Abstract
Leukotrienes (LTs) produced from arachidonic acid by the action of 5-lipoxygenase (5-LO) are classical mediators of inflammatory responses. However, studies published in the literature regarding these mediators are contradictory and it remains uncertain whether these lipid mediators play a role in host defense against the fungal pathogen Paracoccidioides brasiliensis. To determine the involvement of LTs in the host response to pulmonary infection, wild-type and LT-deficient mice by targeted disruption of the 5-lipoxygenase gene (knockout mice) were studied following intratracheal challenge with P. brasiliensis yeasts. The results showed that infection is uniformly fatal in 5-LO-deficient mice and the mechanisms that account for this phenotype are an exacerbated lung injury and higher fungal pulmonary burden. Genetic ablation or pharmacological inhibition of LTs resulted in lower phagocytosis and fungicidal activity of macrophages in vitro, suggesting that deficiency in fungal clearance seems to be secondary to the absence of activation in 5-LO−/− macrophages. Exogenous LTB4 restored phagocytosis and fungicidal activity of 5-LO−/− macrophages. Moreover, P. brasiliensis killing promoted by LTB4 was dependent on nitric oxide (NO) production by macrophages. Taken together, these results reveal a fundamental role for 5-LO-derived LTB4 in the protective response to P. brasiliensis infection and identify relevant mechanisms for the control of fungal infection during the early stages of the host immune response.
Author Summary
Paracoccidioidomycosis is a deep mycosis that is endemic in Latin America, mostly affecting rural workers of Argentina, Colombia, Venezuela and Brazil. Paracoccidioides brasiliensis infection is acquired upon the inhalation of airborne propagules derived from the saprophytic mycelium form of the fungus. Once in the lungs, P. brasiliensis converts to its parasitic yeast form and interacts with alveolar macrophages. This phagocytic cell is specialized in secreting cytokines, lipid mediators, and microbicidal molecules that control the fungal growth. Among the lipid mediators generated by macrophages, 5-LO-derived leukotrienes play an important role in lung innate immunity and are known to be important for neutrophil recruitment and activation. Here, we evaluated whether leukotrienes are involved in the immune response in a murine model of P. brasiliensis infection. 5-LO-deficient mice exhibited high mortality, severe lung pathogenesis and increased fungal burden in the lungs, associated with less activation of macrophages, resulting in lower phagocytosis and P. brasiliensis killing. These results reveal for the first time that endogenous production of leukotrienes, especially LTB4, is important for assembling a protective immune response of the host against P. brasiliensis.
Introduction
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by the dimorphic fungi Paracoccidioides brasiliensis and Paracoccidioides lutzii. PCM is the most prevalent deep mycosis in Latin America [1], [2] and the most important cause of death among systemic mycoses in immunocompetent individuals in Brazil [3]. PCM is considered a neglected infectious disease, as the development of new drugs for the treatment of this mycosis has received little attention [4], [5]. Epidemiological and experimental data suggest that humans probably become infected by inhalation of airborne conidia (the infective form) that reach the lung alveoli, where they transform into yeast cells, the parasitic tissue form [6]. Therefore, it is clear that this route of infection is more relevant experimentally. Clinical forms of the disease range from asymptomatic pulmonary infection to systemic generalized disease [7]. In the asymptomatic form of PCM, T-helper type 1 (Th1) specific immune response occurs, while Th2 immunity is associated with severe disease [8]–[10]. Cellular immune response plays a major role in the host defense against P. brasiliensis and macrophages are the most important effector cells that kill the fungus by oxidative mechanisms and cytokine production [11], [12]. Experimental studies and data from patients with PCM demonstrate that resistance to P. brasiliensis infection is dependent on the activity of T helper cells and mediated by IFN-γ, TNF-α and macrophages/monocytes. The synergistic effect between these cytokines is essential for host resistance and effective fungicidal activity against P. brasiliensis. During the course of infection, CD4+ T lymphocytes synthesize cytokines, such as IFN-γ, TNF-α and IL-12, which provide protection to the host, and prevents fungus spread [13], [14].
In addition to cytokines, lipid mediators such as leukotrienes (LTs) are another class of molecules involved in host defense [15]. LTs are derived from the metabolism of a cell-membrane fatty acid, arachidonic acid (AA), through activation of 5-lipoxygenase (5-LO) enzyme, in concert with its auxiliary protein, 5-LO-activating protein (FLAP). 5-LO catalyzes oxidation of AA to the intermediate 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which is either enzymatically reduced by 5-LO to the unstable epoxide leukotriene A4 (LTA4) or, alternatively, is reduced to 5-hydroxyeicosatetraenoic acid (5-HETE). LTA4 can be hydrolyzed to form leukotriene B4 (LTB4) or can be conjugated with glutathione to form the cysteinyl leukotrienes (cysLTs), LTC4, LTD4 and LTE4 [16]. LTB4 is a potent effector of leukocyte chemotaxis and activation, while the cysLTs increase vascular permeability and smooth muscle tone. The activity of leukotrienes is signaled through two sets of G-protein coupled receptors, BLT1/BLT2 for LTB4 and CysLT1/CysLT2 for cys-LTs [17].
LTs are produced at sites of infection and in vitro and in vivo models have revealed a powerful immunoregulatory role for LTB4 [18]–[27]. However, two recent studies published reported conflicting participation of 5-LO in P. brasiliensis infection. Although 5-LO enzymatic activity was shown to enhance susceptibility during experimental P. brasiliensis infection [28], Balderramas and colleagues demonstrated that leukotriene production was associated with a protective response during the early stages of P. brasiliensis infection in the lungs. LTB4 was shown to induce influx and activation of phagocytes [29]. Therefore, the relevance of 5-LO and its metabolic products during infection by P. brasiliensis remains undefined. In the present study, we evaluated the role of LTs in host defense against the pathogenic fungus P. brasiliensis inoculated in the lungs. As an experimental approach, we compared survival and components of the host response in wild-type and 5-LO-deficient mice, employing a model of intratracheal challenge with P. brasiliensis yeasts, which mimics the infection route of the fungus in human host. We sought to investigate the importance of LTs in phagocytosis and fungicidal activity through genetic and pharmacological tools and in vitro challenge of macrophages with P. brasiliensis yeasts. 5-LO deficiency was associated with macrophage hyporesponsiveness during P. brasiliensis infection, resulting in lower phagocytosis and fungal clearance, leading to increased fungal burden in the lungs and mortality of mice. The results reveal that endogenous 5-LO metabolites, especially LTB4, play a fundamental role in the protective host response to P. brasiliensis infection.
Methods
Animals
Male Sv129 (Wild-type - WT) and 5-LO-deficient mice (5-LO−/−) (6 to 8 weeks old) were used in all experiments. WT mice were obtained from the Animal Care Facilities of Federal University of Minas Gerais, Minas Gerais, Brazil and 5-LO−/− mice were purchased at The Jackson Laboratory, Bar Harbor, Maine, USA [30]. Mice were kept under controlled environmental conditions (temperatures of 24°C and 12 h light/dark cycle) and supplied with sterile food and water in clean bottles, as well as clean bedding.
Ethics statement
Animal experiments were performed in strict accordance with the Brazilian Federal Law 11,794 establishing procedures for the scientific use of animals and approved by the Animal Care and Use Committee of the Federal University of Minas Gerais (protocol no. 194/09).
Fungal culture and quantification
The Pb18 strain, a highly virulent isolate of P. brasiliensis, is part of the strains collection of P. brasiliensis in our laboratory. Pb18 yeast cells were maintained by weekly subcultivation on 2% glucose, 1% peptone, 0.5% yeast extract medium culture (YPD medium). The yeast cells were washed in sterile phosphate-buffered saline (PBS) (pH 7.2) and homogenized. Cells were adjusted to 1×106/ml (for in vivo infection) and 6×104/ml (for in vitro infection) based on hemocytometer counts. Cell viability was determined with Janus Green B vital dye (Merck) and was always higher than 90%.
Reagents
Zileuton, CP105,696 and montelukast were provided by Abbott Pharmaceuticals, Pfizer and Merck, respectively. LTB4 and recombinant interferon-gamma (IFN-γ) was purchased from Cayman Chemical and BD Pharmigen, respectively. Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME), a nitric oxide synthase inhibitor and 4′-Hydroxy-3′-methoxyacetophenone (apocynin), a NADPH oxidase inhibitors were purchased by Sigma Aldrich.
Treatment with zileuton, an inhibitor of 5-lipoxygenase enzyme
The compound was diluted in 100 µl of absolute ethanol plus carboxymethylcellulose (Synth) at 0.5%, in order to get the treatment solution. WT mice were treated daily with 30 mg/Kg/0.2 ml by gavage, the first and last dose administered 1 hour before infection with P. brasiliensis yeasts and before euthanasia of animals, respectively. As a control, mice injected with PBS or infected with P. brasiliensis were treated daily with the same volume of vehicle solution by the same route.
Experimental infection
Mice were anesthetized by intraperitoneal (i.p.) injection of a solution containing ketamine hydrochloride (100 mg) and xylazine (20 mg) (Syntec, São Paulo, Brazil). When deep anesthesia was obtained, an anterior midline incision was made for trachea exposition. WT and 5-LO−/− mice were infected with 106 P. brasiliensis yeast cells, in a volume of 30 µl by surgical intratracheal (i.t.) inoculation, which allowed dispensing of the fungal cells directly into the lungs. Uninfected mice (control) received PBS only. The skin was then sutured, and mice were allowed to recover on a heated plate.
Lung lavage and cell differential count
At different periods after infection, animals were euthanized. The chest cavity of each animal was carefully opened, and the trachea was exposed and catheterized. Lungs were washed after the canulation of trachea with polyethylene tubing, which was attached on a tuberculin syringe. The catheter was tied in place, and sterile PBS was infused in 1 ml aliquots three times. Bronchoalveolar lavage fluid (BAL) was recovered and total cell counts were immediately performed in a Neubauer chamber. Differential counts for leukocyte subsets were obtained by using cytospin preparations.
Immunolabeling and Fluorescence-Activated Cell Sorting Analysis
Bronchoalveolar cells were stained for extracellular molecular expression patterns using monoclonal antibodies against mouse F4/80, CD11c and MHC-II conjugated to fluorophores, and isotype controls (all from BD Pharmingen). Data were acquired on a FACSCanto II (Becton Dickinson) and analyzed by FlowJo 7.5.3 software (TreeStar Inc., Ashland, OR). Limits for the quadrant markers were always set on the basis of negative populations and isotype controls. Results are presented as mean fluorescence intensity (MFI) as indicated.
Fungal burden assessment
The number of viable yeasts in BAL and lungs of infected mice were determined by colony-forming units (CFU) counts. After periods of infection, mice were euthanized, and their lungs were removed, weighed, and homogenized in 1 ml sterile PBS with a tissue grinder. Aliquots (100 µl) of each homogenate and BAL were placed on brain-heart infusion (BHI) agar medium (Difco Laboratories), containing 4% (vol/vol) fetal bovine serum (Cultilab, São Paulo, Brazil), 40 µg/ml gentamicin (Sigma-Aldrich) and 5% P. brasiliensis B339 broth yeast culture filtrate from 2-week-old cultures (supplemented BHI), the latter constituting the source of a growth-promoting factor [31]. The plates, in duplicate, were incubated at 37°C and colonies were counted daily until no increase in counts was observed. The numbers of viable P. brasiliensis per gram of tissue or ml of BAL were expressed as the mean ± the standard error of the mean (SEM).
Myeloperoxidase (MPO) concentrations
The extent of neutrophil accumulation in the lungs was measured by assaying myeloperoxidase activity. Briefly, a portion of the lungs were removed and snap frozen in liquid nitrogen. On thawing and processing, the tissue was assayed for myeloperoxidase activity by measuring the change in optical density (O.D.) at 450 nm using tetramethylbenzidine (Sigma-Aldrich). Results were expressed as the neutrophil index that denotes activity of myeloperoxidase related with casein-elicited murine peritoneal neutrophils processed in the same way.
Measurement of cytokines
The concentrations of murine TNF-α, IFN-γ, IL-1β, IL-6, IL-10 and keratinocyte-derived chemokine (CXCL-1/KC) and macrophage inflammatory protein-2-gamma (CXCL-2/MIP-2) was measured in BAL and lungs samples using commercially available antibodies and according to the procedures supplied by the manufacturer (R&D Systems, Minneapolis, MN). One hundred milligrams of lung were homogenized in 1 ml of PBS containing anti-proteases (0.1 mmol/L phenylmethil sulfonyl fluoride, 0.1 mmol/L benzethonium chloride, 10 mmol/L ethylenediaminetetraacetic acid, and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 minutes at 13000× g and the supernatant stored at −20°C until enzyme-linked immunosorbent assay (ELISA) was performed.
Isolation and cultivation of murine macrophages
Peritoneal macrophages were elicited by i.p. injection of 3% sterile thioglycollate medium (2 mL). After 3 days, mice were euthanized and macrophages were harvested by peritoneal lavage with PBS. Alveolar macrophages were isolated from the bronchoalveolar fluid of animals after the lung lavage protocol described above. Cells (2×105 cells/well in 24-well plates) were either isolated by adherence (4 h at 37°C under 5% CO2) to plastic-bottom tissue culture plates (for fungicidal assays) or plated onto 13-mm-diameter round glass coverlips (for phagocytosis assays). Macrophages were washed to remove nonadherent cells and were cultivated overnight with fresh complete medium (RPMI-1640 tissue culture medium [Cultilab, Brazil] supplemented with 2 mM L-glutamine [Sigma-Aldrich], 10% fetal bovine serum, 20 mM HEPES [Sigma-Aldrich] and 40 µg/ml gentamicin [Sigma-Aldrich]) in the presence or absence of recombinant IFN-γ (100 U/ml).
Infection of murine macrophages cultures with P. brasiliensis
For phagocytosis assays, P. brasiliensis yeasts were added at a ratio of 1∶5 macrophages to fungal cells. The cultures were incubated for 24 h at 37°C under 5% CO2 to allow adhesion and ingestion of fungi. Cells were washed twice with PBS to remove any noningested or nonadherent yeasts, fixed with methanol and stained with Giemsa (Sigma Aldrich). Internalized yeasts were counted by light microscopy and the phagocytic index (PI) was defined by the equation PI = P X F, where P is the percentage of macrophages with internalized yeast and F is the average number of yeast cells per macrophage. At least three independent experiments were carried out in triplicate for each condition [32]. For fungicidal assays, IFN-γ-primed and unprimed macrophages were infected with P. brasiliensis yeasts as described above. After the infection, the supernatants were removed and stored at −20°C and further analyzed for the presence of nitric oxide (NO). The wells were washed with water to lyse macrophages and one hundred microliters of cell homogenates were placed on BHI supplemented agar medium and assayed for the presence of viable yeasts after 7–10 days of incubation at 37°C. Fungicidal assay was normalized according differences in initial ingestion and presented as P. brasiliensis survival. In some experiments, cells were pretreated for 30 minutes before infection with the 5-LO enzyme inhibitor zileuton (10 µM), LTB4 receptor antagonist CP-105,696 (1 µM), cysteinyl-LT receptor antagonist montelukast (10 µM), LTB4 (100 nM), iNOS inhibitor L-NAME (1 mM) or NADPH oxidase inhibitor apocynin (1 mM). Compounds requiring reconstitution were dissolved in either PBS or dimethyl sulfoxide (DMSO). Required dilutions of all compounds were prepared immediately before use, and equivalent quantities of vehicle were added to the appropriate controls.
Measurement of LTB4
For LTB4 measurement, lungs were removed and tissues homogenized, centrifuged at 10000× g for 10 minutes and stored at −80°C until assayed. The supernatant obtained from the phagocytosis assay was also used for LTB4 analysis. Quantification of LTB4 in the samples was performed by specific enzyme immunoassay (Leukotriene B4 EIA Kit, Cayman Chemical, Ann Arbor, Mich.) following the manufacturer's instructions. The sensitivity for LTB4 was 4 pg/ml.
Macrophage nitric oxide production
Nitric oxide (NO) was quantified by the accumulation of nitrite (NO2 −) in the supernatants of macrophage cultures using Griess assay. Briefly, 100 µl of the supernatants collected were mixed with equal volume of Griess reagent (1% sulfanilamide [Sigma-Aldrich] diluted in 5% of phosphoric acid [H3PO4], and 0.1% Naphthylethylenediamine [Sigma-Aldrich]) in 96-well plates and absorbance at 540 nm was determined. Conversion of absorbance to micromolar concentrations of nitrite was obtained using a standard curve of a known concentration of NaNO2 diluted in water. All measurements were performed in duplicate and expressed as micromolar concentrations of nitrite.
Intracellular oxidative burst assay using flowcytometric analysis
The intracellular production of reactive oxygen species (ROS) was assayed by using the substrate dihydrorhodamine 123 (DHR-123, Invitrogen, Eugene, USA) that diffuses into the cells and is oxidized by ROS to the fluorescent Rhodamine 123 [33]. Macrophages were cultivated according to phagocytosis protocol in 96-well plates and after 24 h of infection, the cells were incubated with 5 µM DHR-123 for 30 min at 37°C in the dark. The fluorescence was measured in a flowcytometer (Synergy 2) at an excitation wavelength of 480 nm and at emission wavelength of 530 nm.
Histological analysis
Lungs were removed after infection and tissues were fixed in 10% formalin and embedded in paraffin blocks. Tissue sections (5 µm thick) were stained with hematoxylin and eosin (H&E) for histopathological evaluation. Sections were captured with a digital camera (DEI-470; Optronics, Goleta, CA) connected to a microscope (IX70; Olympus, Center Valley, PA). Inflammation was scored as follows: 0: no inflammation; 1, perivascular cuff inflammatory cells; 2, mild inflammation, extending throughout <25% of the lung; 3, moderate inflammatory covering 25–50% of the lung; 4, severe inflammation over one-half of the lung.
Statistical analysis
The results were analyzed by GraphPad Prism 4 (GraphPad Inc., San Diego, CA, USA). Data were presented as mean ± standard error of the mean (SEM) and were compared using one-way ANOVA test with Newman-Keuls multiple comparisons post-test. P values<0.05 were considered significant.
Results
5-LO deficiency decreased animal survival and pulmonary fungal clearance
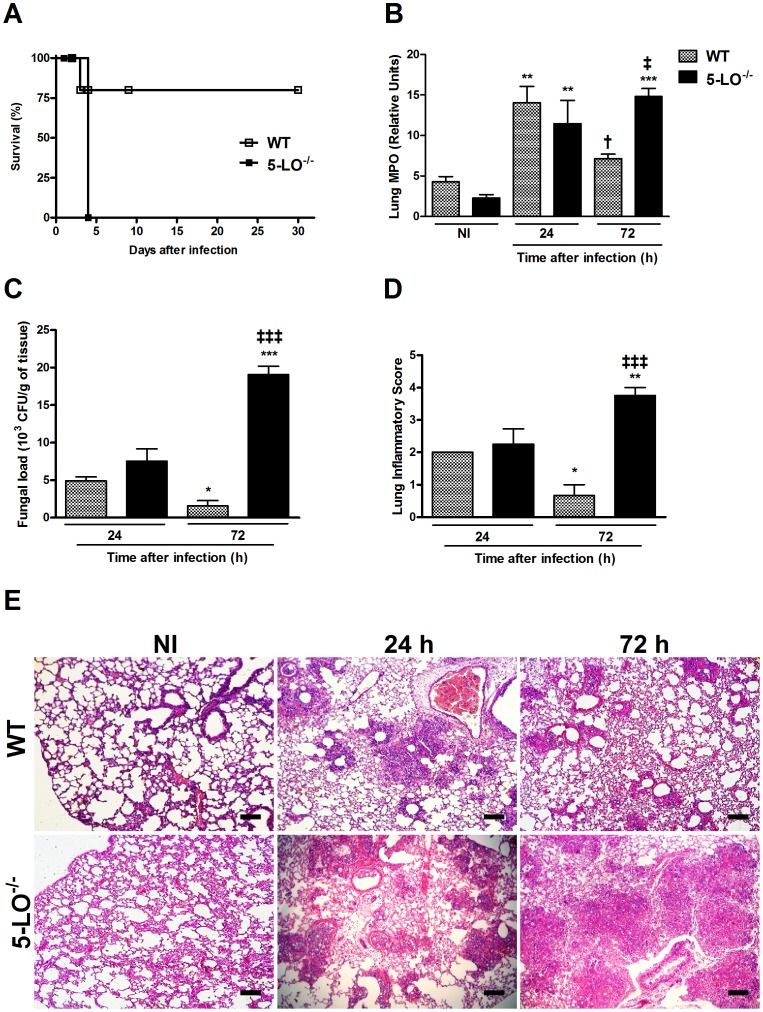
Approximately 75% of WT mice infected with P. brasiliensis survived until day 30 after infection, the end of the observation period, while 100% of 5-LO−/− mice died within four days after infection (Figure 1A). Infection with P. brasiliensis yeasts resulted in increased myeloperoxidase (MPO) activity in WT and 5-LO−/− mice 24 h after intratracheal inoculation when compared with uninfected mice. Neutrophil accumulation peaked after 24 h in the lungs of WT mice and was significantly reduced after 72 h of P. brasiliensis infection (Figure 1B). 5-LO−/− mice had a similar increase of MPO activity 24 h after infection but, unlike WT mice, there was higher MPO activity 72 h after infection.
Figure 1. Role of 5-LO in survival, myeloperoxidase activity, fungal load and histopathological alterations after P. brasiliensis infection.
Groups of WT and 5-LO−/− mice were infected with 106 yeasts. (A) Survival rate was assessed daily until the 30th day of infection. (B) Infiltration of neutrophils in the lungs of mice was determined by MPO activity and expressed in relative units. (C) Pulmonary fungal load in WT and 5-LO−/− mice. Viable yeasts were evaluated by CFU counting in lung homogenates obtained 24 and 72 h after infection. (D) Inflammatory score of lung sections evaluated 24 and 72 h after infection in WT and 5-LO−/− mice. (E) Representative photomicrographs of H&E-stained lung sections from WT (upper line) and 5-LO−/− (bottom line) mice 24 and 72 h after P. brasiliensis infection (bars = 100 µm, original magnification, ×10). Control, uninfected mice (NI) received only sterile PBS. Results were expressed as the mean ± SEM and are representative of two independent experiments (n = 5). Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) when compared to uninfected control or infected mice counterparts at 24 h after infection. Symbol † represents significant difference (†p<0.05) to infected mice counterparts in evaluated periods. Symbol ‡ represents significant difference (‡p<0.05, ‡‡‡p<0.001) between WT and 5-LO−/− mice in the respective period evaluated.
In order to evaluate whether the increased mortality of 5-LO−/− mice was due to fungal burden, viable yeast cells from bronchoalveolar lavage fluid (BAL) and lungs obtained from WT and 5-LO−/− mice infected with P. brasiliensis yeasts were quantified by CFU counts (Figure 1C). At 24 h after infection, viable yeasts were recovered from WT lungs, followed by a significant decrease 72 h after infection. Similar amount of fungal yeasts was recovered after 24 h from lungs of 5-LO−/− mice, but fungal load increased significantly and was about twelve times higher in 5-LO−/− than WT mice 72 h after infection (Figure 1C). The same pattern was observed in BAL of WT and 5-LO−/− mice (data not shown).
5-LO deficiency resulted in exacerbated lung inflammation in response to P. brasiliensis infection
Next, we scored the inflammation in the lungs of infected mice. Semi-quantification of lung inflammation concurred with the overall histopathological findings (Figure 1D–E). As shown in Figure 2E, lungs of uninfected WT and 5-LO−/− mice were similar. At 24 h after infection, there was similar tissue inflammation in WT and 5-LO−/− mice (Figure 1E). At 72 h after infection, lungs from WT mice showed dramatically reduced inflammatory infiltration and alveolar spaces were usually free of inflammation. In contrast, in 5-LO-deficient mice infected with P. brasiliensis, the extent of the inflammatory response was substantially greater with severe, widespread inflammation associated with a decrease in alveolar space and loss of lung architecture (Figure 1E).
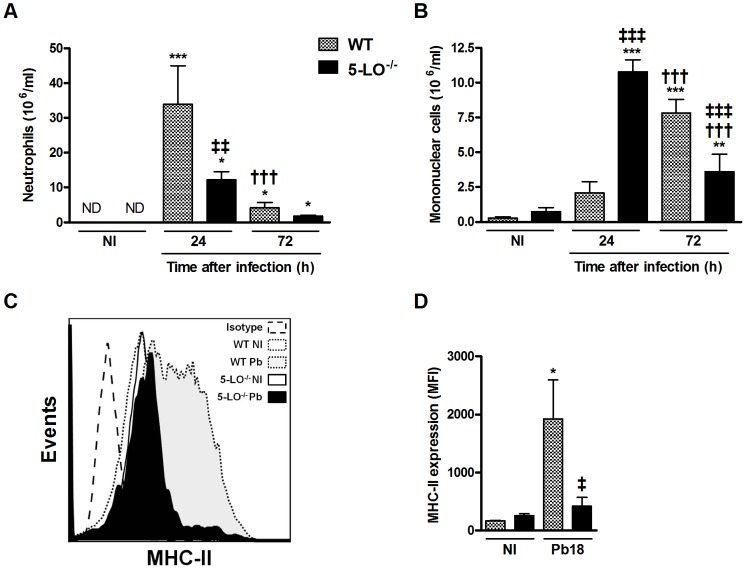
Figure 2. Effect of 5-LO on leukocyte recruitment and activation profile of bronchoalveolar cells.
(A–B) BAL cells were obtained from mice 24 and 72 h after infection. Neutrophils and mononuclear cells were identified and counted after staining. (C) Flow cytometry analyses of bronchoalveolar leukocytes (F4/80+CD11c+ cells) from uninfected (NI), WT and 5-LO−/− mice 24 h after infection with P. brasiliensis. Histograms were generated showing surface expression of MHC-II. (D) Activated F4/80+CD11c+ lymphocytes (macrophages) expression of MHC-II was also evaluated and expressed as MFI. Results were expressed as mean ± SEM and are representative of independent experiments (n = 5). Control, uninfected mice (NI) received only sterile PBS. Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) when compared to uninfected control or infected mice counterparts at 24 h after infection. Symbol † represents significant difference (†††p<0.001) to infected mice counterparts in evaluated periods. Symbol ‡ represents significant difference (‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001) between WT and 5-LO−/− mice in the respective periods evaluated. MFI, mean fluorescence intensity. ND, not detected.
5-LO deficiency impaired inflammatory cell recruitment and macrophage activation
Intratracheal inoculation of P. brasiliensis in WT mice induced significant neutrophil recruitment into the bronchoalveolar space 24 h after infection (Figure 2A). The number of neutrophils decreased in BAL but was still greater compared to uninfected mice 72 h after infection. In 5-LO−/− mice, neutrophils counts was lower than WT mice 24 and 72 h after infection (Figure 2A). Considering the mononuclear cells influx, WT mice showed increased cell recruitment between 24 and 72 h after infection (Figure 2B). In 5-LO−/− mice, mononuclear cells counts were higher at 24 h but greatly reduced 72 h after infection (Figure 2B).
After, we measured the activation of bronchoalveolar lavage cells of WT and 5-LO−/− mice 24 h after infection, a time point when occurs the peak of the inflammatory events in WT mice. The activation of F4/80+CD11c+ alveolar macrophages was evaluated 24 h after infection by measuring surface expression of class II major histocompatibility (MHC-II). After P. brasiliensis infection, there was a significant increase of MHC-II expression on WT macrophages (Figure 2C and 2D). In contrast, there was no difference in the regulation of MHC-II expression in 5-LO−/− macrophages after infection compared to uninfected cells (Figure 2C and 2D).
5-LO deficiency changed cytokines production in BAL and lungs during P. brasiliensis infection
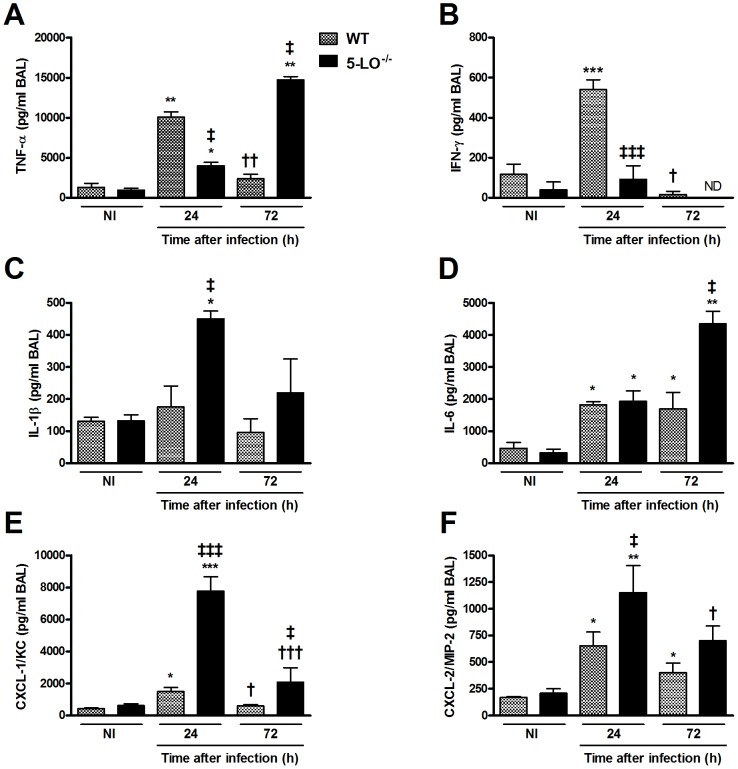
Then, we determined whether 5-LO−/− mice presented altered levels of cytokines previously shown to be involved in inflammatory and immune responses to P. brasiliensis (Figure 3 and Table 1). In WT mice, levels of TNF-α, IFN-γ and CXCL-1 peaked 24 h after infection and decreased to virtually background levels after 72 h in BAL (Figure 3A, B and E). Regarding the cytokine IL-1β, there was no difference in the concentration of this cytokine in WT-infected mice at the different times evaluated compared to control animals (Figure 3C). Considering the concentration of IL-6 and CXCL-2, there was an increase of these mediators after 24 h of infection, and this increase was maintained after 72 h of infection compared to control animals (Figure 3D and F). In 5-LO−/− mice, levels of TNF-α were lower 24 h after infection and higher after 72 h compared with cytokine levels of WT-infected mice (Figure 3A). Levels of IFN-γ were lower 24 h after infection in 5-LO−/− mice and were not detectable after 72 h (Figure 3B). Levels of IL-1β, CXCL-1 and CXCL-2 were higher in 5-LO−/− mice 24 h after infection compared with WT mice (Figure 3C, E and F). Levels of IL-6 and CXCL-1 were higher in BAL of 5-LO−/− mice 72 h after infection compared with the same period in WT mice (Figure 3D and E). Overall, the cytokines and chemokines production in the lungs (Table 1) of 5-LO−/− mice after infection was higher to that found in WT mice 72 h after infection. In addition to the cytokines mentioned above, we could also measure significant amounts of IL-10 in lungs (Table 1), but not in BAL (not detected, data not shown), of P. brasiliensis-infected mice. Levels of IL-10 were high after 24 h and dropped to background levels 72 h after infection in WT mice. Levels of IL-10 remained low and were below background levels in 5-LO−/− mice (Table 1).
Figure 3. Cytokine levels in BAL after P. brasiliensis infection. WT and 5-LO−/− mice were infected with 106 P. brasiliensis yeasts.
Cytokine levels for TNF-α (A), IFN-γ (B), IL-1β (C), IL-6 (D), CXCL-1/KC (E) and CXCL-2/MIP-2 (F) was determined by ELISA in BAL after 24 and 72 h of infection. The data were expressed as mean ± SEM (n = 5). Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) between infected mice compared to respective control group. Symbol † represents significant difference (†p<0.05, ††p<0.01, †††p<0.001) to infected mice counterparts in evaluated periods. Symbol ‡ represents significant difference (‡p<0.05, ‡‡‡p<0.001) between WT and 5-LO−/− mice in the respective periods evaluated. ND, not detected.
Table 1. Cytokines and chemokines concentrations in the lungs of WT and 5-LO−/− mice.
| Cytokine or chemokine | WT | 5-LO−/− | ||||
| NI | 24 h | 72 h | NI | 24 h | 72 h | |
| TNF-α | 18.76±37.52 | 1497±492.7* | 445.2±504.0† | 11.12±19.26 | 526.7±102.8‡ | 2243±518.6* † ‡ |
| IFN-γ | 249.7±139.5 | 858.5±76.02* | 430.6±135.2† | 306.9±103.6 | 279.6±142.9‡ | 621.4±192.8* † ‡ |
| IL-1β | 241.5±104.6 | 5868±422.0* | 2223±1016* † | 239.1±103.6 | 4708±930.5* ‡ | 5813±308.8* † ‡ |
| IL-6 | 59.91±119.8 | 780.2±347.0* | 28.07±47.35† | ND | 1923±123.0* ‡ | 1045±664.9* † ‡ |
| IL-10 | 188.7±51.95 | 559.0±43.64* | 176.3±146.9† | 157.9±84.52 | 30.61±37.54* ‡ | 57.55±40.83 |
| CXCL1/KC | 144.4±38.89 | 3721±1250* | 774.1±470.5† | 419.9±300.9 | 7184±959.2* ‡ | 2840±1352* † ‡ |
| CXCL2/MIP-2 | 252.8±106.7 | 5970±1706* | 650.0±562.0† | 431.5±332.8 | 5676±428.9* | 2916±1606* † ‡ |
Values represent the mean and standard deviation of cytokines and chemokines concentrations (pg/100 mg of tissue) in the lungs of WT and 5-LO−/− mice. NI: not infected. ND: not detected.
p<0.01; p<0.001 Statistical differences between groups of mice infected during the periods of time indicated (24 h or 72 h) compared with NI mice.
p<0.05; p<0.01; p<0.001 Statistical differences between times after infection (24 h and 72 h) within the same group of mice (WT or 5-LO−/−).
p<0.05; p<0.01; p<0.001 Statistical differences between 5-LO−/− mice compared with WT mice in the time period indicated (24 h or 72 h) following infection.
Pharmacological inhibition of 5-LO during infection with P. brasiliensis mimics the phenotype of 5-LO-deficient mice
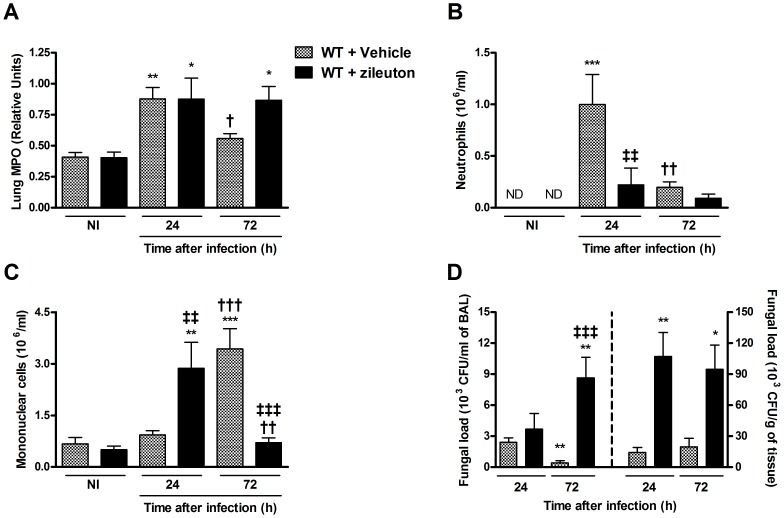
To determine the effects of pharmacological inhibition of 5-LO during P. brasiliensis infection, WT mice were treated daily with zileuton, a compound which inhibits the leukotrienes synthesis by complexing with an iron atom in the site of the 5-LO enzyme [34]. Overall, treatment with zileuton resulted in changes at inflammatory parameters which were remarkably similar to those of 5-LO−/− mice (Figure 4). Indeed, there was greater MPO activity in the lungs (Figure 4A) but fewer neutrophil counts in BAL (Figure 4B), suggesting that neutrophils were trapped into the parenchyma and increased with time. Similarly, number of mononuclear cells early increased and were lower than vehicle-treated mice 72 h after infection (Figure 4C). Similar to that observed in 5-LO−/− mice, zileuton-treated mice presented increased fungal burden in BAL and lung tissue after infection (Figure 4D).
Figure 4. Effect of LT-synthesis blockade in vivo during infection with P. brasiliensis.
WT mice were treated with zileuton (30 mg/Kg/day; 0.2 ml by gavage) before infection with P. brasiliensis yeasts. (A) MPO activity in the lungs of mice inoculated with P. brasiliensis yeasts was expressed in relative units. Control (NI) mice were injected with PBS and treated daily with the same volume of vehicle solution by the same route. BAL cells were obtained from mice 24 and 72 h after infection and neutrophils (B) and mononuclear cells (C) were counted and identified after staining. (D) Fungal burden was evaluated by counting the number of viable yeasts presents in BAL (left) and lung homogenates (right). The results were expressed as the mean ± SEM (n = 5). Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) between infected mice compared to respective control group. Symbol † represents significant difference (†p<0.05, ††p<0.01, †††p<0.001) to infected mice counterparts in evaluated periods. Symbol ‡ represents significant difference (‡‡p<0.01, ‡‡‡p<0.001) between WT and 5-LO−/− mice in the respective periods evaluated. ND, not detected.
Absence or pharmacological inhibition of 5-LO decreased phagocytosis and killing of P. brasiliensis by macrophages
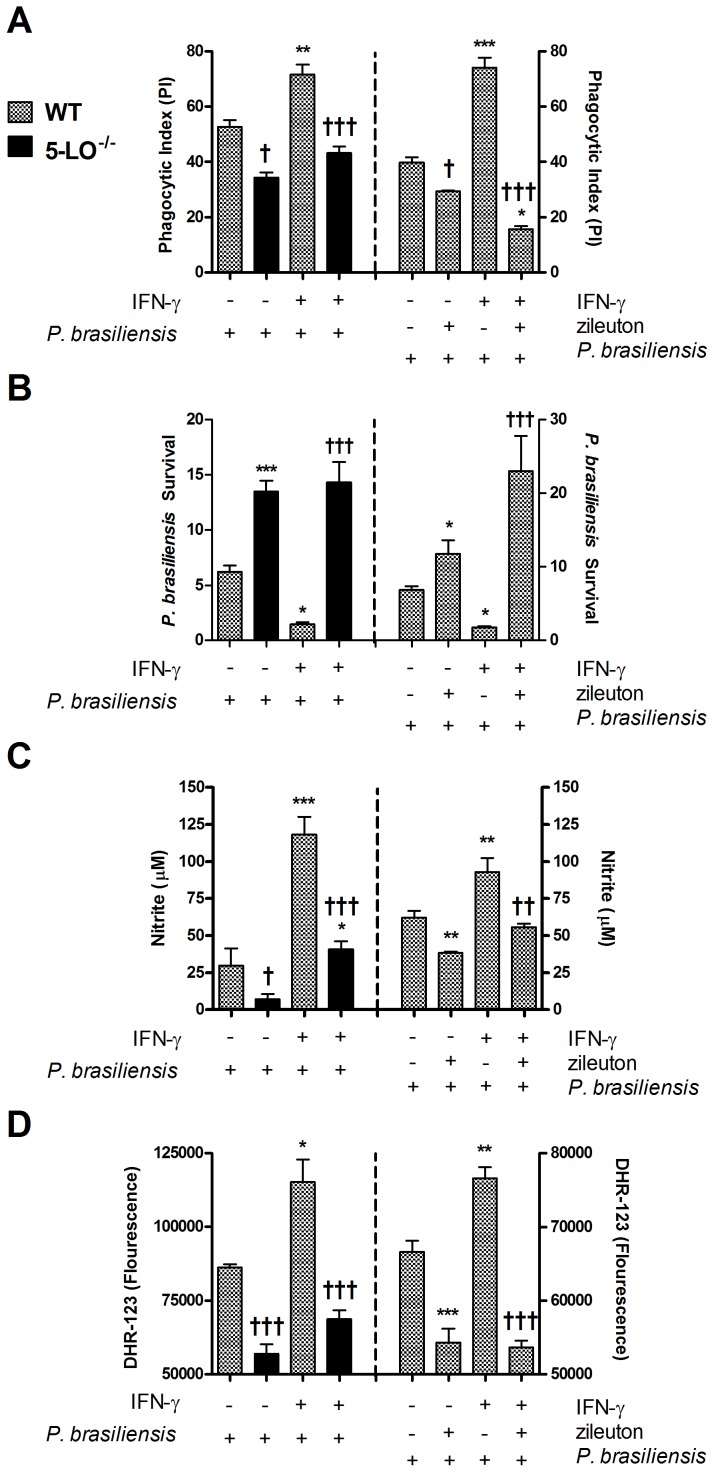
Given the increased fungal burden observed in 5-LO−/− or zileuton-treated mice, we examined the ability of macrophages from these mice to deal with the fungus in vitro. WT macrophages were capable of engulfing P. brasiliensis and this phagocytic activity was enhanced by IFN-γ (Figure 5A). Also, IFN-γ were able to significantly increase fungal killing (Figure 5B), which was associated with an increased NO (Figure 5C) and ROS production by infected macrophages (Figure 5D). In macrophages from 5-LO−/− mice or treated with zileuton, P. brasiliensis phagocytosis was decreased, even in the presence of IFN-γ (Figure 5A). Moreover, decreased spontaneous phagocytosis or in response to IFN-γ was followed by increased fungal burden recovered from the macrophage cultures (Figure 5B) and lower NO and ROS production (Figure 5C and D). Essentially, similar results were also observed in alveolar macrophages from WT and 5-LO−/− mice subjected to the same stimuli (Figure S1).
Figure 5. Phagocytic and fungicidal activity during infection with P. brasiliensis in vitro.
(Left) Macrophages were harvested from the peritoneal cavity of WT and 5-LO−/− mice and infected in vitro with P. brasiliensis yeasts. (Right) Peritoneal WT macrophages were treated with vehicle or zileuton (10 mM) for 30 min before infection in vitro with P. brasiliensis yeasts. Phagocytic index (A) and intracellular killing (B) were calculated 24 h after incubation. Killing assay was normalized according initial ingestion (PI) and presented as P. brasiliensis survival. The supernatants were harvested and nitrite concentrations (C) were measured. (D) ROS concentrations were determined by mean fluorescence intensity (DHR-123) in macrophage cultures after infection. Each value represents the mean ± SEM of triplicate cultures from two independent experiments. Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) compared to WT infected group. Symbol † represents significant difference (†p<0.05, ††p<0.01, †††p<0.001) between the same experimental group compared to different culture conditions.
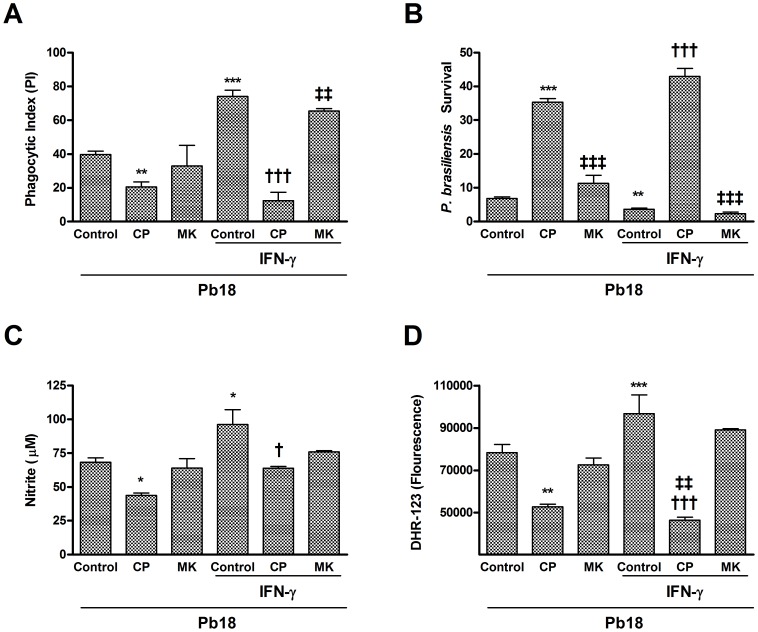
LTB4 receptor antagonist decreased phagocytosis and fungicidal activity of macrophages infected with P. brasiliensis
Our previous experiments established a role for 5-LO pathway products but did not identify the metabolite responsible for the endogenous antifungal activity. Therefore, we used a strategy involving selective receptor antagonists (LTB4 receptor antagonist - CP-105,696 or cysteinyl-LT receptor antagonist - montelukast) to determine the individual contributions of LTB4 and cysLTs, specific 5-LO metabolites, in phagocytosis and fungicidal activity. As shown in Figure 6, treatment of macrophages with the LTB4 receptor antagonist, but not the cysteinyl-LT receptor antagonist, decreased phagocytosis (either spontaneous or enhanced by IFN-γ) and P. brasiliensis killing (Figure 6A and B). Also, there was reduced production of NO and ROS after treatment of macrophages with LTB4 receptor antagonist, fact that was not observed in macrophages treated with the cysteinyl-LT receptor antagonist (Figure 6C and D).
Figure 6. Effect of LTB4- or cysLT-receptor antagonists on macrophage phagocytosis and fungicidal activity against P. brasiliensis.
Peritoneal WT macrophages were treated with vehicle, LTB4 receptor antagonist CP-105,696 (CP) (1 mM) or cysteinyl-LT receptor antagonist montelukast (MK) (10 mM) for 30 min before the addition of P. brasiliensis yeasts. Phagocytic index (A) and intracellular killing (B) were calculated 24 h after incubation. Killing assay was normalized according initial ingestion (PI) and presented as P. brasiliensis survival. The supernatants were harvested and nitrite concentrations (C) were measured. (D) ROS concentrations were determined by mean fluorescence intensity (DHR-123) in macrophage cultures after infection. Each value represents the mean ± SEM of triplicate cultures from two independent experiments. Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) compared to control group (macrophages that were treated with vehicle and infected with P. brasiliens yeasts). Symbol † represents significant difference (†p<0.05, †††p<0.001) compared to IFN-γ control group (macrophages that were activated with IFN-γ, treated with vehicle and infected with P. brasiliens yeasts). Symbol ‡ represents significant difference (‡‡p<0.01) between the same experimental group compared to different culture conditions.
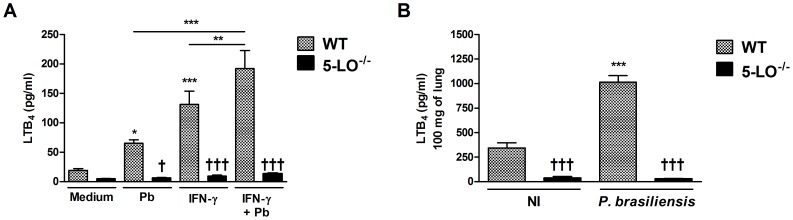
P. brasiliensis infection induces LTB4 synthesis
Having established the importance of endogenously produced LTB4 for phagocytic capacity of macrophages at levels similar to those observed after stimulation with IFN-γ, we next investigated if P. brasiliensis phagocytosis would result in the LTB4 synthesis, in the presence or not of IFN-γ. As indicated in Figure 7A, control cultures (only medium) had very low LTB4 levels, and these were similar in WT and 5-LO−/− macrophages. However, WT-infected macrophages showed increased LTB4 production 24 h after P. brasiliensis-macrophages interaction and this level was significantly enhanced in presence of IFN-γ stimulation alone or after stimulation and interaction with P. brasiliensis. Macrophage production of LTB4 for all conditions evaluated was markedly reduced in 5-LO-deficient macrophages.
Figure 7. LTB4 production after fungal infection.
(A) Enzyme immunoassay quantification of LTB4 concentrations in alveolar macrophages cultures after 24 h of incubation with P. brasiliensis yeasts. Data are presented as mean ± SEM and are representative of one experiment (n = 8). Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) when compared to uninfected cultures (only medium) or between indicated groups. Symbol † represents significant difference (†††p<0.001) between WT and 5-LO−/− macrophages under the conditions mentioned. (B) Lung LTB4 concentration in WT and 5-LO−/− mice. Quantification of LTB4 in lungs from uninfected- (control - NI) or infected mice with P. brasiliensis evaluated 24 h after inoculation. Leukotriene concentrations in supernatant of homogenized lungs (100 mg) were expressed as pg/ml. Data were expressed as the mean ± SEM and are representative of one experiment (n = 6). Symbol * represents significant difference (***p<0.001) when compared to uninfected mice. Symbol † represents significant difference (†††p<0.001) between WT and 5-LO−/− mice under the conditions mentioned.
Further, we measured LTB4 production in the lungs of infected mice (Figure 7B). WT and 5-LO−/− mice were infected with P. brasiliensis yeast cells and the production of this lipid mediator was determined 24 h after infection. Uninfected WT mice displayed low levels of LTB4 whereas infection with P. brasiliensis induced increased lung LTB4 production. LTB4 levels were significantly lower in the lungs of 5-LO-deficient mice.
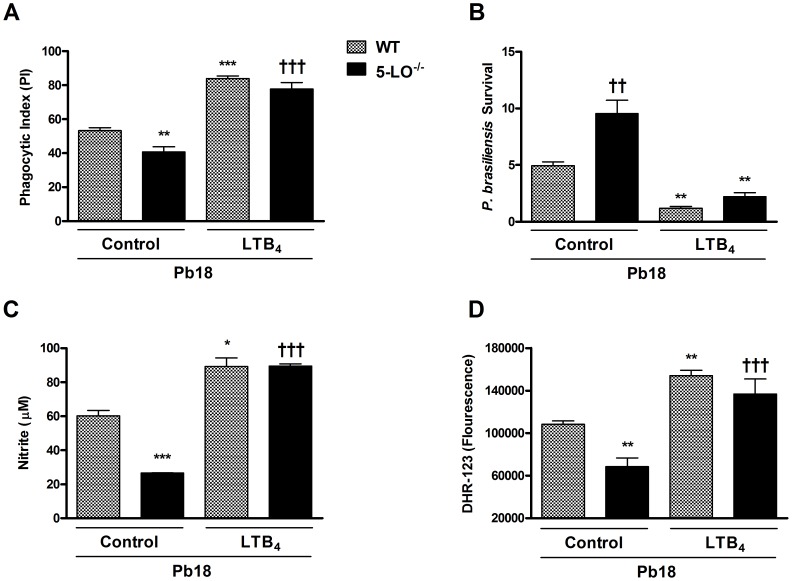
Addition of LTB4 restores phagocytosis and fungicidal activity of macrophages
Previous experiments suggested that the LTB4 receptor was the major signaling pathway that accounted for the action of 5-LO derived products in the context of P. brasiliensis infection. We therefore evaluated whether addition of LTB4 could induce phagocytosis and fungicidal activity directly and overcome the phenotype of 5-LO-deficient mice. As shown in Figure 8, pretreatment of WT macrophages with LTB4 enhanced the phagocytic activity, P. brasiliensis killing and NO and ROS production. More importantly, addition of LTB4 reversed the phenotype observed in 5-LO−/− macrophages, causing greater phagocytosis, fungal killing and NO and ROS production (Figure 8). The same results were observed with alveolar macrophages (Figure S1).
Figure 8. Effects of exogenous LTB4 on phagocytosis and fungicidal activity in vitro during infection with P. brasiliensis.
Peritoneal WT and 5-LO−/− macrophages were treated with LTB4 (100 mM) for 30 min before the addition of P. brasiliensis yeasts. Phagocytic index (A) and intracellular killing (B) were calculated 24 h after incubation. Killing assay was normalized according initial ingestion (PI) and presented as P. brasiliensis survival. The supernatants were harvested and nitrite concentrations (C) were measured. (D) ROS concentrations were determined by mean fluorescence intensity (DHR-123) in macrophage cultures after infection. Each value represents the mean ± SEM of triplicate cultures from two independent experiments. Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) compared to WT infected group. Symbol † represents significant difference (††p<0.01, †††p<0.001) between the same experimental group compared to different culture conditions.
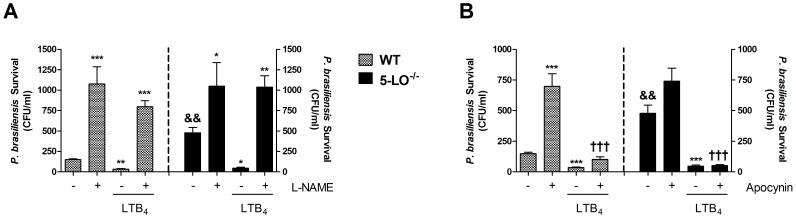
LTB4 increases nitric oxide-dependent killing of P. brasiliensis
As the results described above indicated that decreased NO and ROS generation was associated with a reduction in the ability of macrophages to kill P. brasiliensis yeasts, we evaluated the effect of LTB4 in modulating macrophage antifungal activity by these reactive species. For these experiments, macrophages were pretreated with L-NAME (1 mM), a NO synthase inhibitor or apocynin (1 mM), a NADPH oxidase inhibitor, and then stimulated with LTB4. As shown in Figure 9A and B, inhibition of NO or ROS production abolished the ability of WT- or 5-LO−/− macrophages to control P. brasiliensis infection, increasing the number of viable yeasts. In the presence of L-NAME, exogenous LTB4 failed to overcome this defect (Figure 9A). However, inhibition of ROS by apocynin increased P. brasiliensis survival in macrophages and this phenotype was reversed by treatment with LTB4 (Figure 9B). Taken together, these data suggest that NO generated by iNOS activity plays an important role in the enhanced killing induced by LTB4 in macrophages.
Figure 9. Role of NO and ROS in the fungal killing induced by LTB4 in macrophages.
Alveolar macrophages were pretreated with the iNOS inhibitor L-NAME (1 mM) (A) or the NADPH oxidase inhibitor apocynin (1 mM) (B) for 30 minutes before the addition of P. brasiliensis yeasts. Cultures were added with or without LTB4 (100 mM) 30 minutes later. Fungicidal assay was performed and fungal survival was represented as number of colony forming units (CFU) per ml of culture. Data are expressed as the mean ± SEM of triplicate cultures from one experiment. Symbol * represents significant difference (*p<0.05, **p<0.01, ***p<0.001) compared to untreated macrophages. Symbol † represents significant difference (†††p<0.001) compared to macrophages culture treated with the respective inhibitor. Symbol & represents significant difference (&&p<0.01) compared with the same culture condition in WT macrophages.
Discussion
The present study demonstrates the effects and mechanisms by which 5-LO and its metabolic products positively participate in host defense against the pathogenic fungus P. brasiliensis. Infection with P. brasiliensis was uniformly fatal in 5-LO-deficient mice and the mechanisms accounting for this phenotype were associated with exacerbated lung pathology and higher pulmonary fungal load. The susceptibility of 5-LO−/− mice was also correlated with lower macrophage activation, resulting in reduced phagocytosis and NO dependent-killing of P. brasiliensis yeasts. Exogenous LTB4 restored these functions in 5-LO-deficient macrophages. Previous studies have shown that in a model of Histoplasma capsulatum infection, another important pathogenic dimorphic fungus, reductions in leukotriene synthesis caused by MK 886 administration, a 5-LO pathway inhibitor [35], or in 5-LO-deficient mice [36], resulted in significant mortality in mice and increased lung CFU at 7 and 14 days post infection. These results are consistent with a pivotal role of 5-LO metabolites in the protective host response seen in this model of P. brasiliensis infection.
Leukotrienes are chemotactic for neutrophils [37] and T lymphocytes [38] and can also contribute to leukocyte recruitment indirectly by augmenting cytokines and chemokines production [39]. Thus, we assessed whether the increased susceptibility of 5-LO−/− mice was associated with the leukocyte recruitment profile to the site of infection. While in WT mice neutrophil influx into pulmonary parenchyma peaked at 24 h after infection, in 5-LO−/− mice this influx was continued, resulting in exacerbated lung inflammation and increased pulmonary tissue damage. Previous studies have demonstrated that neutrophil recruitment into the lungs occurs in a sequence of events involving their sequestration in pulmonary vessels, migration from blood to interstitium and then migration from the interstitium to alveolar spaces across epithelial cells. Cytokines (TNF-α, IL-1β, and IL-6), chemokines (CXCL-1 and CXCL-2) and lipid mediators such LTB4 and platelet-activating factor (PAF), triggered by activation of resident cells in the inflamed area, regulate this migration in concert with cell adhesion molecules [40]. It is clear that different mechanisms account for the accumulation of neutrophils in the interstitium and in airspaces in models of pulmonary inflammation [41]. Therefore, one can envisage the situation observed in the current study in which neutrophil accumulation in airspace is decreased, but not in lung tissue. These results demonstrate clearly that 5-LO-derived molecules, likely LTB4, are crucial for transepithelial migration but not to accumulation in the lung parenchyma.
Neutrophil and macrophage recruitment into the pulmonary parenchyma was not sufficient to control P. brasiliensis, as 5-LO−/− mice had greater number of viable yeasts recovered in the lungs. It is interesting that, despite the high number of macrophages in lung tissues of 5-LO−/− mice 24 h after infection, these cells seemed to be less activated. Hence, macrophages of 5-LO−/− mice did not up-regulate MHC-II expression upon P. brasiliensis infection as their WT counterparts. Macrophages are believed to be important in the initial containment of the microorganisms through nonspecific or natural immune mechanisms, and the expression of MHC-II in macrophages is indicative of a preserved and active macrophage function in patients with PCM, in addition to its essential function in antigen presentation [42], [43]. Therefore, these data suggest that 5-LO-derived metabolites are essential for macrophage activation and function during interaction with P. brasiliensis yeasts.
Cytokine production during acute infection in murine PCM is extremely important because it influences the fate of immune response, which may lead to susceptibility or resistance [44]–[50]. Experimental studies and patients with PCM indicate that resistance to infection is dependent on the activity of T helper cells and macrophages/monocytes mediated by IFN-γ and TNF-α. The synergistic effect between these two cytokines is essential for the activation of the host cells and efficiently fungicidal activity against P. brasiliensis [51], [52]. In accordance, our results suggested that after 24 h of infection, there is lower TNF-α and IFN-γ production in alveolar space of 5-LO−/− mice. These decreased cytokines production may have contributed to increased susceptibility to P. brasiliensis infection, associated with minor cell activation (lower expression of MHC-II in 5-LO−/− macrophages) and failure in fungicidal activity. These latter defects in combination would result in elevated pulmonary fungal burden. It was interesting to note that there was a delayed production of TNF-α in 5-LO−/− mice. If lack of early response may contribute to inability to deal with infection, late production may have contributed to the enhanced inflammatory response and tissue injury. Although TNF-α is predominantly produced by activated mononuclear phagocytes in vivo, a number of other resident and inflammatory cells, including endothelial cells, mast cells, smooth muscle cells, B cells, and T cells, can secrete TNF-α [53]. Based on our findings, it appears that if the initial response by macrophages fails, other pulmonary cells may release this and potentially other cytokines in response to high fungal burden in the lungs of 5-LO−/− mice.
Conversely, we observed an important increase of cytokines production, TNF-α, IFN-γ, IL-1β, IL-6 and CXCL-1/2 in lung tissue of 5-LO−/− mice. Therefore, the higher amounts of these cytokines and chemokines, together with the neutrophil accumulation in the lungs of 5-LO-deficient mice, might contribute to exacerbation of inflammatory response leading to tissue damage and loss of lung functions. IL-10 is considered the most important anti-inflammatory cytokine since it may reduce the production of inflammatory mediators and suppress effector functions of macrophages and other immune cells. Given that our results showed reduced concentration of IL-10 in the lungs homogenates of 5-LO−/− mice during infection with P. brasiliensis, this may have contributed to the exacerbated production of pro-inflammatory cytokines and chemokines observed in these animals.
Lipoxins, such as lipoxin A4 (LXA4), are 5-LO-derived products which decrease macrophage function and which have known anti-inflammatory activity in vivo. In the absence of 5-LO, there could also be decreased LXA4 production and consequent facilitation of inflammation. This molecule was not investigated here as we focused on the leukotriene pathway and we, therefore, cannot discard the involvement of LXA4 in our system. Specific blockers of LXA4 production or action are not widely available, making it difficult to examine the exact role of this molecule in vivo. Future studies should define the role of LXA4 as specific tools become available.
In this study, we used both genetic and pharmacologic approaches to demonstrate that endogenously produced LTB4 promoted macrophage phagocytosis and P. brasiliensis killing. We observed that both macrophages elicited from the peritoneum or derived from the lungs of 5-LO−/− mice exhibited reduced phagocytosis and fungal killing. The same phenotype was found when peritoneal macrophages were pretreated with zileuton, a drug that block 5-LO activity. It is likely that LTB4 is the endogenous 5-LO product responsible for this effect, since LTB4 receptor antagonist, but not cysteinyl-LT receptor one, also reduced macrophage activities. Moreover, exogenous LTB4 was capable of restoring antifungal functions of macrophages to levels similar to observed in WT macrophages. Our results are consistent with published data showing the capacity of LTB4 to induce polymorphonuclear neutrophils and macrophages phagocytosis [44]–[46]. Recently, it was demonstrated that LTB4 and its high affinity receptor BLT1 signaling are central determinants of dectin-1 expression and of host responses to fungi [54]. Dectin-1, a C-type lectin, is the major receptor on macrophages for β-1,3-glucan, a polymer of glucose present in the fungal cell wall that stimulates phagocytosis, production of inflammatory cytokines and reactive species [55]. Dectin-1 engagement also activates phospholipase A2 with subsequent production of eicosanoid lipid mediators including cyclooxygenase-derived prostanoids and 5-LO-derived leukotrienes such as LTB4 [56], [57]. Previous studies highlighted the participation of dectin-1 in P. brasiliensis recognition, internalization and consequent activation of the immune response against the fungus [58]. Future research should detail whether the expression of this receptor is controlled by 5-LO and its metabolites in macrophages and in vivo.
The initial contact of P. brasiliensis to the host is handled by phagocytic cells, which constitute an important part of innate defense. The fungus is internalized by macrophages in vivo and in vitro, and multiplies in monocytes and macrophages that are not activated. Only monocytes and macrophages activated with IFN-γ and TNF-α have fungicidal activity against P. brasiliensis [59]. The results presented here demonstrated that P. brasiliensis-macrophage interaction induced high LTB4 production. Interestingly, we verified that IFN-γ-primed macrophages enhanced LTB4 levels. Also, it appears that IFN-γ and P. brasiliensis had a synergistic effect on macrophages since the two stimuli together enhanced LTB4 concentrations in cultures. Our study demonstrates for the first time that IFN-γ induces and facilitates production of LTB4 by macrophages during P. brasiliensis infection in vitro. Therefore, our work contributes substantially to the knowledge of the literature, indicating that the participation of IFN-γ in the control mechanisms of infection by macrophages was partly promoted by the production of LTB4.
One of the characteristics of activated macrophages is the increase in nitric oxide (NO) production. NO, synthesized by induced NO synthase (iNOS), possesses known antimicrobial activity against P. brasiliensis [60]. Through reaction of NO with superoxide anion (O2 −), peroxynitrite (ONOO−) is produced, which is cytotoxic and important for the cellular immunity. Macrophages also produce hydrogen peroxide (H2O2), which is an important fungicidal agent [61]. Previous studies have shown that leukotrienes enhance reactive species production in macrophages infected with many microorganisms [19], [36], [44], [62]. A significant increase in NO and ROS production was observed in WT macrophage cultures incubated with P. brasiliensis after activation with IFN-γ. There was decreased levels of NO and ROS in infected 5-LO-deficient macrophages. This phenotype observed in 5-LO−/− macrophages was reversed by LTB4. We also observed increased NO and ROS production in WT macrophages cultures after LTB4 addition at levels similar to macrophages activated with IFN-γ. Therefore, our data show that activation of 5-LO contributed to enhanced macrophage activity by facilitating the generation of NO and ROS. Interestingly, we showed that clearance of P. brasiliensis is dependent on NO production only, as macrophages treated with apocynin (NADPH oxidase inhibitor) but not with L-NAME (iNOS inhibitor) reversed the killing after LTB4 addition in cultures. Therefore, our data suggest that NO generated by iNOS activity plays an important role for the enhanced antifungal activity induced by LTB4 in macrophages. ROS production is also dependent on 5-LO-induced LTB4 but is not important for killing of the fungus.
Recently, two studies have been published investigating the involvement of the LT pathway in P. brasiliensis infection. These studies differ with respect to the role of these mediators during infection. The first one showed that levels of leukotrienes and lipoxins were increased in WT-infected mice and that 5-LO−/− mice exhibited diminished fungal growth and increased survival rate compared to WT mice. These data indicate that 5-LO derived products might have a critical role in the exacerbation and severity of PCM [28]. Another study investigated the involvement of leukotrienes in the early stages of experimental PCM in selected mouse lines endowed with maximal or minimal acute inflammatory reactivity, and designated AIRmax and AIRmin, respectively [29]. Mice treated with the 5-LO inhibitor (MK 886) presented increased fungal recovery, showing that endogenous production of leukotrienes is associated with a protective immune response. Our study differs in several aspects to the first one [28], especially regarding the route of infection. Our murine experimental model used the intratracheal route that better mimics the infection in the human host. This experimental design allowed us to evaluate the acute inflammatory response triggered against the fungus while the model described in the previous study, developed from the intravenous infection, allowed to investigate chronic/systemic aspects of P. brasiliensis infection. Therefore, it appears that innate immune response differs depending on the initial organ infected by the microorganism and the route of inoculation is important to determine this response in host. Also, our results reinforce the data presented in the second study [29] since we found that leukotrienes, especially LTB4, are involved in the mechanisms that control fungal infection. We verified that 5-LO products play an important role in inflammatory cell recruitment and cytokines production. Importantly, LTB4 released in the lungs could activate resident cells and enhance macrophages function, like phagocytosis and NO-dependent fungal killing, contributing to resistance of host against P. brasiliensis infection. In addition, our study provides important mechanistic insights into the participation of 5-LO-derived products during P. brasiliensis infection.
In summary, we have shown that the absence of 5-LO resulted in death of the host infected with P. brasiliensis. Increased mortality was associated with changes in the recruitment of inflammatory cells in the early stages of infection, fungal proliferation and exacerbated lung inflammation. Absence or blockade of 5-LO decreased phagocytosis and NO-dependent fungicidal activity of macrophages. Furthermore, it is suggested that LTs, especially LTB4, are the major 5-LO-dependent mediator of P. brasiliensis phagocytosis and killing. These findings indicate that an intact 5-LO system is required for efficient pulmonary antifungal host defense against P. brasiliensis infection.
Supporting Information
Phagocytic and fungicidal activity during infection with P. brasiliensis in vitro . Alveolar macrophages were harvested from lung lavage of WT and 5-LO−/− mice and infected in vitro with P. brasiliensis yeasts. Phagocytic index (A) and intracellular killing (B) were calculated 24 h after incubation. Survival was calculated considering the remaining colony forming units (CFU) from the killing assay in function of the phagocytic index (PI). The supernatants were harvested and nitrite concentrations (C) were measured. (D) ROS concentrations were determined by mean fluorescence intensity (DHR-123) in macrophage cultures after infection. Each value represents the mean ± SEM of triplicate cultures from one experiment. Symbol * represents significant difference (**p<0.01, ***p<0.001) compared to WT infected group. Symbol † represents significant difference (†p<0.05, ††p<0.01, †††p<0.001) between WT and 5-LO−/− macrophages at the same culture conditions.
(TIF)
Acknowledgments
We thank Mirla Carolina Braga, Ilma Marçal and Gilvânia Ferreira da Silva (ICB/UFMG) for technical assistance.
Funding Statement
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PROEX project 0202088), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (PPM-00485-09), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (303084/2012-0) and Instituto Nacional de Ciência e Tecnologia em Dengue (INCT-Dengue)(573876/2008-8). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brummer E, Castaneda E, Restrepo A (1993) Paracoccidioidomycosis: an update. Clin Microbiol Rev 6: 89–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theodoro RC, Teixeira M de M, Felipe MS, Paduan K dos S, Ribolla PM, et al. (2012) Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS One 7 5: e37694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prado M, Da Silva MB, Laurenti R, Travassos LR, Taborda CP (2009) Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 104 3: 513–521. [DOI] [PubMed] [Google Scholar]
- 4. Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR (2008) The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a road map for control and elimination. PLoS Negl Trop Dis 2 9: e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matinez R (2010) Paracoccidioidomycosis: the dimension of the problem of a neglected disease. Rev Soc Bras Med Trop 43 4: 480. [DOI] [PubMed] [Google Scholar]
- 6. McEwen JG, Bedoya V, Patino MM, Salazar ME, Restrepo A (1987) Experimental murine paracoccidioidomycosis induced by the inhalation of conidia. J Med Vet Mycol 25: 165–175. [DOI] [PubMed] [Google Scholar]
- 7. Ramos ESM, Saraiva LE (2008) Paracoccidioidomycosis. Dermatol Clin 26: 257–269. [DOI] [PubMed] [Google Scholar]
- 8. Oliveira SJ, Mamoni RL, Musatti CC, Papaiordanou PM, Blotta MH (2002) Cytokines and lymphocyte proliferation in juvenile and adult forms of paracoccidioidomycosis: comparison with infected and non-infected controls. Microbes Infect 4: 139–144. [DOI] [PubMed] [Google Scholar]
- 9. Karhawi AS, Colombo AL, Salomao R (2000) Production of IFN-gamma is impaired in patients with paracoccidioidomycosis during active disease and is restored after clinical remission. Med Mycol 38: 225–229. [DOI] [PubMed] [Google Scholar]
- 10. Benard G, Romano CC, Cacere CR, Juvenale M, Mendes-Giannini MJ, et al. (2001) Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosupression associated with human paracoccidioidomycosis. Cytokine 13: 248–252. [DOI] [PubMed] [Google Scholar]
- 11. Kashino SS, Fazioli RA, Cafalli-Favati C, Meloni-Bruneri LH, Vaz CA, et al. (2000) Resistance to Paracoccidioides brasiliensis infection is linked to a preferential Th1 immune response, whereas susceptibility is associated with absence of IFN-gamma production. J Interferon Cytokine Res 20: 89–97. [DOI] [PubMed] [Google Scholar]
- 12.Calich VL, Blotta MHSL (2005) Pulmonary paracoccidioidomycosis. In: Fidel PL, Huffnagle GB, editors. Fungal Immunology. New York, Springer. pp. 201–227.
- 13. Benard G (2008) An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 165: 209–221. [DOI] [PubMed] [Google Scholar]
- 14. Calvi SA, Peraçoli MT, Mendes RP, Marcondes-Machado J, Fecchio D, et al. (2003) Effect of cytokines on the in vitro fungicidal activity of monocytes from paracoccidioidomycosis patients. Microbes Infect 5: 107–13. [DOI] [PubMed] [Google Scholar]
- 15. Peters-Golden M, Henderson WRJ (2007) Leukotrienes. N Engl J Med 357: 1841–1854. [DOI] [PubMed] [Google Scholar]
- 16. Peters-Golden M, Brock TG (2003) 5-Lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids 69: 99–109. [DOI] [PubMed] [Google Scholar]
- 17. Rinaldo-Mattis A, Haeggström JZ (2010) Structures and mechanisms of enzymes in the leukotriene cascade. Biochimie 92: 676–681. [DOI] [PubMed] [Google Scholar]
- 18. Peters-Golden M, Canetti C, Mancuso P, Coffey MJ (2005) Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 174: 589–594. [DOI] [PubMed] [Google Scholar]
- 19. Mancuso P, Marshall T, Standiford T, Peters-Golden M (1998) 5-Lipoxigenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumonia . Infect Immun 66: 5140–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Medeiros AI, Silva CL, Malheiro A, Maffei CML, Faccioli LH (1999) Leukotrienes are involved in leukocyte recruitment induced by live Histoplasma capsulatum or by the beta-glucan present in their cell wall. Br J Pharmacol 128: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcinkiewicz J, Grabowska A, Bryniarski K, Chain BM (1997) Enhancement of CD4+ T-cell-dependent interleukin-2 production in vitro by murine alveolar macrophages: the role of leukotriene B4. Immunology 91: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter RM, et al. (1996) Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocitic and bactericidal activities. J Immunol 157: 5221–5224. [PubMed] [Google Scholar]
- 23. Mancuso P, Casey L, Serezani CH, Goel D, Peters-Golden M (2010) Intrapulmonary administration of leukotriene B4 enhances pulmonary host defence against pneumococcal pneumonia. Infect Immun 78: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peres CM, De Paula L, Medeiros AI, Sorgi CA, Soares EG, et al. (2007) Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis . Microbes Infect 9 4: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Medeiros AI, Sa-Nunes A, Turato WM, Secatto A, Frantz FG, et al. (2008) Leukotrienes are potent adjuvant during fungal infection: effects on memory T cells. J Immunol 181: 8544–8551. [DOI] [PubMed] [Google Scholar]
- 26. Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S (2006) Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J Immunol 177: 3201–3208. [DOI] [PubMed] [Google Scholar]
- 27. Panis C, Mazzuco TL, Costa CZF, Victorino VJ, Tatakihara VLH, et al. (2011) Trypanosoma cruzi: effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on levels of cytokines, nitric oxide and iNOS expression in cardiac tissue in the acute phase of infection in mice. Experimental Parasitology 127: 58–65. [DOI] [PubMed] [Google Scholar]
- 28. Tristão FS, Rocha FA, Moreira AP, Cunha FQ, Rossi MA, et al. (2013) 5-Lipoxygenase activity increases susceptibility to experimental Paracoccidioides brasiliensis infection. Infect Immun 81 4: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balderramas HA, Ribeiro OG, Soares AMVC & Oliveira LO (2013) The role of leukotriene B4 in early stages of experimental paracoccidioidomycosis induced in phenotypically selected mouse strains. Medical Mycology Early Online: 1–10. [DOI] [PubMed]
- 30. Chen XS, Sheller JR, Jonhson EM, Funk CD (1994) Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature 372: 179–182. [DOI] [PubMed] [Google Scholar]
- 31. Kurita N, Sano A, Coelho KI, Nishimura K, Miyaji M (1993) An improved culture médium for detecting live yeast phase cells of Paracoccidiodies brasiliensis . J Med Vet Mycol 31: 201–205. [DOI] [PubMed] [Google Scholar]
- 32. Buissa-Filho R, Puccia R, Marques AF, Pinto FA, Muñoz JE, et al. (2008) The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus. Infect Immun 76 7: 3321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kooy NW, Royall JA, Ischiropoulos H, Beckman JS (1994) Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16: 149–156. [DOI] [PubMed] [Google Scholar]
- 34. Carter G, Young P, Albert D, Bouska J, Dyer R, et al. (1991) 5-lypoxigenase inhibitory activity of zileuton. J Pharmacol Exp Ther 256: 929–937. [PubMed] [Google Scholar]
- 35. Medeiros AI, Sa-Nunes A, Soares EG, Peres CM, Silva CL, et al. (2004) Blockade of endogenous leukotrienes exarcebates pulmonary histoplasmosis. Infect Immun 72: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Secatto A, Rodrigues LC, Serezani CH, Ramos SG, Dias-Baruffi M, et al. (2012) 5-Lipoxygenase Deficiency Impairs Innate and Adaptive Immune Responses during Fungal Infection. PLoS One 7 3: e31701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, et al. (2003) Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment,. Nature Immunol 4: 982–990. [DOI] [PubMed] [Google Scholar]
- 38. Gagnon L, Filion LG, Dubois C, Rola-Pleszczinski M (1989) Leukotrienes and macrophage activation: augmented cytotoxic activity and enhanced interleukin 1, tumor necrosis factor and hydrogen peroxide production. Agents Actions 26: 141–147. [DOI] [PubMed] [Google Scholar]
- 39. Huang L, Zhao A, Wong F, Ayala JM, Struthers M, et al. (2004) Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol 24: 1783–1788. [DOI] [PubMed] [Google Scholar]
- 40. Perreti M, Getting SJ (2003) Migration of specific leukocyte subsets in response to cytokine or chemokine application in vivo . Methods Mol Biol 225: 339–346. [DOI] [PubMed] [Google Scholar]
- 41. Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffê E, et al. (2009) Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol 40 4: 410–21. [DOI] [PubMed] [Google Scholar]
- 42. Bocca AL, Silva MF, Silva CL, Cunha FQ, Figueiredo F (1999) Macrophage expression of class II major histocompatibility complex gene products in Paracoccidioides brasiliensis–infected mice. Am J Trop Med Hyg 61 2: 280–287. [DOI] [PubMed] [Google Scholar]
- 43. Gordon S (2003) Alternative activation of macrophages. Nature Reviews Immunology 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 44. Talvani A, Machado FS, Santana GC, Klein A, Barcelos L, et al. (2002) Leukotriene B4 induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infect Immun 70: 4247–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mancuso P, Nana-Sinkan P, Peters-Golden M (2001) Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae . Infect Immun 69: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morato-Marques M, Campos MR, Kane S, Rangel AP, Lewis C, et al. (2011) Leukotrienes target F-actin/Cofilin-1 to enhance alveolar macrophage anti-fungal activity. J Biol Chemistry 286: 28902–28913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, et al. (2000) Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol 156: 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souto JT, Aliberti JC, Campanelli AP, Livonesis MC, Maffei CM, et al. (2003) Chemokine production and leukocyte recruitment to the lungs of Paracoccidioides brasiliensis-infected mice is modulated by interferon-gamma. Am J Pathol 163: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singer-Vermes LM, Caldeira CB, Burger E, Calich LG (1993) Experimental murine paracoccidioidomycosis: relationship among the dissemination of the infection, humoral and cellular immune responses. Clin Exp Immunol 94: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pina A, Saldiva PH, Restrepo LE, Calich VL (2006) Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol 79: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 51. Calvi SA, Peraçoli MT, Mendes RP, Marcondes-Machado J, Fecchio D, et al. (2003) Effect of cytokines on the in vitro fungicidal activity of monocytes from paracoccidioidomycosis patients. Microbes Infect 5: 107–13. [DOI] [PubMed] [Google Scholar]
- 52. Kurokawa CS, Araujo JP Jr, Soares AM, Sugizaki MF, Peraçoli MT (2007) Pro- and anti-inflammatory cytokines produced by human monocytes challenged in vitro with Paracoccidioides brasiliensis . Microbiol Immunol 51: 421–8. [DOI] [PubMed] [Google Scholar]
- 53. Calich VL, Vaz CAC, Burguer E (1985) PMN chemotatic factor produced by glass-adherent cells in the acute inflammation caused by Paracoccidiodies brasiliensis . J Exp Pathol 66: 585–594. [PMC free article] [PubMed] [Google Scholar]
- 54. Serezani CH, Kane S, Collins L, Morato-Marques M, Osterholzer JJ, et al. (2012) Macrophage dectin-1 expression is controlled by leukotriene B4 via a GM-CSF/PU.1 axis. J Immunol 189 2: 906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reid DM, Gow NA, Brown GD (2009) Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol 21: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, et al. (2006) Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J Biol Chem 281: 6691–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morato-Marques M, Campos MR, Kane S, Rangel AP, Lewis C, et al. (2011) Leukotrienes target F-actin/cofilin-1 to enhance alveolar macrophage antifungal activity. J Biol Chem 286: 28902–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonfim CV, Mamoni RL, Blotta MH (2009) TLR-2, TLR-4 and dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis . Med Mycol 47 7: 722–33. [DOI] [PubMed] [Google Scholar]
- 59. Brummer E, Hanson LH, Restrepo A, Stevens DA (1988) In vivo and in vitro activation of pulmonary macrophages by IFN-gamma for enhanced killing of Paracoccidioides brasiliensis or Blastomyced dermatitides . J Immunol 140: 2786–2789. [PubMed] [Google Scholar]
- 60. González A, De Gregori W, Vélez D, Restrepo A, Cano LE (2000) Nitric oxide participation in the fungicidal mechanism of gamma-interferon-activated murine macrophages against Paracoccidioides brasiliensis conidia. Infect Immun 68: 2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moreira AP, Dias-Melicio LA, Peraçoli MTS, Calvi SA, Soares AMVC (2008) Killing of Paracoccidiodies brasiliensis yeast cells by IFN-gamma and TNF-alpha activated murine peritoneal macrophages: evidence of H2O2 and NO effector mechanisms. Mycopathologia 166: 17–23. [DOI] [PubMed] [Google Scholar]
- 62. Larfars G, Lantoine F, Devynck MA, Palmblad J, Gyllenhammar H (1999) Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood 93: 1399–1405. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phagocytic and fungicidal activity during infection with P. brasiliensis in vitro . Alveolar macrophages were harvested from lung lavage of WT and 5-LO−/− mice and infected in vitro with P. brasiliensis yeasts. Phagocytic index (A) and intracellular killing (B) were calculated 24 h after incubation. Survival was calculated considering the remaining colony forming units (CFU) from the killing assay in function of the phagocytic index (PI). The supernatants were harvested and nitrite concentrations (C) were measured. (D) ROS concentrations were determined by mean fluorescence intensity (DHR-123) in macrophage cultures after infection. Each value represents the mean ± SEM of triplicate cultures from one experiment. Symbol * represents significant difference (**p<0.01, ***p<0.001) compared to WT infected group. Symbol † represents significant difference (†p<0.05, ††p<0.01, †††p<0.001) between WT and 5-LO−/− macrophages at the same culture conditions.
(TIF)