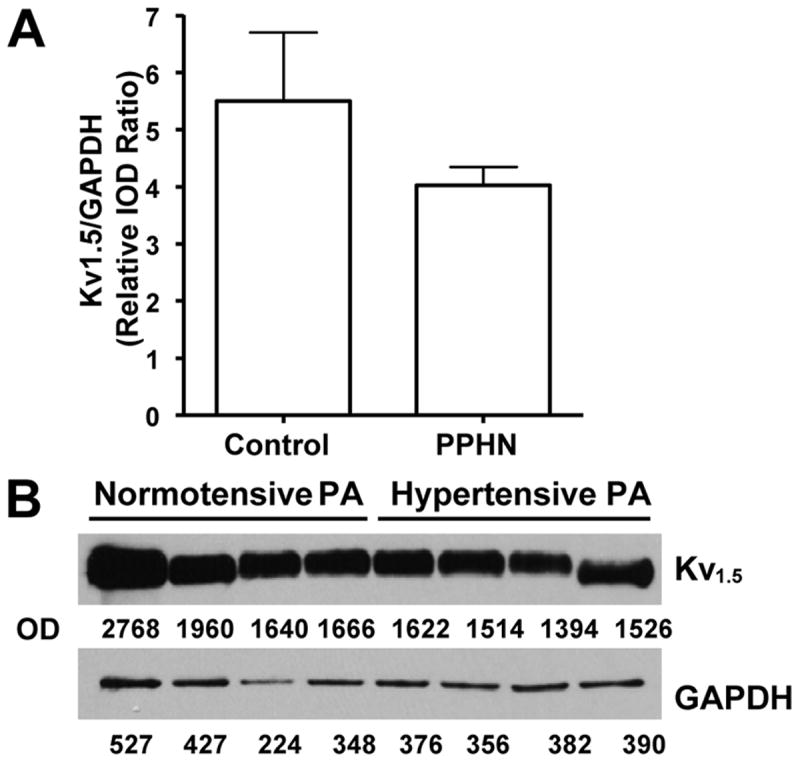
Summarized data (A) and sample blot (B) for Kv 1.5 channel protein levels assessed by immunoblotting with specific antibody. Summarized data from control and PPHN pulmonary artery homogenates are shown in A as mean±SD of IOD ratios for Kv 1.5 channel protein and GAPDH, used as internal control. Sample blots from control and PPHN pulmonary arteries are shown in B. No significant difference (P=0.07) was noted between control and PPHN groups (A).
Effect of superoxide and SOD mimetic on Kv current
VSMC from control fetal pulmonary arteries showed K+ current which was inhibited by 3mM 4-AP (Figure 4). Addition of xanthine+xanthine oxidase but not xanthine alone inhibited this Kv current in control VSMC (Figure 5). Further addition of 4-AP to VSMC in the presence of xanthine+xanthine oxidase had no effect on this current, indicating that Kv current was already inhibited by O2•− (Figure 5). Catalase was added to xanthine+xanthine oxidase to differentiate the effects of O2•− from its metabolite, H2O2. K+ current of VSMC from PPHN lambs was not inhibited by 4-AP, indicating attenuation of Kv current at basal level (Figure 6) in PPHN. Addition of tiron (10-4M) restored the 4-AP sensitive K+ current, indicating increase in Kv current (Figure 6). These data together suggest that oxidative stress in PPHN impairs Kv channel function in VSMC.
Figure 4.
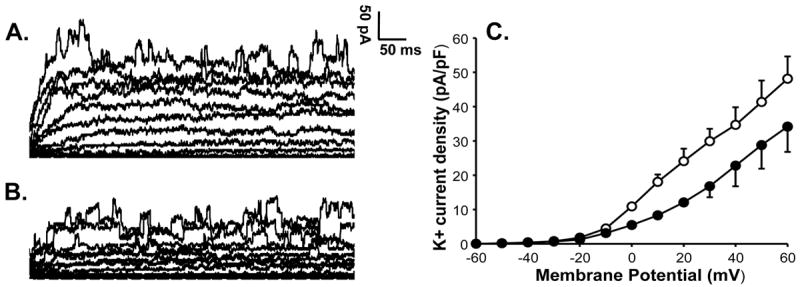
K+ current of a pulmonary artery VSMC from a control lamb is shown at basal level (A) and after application of 4-AP (B). K+ current shows significant suppression by 3 mM 4-AP indicating the presence of Kv channel activity in a control cell. Summarized data from 4 cells is shown to the right in panel C for K+ current density in the absence (-○-) or presence of 4-AP (-●-) and demonstrates inhibition of the current by 4-AP.
Figure 5.
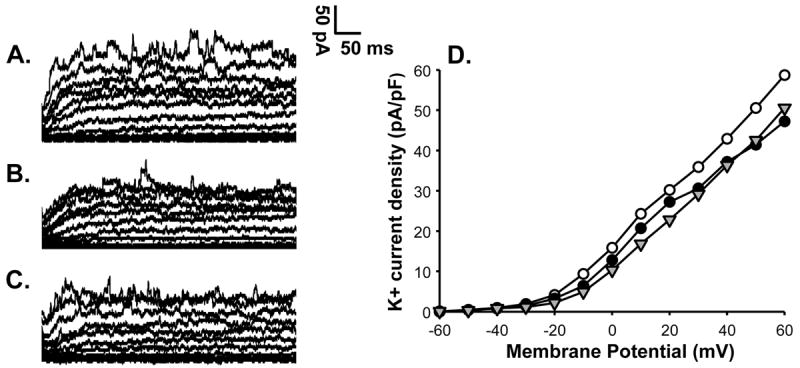
K+ current from control pulmonary artery VSMC in the presence of xanthine alone (A), xanthine+xanthine oxidase to generate superoxide (B) and xanthine+xanthine oxidase+4-AP (C). Xanthine alone (A) did not alter the K+ current. Addition of xanthine+xanthine oxidase resulted in suppression of K+ current (B). Further addition of 4-AP fails to alter K+ current in the presence of xanthine+xanthine oxidase (C), suggesting inhibition of Kv current by superoxide. Summary data in panel D show that xanthine+xanthine oxidase (-●-) attenuates the K+ current density compared to xanthine alone (-○-) and addition of 4-AP to xanthine+xanthine oxidase (-△-) does not attenuate the K+ current further.
Figure 6.
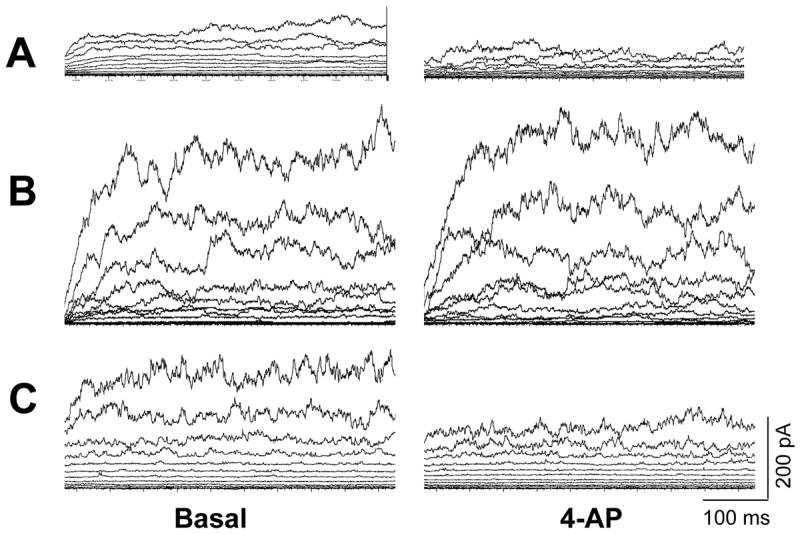
K+ channel tracings of smooth muscle cells from control (A) and PPHN (B) cells and PPHN cells treated with tiron (C). Suppression of K+ current by 3 mM 4-AP was used to define Kv channel current. The control smooth muscle cell (A) shows Kv channel current; this was attenuated in PPHN cell (B). Superoxide scavenger, tiron restores 4-AP sensitive current to PPHN smooth muscle cell (C).
Discussion
Our study provides the evidence that Kv channel function is impaired by oxidative stress in pulmonary arteries in PPHN. Since Kv channels contribute to basal vascular tone and mediate the response of vascular smooth muscle to a number of vasodilators, their impaired function may result in an altered adaptation of pulmonary circulation at birth. Our study also provides evidence that scavenging O2•−restores the Kv channel function in this model of PPHN.
Vascular K+ channels play a major role in maintaining the basal tone and in the regulation of responses to vasoactive mediators. The K+ channels are a heterogeneous group with different roles in mediating physiological responses. The Kv channels and BKCa channels contribute the majority of resting K+ current in VSMC (15). BKCa channels regulate the capacitive Ca2+ entry and play an important role in the O2 induced pulmonary vasodilation in fetal lambs (26). These channels also undergo maturational changes during gestation (16). Olschewski et al demonstrated that the contribution of KCa channel to membrane potential and O2 sensitivity are decreased in VSMC from lambs with PPHN induced by ductal ligation (17). However, alteration in the functional responses of Kv channels in PPHN remains unclear. Previous studies in adult animals demonstrated that the Kv channels, in particular Kv 1.5 and Kv 2.1 play a significant role in the hypoxic pulmonary vasoconstriction (27). Hypoxia induced pulmonary hypertension in rats is associated with a decrease in Kv 1.5 channel protein (28). The distribution of Kv channels also demonstrates a segmental heterogeneity with the distal resistance vessels showing predominance of Kv 1.5 channel protein (28). Gene therapy with Kv 1.5 channel protein ameliorates hypoxia induced pulmonary hypertension in rats (29). Based on the significance of resistance arteries in the regulation of pulmonary vascular tone and the role of Kv 1.5 channel in mediating pulmonary vasoconstriction, we investigated the alteration in this channel in the distal resistance vessels. We observed that the expression of this channel protein is not significantly altered in our model of PPHN induced by ductal ligation for 8 days. Our results are similar to previous observation by Linden et al that the Kv channel mRNA levels assessed by real time PCR are not altered in this model of PPHN (25). Whether longer exposure to pulmonary hypertension will result in decreased channel expression is unknown and requires further investigation. However, the function of Kv channels in smooth muscle cells from resistance vessels is altered in PPHN.
Our study used ATP to test the relaxation response of pulmonary arteries since ATP causes both NO-mediated and NO- independent vasodilation (4-6). Our previous studies in rabbit pulmonary arteries showed that the relaxation response to ATP in L-NAME treated and endothelium denuded pulmonary artery rings is independent of prostaglandin and cytochrome P450 pathways (6). Our new data suggest that the NO-independent response to ATP is mediated in part by Kv channels and not by KCa or KATP channels. These data also support our previous observation in intact fetal lambs that glybenclamide, a KATP channel antagonist, does not attenuate the vasodilator response to ATP (4).
Previous studies in adult animal models demonstrated that Kv channel function is impaired by vascular oxidative stress induced by high glucose or pulmonary hypertension (18-20). Since oxidative stress impairs vasodilation in PPHN (9-10), we speculated that inhibition of Kv channel function by superoxide contributes to vascular dysfunction. We used xanthine+xanthine oxidase to generate O2•− in our studies, as previously reported (19). Since xanthine+xanthine oxidase may generate variable levels of O2•− based on enzyme activity in different preparations, we used menadione as an alternate source of O2•− to verify our results (21,22). We observed that both oxidant generating systems caused similar attenuation of the response to 4-AP in pulmonary artery rings. Our studies suggest that O2•− impairs Kv channel function in the fetal pulmonary arteries by inhibition of Kv channel current. These observations are similar to the inhibitory effect of O2•− on the rat coronary artery smooth muscle cells in response to high glucose (18). The mechanism by which O2•− induces Kv channel dysfunction is not apparent from our studies. Although we used catalase in the studies done with xanthine+xanthine oxidase to remove H2O2 in control VSMC, the cell permeability of catalase in our preparation is uncertain. We did not use PEG catalase, which has greater cell permeability. Therefore, we cannot exclude the contribution of intra-cellular H2O2 to the inhibition of Kv channel function in these studies. Nitration of Kv channel protein was reported by other investigators in vascular dysfunction secondary to high glucose (30). Whether nitrosative stress contributes to impaired function of Kv channels in PPHN requires further study.
Although an improvement in the relaxation response to ATP by inhibition of eNOS appears incongruous, our previous studies demonstrated that eNOS is uncoupled and becomes a source of O2•− in PPHN (10). The improved vasodilation observed in L-NAME treated pulmonary arteries appears to be mediated in part by restoration of Kv channel function, based on inhibition of this response by 4-AP in vascular rings. Whether the improvement in Kv channel function by anti-oxidants restores response to other vasodilators requires further investigation. O2•− also appears to contribute to altered Kv channel function in isolated VSMC from PPHN lambs; however, the source of this O2•− in VSMC is not clear from our studies. NADPH oxidase and mitochondria are important sources of O2•− in vascular cells (31) and their contribution to impaired Kv channel activity in VSMC in PPHN requires further investigation.
The significance of our observations is that the impaired Kv channel function was improved by a scavenger of O2•−, tiron. Recombinant human SOD has been shown to improve pulmonary vasodilation and oxygenation in fetal lambs with PPHN (12,32). An improvement in the response to exogenous NO was also noted in this model following treatment with SOD (12,32). Our previous in vitro studies on pulmonary arteries isolated from PPHN lambs demonstrated that scavenging O2•− by tiron or inhibition of uncoupled eNOS by L-NAME improves the relaxation response to ATP (10). Our present study demonstrated that the O2•− scavenger, tiron restores the Kv channel current in VSMC from PPHN pulmonary arteries. Whether O2•− scavengers will have a role in the treatment of PPHN requires further investigation in the animal models and in babies with PPHN.
Acknowledgments
Financial Support: Supported by RO1 HL 57268 from NHLBI and a grant- in- aid from American Heart Association Greater Midwest affiliate.
Abbreviations
- Kv channel
Voltage gated potassium channel
- BKCa
High conductance Ca2+ activated channels
- GAPDH
Glyceraldehyde 3 phosphate dehydrogenase
- H2O2
Hydrogen peroxide
- O2•−
superoxide
- PPHN
Persistent pulmonary hypertension of newborn
- SOD
Superoxide dismutase
- VSMC
Vascular smooth muscle cell
References
- 1.Konduri GG, Gervasio CT, Theodorou AA. Role of adenosine triphosphate and adenosine in oxygen induced pulmonary vasodilation in fetal lambs. Pediatr Res. 1993;33:533–539. doi: 10.1203/00006450-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Konduri GG, Mattei J. Role of oxidative phosphorylation and ATP release in birth related pulmonary vasodilation in fetal lambs. Am J Physiol Heart Circ Physiol. 2002;283:H1600–H1608. doi: 10.1152/ajpheart.00245.2002. [DOI] [PubMed] [Google Scholar]
- 3.Konduri GG, Mital S, Gervasio CT, Rotta AT, Forman K. Purine nucleotides contribute to pulmonary vasodilation caused by birth related stimuli in the ovine fetus. Am J Physiol. 1997;272:H2377–H2384. doi: 10.1152/ajpheart.1997.272.5.H2377. [DOI] [PubMed] [Google Scholar]
- 4.Konduri GG, Mital S. Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilation in fetal lambs. Biol Neonate. 2000;78:220–229. doi: 10.1159/000014274. [DOI] [PubMed] [Google Scholar]
- 5.Fineman JR, Heymann MA, Soifer SJ. N omega-nitro-L-arginine attenuates endothelium-dependent pulmonary vasodilation in lambs. Am J Physiol. 1991;260:H1299–H1306. doi: 10.1152/ajpheart.1991.260.4.H1299. [DOI] [PubMed] [Google Scholar]
- 6.Konduri GG, Bakhutashvili I, Frenn R, Chandrasekhar I, Jacobs ER, Khanna AK. P2Y purine receptor responses and expression in the pulmonary circulation of juvenile rabbits. Am J Physiol Heart Circ Physiol. 2004;287:H157–H164. doi: 10.1152/ajpheart.00617.2003. [DOI] [PubMed] [Google Scholar]
- 7.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol. 1997;272:L1005–L1012. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- 8.Steinhorn RH, Russell JA, Morin FC., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 9.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 10.Konduri GG, Bakhutashvili I, Eis A, Pritchard K., Jr Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1812–H1820. doi: 10.1152/ajpheart.00425.2006. [DOI] [PubMed] [Google Scholar]
- 11.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 12.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 13.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 15.Leblanc N, Wan X, Leung PM. Physiological role of Ca2+-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994;266:C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- 16.Cornfield DN, Saqueton CB, Porter VA, Herron J, Resnik E, Haddad IY, Reeve HL. Voltage-gated K(+)-channel activity in ovine pulmonary vasculature is developmentally regulated. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1297–L1304. doi: 10.1152/ajplung.2000.278.6.L1297. [DOI] [PubMed] [Google Scholar]
- 17.Olschewski A, Hong Z, Linden BC, Porter VA, Weir EK, Cornfield DN. Contribution of the K(Ca) channel to membrane potential and O2 sensitivity is decreased in an ovine PPHN model. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1103–L1109. doi: 10.1152/ajplung.00100.2002. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 19.Bubolz AH, Li H, Wu Q, Liu Y. Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1873–H1880. doi: 10.1152/ajpheart.00357.2005. [DOI] [PubMed] [Google Scholar]
- 20.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional Voltage-Gated K+ Channels in Pulmonary Artery Smooth Muscle Cells of Patients With Primary Pulmonary Hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 21.Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem. 2006;281:40485–40492. doi: 10.1074/jbc.M607704200. [DOI] [PubMed] [Google Scholar]
- 22.Sampath V, Radish AC, Eis AL, Broniowska K, Hogg N, Konduri GG. Attenuation of lipopolysaccharide-induced oxidative stress and apoptosis in fetal pulmonary artery endothelial cells by hypoxia. Free Radic Biol Med. 2009;46:663–671. doi: 10.1016/j.freeradbiomed.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–40. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 24.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 25.Linden BC, Resnik ER, Hendrickson KJ, Herron JM, O’Connor TJ, Cornfield DN. Chronic intrauterine pulmonary hypertension compromises fetal pulmonary artery smooth muscle cell O2 sensing. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1354–L1361. doi: 10.1152/ajplung.00091.2003. [DOI] [PubMed] [Google Scholar]
- 26.Cornfield DN, Reeve HL, Tolarova S, Weir EK, Archer S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci USA. 1996;93:8089–8094. doi: 10.1073/pnas.93.15.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archer SL, Wu XC, Thébaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential Expression and Function of Voltage-Gated, O2-Sensitive K+ Channels in Resistance Pulmonary Arteries explains Regional Heterogeneity in Hypoxic Pulmonary Vasoconstriction: Ionic Diversity in smooth Muscle Cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 29.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- 31.Mueller CF, Laude K, McNally JS, Harrison DG. Redox Mechanisms in Blood Vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 32.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


