Abstract
This study was carried out to dissect the mechanism by which β1 integrins promote resistance to radiation. For this purpose, we conditionally ablated β1 integrins in the prostatic epithelium of transgenic adenocarcinoma of mouse prostate (TRAMP) mice. The ability of β1 to promote resistance to radiation was also analyzed by using an inhibitory antibody to β1, AIIB2, in a xenograft model. The role of β1 integrins and of a β1 downstream target, c-Jun amino-terminal kinase 1 (JNK1), in regulating radiation-induced apoptosis in vivo and in vitro was studied. We show that β1 integrins promote prostate cancer (PrCa) progression and resistance to radiation in vivo. Mechanistically, β1 integrins are shown here to suppress activation of JNK1 and, consequently apoptosis, in response to irradiation. Downregulation of JNK1 is necessary to preserve the effect of β1 on resistance to radiation in vitro and in vivo. Finally, given the established cross-talk between β1 integrins and type 1 insulin-like growth factor receptor (IGF-IR), we analyzed the ability of IGF-IR to modulate β1 integrin levels. We report that IGF-IR regulates the expression of β1 integrins, which in turn confer resistance to radiation in PrCa cells. In conclusion, this study demonstrates that β1 integrins mediate resistance to ionizing radiation through inhibition of JNK1 activation.
Keywords: TRAMP mice, Prostate cancer, Apoptosis, Insulin-like growth factor receptor
Introduction
Irradiation is the therapeutic option for more than 50% of all cancer patients. Both chemo- and radiotherapy are currently used in aggressive, advanced cancer but resistance to these treatments often develops. In vitro experiments have shown that resistance to radiotherapy is modulated by mechanisms that promote cell survival and is supported by deregulated interactions between extracellular matrix (ECM) and its receptors, integrins (Sethi et al., 1999; Hynes, 2002; Brakebusch and Fassler, 2005; Fitzgerald et al., 2008). Selective upregulation of integrins in prostate cancer (PrCa) has been associated with resistance to radiation and thus, adhesion modulation coupled with radiotherapy has been suggested to be a promising translational science approach (Fitzgerald et al., 2008; Wang et al., 2011). β1 integrins, whose expression has been associated with poor prognosis of breast cancer (Yao et al., 2007), have been shown to affect mammary tumor induction and pancreatic tumor growth (White et al., 2004; Kren et al., 2007) as well as proliferation of metastatic mammary carcinoma cells disseminated in the lungs (Shibue and Weinberg, 2009). These effects are shown to be mediated by regulation of proliferation or senescence. The type 1 insulin-like growth factor receptor (IGF-IR), a trans-membrane tyrosine-kinase receptor, is known to play an essential role in the development and progression of cancer by regulating cell proliferation, differentiation, apoptosis and metastasis (Baserga et al., 2003). In addition, IGF-IR signaling is known to mediate resistance to cytotoxic chemotherapy and radiotherapy (Allen et al., 2007). The established functional crosstalk between integrins and IGF-IR (Goel et al., 2004; Goel et al., 2005; Alam et al., 2007; Sayeed et al., 2012) supports the hypothesis that integrins and IGF-IR together, may play a concerted role in radioresistance in cancer cells.
Recent advances in genetic engineering enable scientists to investigate the role of genes in regulating the response of normal tissues and tumors to radiation (Kirsch et al., 2005). Here we report for the first time that conditional ablation of β1 integrins improves survival and delays PrCa progression in a mouse model of PrCa. We identify c-Jun amino-terminal kinase 1 (JNK1) as a mediator of radiation-induced apoptosis and demonstrate that β1 integrins elicit resistance to radiation in PrCa by effectively suppressing JNK1 activation in vivo. We report for the first time that the expression of β1 integrins is tightly regulated by IGF-IR, suggesting a vital crosstalk between these receptors to induce radiation resistance in PrCa.
Materials and methods
Reagents and antibodies (Abs)
IGF-I was purchased from R&D Systems Inc., synthetic androgen R1881 from Perkin-Elmer and etoposide from Sigma. Murine monoclonal (m) Abs against the following antigens were used: human β1, TS2/16 (ATCC); all β1, clone-18; JNK1/JNK2; RACK1; PARP (BD Biosciences); human αv integrin, L230; hemagglutinin, HA, 12CA5 (ATCC); c-src and phospho (p)-ERK (Cell Signaling); p-histone H2AX (Millipore). Rabbit polyclonal Abs against the following antigens were used: IGF-IR (IGF-IR-β sc713); survivin (Novus Biologicals); AKT; focal adhesion kinase (FAK); c-Jun; JNK1; p38 and ERK1/2 (Santa Cruz); Histone H2AX (Millipore); p-JNK; p-MAP kinase kinase (MKK) 4; p-MKK7; MKK4; MKK7; cleaved caspase-3; p-p38; p-AKT; AKT; p-src Tyr416 (Cell Signaling) and von Willebrand factor (vWF) (Dako). Non-immune mouse IgG (ni-mIgG) and rat immunoglobulin (rtIgG) were purchased from Pierce. Inhibitory rat mAb to human β1, AIIB2, was purchased from Aragen Bioscience. SP600125, a cell-permeable, selective and reversible inhibitor of JNK, was purchased from Sigma Aldrich.
Cell lines and transfectants
PC3, LNCaP, C4-2B, DU145, transgenic adenocarcinoma of mouse prostate (TRAMP)-C2 (a cell line established from a TRAMP mouse prostate tumor) PrCa cells were purchased from ATCC. PC3, LNCaP and C4-2B were grown at 37°C and 5% CO2 in RPMI-1640 supplemented with either 10% (PC3) or 5% (LNCaP, C4-2B) FBS. Medium for LNCaP and C4-2B cells was further supplemented with 1% each of sodium pyruvate, HEPES and non-essential amino acids. TRAMP-C2 cells were cultured as described previously (Sayeed et al., 2012). Authentication of the cell lines was provided with their purchase from ATCC. PC3 and DU145 cells stably transfected with β1-shRNA, β6-shRNA (PC3/cont-shRNA), vector (designated as mock) were generated as described (Goel et al., 2010). PC3 cells stably transfected with JNK1-shRNA (TRCN0000010580, Open Biosystems), TROP1-shRNA (used as a control shRNA, since these cells do not express TROP1, TRCN0000073733), or pLKO (empty vector) were generated by selecting the populations in the presence of puromycin (2 µg/ml). Immortalized mouse fibroblasts from JNK1/JNK2 double knockout mice (designated as JNK-null) or from JNK1/JNK2 positive mice (designated as JNK-wt) were cultured in DMEM with 10% FBS (Ventura et al., 2004). PC3 cells were stably transfected with either scrambled or IGF-IR-shRNA constructs in retroviral pRS vector (Origene). The target sequence of IGF-IR-shRNA construct is 5'-CCTCAAGGATGGAGTCTTCACCACCACTTACT-3' corresponding to 3599–3627 of the human IGF-IR mRNA. The cells were selected in puromycin (2 µg/ml) selection medium and pooled populations were maintained in the same medium.
Flow cytometry
Surface expression of human β1 or αv integrins in DU145 stable transfectants was analyzed by FACS using TS2/16, L230 or 12CA5 Abs as described (Fornaro et al., 2003).
Prostate xenografts
PC3 parental or PC3 transfectants (JNK1-sh, TROP1-sh or mock) were inoculated subcutaneously into the right flank of seven-week-old male athymic nu/nu mice (Charles River). Once the tumors reached 100 mm3 (day 0), AIIB2 Ab or non-specific rtIgG was injected intraperitoneally (5 mg/Kg) on day 0 and day 14. The tumors were irradiated using 6 MeV Varian 2300CD linear accelerator 6 (Varian Medical Systems) at a dose of 10 Gy or non-irradiated. Tumor size was measured as described before (Goel et al., 2004). Tumors were lysed and immunoblotted using Abs to p-JNK, JNK, p-p38, p38, p-ERK or ERK.
Apoptosis
DU145/β1-shRNA or DU145/mock cells were irradiated (5 or 10 Gy) or non-irradiated in the presence or absence of carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-fmk) (20 µM) or after infection with retroviral particles expressing either wt-JNK or JNK inhibitor, JNK-binding domain (JBD) of JNK interacting protein-1 (JBD-JIP1). JNK-null or wt-JNK cells were transiently transfected with β1-siRNA. Cells were irradiated (5, 10 or 15 Gy) or non-irradiated. Apoptosis in all cells was analyzed 24 h post-irradiation using the Cell Death Detection Enzyme-linked Immunosorbent Assay kit (Roche Applied Science) according to the manufacturer’s instructions as described previously (Fornaro et al., 2003). TRAMP-C2 cells transfected with either siRNA to β1A or β1C (cytoplasmic variant known to be expressed in normal prostate, used as a control), were serum-starved for 24 h. Cells were irradiated (10 Gy) or non-irradiated. 24 h after irradiation, cells were detached and caspase-3 activity was measured using CaspaseTag™ kit (Chemicon) as per manufacturer’s instructions. PC3/β1-shRNA or PC3/mock were embedded in Matrigel as single cells and cultured for 12 days. Colonies were irradiated with 6 MeV x-rays at a dose of 10 Gy or non-irradiated. Colonies were smeared and apoptosis was measured using Apoptag Cell Death Detection kit from Chemicon.
Immunoprecipitation (IP)
DU145 cells were serum-starved for 24 h. Cells were lysed and immunoprecipitated using Ab to JNK1 and protein A-Sepharose. Immunoprecipitates were immunoblotted using Ab to RACK1 or JNK1.
Mice
TRAMP, expressing SV40 large T antigen into the prostatic epithelium (B6), β1loxP/loxP (B6;129) and PB-Cre4 (B6.D2) mice were generated and characterized as described (Greenberg et al., 1995; Raghavan et al., 2000; Wu et al., 2001). β1loxP/loxP/TRAMP/PB-Cre4 (TRAMP mice carrying conditional ablation of β1) and β1loxP/loxP/TRAMP (TRAMP mice expressing wt β1) were generated as described in the Supplementary Methods and designated as: β1pc−/−/TRAMP and β1wt/TRAMP, respectively. All mice were maintained under specific pathogen-free conditions. Care and handling of animals was in compliance with standards established by Animal Use and Care Committee of the NCI and experimental protocols were approved by the IACUC. Histological analysis of metastases to lungs, lymph nodes and liver was performed by IL and DG.
Laser Capture Microdissection (LCM)
To confirm downregulation of β1 in prostatic intraepithelial neoplasia (PIN) lesions of β1pc−/−/TRAMP mice, dorsolateral prostate lobes were obtained from 10% formalin-fixed paraffin-embedded sections. Hematoxylin and Eosin (H&E) stained sections were selected from representative prostate glands to perform LCM using a Pixcell 11 instrument (Arcturus). Genomic DNA was extracted from the collected tissue samples and used for PCR to confirm the successful conditional removal of exon 3 in prostate as described above.
Immunohistochemistry (IHC)
For histopathological analysis, different prostate lobes from β1wt/TRAMP and β1pc−/−/TRAMP mice were isolated, fixed in buffer-neutral formalin and embedded in paraffin. Sections were stained with H&E or with an Ab to vWF, expression of which is a measure of angiogenesis to study the vascularization of prostate glands in β1wt/TRAMP and β1pc−/−/TRAMP mice. Vessels were counted and their density was measured in a blinded manner in 20 different microscopic fields by two investigators (LRL and HLG).
Transient transfection
Transfection of cells with siRNA oligonucleotides (Thermo Scientific) was performed as previously described (Goel et al., 2005) using oligofectamine (Invitrogen). To downregulate IGF-IR, the sequences of sense strands of duplex siRNAs used are as follows: IGF-IR-siRNA: 5'-CGACUAUCAGCAGCUGAAGUUdTdT-3'; inverted control IGF-IR-siRNA: 5'-GAAGUCGACGACUAUCAGCUUdTdT-3'.
Immunoblotting (IB)
Cell lysates were separated on reducing SDS-PAGE and immunoblotted using specific Abs as detailed in each figure legend.
Clonogenic assay
PC3 cells stably expressing scrambled or IGF-IR-shRNA were treated with various doses of ionizing radiation. Two h later, cells were trypsinized and plated at 5,000 cells / well in 6-well plates in duplicate sets. Colonies were allowed to grow for 10 days and fixed and stained with crystal violet solution before being counted by naked eye. Activation of histone H2AX was measured in parallel lysates by IB to confirm the radiation response.
Statistical analysis
Student’s t-test was used to compare the averaged tumor volumes between different study groups. Kaplan-Meier survival curve with Log-rank test was used to compare survival of β1pc−/−/TRAMP versus β1wt/TRAMP in one experiment or β1pc−/−/TRAMP, irradiated β1wt/TRAMP, irradiated β1pc−/−/TRAMP versus β1wt/TRAMP mice in another experiment. Fisher’s exact test was used to compare the incidences of cancer in 20-week old mice, and of metastasis in 24–26 week old β1wt/TRAMP and β1pc−/−/TRAMP mice. Two-tailed P-values were reported according to the original study hypotheses. Student’s t-test was also used to compare the average number of colonies and one-tailed P-values were used to calculate the significance. P-value < 0.05 was considered as significant.
Results
Conditional ablation of β1 integrins improves survival and delays PrCa progression in TRAMP mice
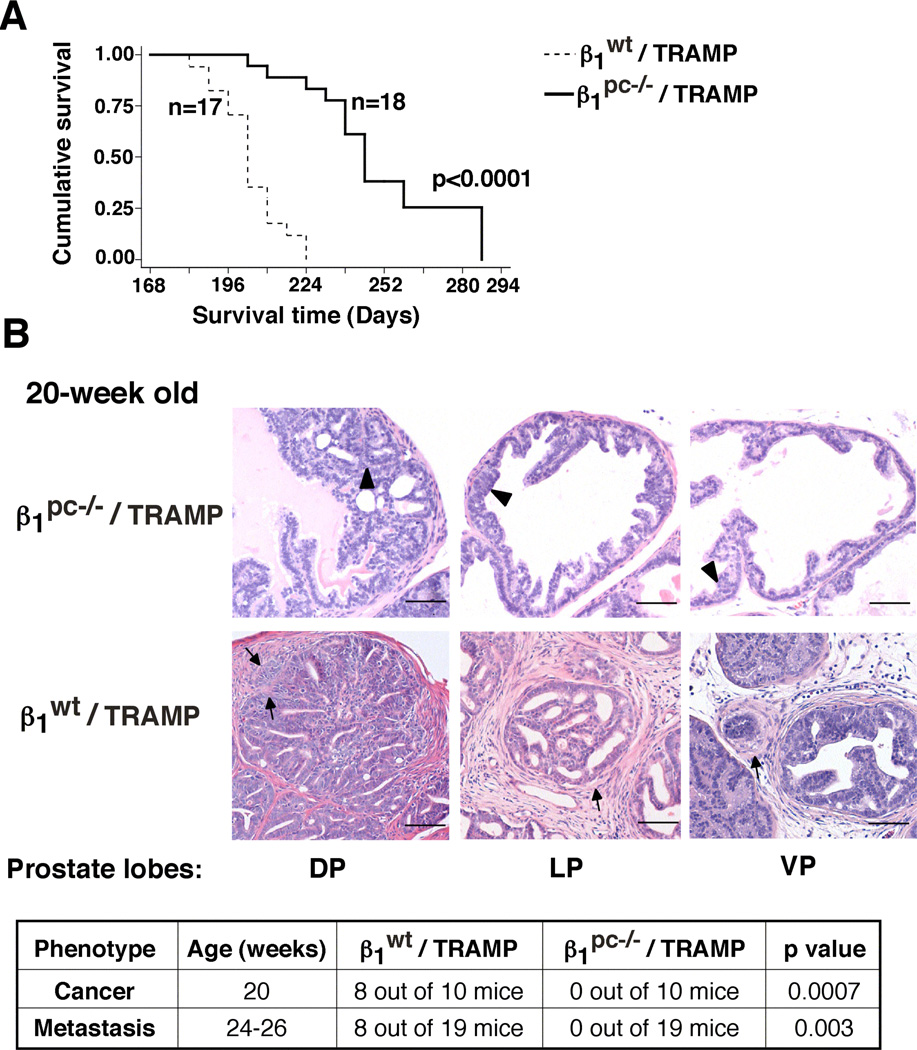
To analyze the role of β1 integrins in inhibition of apoptosis induced in response to irradiation, we generated a mouse model of PrCa (β1pc−/−/TRAMP mice), wherein β1 integrins were conditionally downregulated in the prostatic epithelium (Supplementary Fig. S1), as confirmed by LCM (Supplementary Fig. S2). Details concerning generation of β1pc−/−/TRAMP (as well as β1wt/TRAMP) mice are described in the supplementary materials section. β1pc−/− mice are fertile and able to generate progeny effectively, indicating that the loss of β1 does not cause any defect in the development of the prostate gland (Supplementary Fig. S3). Possible explanations are that either other members of the integrin family can compensate for β1, or β1 may not be required for prostate functions during development. β1 downregulation significantly delays mortality (Fig. 1A) and prolongs the onset of cancer and metastasis. In fact, neither tumors nor metastasis are observed in β1pc−/−/TRAMP, as compared to age-matched β1wt/TRAMP mice (Fig. 1B). Ablation of β1 does not affect emergence of PIN lesions or rates of vascularization (data not shown). These results show that β1 integrins regulate PrCa progression.
Fig. 1. Conditional ablation of β1 integrins improves survival and delays PrCa progression in TRAMP mice.
A: Kaplan-Meier survival analysis. A statistically significant increase in lifespan was found in the β1pc−/−/TRAMP (n=18) cohort as compared to the β1wt/TRAMP (n=17) cohort (P=0.0001). B: Histopathological analysis of different prostate lobes from β1wt/TRAMP and β1pc−/−/TRAMP mice stained with H&E (upper panels). Arrows, adenocarcinoma; arrowheads, PIN lesions. Prostate lobes: DP: dorsal; LP: lateral; VP: ventral. The table in the bottom panel shows the incidence (number of mice) of cancer (adenocarcinoma and neuroendocrine) in 20-week old and of metastasis in 24–26-week old β1wt/TRAMP and β1pc−/−/TRAMP mice. β1pc−/−/TRAMP mice show a statistically significant decrease in incidence of cancer (P=0.0007) and of metastasis (P=0.003) as compared to age-matched β1wt/TRAMP mice. Statistical analysis was performed using Fisher’s exact test. Scale bar 50 µm.
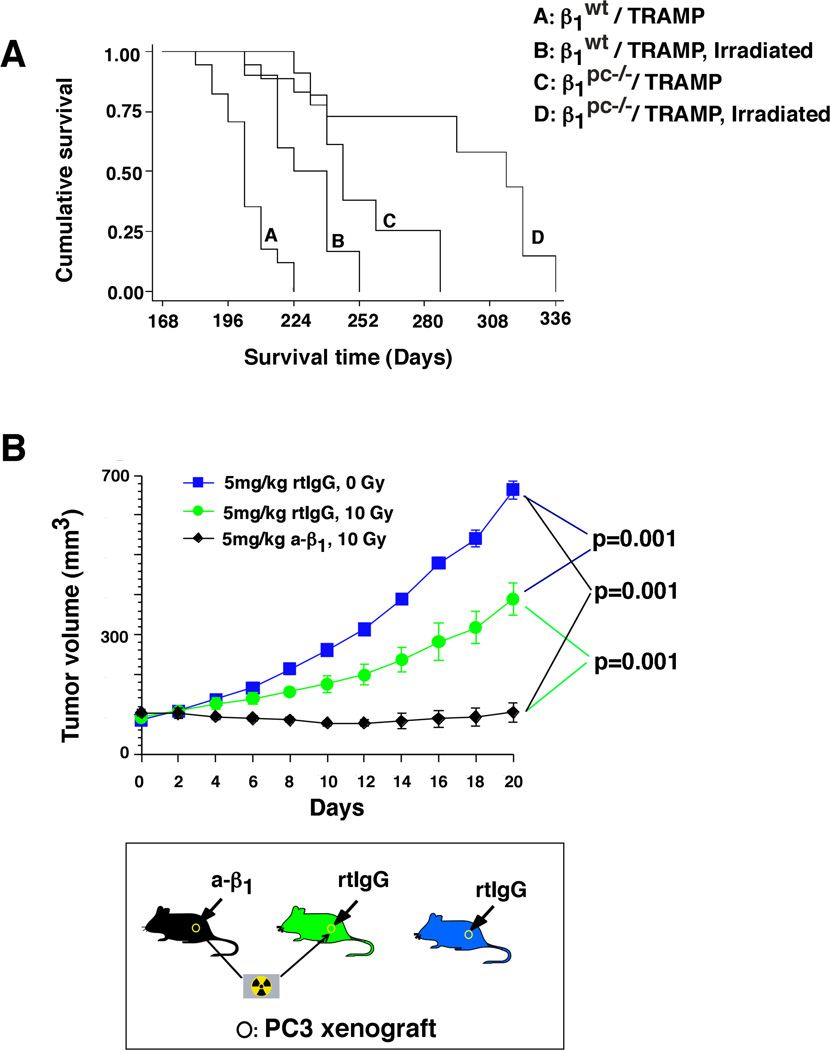
We then analyzed in vivo the effect of fractionated doses of radiation on β1pc−/−/TRAMP mice. Survival analysis demonstrate that irradiated β1pc−/−/TRAMP mice significantly live longer compared to either irradiated β1wt/TRAMP or non-irradiated β1pc−/−/TRAMP mice (Fig. 2A). In support of these studies, we also inhibited β1 functions using AIIB2, a neutralizing mAb to β1, that has been previously shown to partially inhibit subcutaneous PC3 tumor growth (Goel et al., 2009). Similarly, irradiated PC3 tumors show partial decrease in tumor volume as compared to non-irradiated tumors. However, AIIB2 completely blocks prostate tumor growth upon irradiation (Fig. 2B). All these results confirm that β1 integrins promote resistance to radiation in vivo.
Fig. 2. β1 integrins promote resistance to fractionated doses of radiation in vivo.
A: Kaplan-Meier survival analysis. β1pc−/−/TRAMP cohort (n=11) shows a statistically significant increase in lifespan as compared to the β1wt/TRAMP cohort (n=10, P=0.0035) upon irradiation. B: PC3 cell xenograft-bearing nude mice were analyzed. Once the tumors reached 100 mm3 (day 0), AIIB2 Ab or non-specific rtIgG was injected intraperitoneally (5 mg/Kg) on day 0 and day 14. Twenty-four h after first injection, tumors were irradiated (0 or 10 Gy) and tumor volume was measured up to 20 days. Data are the mean ± SEM of 6 animals per group. The differences in tumor volume during the time frame of 10 to 20 days after injection are statistically significant at each time point as indicated in the figure (P<0.001). The graph shows kinetics of tumor growth (upper panel). The lower panel shows a schematic representation of the experiment. Statistical analysis was conducted using the Student's t-test.
β1 downregulation induces caspase-3-dependent apoptosis upon irradiation
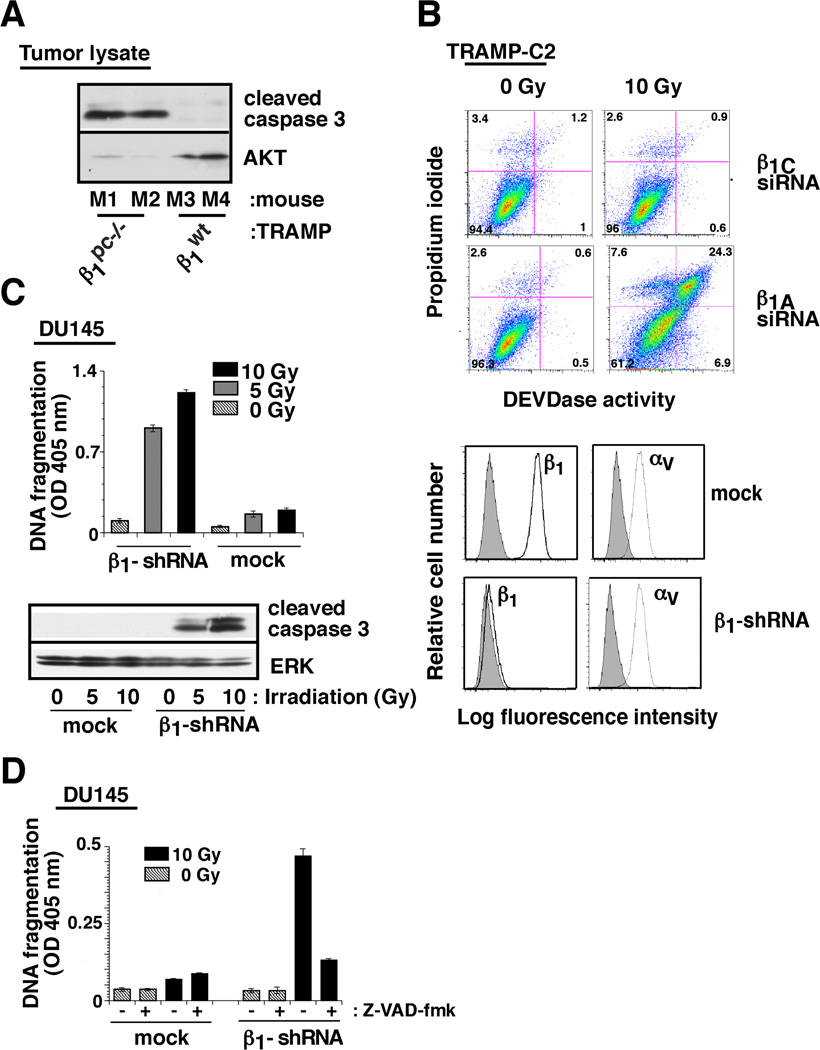
Since survival of mice or prostate tumor growth is significantly affected by β1 integrins upon irradiation, we next investigated the mechanism underlying β1 induced resistance to radiation of prostate cancer. The analysis was performed in vivo using either β1wt/TRAMP and β1pc−/−/TRAMP mice or also in vitro upon β1 downregulation in PrCa cells. In vivo, prostatic areas of β1wt/TRAMP and β1pc−/−/TRAMP mice were irradiated (up to 50 Gy) with fractionated doses of ionizing radiation. We demonstrate significant induction of apoptosis (measured by caspase-3 cleavage) only in β1pc−/−/TRAMP mice (Fig. 3A). In vitro, using mouse TRAMP-C2 cells, we show that abrogation of β1 integrin expression significantly induces apoptosis (evaluated by caspase-3 activity) upon irradiation (Fig. 3B). Similar results were obtained using DU145/β1-shRNA, human PrCa cells in which the expression of β1 integrins was significantly decreased as compared to vector-transfected cells (mock). To ensure specificity of apoptotic response by abrogation of β1, we demonstrate that β1 downregulation does not change the expression of αv (Fig. 3C, right panel), another integrin, which has been shown to promote prostate tumor growth (Bisanz et al., 2005). We show that irradiation induces apoptosis in DU145/β1-shRNA, but not in DU145/mock cells as measured by DNA fragmentation and caspase-3 cleavage (Fig. 3C, left panel). Moreover, blocking caspase cleavage using Z-VAD-fmk, an inhibitor of caspase cleavage, rescues β1-shRNA expressing cells from apoptosis induced by irradiation (Fig. 3D). The observation that β1 downregulation induces apoptosis upon irradiation was validated by using three different siRNAs to β1 (Supplementary Fig. S4A). All the observed effects were obtained using cells in two-dimensional (2-D) cultures are not due to changes in cell morphology or proliferation (data not shown) and the results appear to be reproducible in three-dimensional (3-D) cultures (Supplementary Fig. S4B). Taken together, these results demonstrate in vivo and in vitro that β1 integrins protect cancer cells from irradiation-induced apoptosis.
Fig. 3. β1 downregulation induces caspase-3-dependent apoptosis upon irradiation.
A: β1pc−/−/TRAMP or β1wt/TRAMP mice were irradiated (20 Gy). 24 h after irradiation, prostate tumors were isolated from 4 mice (M1–M4), lysed and immunoblotted using Abs to cleaved caspase-3 or to AKT. B: TRAMP-C2 cells transfected with either β1A or β1C-siRNA, were serum-starved for 24 h. Cells were irradiated (10 Gy) or non-irradiated (0 Gy); 24 h after irradiation, cells were detached and caspase-3 (DEVDase) activity was measured. C: DU145/β1-shRNA and DU145/mock transfectants were irradiated (5 or 10 Gy) or non-irradiated, detached and either analysed for DNA fragmentation (upper left panel) or lysed and immunoblotted with an Ab to cleaved caspase-3 or to ERK1/2 (lower left panels). DU145/β1-shRNA and DU145/mock transfectants were analyzed by FACS (right panel) using Ab to β1 (TS2/16, solid black line), αv (L230, dotted line), and hemagglutinin (12CA5, filled grey) as a negative control. D: Cells were irradiated (10 Gy) or non-irradiated in the presence or absence of Z-VAD-fmk, detached and DNA fragmentation was measured.
β1 integrins mediate resistance to radiation by preventing JNK1 activation
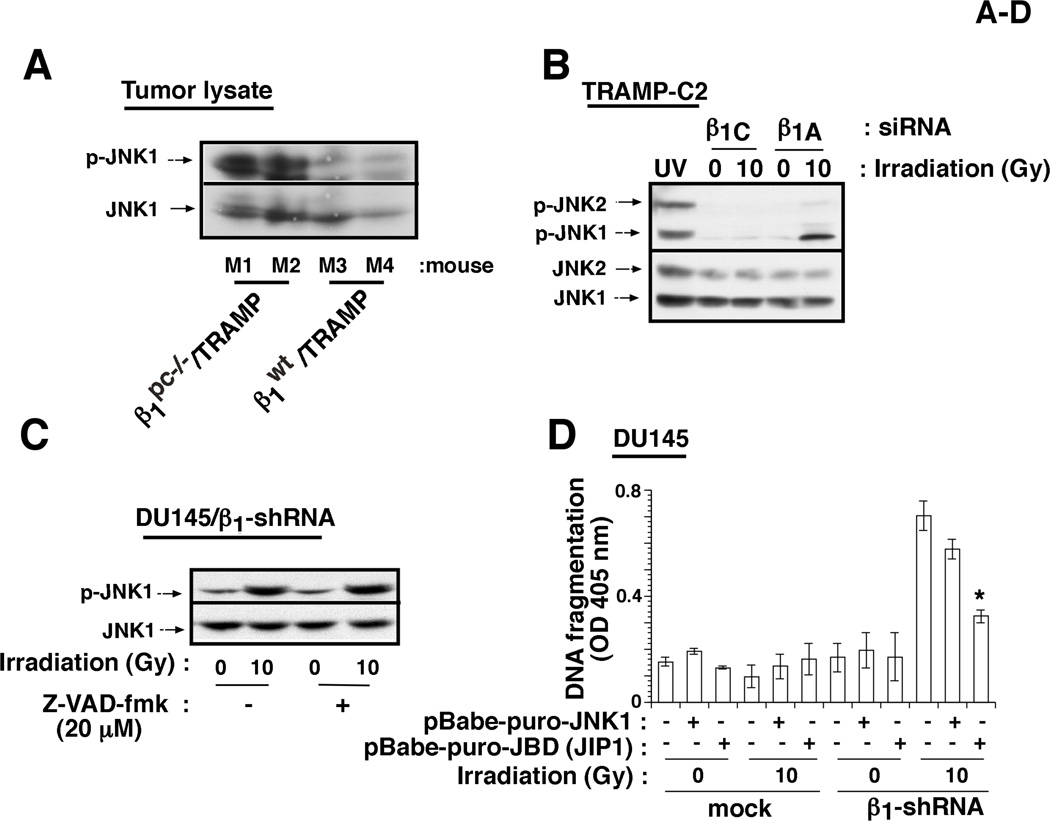
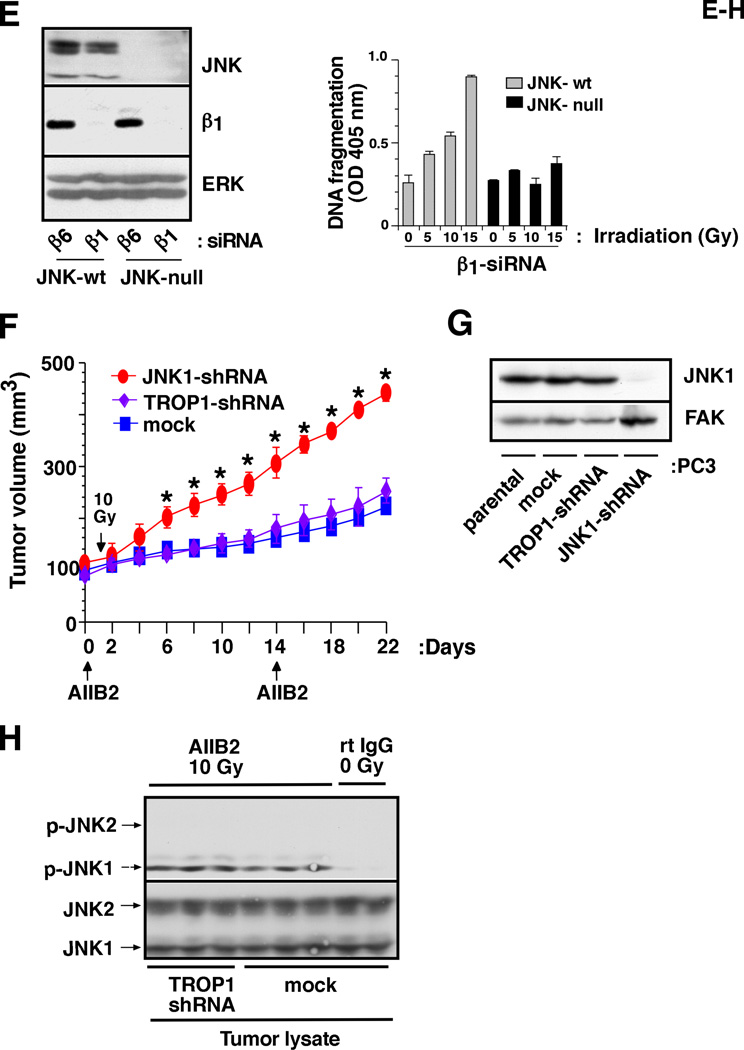
β1 integrins have been shown to regulate several signaling pathways (Hynes, 2002). We analyzed the activation of src kinase, AKT, FAK, sonic hedgehog/GLI and mitogen-activated protein kinase (MAPK) pathways. Activation of src (Supplementary Fig. S5A) and ERK (data not shown) is not affected by either β1 downregulation, irradiation or by β1 downregulation in irradiated cells. Irradiation does not elicit any change in AKT activation upon β1 downregulation (Supplementary Fig. S5B). Activation levels of FAK and GLI1 (Goel et al., 2010) are reduced upon β1 downregulation, but irradiation does not cause any further change (data not shown). However, we observe that β1 downregulation increases activation of MKK4, an upstream regulator of JNK, and JNK1, but not JNK2, upon irradiation (Fig. 4A–C, Supplementary Figs. S5A and S5C) in comparison to either of the two single treatments. JNK1, a known stress-activated protein kinase (Weston and Davis, 2007), is activated upon β1 downregulation in irradiated β1pc−/−/TRAMP mice, as well as in TRAMP-C2, DU145 and PC3 cells (Fig. 4A–C and Supplementary Fig. S5). Moreover, JNK1 activation appears to be the cause rather than the result of undergoing cell death, since JNK1 is activated even in the presence of the caspase inhibitor, Z-VAD-fmk (Fig. 4C). We also studied activation of JNK1 in tumor lysates from mice injected with either rtIgG or AIIB2 Ab in the presence or absence of irradiation. JNK1, but not ERK or p38, activation is observed upon irradiation in the presence of AIIB2 in vivo (Supplementary Fig. S6). These results show that β1 inhibition activates JNK1 in response to irradiation in vitro and in vivo.
Fig. 4. β1 integrins mediate resistance to radiation by preventing JNK1 activation.
A: β1pc−/−/TRAMP or β1wt/TRAMP mice (M1-M4) were irradiated (40 Gy). Prostate tumors were isolated 24 h after irradiation, lysed and immunoblotted using Abs to p-JNK or to JNK. B: TRAMP-C2 cells transiently transfected with either β1A or β1C-siRNA, were serum-starved for 24 h. Cells were irradiated (10 Gy) or non-irradiated. After 24 h, cells were lysed and immunoblotted using Abs to p-JNK or JNK. Cells irradiated with UV were used as a positive control for JNK activation. C: DU145/β1-shRNA cells were irradiated (10 Gy) or non-irradiated in the presence or absence of Z-VAD-fmk, lysed and immunoblotted with an Ab to p-JNK, or JNK. D: DU145/β1-shRNA or DU145/mock cells expressing either JBD-JIP1, a JNK inhibitor or wt-JNK were irradiated (10 Gy) or non-irradiated, detached and DNA fragmentation was measured. E: JNK-null and JNK-wt mouse fibroblasts, transiently transfected with β1 or β6-siRNA, were lysed and immunoblotted using Abs to JNK, β1 or ERK1/2 (left panels). JNK-null and JNK-wt cells transiently transfected with β1-siRNA, were irradiated (5, 10 or 15 Gy) or non-irradiated, detached and DNA fragmentation was measured (right panel). F: Nude mice bearing PC3 transfectant (JNK1-shRNA, TROP1-shRNA or mock-shRNA) xenografts (100 mm3) were analyzed. Once the tumors reached 100 mm3 (day 0), AIIB2 Ab or non-specific rtIgG was injected intraperitoneally (5 mg/Kg) on day 0 and day 14; 24 h after first AIIB2 injection, tumors were irradiated (10 Gy) and tumor growth was measured up to 22 days. Data are expressed as tumor volume. Data are the mean ± SEM of 6 animals per group. The differences in tumor volume are statistically significant between the PC3/JNK1-shRNA xenograft and either the PC3/mock (P=0.00005 on day 22) or PC3/TROP1-shRNA (P=0.00007 on day 22) xenograft. The graph shows kinetics of tumor growth. G: Cell lysates from PC3 (parental, mock, TROP1-shRNA and JNK1-shRNA) were immunoblotted using Abs to JNK1 or to FAK, as a loading control. H: PC3 tumor lysates (mock or TROP1-shRNA xenografts) from the experiment described in Figure 4F were immunoblotted using Abs to p-JNK or to JNK.
To further confirm whether JNK1 activation induced by concurrent β1 downregulation and irradiation, is a consequence or a mediator of apoptosis, we analyzed apoptosis in cells infected with a retrovirus expressing the JBD-JIP1 which is known to act as dominant negative and suppress JNK signaling (Dickens et al., 1997). JBD-JIP1 induction is proven to be effective, since its expression inhibits UV-induced c-Jun phosphorylation in DU145 cells (data not shown). In addition, JBD-JIP1 expression significantly inhibits apoptosis induced by irradiation in DU145/β1-shRNA cells (Fig. 4D). Similar to JBD-JIP1, inhibition of apoptosis is observed upon treatment with SP600125, a selective and reversible JNK inhibitor (data not shown). We further confirm the role of JNK signaling in β1-regulated apoptosis by transfecting β1-shRNA into JNK-null mouse fibroblasts, using β6-shRNA as control (Fig. 4E, left panel). The results show that irradiation significantly induces apoptosis only in the presence of JNK (Fig. 4E, right panel). Our results show that JNK1 is required for apoptosis induced by β1 inhibition upon irradiation. To validate the role of JNK1 in tumor growth inhibition after treatment with AIIB2 and irradiation, we downregulated JNK1 in PC3 cells using JNK1-shRNA. We show that JNK1-mediated apoptosis is responsible for the observed effect on tumor growth upon β1 inhibition and irradiation (Fig. 4F); we confirm downregulation of JNK1 in tumors and in PC3/JNK1-shRNA cells using IB (Supplementary Fig. S7 and Fig. 4G). In these experiments, we demonstrate that downregulation of JNK1 does not affect PC3 tumor growth (data not shown), but significantly suppresses the inhibitory effect of AIIB2 on irradiated tumors (Fig. 4F). Control experiments demonstrate that JNK1 is activated in irradiated tumors from mice treated with AIIB2 Ab (Fig. 4H).
IGF-IR regulates β1 integrin expression in PrCa cells
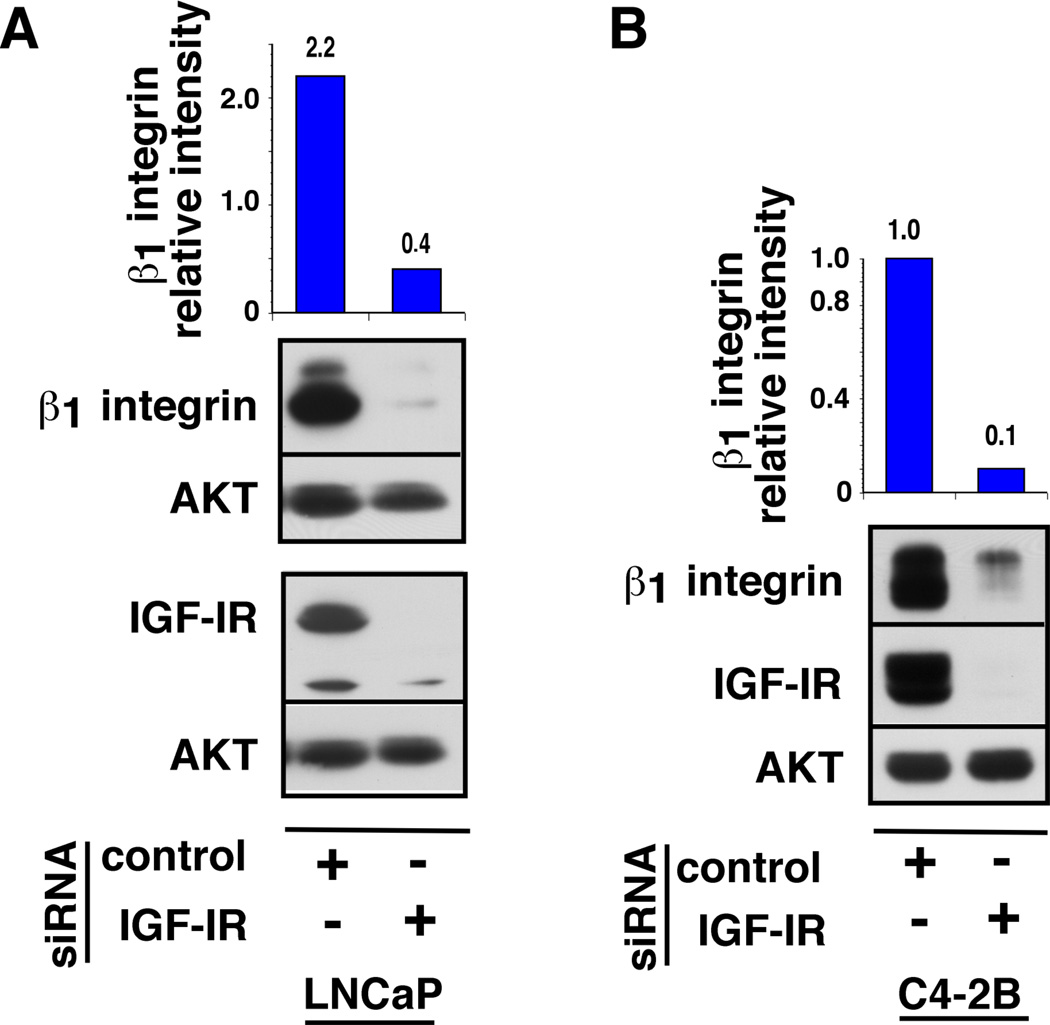
To determine whether the IGF-IR may play a role in regulating the expression of the β1 integrin subunit, LNCaP and C4-2B cells were depleted of endogenous IGF-IR by siRNA approach and stimulated with R1881. As previously demonstrated (Sayeed et al., 2012), androgen treatment significantly upregulates IGF-IR and β1 integrin subunit expression in both LNCaP and C4-2B cells. Notably, a significant decrease in β1 integrin subunit expression is observed upon IGF-IR depletion compared to control-siRNA transfected cells (Fig. 5A–B).
Fig. 5. IGF-IR regulates the expression of β1 integrins in PrCa cells.
LNCaP (A) and C4-2B (B) cells were transfected with IGF-IR-siRNA or control inverted IGF-IR-siRNA. The cells were grown in medium containing 2% charcoal stripped serum (CSS) for 24 h and treated with 1 nM R1881 for additional 24 h. Cell lysates were analyzed for expression of β1 integrin subunit and IGF-IR by IB. AKT was used as a loading control. The band intensities of β1 integrin subunit and AKT were quantified by ImageJ analysis and normalized expression of β1 integrin subunit is represented in the top panels. Relative intensity is expressed in arbitrary units.
IGF-IR confers resistance to radiation in PrCa cells
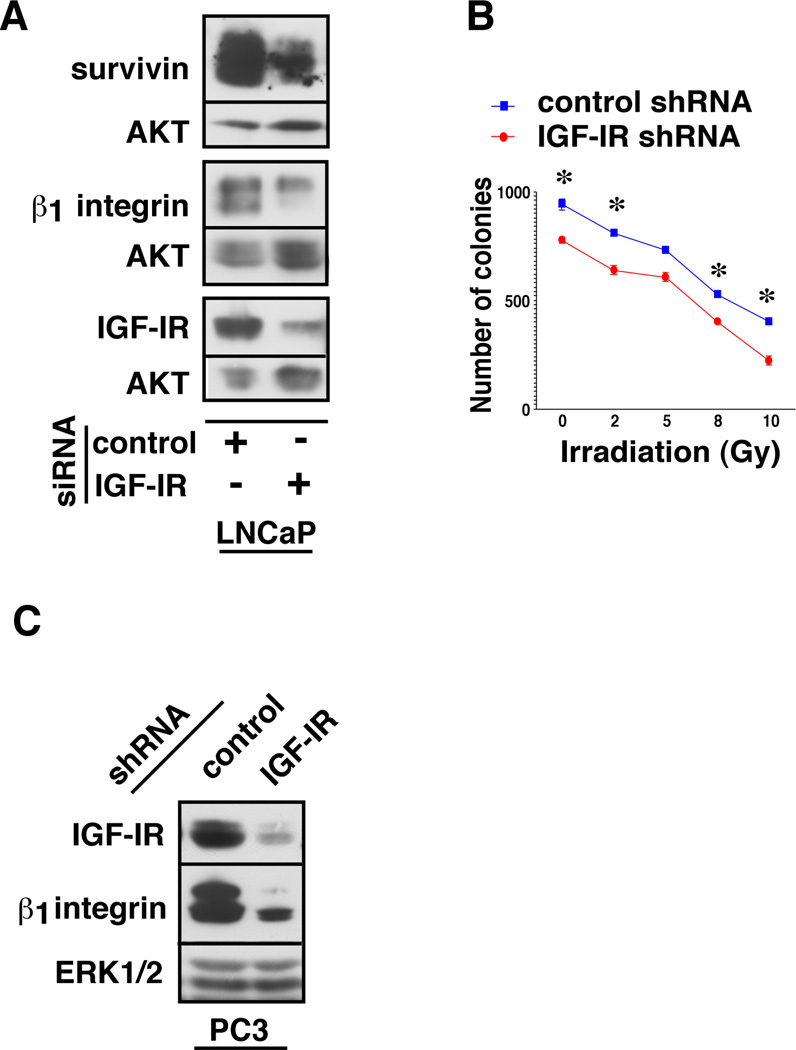
In order to study the effect of IGF-IR ablation on cell survival, LNCaP cells were transiently transfected with either a control-siRNA or an IGF-IR-siRNA. Cell lysates were analyzed for survivin expression in addition to the levels of IGF-IR and the β1 integrin subunit. A substantial reduction in survivin levels is detected upon the loss of IGF-IR and β1 integrin subunit (Fig. 6A), suggesting a direct regulatory role of these molecules on cancer cell survival and proliferation. To further characterize their role in presence of radiation, endogenous IGF-IR was downregulated in androgen receptor (AR)-negative, PC3 cell stable-transfectants expressing IGF-IR-shRNA and treated with various doses of ionizing radiation followed by clonogenic assay. Irradiated cells were plated at 5,000 cells / dish, cultured for 10 days and colonies were counted using crystal violet staining. Irradiated cells show a significant reduction in their clonogenic ability upon IGF-IR knockdown (Fig. 6B). As assessed by IB analysis in PC3 cells, depletion of endogenous IGF-IR is again associated with a significant decrease of β1 integrins (Fig. 6C), suggesting an AR-independent regulation of β1 integrins by IGF-IR. To confirm the efficacy of various doses of radiation in the clonogenic assay, PC3 cells were treated with increasing doses of radiation and analyzed for the expression of total histone variant H2AX and phosphorylated form of H2AX (γH2AX) (not shown).
Fig. 6. The IGF-IR/β1 integrin signaling pathway promotes survival of PrCa cells.
A: LNCaP cells were transiently transfected with control or IGF-IR-siRNA and allowed to grow in medium containing 2% CSS for 48 h followed by analysis of survivin, IGF-IR and β1 integrin subunit expression by IB. AKT was used as a loading control. B: PC3 cells stably transfected with control or IGF-IR-shRNA, treated with 0, 2, 5, 8 or 10 Gy ionizing radiation and plated at 5,000 cells/well in a 6-well plate for clonogenic assays. After 10 days, colonies were fixed and stained with crystal violet solution and colonies counted. Each experiment was performed in duplicate and error bars represent standard deviations, *p<0.01 (one-tailed t-test). C: PC3 cells stably transfected with either control-shRNA or IGF-IR-shRNA were analyzed for the expression of IGF-IR and β1 integrin subunit by IB. ERK1/2 was used as a loading control.
Discussion
Here we report for the first time that conditional ablation of β1 integrins improves survival and delays PrCa progression in TRAMP mice. We identify JNK1 as a mediator of radiation-induced apoptosis and demonstrate that β1 integrins elicit resistance to radiation in PrCa by effectively suppressing JNK1 activation in vivo. We report for the first time that the expression of β1 integrins is tightly regulated by IGF-IR suggesting a vital signaling crosstalk between these receptors to induce radiation resistance in PrCa.
We demonstrate that β1 integrin expression is crucial for PrCa progression in the TRAMP model system and that β1 integrins promote resistance to radiation in vivo. While this manuscript was in preparation, another study reported progression of PrCa upon conditional deletion of β1 integrins in TRAMP mice (Moran-Jones et al., 2012) suggesting, in contrast, a role of β1 integrins in tumor cell differentiation. However, the observation that β1 integrins play a prominent role in the progression of cancer is reinforced by significant literature in this field. We have previously reported that β1 integrins are expressed in normal mouse prostate with protein levels increasing during the progression from PIN into well-differentiated carcinoma (Goel et al., 2005). An inhibitory Ab to β1 integrins significantly affects in vitro and in vivo growth of human breast cancer cells (Park et al., 2006); a neutralizing mAb against integrin α5β1 inhibits angiogenesis and impedes tumor growth (Bhaskar et al., 2007). Furthermore, β1 integrin expression is known to correlate with actin filament-associated protein AFAP-110 which plays a pivotal role in regulating focal adhesions and cell migration in PrCa (Zhang et al., 2007). Overall, these studies clearly highlight the tumor-promoting role of β1 integrins and are consistent with our observations that conditional deletion of β1 integrins in TRAMP mice significantly improves survival and delays progression of PrCa.
We describe in vivo a mechanism of resistance to radiation mediated by integrins via inhibition of JNK1. There are two pathways potentially able to inhibit JNK in an integrin-dependent manner: 1) β1 integrins may inhibit, by direct interaction, RACK1 (receptor for activated C kinase 1) which has been shown to play an important role in the activation of JNK in response to UV irradiation, tumor promoting agent, 12-O-tetradecanoylphorbol-13-acetate (TPA) or TNF-α (Lopez-Bergami et al., 2005). However, our data show that the association between RACK1 and JNK1 is not affected upon β1 downregulation in irradiated cells (Supplementary Fig. S8); thus this pathway is unlikely to mediate the observed integrin-dependent inhibition of JNK1. 2) β1 integrins stimulate the activity of PI3-kinase (PI3-K), consequently activating AKT, Cas and paxillin, which in turn prevent activation of JNK (Levresse et al., 2000; Seidler et al., 2005). However, irradiation does not influence AKT activity in our system. Thus, AKT signaling may not play a crucial role in β1-mediated radiation resistance in PrCa although it has been reported that both neutralizing Ab to β1, AIIB2, and ATN-161 (an inhibitor of α5β1 and αvβ3) increase apoptosis after irradiation by regulating AKT in breast cancer cells (Nam et al., 2010; Park et al., 2008). The observed differences are likely to be a reflection of inherent aberrations in two solid tumor types. Finally, recent reports have indicated that phosphatase and tensin homolog (PTEN) loss, which causes AKT activation, may affect PrCa progression. In this pathological condition, JNK1/2 signaling was recently reported to reduce the development of invasive adenocarcinoma in a PTEN conditional deletion model of PrCa (Hubner et al., 2012). In contrast, Vivanco et al (Vivanco et al., 2007) show that PTEN loss is associated with higher activity of JNK. Since PTEN loss has been recently shown to be prognostic for biochemical relapse following radiotherapy in PrCa (Zafarana et al., 2012), and to promote a complex pattern of sensitivity to DNA damaging agents (Fraser et al., 2012) further investigations are recommended to define the precise mechanism through which radiation and β1 integrins orchestrate JNK1 activation in the absence of PTEN.
There are conflicting reports in the literature regarding the role of JNK in cancer (Das et al., 2011). In some reports it has been suggested to promote cancer cell proliferation, while in others, it is known to be crucial for induction of apoptosis (Whitmarsh and Davis, 2007). In normal prostate, high levels of MKK4, which is upstream of JNK, have been observed in the epithelial compartment, whereas in neoplastic prostate tissues, the levels of MKK4 are reduced. Besides, an inverse correlation between MKK4 expression and metastatic potential has been reported (Kim et al., 2001). Another study reports somatic mutations in MKK4 and shows a dominant negative effect of these mutations on anchorage-independent growth (Kan et al., 2010). We demonstrate that JNK1 activation is tightly regulated by β1 integrins and associated with apoptosis in response to ionizing radiation. JNK is already known to be activated during apoptosis, designated anoikis, upon loss of integrin-mediated contacts with the ECM; however, JNK activation during this process requires caspase activity (Cardone et al., 1997). In contrast, our data show that JNK1 activation is a caspase-independent event, suggesting that the mechanism behind β1-mediated resistance to radiation is different from anoikis.
β1-mediated signaling through FAK/cortactin/JNK1 pathway was recently reported to be the mechanism of resistance to radiation in head and neck cancer cells (Eke et al., 2012). In this report, it was demonstrated that inhibition of β1 integrins results in dissociation of a FAK/cortactin protein complex, which in turn downregulates JNK signaling leading to radiosensitization, thus implying a positive regulation of JNK by β1 integrins. However, in our system, we demonstrate that radiation selectively induces JNK1 activation upon β1 integrin targeting; we also show that inhibition of JNK1 in a xenograft model system induces resistance to radiation and that β1 integrins suppress JNK1 activation.
Our findings also demonstrate that β1 integrins are downstream targets of IGF-IR. Prostate epithelial-specific deletion of IGF-IR has been reported to enhance the emergence of aggressive PrCa under the conditions of compromised p53 activity suggesting that IGF-IR inhibits differentiation in this system (Sutherland et al., 2008). Our previous studies had demonstrated an androgen-dependent regulation of both β1 integrins and IGF-IR in PrCa (Sayeed et al., 2012); this may explain the successful combination of androgen-deprivation therapy and low-dose-rate brachytherapy strategy, recently reported to decrease biochemical failure and PrCa death (Shilkrut et al., 2012). In addition, clinical trials have demonstrated better outcomes when neoadjuvant hormonal therapy was combined with radiation therapy compared with radiation therapy alone (Lawton et al., 2007). Androgen- and IGF-IR - dependent regulation of β1 integrins in PrCa implies that AR, β1 and IGF-IR may act in concert to play a crucial role in the progression towards aggressive phases of the disease and provides an additional rational basis for the development of combined strategies directed against β1 and IGF-IR together with androgen-deprivation and radiation.
Our study shows that β1 downregulation or inhibition can play an important role as a therapeutic strategy to increase PrCa sensitivity to radiotherapy. Because mice lacking β1 die as embryos (Fassler and Meyer, 1995), there is considerable concern about the potential toxicity of β1 inhibitors as systemic agents in cancer patients. However, toxicity studies of several relevant anti-integrin therapies using blocking Abs (volociximab specific for α5β1, CNTO 95 specific for αv) have established the safety of these agents when given systemically (Chu et al., 2011; Park et al., 2006; Ricart et al., 2008). Recently, either conjugation or co-administration of anti-cancer drugs with a RGD based peptide was reported to significantly increase the efficacy of these drugs (Sugahara et al., 2009; Sugahara et al., 2010). Our data using etoposide show similar levels of apoptosis in the presence or absence of β1 integrins (Supplementary Fig. S9) suggesting that the effects observed in this study should not be extrapolated to other DNA damaging agents. In conclusion, this study highlights the importance of IGF-IR and β1 integrins in resistance to radiation through JNK1 inhibition, and describes a strategy uniquely poised to increase cancer sensitivity to radiotherapy.
Supplementary Material
Acknowledgments
We thank Drs R. Baserga and N. Greenberg for helpful suggestions. We are grateful to Mr. Varun Saluja and Mr. Thomas K. Sawyer for technical assistance. We would like to thank Thomas Lozeau, Diane Safer and other radiotherapists, Department of Radiation Oncology, University of Massachusetts Medical School for technical assistance. We would like to thank members of the Languino laboratory: Tiziana DeAngelis, Anindita Dutta, Carmine Fedele, Kirat Ganguly and Marco Trerotola, for suggestions.
Contract grant sponsor: NIH;
Contract grant number: R01 CA-89720 and CA-109874 (to LRL), P01 CA-140043 (to LRL and DCA), R01 CA-65861 (to RJD), R01 CA-164462 (to AM); Diabetes Endocrinology Research Center DK32520, Core resources supported by this grant were also used.
Contract grant sponsor: ACS;
Contract grant number: ACS Institutional Research Grant, IRG-93-033 (to HLG).
Contract grant sponsor: Pennsylvania Department of Health. This project is also funded, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
No potential conflicts of interest were declared by authors.
References
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Allen GW, Saba C, Armstrong EA, Huang SM, Benavente S, Ludwig DL, Hicklin DJ, Harari PM. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67:1155–1162. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Bhaskar V, Zhang D, Fox M, Seto P, Wong MH, Wales PE, Powers D, Chao DT, Dubridge RB, Ramakrishnan V. A function blocking anti-mouse integrin alpha5beta1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J Transl Med. 2007;5:61–71. doi: 10.1186/1479-5876-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz K, Yu J, Edlund M, Spohn B, Hung MC, Chung LW, Hsieh CL. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Mol Ther. 2005;12:634–643. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. β1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 2005;24:403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Chu FM, Picus J, Fracasso PM, Dreicer R, Lang Z, Foster B. A phase 1, multicenter, open-label study of the safety of two dose levels of a human monoclonal antibody to human α(v) integrins, intetumumab, in combination with docetaxel and prednisone in patients with castrate-resistant metastatic prostate cancer. Invest New Drugs. 2011;29:674–679. doi: 10.1007/s10637-010-9388-4. [DOI] [PubMed] [Google Scholar]
- Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, Shevchenko A, Sandfort V, Cordes N. β1 Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest. 2012;122:1529–1540. doi: 10.1172/JCI61350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fitzgerald TJ, Wang T, Goel HL, Huang J, Stein G, Lian J, Davis RJ, Doxsey S, Balaji KC, Aronowitz J, Languino LR. Prostate carcinoma and radiation therapy: therapeutic treatment resistance and strategies for targeted therapeutic intervention. Expert Rev Anticancer Ther. 2008;8:967–974. doi: 10.1586/14737140.8.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor α-induced apoptosis via the AKT/Survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- Fraser M, Zhao H, Luoto KR, Lundin C, Coackley C, Chan N, Joshua AM, Bismar TA, Evans A, Helleday T, Bristow RG. PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res. 2012;18:1015–1027. doi: 10.1158/1078-0432.CCR-11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. β1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Moro L, Murphy-Ullrich JE, Hsieh CC, Wu CL, Jiang Z, Languino LR. β1 integrin cytoplasmic variants differentially regulate expression of the antiangiogenic extracellular matrix protein thrombospondin 1. Cancer Res. 2009;69:5374–5382. doi: 10.1158/0008-5472.CAN-09-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Underwood JM, Nickerson JA, Hsieh CC, Languino LR. β1 integrins mediate cell proliferation in three-dimensional cultures by regulating expression of the sonic hedgehog effector protein, GLI1. J Cell Physiol. 2010;224:210–217. doi: 10.1002/jcp.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Mulholland DJ, Standen CL, Karasarides M, Cavanagh-Kyros J, Barrett T, Chi H, Greiner DL, Tournier C, Sawyers CL, Flavell RA, Wu H, Davis RJ. JNK and PTEN cooperatively control the development of invasive adenocarcinoma of the prostate. Proc Natl Acad Sci U S A. 2012;109:12046–12051. doi: 10.1073/pnas.1209660109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, Moorhead M, Chaudhuri S, Tomsho LP, Peters BA, Pujara K, Cordes S, Davis DP, Carlton VE, Yuan W, Li L, Wang W, Eigenbrot C, Kaminker JS, Eberhard DA, Waring P, Schuster SC, Modrusan Z, Zhang Z, Stokoe D, de Sauvage FJ, Faham M, Seshagiri S. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Kim HL, Vander Griend DJ, Yang X, Benson DA, Dubauskas Z, Yoshida BA, Chekmareva MA, Ichikawa Y, Sokoloff MH, Zhan P, Karrison T, Lin A, Stadler WM, Ichikawa T, Rubin MA, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61:2833–2837. [PubMed] [Google Scholar]
- Kirsch DG, Haigis K, Santiago P, Tukeshia S, Korsmeyer S, Jacks T. Using Mouse Genetics to Dissect the Cellular Target of Radiation in the GI Syndrome. Int J Radiat Oncol Biol Phys. 2005;63:S469. [Google Scholar]
- Kren A, Baeriswyl V, Lehembre F, Wunderlin C, Strittmatter K, Antoniadis H, Fassler R, Cavallaro U, Christofori G. Increased tumor cell dissemination and cellular senescence in the absence of β1-integrin function. EMBO J. 2007;26:2832–2842. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, Rotman M, Jones C, Asbell S, Valicenti R, Hahn S, Thomas CR., Jr An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levresse V, Butterfield L, Zentrich E, Heasley LE. Akt negatively regulates the cJun N-terminal kinase pathway in PC12 cells. J Neurosci Res. 2000;62:799–808. doi: 10.1002/1097-4547(20001215)62:6<799::AID-JNR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C. Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Jones K, Ledger A, Naylor MJ. β1 integrin deletion enhances progression of prostate cancer in the TRAMP mouse model. Sci Rep. 2012;2:526. doi: 10.1038/srep00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JM, Onodera Y, Bissell MJ, Park CC. Breast Cancer Cells in Three-dimensional Culture Display an Enhanced Radioresponse after Coordinate Targeting of Integrin α5β1 and Fibronectin. Cancer Res. 2010;70:5238–5248. doi: 10.1158/0008-5472.CAN-09-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. β1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, Wilding G. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14:7924–7929. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed A, Alam N, Trerotola M, Languino LR. Insulin-like growth factor 1 stimulation of androgen receptor activity requires β1A integrins. J Cell Physiol. 2012;227:751–758. doi: 10.1002/jcp.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler J, Durzok R, Brakebusch C, Cordes N. Interactions of the integrin subunit β1A with protein kinase B/Akt, p130Cas and paxillin contribute to regulation of radiation survival. Radiother Oncol. 2005;76:129–134. doi: 10.1016/j.radonc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilkrut M, Merrick GS, McLaughlin PW, Stenmark MH, Abu-Isa E, Vance SM, Sandler HM, Feng FY, Hamstra DA. The addition of low-dose-rate brachytherapy and androgen-deprivation therapy decreases biochemical failure and prostate cancer death compared with dose-escalated external-beam radiation therapy for high-risk prostate cancer. Cancer. 2013;119:681–690. doi: 10.1002/cncr.27784. [DOI] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating Peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland BW, Knoblaugh SE, Kaplan-Lefko PJ, Wang F, Holzenberger M, Greenberg NM. Conditional deletion of insulin-like growth factor-I receptor in prostate epithelium. Cancer Res. 2008;68:3495–3504. doi: 10.1158/0008-5472.CAN-07-6531. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Wang T, Languino LR, Lian J, Stein G, Blute M, Fitzgerald TJ. Molecular targets for radiation oncology in prostate cancer. Front Oncol. 2011;1:1–11. doi: 10.3389/fonc.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased β1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- Zafarana G, Ishkanian AS, Malloff CA, Locke JA, Sykes J, Thoms J, Lam WL, Squire JA, Yoshimoto M, Ramnarine VR, Meng A, Ahmed O, Jurisca I, Milosevic M, Pintilie M, van der Kwast T, Bristow RG. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer. 2012;118:4053–4062. doi: 10.1002/cncr.26729. [DOI] [PubMed] [Google Scholar]
- Zhang J, Park SI, Artime MC, Summy JM, Shah AN, Bomser JA, Dorfleutner A, Flynn DC, Gallick GE. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest. 2007;117:2962–2973. doi: 10.1172/JCI30710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.