Abstract
Cross-talk between the estrogen and the EGFR/HER signalling pathways has been suggested as a potential cause of resistance to endocrine therapy in breast cancer. Here, we determined HER1-4 receptor and neuregulin-1 (NRG1) ligand mRNA expression levels in breast cancers and corresponding normal breast tissue from patients previously characterized for plasma and tissue estrogen levels. In tumours from postmenopausal women harbouring normal HER2 gene copy numbers, we found HER2 and HER4, but HER3 levels in particular, to be elevated (2.48, 1.30 and 22.27 –fold respectively; P<0.01 for each) compared to normal tissue. Interestingly, HER3 as well as HER4 were higher among ER+ as compared to ER- tumours (P=0.004 and P=0.024, respectively). HER2 and HER3 expression levels correlated positively with ER mRNA (ESR1) expression levels (r=0.525, P=0.044; r=0.707, P=0.003, respectively). In contrast, EGFR/HER1 was downregulated in tumour compared to normal tissue (0.13-fold, P<0.001). In addition, EGFR/HER1 correlated negatively to intra-tumour (r=-0.633, P=0.001) as well as normal tissue (r=-0.556, P=0.006) and plasma estradiol levels (r=-0.625, P=0.002), suggesting an inverse regulation between estradiol and EGFR/HER1 levels. In ER+ tumours from postmenopausal women, NRG1 levels correlated positively with EGFR/HER1 (r=0.606, P=0.002) and negatively to ESR1 (r=-0.769, P=0.003) and E2 levels (r=-0.542, P=0.020). Our results indicate influence of estradiol on the expression of multiple components of the HER system in tumours not amplified for HER2, adding further support to the hypothesis that cross-talk between these systems may be of importance to breast cancer growth in vivo.
Introduction
Breast cancer is the most frequent cancer among women world-wide. Estradiol (E2) stimulation through the estrogen receptor (ER) and constitutional HER2 proto-oncogene hyperactivity represent two pivotal pathways regulating breast cancer growth. Breast cancers expressing the ER in general belongs to either the luminal A or B sub-class, each characterized by a distinct gene expression profile [1]. Moreover, between 16 and 20% of all breast cancers are amplified for the HER2 proto-oncogene; these tumours in general belong to the so-called “HER2” class, again characterized by a distinct gene expression profile [1]. The importance of ER and HER2 activation to breast cancer growth is underlined by improved outcome for patients with advanced ER+ and HER2 amplified early breast cancers treated with an aromatase inhibitor and anti-HER2 therapy in concert [2,3].
Similar to what has been recorded for chemotherapy, de novo and acquired drug resistance become the main obstacles to cure by anti-hormonal as well as anti-HER2 therapies. Cross-talk between the HER2- and ER-downstream gene activation pathways has been suggested as a cause of endocrine resistance [4]. Notably, two studies [2,3] revealed improved time to progression for ER+ HER2 amplified metastatic breast cancers having either trastuzumab or lapatinib added to treatment with an aromatase inhibitor. However the benefit of HER2 targeting treatment in HER2 non-amplified breast cancer is not well known. In vitro models of acquired endocrine resistance show modest upregulation of HER2 [5,6] and, interestingly, lapatinib has been shown to restore endocrine sensitivity in these cell lines [5]. Moreover, previous data from our group indicate that HER2 may be upregulated during estrogen deprivation in breast tumours [7]. Further, experimental evidence has implicated EGFR/HER1 as well as HER3 and HER4 to endocrine resistance [8,9]. In general, ER and HER2 positivity are known to be inversely correlated [10–12]. However, while most ER+ tumours are non-amplified for HER2, nearly 50% of all HER2 amplified tumours express ER at moderate to low concentrations [13,14], and recently, HER2 and ER have been shown to be positively correlated in HER2 non-amplified tumours [15]. Taken together, these findings indicate a potential role for HER2 as well as other components of the HER-receptor family in HER2 non-amplified breast cancer and endocrine resistance [16].
In this study, we aimed to further explore potential associations between estrogen levels and expression levels of the HER-family members in HER2 non-amplified breast cancer and in normal breast tissue. To do so, we analysed HER-1–4 and ligand NRG1 mRNA expression pattern in breast cancer and normal breast tissue and correlated findings to ER mRNA expression (ESR1) and plasma, normal tissue and breast cancer tissue estrogen levels previously determined [17,18]. Our findings add novel information, and provides a better understanding of the potential interactions in-between members of the HER system and their regulation by estradiol.
Materials and Methods
Ethics Statements
The study was presented and exempted from review by the Regional Committee for Medical and Health Research Ethics (REK) at the time of collection. All patients provided written informed consent, and the study was performed in accordance to Norwegian law and regulations. After the samples had been collected, each patient was allocated a trial number, demographic data collected, and the database anonymised.
Study population and sample collection
The breast cancer patients included in this study (n = 42) have been described previously [18]. In short, pre- and postmenopausal women with ER+ or ER- breast cancer, selected for mastectomy at the Department of Surgery, Haukeland University Hospital, Bergen, Norway were included. Patients that had taken any hormone replacement therapy within the 4 weeks pre-surgical period were excluded. Tissues obtained from mastectomy specimens, both normal and tumour tissue, were removed and immediately snap-frozen in liquid nitrogen in the operating theatre, before storage in liquid nitrogen until use. Normal tissue was isolated from the breast quadrant farthest from the tumour-containing quadrant in the breast. Blood samples for plasma measurements were obtained at the day of surgery after fasting overnight, and stored at -20°C until use. Normal tissue was available from all but one patient and tumour tissues were available from all but two other patients, leaving 39 patients for statistical comparisons between tumour and normal tissue.
Multiplex Ligation-dependent Probe Amplification (MPLA)
Gene-amplifications of EGFR/HER1 and HER2 were analysed by MLPA using the SALSA MLPA Breast tumour kit (P078-B1; MRC-Holland, Amsterdam, The Netherlands) according to the manufacturer’s instructions. In the patient samples, the peak areas of all MLPA products resulting from EGFR/HER1 and HER2 specific probes were first normalized by the average of peak areas resulting from control probes specific for locations outside of chromosomes 7/17. A ratio was then calculated where this normalized value was divided by the corresponding value from a sample consisting of pooled DNA from 10 healthy individuals. A sample was scored as having a reduced copy number at a specific location if this ratio was below 0.75, and as having an increased copy number if the ratio was above 1.25.
Real time PCR quantification
Total RNA was extracted from ~25 mg tissue using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. The RNA was re-suspended in PCR-grade water and concentrations were estimated by optical density (OD) measurement using the Nanodrop (Saveen Werner, Copenhagen, Denmark). For each sample, 1 μg total RNA was reversely transcribed by the 1st Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) using random primers. The cDNA was diluted 1/10 in PCR-grade water and stored at -20°C until use.
Real time PCR analyses were performed in three parallel runs on a Light Cycler 480 (LC480) thermo cycler (Roche, Basel, Switzerland) and a negative control without any cDNA was included in each run. Gene specific primers and probes were designed using the Universal Probe Library (UPL, Roche, Basel, Switzerland), and all analyses were run in duplex with the TATA-box binding protein (TBP) reference analyse kit using the Probe Master kit (Roche, Basel, Switzerland). Assay with primer sequences and UPL probes are given in Table 1. The amplification reaction mixture consisted of 2.5 µL diluted cDNA, 10 µL LC480 Probe Master mix, 0.4 µmol/L of each target primer, 0.2 µmol/L of target UPL probe, 0.2 µmol/L of TBP reference primers and 0.1 µmol/L TBP reference probe in a total volume of 20µL. Termocycling conditions used were pre-incubation at 95 °C for 10 minutes followed by 45 cycles with denaturation at 95 °C for 10 seconds, primer annealing at 60 °C for 30 seconds and DNA sequence extension at 72 °C for 1 second followed by fluorescence measurement. The PCR products were then cooled at 40 °C. Crossing points (Cp) for both target gene and TBP and the efficiency from standard curves from a serially diluted cDNA sample were used to quantify relative expression levels of each target gene separately. The relative mRNA expression levels are presented as the mRNA expression level of target gene divided by the mRNA expression levels of the reference gene TBP in each single sample.
Table 1. Primer sequences and UPL probes used for real-time PCR.
| Gene | Forward (left) primer | Reverse (right) primer | UPL probe† |
|---|---|---|---|
| EGFR/HER1 | 5'-cagccacccatatgtaccatc-3' | 5'-aactttgggcgactatctgc-3' | 42 |
| HER2 | 5'-ccctgacctgctggaaaag-3' | 5'-ggccgacattcagagtcaat-3' | 43 |
| HER3 | 5'-acagccccagatctgcac-3' | 5'-gttgggcgaatgttctcatc-3' | 9 |
| HER4 | 5'-ttccactttaccacaacatgcta-3' | 5'-cagaatgaagagcccacca-3' | 78 |
| NRG1 | 5'-gatcagcaaattaggaaatgacag-3' | 5'-ggcataccagtgatgatctcg-3' | 53 |
† Universal probe library (UPL) probe number (Roche, Basel, Switzerland).
ESR1 mRNA expression levels in tumours from 28 (9 pre- and 19 postmenopausal) of the 42 patients have been analysed and reported previously [17].
Measurement of estrogen levels
Estrogen levels measured in plasma and the matched normal and tumour tissue samples from 13 premenopausal and 29 postmenopausal women have been reported previously [18]. In brief, estrogen fractions were measured with highly sensitive RIA methods subsequent to pre-analytical purification through LH20 column (plasma) or HPLC (tissue) chromatography [19,20]. Sensitivity limits for the different analysis were 1.14 pmol/L for E1, 0.67 pmol/L for E2, and 0.55 pmol/L for E1S [20].
Statistical analysis
The mRNA expression levels are presented as geometric mean with 95% confidence interval (CI) of the mean. Boxplot and stem-and-leaf plot were used to present median mRNA expression levels, quartiles and outliers within each group. Correlation analyses of the expression of HER-receptors and NRG1 in normal and tumour tissue and the levels of E1, E2 and E1S in normal and tumour tissue and plasma were analysed using the Spearman Rank test. Differences in mRNA expression between related tumour and normal-tissue samples were analysed using the non-parametric Wilcoxon test. Differences in mRNA expression levels between ER+ and ER- or HER2 amplified and non-amplified subjects were analysed using non-parametric Mann-Whitney U rank test of independent samples. All P-values are two-sided and the threshold P-value for statistical significance was 0.05. All analyses were performed using the software SPSS Statistics version 19 (IBM SPSS Statistics).
Results
Patient characteristics and tissue specimens
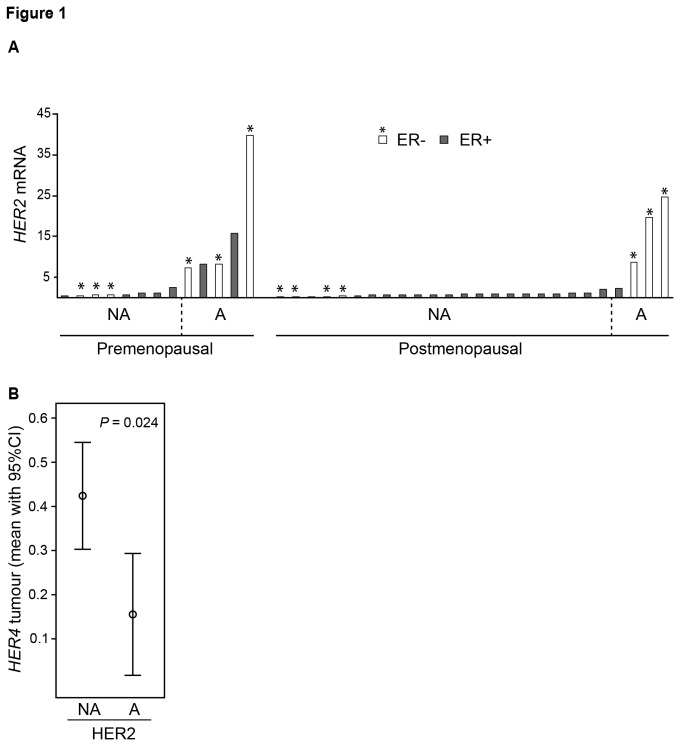
The study population including 42 pre- and postmenopausal breast cancer patients with ER+ and ER- disease (invasive carcinomas) has been described in detail previously (Table 2 [18]). Breast cancer and normal tissue from the same breast were available from 39 of the 42 patients. Nine tumours were amplified (>2 alleles) for the HER2 gene (range 3-14 alleles) and one tumour was amplified for the EGFR/HER1 gene. Tumours harbouring an elevated number of HER2 alleles in general presented high mRNA expression levels of HER2 (Figure 1A). Even though both ER+ (P=0.002) and ER- (P=0.001) tumours exhibit a significant higher HER2 mRNA levels in HER2 amplified compared to non-amplified tumours, the difference in HER2 mRNA levels between amplified and non-amplified tumours were most evident among ER- tumours (Figure 1A). Interestingly, we also observed a decreased HER4 mRNA expression in tumours with HER2 gene amplification compared to non-amplified tumours (P=0.024, Figure 1B) suggesting that increased levels of HER2 may be associated with HER4 suppression.
Table 2. Patient and tumour characteristics.
| Premenopausal (n = 13) | Postmenopausal (n = 29a) | ||
|---|---|---|---|
| Median age (range), y | 41 (31-49) | 61 (44-81) | |
| IHC ER, n | ER+ | 7 | 20 |
| ER- | 6 | 9 | |
| IHC PR, n | PR+ | 7 | 17 |
| PR- | 6 | 12 | |
| MLPA, EGFR/HER1, n | Ampl* | 0 | 1 |
| Non-ampl | 13 | 25 | |
| MLPA, HER2, n | Amplb | 5 | 4 |
| Non-ampl | 8 | 22 |
Abbreviations: IHC, Immunohistochemistry; ER, estrogen receptor; PR, progesterone receptor
a Normal tissue was not available for MLPA and mRNA analyses from one patient and tumour tissues were not available from two other patients
b More than 2 alleles of either HER2 or EGFR were considered amplified.
Figure 1. HER2 tumour levels and classification of HER2 amplified and non-amplified cancers.
A) Relative mRNA expression levels of HER2 in estrogen receptor positive (ER+; black bars) and ER negative (- ; white bars with asterisk) breast tumours from pre- and postmenopausal women. Tumours identified to be HER2 non-amplified (NA) and amplified (A) by MPLA are indicated. B) Relative HER4 levels in HER2 NA and A tumours. Significant difference between A and NA tumours is presented using the Mann-Whitney U test.
Expression of HER receptors and NRG1 in tumour and normal tissue
Considering HER2 amplified tumours as a distinct class, we restricted this analysis to tumours harbouring a normal HER2 copy number. The mRNA expression levels of all analysed genes were found to be log normally distributed except for EGFR/HER1 in normal tissue and HER4 in tumour tissue that were found to be normally distributed.
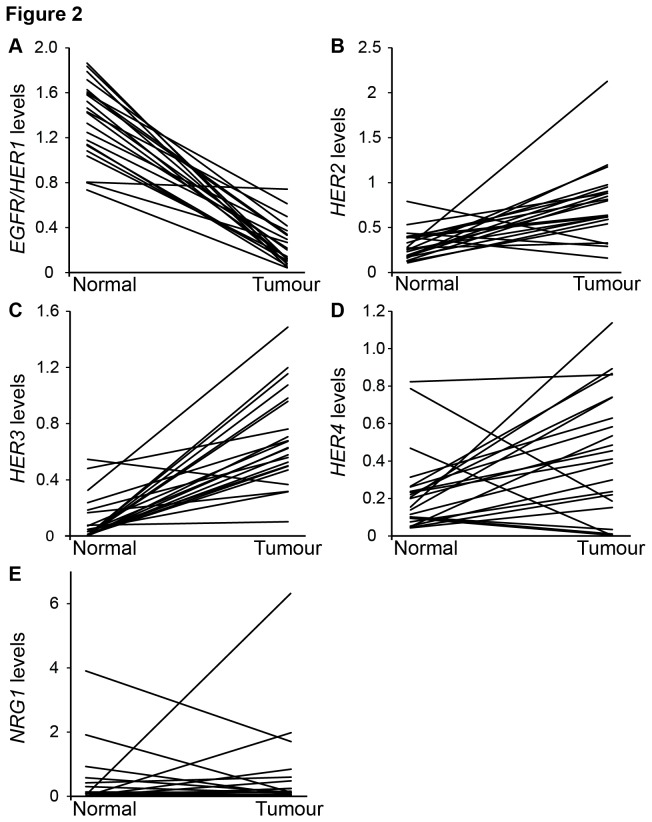
Expression levels of EGFR/HER1, HER2, HER3, HER4 and NRG1 in normal and breast cancer tissue are presented in Table 3 and Figure 2. Comparing paired tumour and normal tissue samples, we found a significantly lower level of EGFR/HER1 in tumour compared to normal tissue both among premenopausal (8 of 8 patients, individual ratio 0.10 (95% CI: 0.047-0.23), P=0.012) as well as postmenopausal (22 of 22, individual ratio: 0.13; CI: 0.09-0.20, P<0.001) women. In contrast, HER2 and HER3 expression levels were higher in tumours compared to normal tissue. Thus, HER2 was elevated in 7 of 8 premenopausal tumours (individual ratio: 2.64; CI: 1.50-4.63, P=0.017) and HER3 in 8 of 8 individuals (individual ratio: 16.96; CI: 4.02-71.52, P=0.012). In postmenopausal tumours, HER2 was elevated in 19 of 22 (individual ratio: 2.48; CI: 1.70-3.65, P<0.001) and HER3 in 21 out of 22 (individual ratio: 22.27; CI: 9.03-54.92, P<0.001). HER4 expression levels were significantly higher in tumours compared to normal tissue among postmenopausal women only (17 of 22, individual ratio: 1.30 CI: 0.54-3.16, P=0.006). No significant difference in NRG1 levels between normal breast and cancer tissue was recorded.
Table 3. HER1-4 and NRG1 levels in normal and breast cancer tissue among postmenopausal patients.
| Gene | Menopause stage | Normal tissue | Tumour tissue | Fold change (95% CI) | Tumour versus normal tissuea | P for changeb |
|---|---|---|---|---|---|---|
| EGFR/HER1 | Pre | 1.31 (1.07-1.62)c | 0.14 (0.058-0.32) | 0.10 (0.047-0.23) | 0↑ 8↓ | P=0.012 |
| Post | 1.32 (1.17-1.48) | 0.18 (0.12-0.26) | 0.13 (0.09-0.20) | 0↑ 22↓ | P≤0.001 | |
| HER2 | Pre | 0.31 (0.20-0.50) | 0.83 (0.52-1.33) | 2.64 (1.50-4.63) | 7↑ 1↓ | P=0.017 |
| Post | 0.27 (0.21-0.34) | 0.66 (0.52-0.85) | 2.48 (1.70-3.65) | 19↑ 3↓ | P≤0.001 | |
| HER3 | Pre | 0.062 (0.015-0.26) | 1.05 (0.45-2.43) | 16.96 (4.02-71.52) | 8↑ 0↓ | P=0.012 |
| Post | 0.027 (0.012-0.061) | 0.60 (0.47-0.78) | 22.27 (9.03-54.92) | 21↑ 1↓ | P≤0.001 | |
| HER4 | Pre | 0.16 (0.10-0.27) | 0.25 (0.096-0.67) | 1.54 (0.69-3.45) | 5↑ 3↓ | P=0.123 |
| Post | 0.16 (0.11-0.23) | 0.23 (0.11-0.50) | 1.30 (0.54-3.16) | 17↑ 5↓ | P=0.006 | |
| NRG1 | Pre | 0.025 (8.76*10-4-0.72) | 0.071 (0.031-0.16) | 2.86 (0.11-77.45) | 4↑ 4↓ | P=0.263 |
| Post | 0.013 (0.0026-0.062) | 0.11 (0.045-0.29) | 8.97 (1.90-42.40) | 14↑ 8↓ | P=0.506 |
a Number of patients with higher (↑) or lower (↓) tumour compared to normal tissue mRNA levels.
b P value from Wilcoxon Signed Ranks test for paired samples.
c mRNA levels presented as geometric mean (95% CI).
Figure 2. Changes in HER1-4 and NRG1 expression levels from normal to tumour breast tissue.
Individual mRNA levels of EGFR/HER1(A), HER2 (B), HER3 (C), HER4 (D) and NRG1 (E) from normal and tumour tissue from each postmenopausal patient.
HER3 expression correlated positively to HER2 (r=0.532, P=0.009) as well as HER4 (r=0.480, P=0.020), but negatively to EGFR/HER1 (r=-0.450, P=0.031, Table 4) in tumour tissue from postmenopausal patients. Interestingly we observed a strong positive correlation between intratumour EGFR/HER1 and NRG1 levels (r=0.606, P=0.002).
Table 4. Correlations between HER-receptors and NRG1 in normal and breast tumour tissue among postmenopausal women.
| Normal tissue |
Tumour |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2 | HER3 | HER4 | NRG1 | EGFR/HER1 | HER2 | HER3 | HER4 | NRG1 | |||
| Normal tissue | EGFR/HER1 | Totala | -0.371 | -0.211 | -0.197 | -0.221 | -0.004 | 0.353 | 0.307 | 0.019 | 0.125 |
| ER+ | -0.183 | -0.093 | -0.153 | -0.154 | 0.017 | 0.290 | 0.222 | -0.195 | 0.082 | ||
| HER2 | Total | 0.616** | 0.444* | 0.681** | 0.199 | -0.205 | -0.398 | -0.103 | 0.106 | ||
| ER+ | 0.527* | 0.488* | 0.665** | 0.084 | -0.001 | -0.294 | 0.106 | 0.162 | |||
| HER3 | Total | 0.182 | 0.817** | 0.343 | -0.197 | -0.269 | -0.220 | 0.091 | |||
| ER+ | 0.212 | 0.911** | 0.232 | -0.055 | -0.137 | 0.121 | 0.189 | ||||
| HER4 | Total | 0.324 | 0.135 | -0.133 | 0.193 | 0.372 | 0.153 | ||||
| ER+ | 0.298 | 0.096 | -0.152 | 0.216 | 0.482* | 0.127 | |||||
| NRG1 | Total | 0.318 | -0.031 | -0.042 | -0.038 | 0.286 | |||||
| ER+ | 0.219 | 0.008 | 0.004 | 0.115 | 0.215 | ||||||
| Tumour | EGFR/HER1 | Total | -0.318 | -0.450* | -0.334 | 0.606** | |||||
| ER+ | -0.271 | -0.321 | -0.092 | 0.744** | |||||||
| HER2 | Total | 0.532** | 0.204 | 0.076 | |||||||
| ER+ | 0.564* | 0.005 | -0.222 | ||||||||
| HER3 | Total | 0.480* | -0.144 | ||||||||
| ER+ | 0.183 | -0.327 | |||||||||
| HER4 | Total | 0.244 | |||||||||
| ER+ | 0.253 | ||||||||||
a The total group of postmenopausal women and the subgroup of ER+ postmenopausal women.
Spearman rank correlation (two-tailed):
*P≤ 0.05
** P≤ 0.01
Correlations between HER receptors / NRG1 expression levels versus ER-status and plasma and tissue estradiol levels
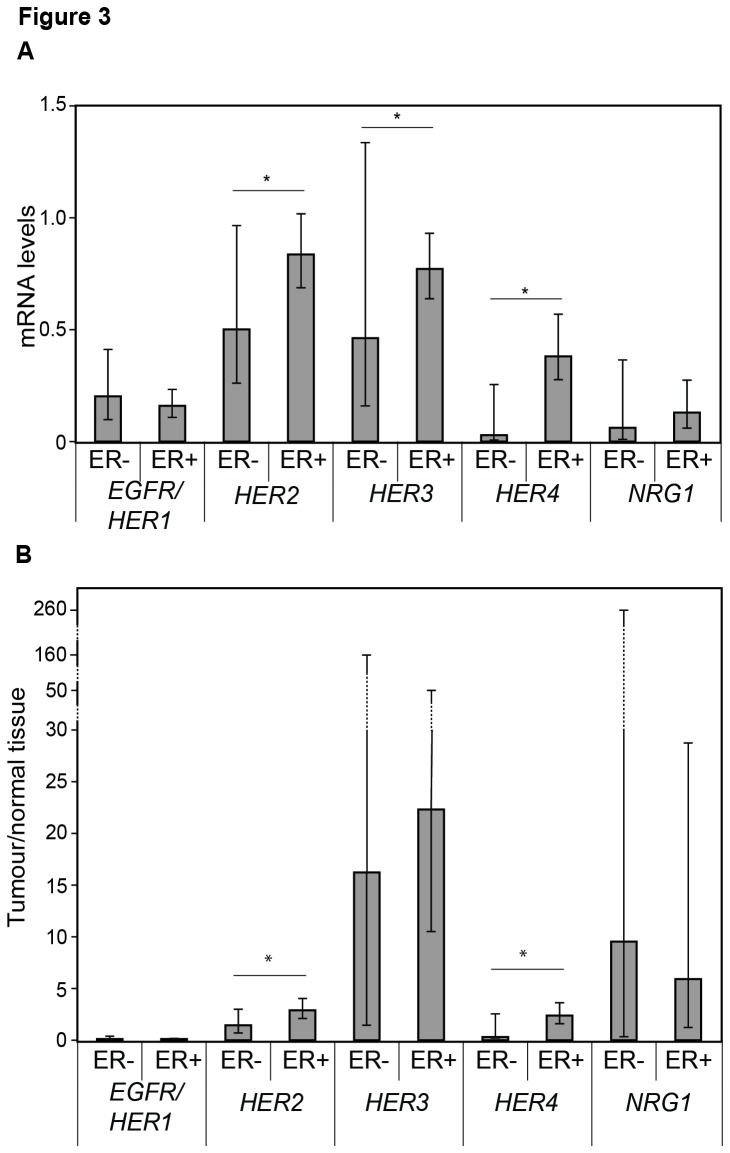
Among all HER2 non-amplified tumours, we found HER2 (P=0.026) in addition to HER3 (P=0.030) and HER4 (P=0.007) to be higher among ER+ as compared to ER- tumours (Figure 3A). Moreover, a higher tumour to normal tissue concentration ratio was observed for HER2 (P=0.042) as well as HER4 (P=0.012) among ER+ tumours as compared to ER- tumours (Figure 3B). In addition, both HER2 (r=0.547, P=0.001, data not shown) and HER4 (r=0.513, P=0.017) correlated positively with ESR1 expression levels in these HER2 non-amplified tumours including both pre- and postmenopausal women. ESR1 mRNA expression levels were obtained from a previous study were high ESR1 expression levels were associated with ER+ tumours [17].
Figure 3. HER1-4 and NRG1 levels related to estrogen receptor status.
Geometric mean with 95% confidence intervals of the HER-receptors and NRG1 in estrogen receptor positive (ER+) and ER negative (-) tumours (A) and intervals of tumour to normal tissue ratio (B) among all patients with HER2 non-amplified disease. Significant differences between ER+ and ER- tumours are presented using the Mann-Whitney U test.
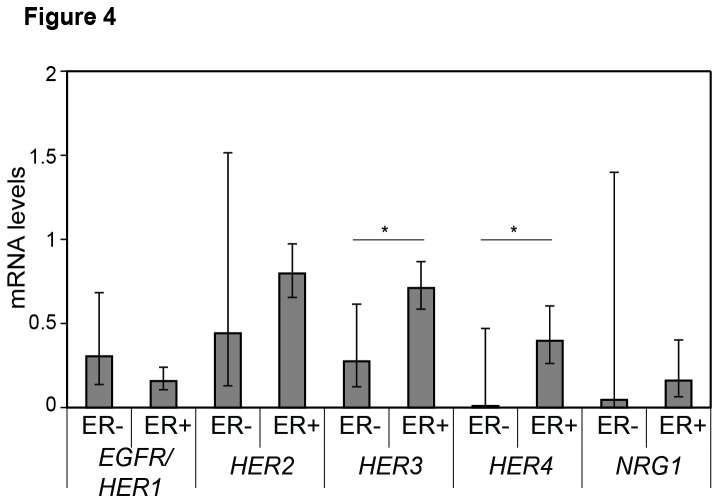
From the subgroup of postmenopausal women, HER3 (P=0.004) and HER4 (P=0.024) were higher among ER+ as compared to ER- tumours (Figure 4). In addition, HER2 (r=0.525, P=0.044, Figure 5A) and HER3 (r=0.707, P=0.003, Figure 5B) correlated positively with ESR1 expression levels in postmenopausal women, and there was a correlation between intratumoural HER3 and E2 levels (r=0.544, P= 0.007, Table 5). Taken together, these data indicate breast cancer tissue HER3 and, potentially, HER2 and HER4 to be associated with estrogen stimulation.
Figure 4. HER1-4 and NRG1 intratumoural levels related to estrogen receptor status among postmenopausal women.
Geometric mean with 95% confidence intervals of EGFR/HER1, HER2, HER3 and HER4 levels in estrogen receptor positive (ER+) and ER negative (-) HER2 non-amplified tumours from postmenopausal women. Significant differences between ER+ and ER- tumours are presented using the Mann-Whitney U test.
Figure 5. Intratumoural correlations of growth factor receptors with estrogen receptor mRNA levels (ESR1).
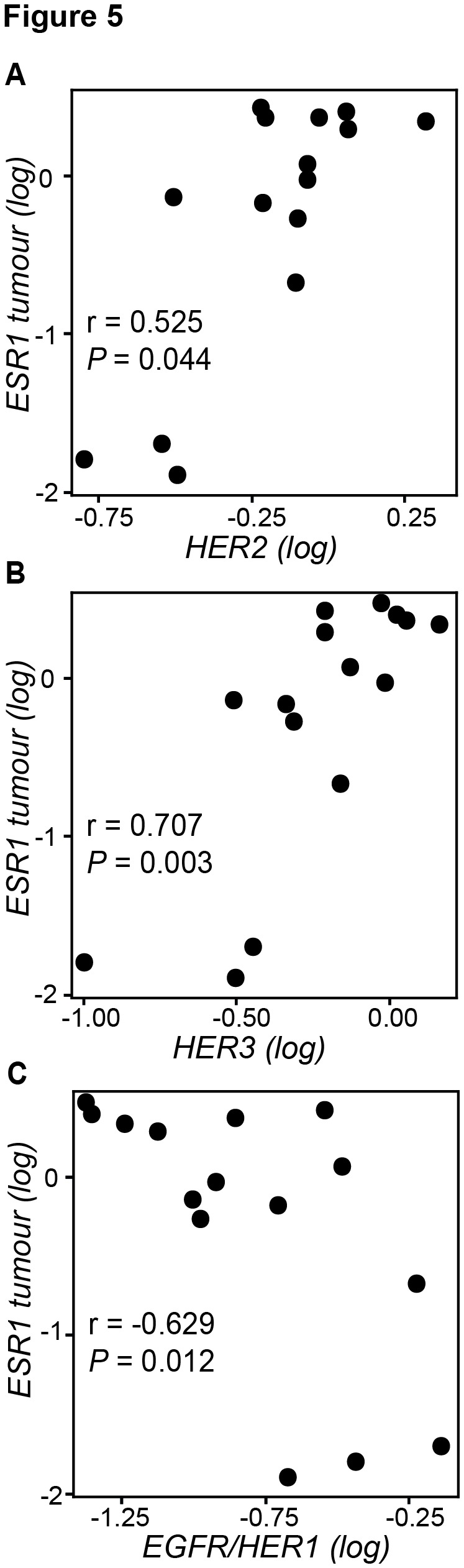
Scatterplots illustrate correlations of HER2 (A), HER3 (B) and EGFR/HER1 (C) with ESR1 among postmenopausal women. HER2 amplified tumours are excluded. Significant correlations were evaluated using the Spearman rank (two-tailed) test.
Table 5. Correlations of HER-receptors and NRG1 with estrogen levels in normal tissue, tumour tissue and plasma.
|
|
Tumour |
|||||||
|---|---|---|---|---|---|---|---|---|
| EGFR/HER1 | HER2 | HER3 | HER4 | NRG1 | ||||
| Normal tissue | E1 | Totala | -0.458* | 0.084 | 0.367 | 0.276 | -0.315 | |
| ER+ | -0.498* | 0.276 | 0.423 | 0.370 | -0.236 | |||
| E2 | Total | -0.556** | 0.086 | 0.412 | 0.403 | -0.473* | ||
| ER+ | -0.494* | 0.187 | 0.327 | 0.291 | -0.437 | |||
| E1S | Total | 0.223 | 0.081 | 0.114 | 0.025 | 0.196 | ||
| ER+ | 0.211 | 0.049 | 0.147 | 0.004 | 0.158 | |||
| Tumour | E1 | Total | -0.148 | 0.136 | 0.200 | 0.238 | -0.106 | |
| ER+ | -0.343 | 0.257 | 0.354 | 0.463 | -0.214 | |||
| E2 | Total | -0.633** | 0.289 | 0.544** | 0.298 | -0.280 | ||
| ER+ | -0.676** | 0.164 | 0.261 | -0.115 | -0.542* | |||
| E1S | Total | 0.039 | 0.057 | 0.133 | 0.269 | 0.336 | ||
| ER+ | -0.029 | 0.015 | 0.220 | 0.394 | 0.320 | |||
| Plasma | E1 | Total | -0.509* | 0.464* | 0.336 | 0.187 | -0.331 | |
| ER+ | -0.529* | 0.245 | 0.255 | 0.047 | -0.475 | |||
| E2 | Total | -0.625** | 0.285 | 0.171 | 0.215 | -0.379 | ||
| ER+ | -0.647** | 0.140 | 0.206 | 0.194 | -0.436 | |||
| E1S | Total | -0.417 | 0.128 | 0.115 | 0.059 | -0.257 | ||
| ER+ | -0.321 | -0.069 | 0.034 | 0.017 | -0.228 | |||
a The total group of postmenopausal women and the subgroup of ER+ postmenopausal women
Spearman rank correlation (two tailed):
* P≤0.05
** P≤0.01
In contrast, EGFR/HER1 expression levels correlated negatively with ESR1 tumour levels (r=-0.629, P=0.012, Figure 5C) in addition to intratumour (r=-0.633, P=0.001, Table 5), normal tissue (r=-0.556, P=0.005) and plasma (r=-0.625, P=0.002) E2 levels. These negative correlations were also significant when restricting the analysis to ER+ tumours only (Table 5). Interestingly, we also observed a trend of negative correlations between tumour NRG1 levels and estrogens in tissues and plasma where tumour NRG1 correlated negatively with E2 in normal tissue (r=-0.473, P=0.023, Table 5). Moreover, in ER+ tumours we observed significantly negative correlations between intratumour NRG1 and ESR1 levels in premenopausal (r=-0.604, P=0.017) as well as among postmenopausal women (r=-0.769, P=0.003, data not shown). In postmenopausal women harbouring ER+ tumours, we also recorded a negative correlation between NRG1 and tumour E2 levels (r=-0.542, P=0.020, Table 5). These results suggest an association between elevated estradiol and suppression of EGFR/HER1 and potentially NRG1 in tumours from postmenopausal women.
Discussion
In this study we have shown correlations in-between members of the HER-receptor family as well as between members of the HER-receptor family and plasma and tumour tissue estradiol levels in breast cancer patients. The study design provides a unique sample set with matched tumour and normal breast tissue from the same breast together with plasma samples collected synchronously [18]. To our knowledge, this is the first mRNA-expression analysis of all members of the HER-receptor family in addition to the ligand for HER3/4, neuregulin-1 (NRG1) in tumour and normal tissue from breast cancer patients where tissue estrogen levels have been determined in concert.
Experimental studies have shown that resistance to endocrine therapy involve a switch from ER dependent- to growth factor-dependent growth, promoting cross-talks between ER and growth factors, in particular HER2 [21–24]. Notably, among patients with ER positive tumours, overexpression of HER2 has been associated with higher relapse rate during endocrine treatment [25–27]. However, our knowledge about growth factor signalling during development of resistance is limited. EGFR/HER1 and HER2 may play important roles, and these receptors have been found upregulated in response to endocrine treatment in ER positive breast cancer cell lines [28–32]. While clinical evidence is lacking, conflicting evidence have linked HER3 and HER4 status to resistance toward different types of endocrine manipulation in vitro [8,33,34]. Moreover, recent findings that combined therapies with trastuzumab and either lapatinib [35] or pertuzumab [36] may improve therapeutic efficacy as compared to trastuzumab monotherapy provides indirect evidence in support of cross-talks between different components of the HER-family.
Clinical studies have reported the benefit of adding HER-targeted drugs to an aromatase inhibitor in ER+ HER2 amplified tumours [3,37], but a potential biological role of the HER-receptor family in tumours not amplified for HER2 remains poorly understood. In 2009, Johnston et al [2] reported lapatinib to improve therapeutic efficacy of aromatase inhibition in a small subgroup of patients with poor prognosis ER+ tumours harbouring normal HER2 gene copy numbers. Moreover, in a preclinical study, lapatinib restored endocrine sensitivity in ER+ HER2 non-amplified cells exhibiting endocrine resistance [5]. More recently, in the MAPLE pre-surgical trial, lapatinib was shown to have antiproliferative effects in both HER2 positive and negative breast cancer [38].
Here, we observed increased levels of HER2, HER3 and HER4 in ER+ HER2 non-amplified tumours compared to normal tissue, implying a role of these receptors in HER2 non-amplified breast cancer [39]. Conflicting evidence has linked estrogen signalling to HER-receptors transcriptional activity [28,40–45]. Our findings support the existence of a cross-talk between estrogen/ER-signalling and growth factors in ER+ HER2 non-amplified tumour implicating a greater potential of increased growth-factor dependent signalling in tumours compared to normal breast tissue. The strongest difference between normal breast tissue and breast tumours was observed for HER3. Together with HER2, HER3 generates the most mitogenic dimer in the HER-family with the capacity to signal both through the mitogen-activated protein kinase (MAPK) pathway for cell proliferation and through the phosphatidylinositol-3´-kinase (PI3K)-Akt pathway for cell survival [46]. HER3 signalling has been shown to play a central role in HER2 amplified disease [47], however the prognostic value of HER3 in these tumours is unclear [48–50]. Interestingly, HER3 overexpression has been shown to have negative effects on breast cancer survival among patients with EGFR/HER1 and HER2 non-amplified tumours [51]. Little is known about the potential direct or indirect effects of estrogens on the transcriptional regulation of HER3, and the mechanisms regulating the activity of HER3 in ER+ HER2 non-amplified tumours should be analysed more in detail. Notably, HER4 has been demonstrated as an estrogen-inducible gene containing estrogen-responsive elements in the promoter, and it also serves as an ER coregulator promoting tumor cell proliferation [45].
Recently, positive correlation between HER2 and ER was observed in HER2 negative tumours [15]. Our results confirm these observations and point out an important difference in biology between HER2 amplified and non-amplified tumours concerning their relationship to ER. Moreover, our results support the observation that ER- tumours distinguish between HER2 positive- and HER2 negative tumours more clearly than ER+ tumours. All together these findings are important when considering treatment of ER+ breast cancer where HER2 status may not be clearly defined.
NRG1, also known as HRG-beta, is a ligand for HER3 and HER4 and is known to mediate an autocrine signalling loop activating HER3 that stimulates the cell proliferation [52,53]. HER3 is essential for HER2 driven tumourigenesis [47], and patients with NRG1 driven HER2 non-amplified tumours have been suggested to derive clinical benefit from HER2: HER3-directed therapies [53]. On the other hand, NRG1 has been shown to be silenced by methylation in breast cancers, in which case tumour cells may be deprived of an important growth factor [54]. We did not observe any significant difference in NRG1 mRNA levels between breast cancer and normal tissue; thus, further studies are required to understand NRG1s role or function in endocrine breast cancer and treatment.
We observed lower tumour compared to normal tissue levels of EGFR/HER1. Experimental studies have found EGFR/HER1 in general to be low in ER+ breast cancer cell lines probably due to downregulation by estrogens [32,42,55–57]. EGFR/HER1 is known as an estrogen-responsive gene transcriptionally repressed by estrogens in ER+ breast cancer cells [58,59]. In the clinical setting it has been demonstrated that ER+ tumours have lower levels of EGFR/HER1 protein than ER- tumours [57]. Thus, although our data are based on a relatively small number of patients, they clearly support the in vitro findings that EGFR/HER1 is suppressed by estrogen in tumours, leading to an inverse relationship between ER and EGFR/HER1.
The present study has some limitations. Even though robust statistical interpretation has been obtained for several of the correlations analyses, the number of paired normal and tumour samples is limited, especially when we are restricting analyses to postmenopausal women only. We present quantitative data based on mRNA expression levels rather than protein levels. From a clinical point of view protein levels are important since treatment decisions are based on immunohistochemistry data. Correlation between protein and mRNA levels may vary depending on the methods that are used, however ESR1 mRNA levels are shown to be upregulated in ER+ tumors [17]. Moreover, previous reports that have included analyses of EGFR and HER2 mRNA and protein in the same samples demonstrate high degrees of correlation between the levels of mRNA and protein [48,60,61]. All patients enrolled in this study had tumours distinct palpable in the mastectomy specimen. Since, the tumours may affect the surrounding tissue, it should be noted that the normal tissue was removed from each breast quadrant at significant distance from the primary tumour. While each normal tissue specimen did not undergo histological examination, the breasts had been subject to pre-operative mammography excluding multifocal disease including microcalcifications indicative of cancer in situ.
In summary, the present study demonstrates that EGFR/HER1 is suppressed and negatively associated with estradiol and ER, whereas HER3, and potentially HER2 and HER4, are elevated and positively associated with estradiol in HER2 non-amplified breast tumours from postmenopausal women. Further studies on the effects of endocrine therapy on HER-receptors and ligands should provide more information about the relationship between estrogens and HER-signalling in vivo.
Acknowledgments
We thank Anne Merete Sellevold and Anita Ivarsflaten for their excellent technical assistance
Funding Statement
Funding for this project was provided by the Norwegian Cancer Society (kreftforeningen.no), Samarbeidsorganet Helse Vest RHF (www.helse-bergen.no/forskning/samarbeidsorganet), Inger R. Haldorsens legat (irhaldorsen.no/haldorsen.html), Michael Irgens Flocks legat (www.stiegler.no) and Odd Fellow Medisinsk Vitenskapelig Forskningsfond (oddfellow.no). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98: 10869-10874. doi:10.1073/pnas.191367098. PubMed: 11553815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston S, Pippen J Jr., Pivot X, Lichinitser M, Sadeghi S et al. (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27: 5538-5546. doi:10.1200/JCO.2009.23.3734. PubMed: 19786658. [DOI] [PubMed] [Google Scholar]
- 3. Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A et al. (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27: 5529-5537. doi:10.1200/JCO.2008.20.6847. PubMed: 19786670. [DOI] [PubMed] [Google Scholar]
- 4. Arpino G, Wiechmann L, Osborne CK, Schiff R (2008) Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 29: 217-233. PubMed: 18216219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE et al. (2010) Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res 16: 1486-1497. doi:10.1158/1078-0432.CCR-09-1764. PubMed: 20179226. [DOI] [PubMed] [Google Scholar]
- 6. Martin LA, Farmer I, Johnston SR, Ali S, Marshall C et al. (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278: 30458-30468. doi:10.1074/jbc.M305226200. PubMed: 12775708. [DOI] [PubMed] [Google Scholar]
- 7. Flågeng MH, Moi LL, Dixon JM, Geisler J, Lien EA et al. (2009) Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br J Cancer 101: 1253-1260. doi:10.1038/sj.bjc.6605324. PubMed: 19755984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutcheson IR, Goddard L, Barrow D, McClelland RA, Francies HE et al. (2011) Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes oestrogen receptor-positive breast cancer cells to heregulin beta1. Breast Cancer Res BCR 13: R29. doi:10.1186/bcr2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sommer A, Hoffmann J, Lichtner RB, Schneider MR, Parczyk K (2003) Studies on the development of resistance to the pure antiestrogen Faslodex in three human breast cancer cell lines. J Steroid Biochem Mol Biol 85: 33-47. doi:10.1016/S0960-0760(03)00139-0. PubMed: 12798355. [DOI] [PubMed] [Google Scholar]
- 10. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C et al. (1998) neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16: 1340-1349. [DOI] [PubMed] [Google Scholar]
- 11. Quénel N, Wafflart J, Bonichon F, de Mascarel I, Trojani M et al. (1995) The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat 35: 283-291. doi:10.1007/BF00665980. PubMed: 7579499. [DOI] [PubMed] [Google Scholar]
- 12. Tagliabue E, Ménard S, Robertson JF, Harris L (1999) c-erbB-2 expression in primary breast cancer. Int J Biol Markers 14: 16-26. PubMed: 10367245. [DOI] [PubMed] [Google Scholar]
- 13. Purdie CA, Baker L, Ashfield A, Chatterjee S, Jordan LB et al. (2010) Increased mortality in HER2 positive, oestrogen receptor positive invasive breast cancer: a population-based study. Br J Cancer 103: 475-481. doi:10.1038/sj.bjc.6605799. PubMed: 20664587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A et al. (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19: 3808-3816. PubMed: 11559718. [DOI] [PubMed] [Google Scholar]
- 15. Pinhel I, Hills M, Drury S, Salter J, Sumo G et al. (2012) ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Breast Cancer Res 14: R46. doi:10.1186/bcr3145. PubMed: 22417870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen MS, Bjerre K, Lykkesfeldt AE, Giobbie-Hurder A, Laenkholm AV et al. (2012) Activated HER-receptors in predicting outcome of ER-positive breast cancer patients treated with adjuvant endocrine therapy. Breast 21: 662-668. doi:10.1016/j.breast.2012.07.005. PubMed: 22854050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haynes BP, Straume AH, Geisler J, A’Hern R, Helle H et al. (2010) Intratumoral estrogen disposition in breast cancer. Clin Cancer Res 16: 1790-1801. doi:10.1158/1078-0432.CCR-09-2481. PubMed: 20215536. [DOI] [PubMed] [Google Scholar]
- 18. Lønning PE, Helle H, Duong NK, Ekse D, Aas T et al. (2009) Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol 117: 31-41. doi:10.1016/j.jsbmb.2009.06.005. PubMed: 19591931. [DOI] [PubMed] [Google Scholar]
- 19. Geisler J, Berntsen H, Lonning PE (2000) A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and estrone sulphate levels in breast cancer tissue. J Steroid Biochem Mol Biol 72: 259-264. doi:10.1016/S0960-0760(00)00036-4. PubMed: 10822015. [DOI] [PubMed] [Google Scholar]
- 20. Geisler J, Ekse D, Helle H, Duong NK, Lønning PE (2008) An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J Steroid Biochem Mol Biol 109: 90-95. doi:10.1016/j.jsbmb.2007.12.011. PubMed: 18242079. [DOI] [PubMed] [Google Scholar]
- 21. Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG et al. (2005) Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res 65: 5380-5389. doi:10.1158/0008-5472.CAN-04-4502. PubMed: 15958587. [DOI] [PubMed] [Google Scholar]
- 22. Johnston SR, Martin LA, Leary A, Head J, Dowsett M (2007) Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol 106: 180-186. doi:10.1016/j.jsbmb.2007.05.019. PubMed: 17624764. [DOI] [PubMed] [Google Scholar]
- 23. Masri S, Phung S, Wang X, Chen S (2010) Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J Steroid Biochem Mol Biol 118: 277-282. doi:10.1016/j.jsbmb.2009.10.011. PubMed: 19897035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J et al. (2005) Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 23: 2469-2476. doi:10.1200/JCO.2005.01.172. PubMed: 15753463. [DOI] [PubMed] [Google Scholar]
- 25. De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C et al. (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11: 4741-4748. doi:10.1158/1078-0432.CCR-04-2569. PubMed: 16000569. [DOI] [PubMed] [Google Scholar]
- 26. De Placido S, De Laurentiis M, Carlomagno C, Gallo C, Perrone F et al. (2003) Twenty-year results of the Naples GUN randomized trial: predictive factors of adjuvant tamoxifen efficacy in early breast cancer. Clin Cancer Res 9: 1039-1046. PubMed: 12631604. [PubMed] [Google Scholar]
- 27. Dowsett M, Houghton J, Iden C, Salter J, Farndon J et al. (2006) Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 17: 818-826. doi:10.1093/annonc/mdl016. PubMed: 16497822. [DOI] [PubMed] [Google Scholar]
- 28. Bates NP, Hurst HC (1997) An intron 1 enhancer element mediates oestrogen-induced suppression of ERBB2 expression. Oncogene 15: 473-481. doi:10.1038/sj.onc.1201368. PubMed: 9242384. [DOI] [PubMed] [Google Scholar]
- 29. Gee JM, Shaw VE, Hiscox SE, McClelland RA, Rushmere NK et al. (2006) Deciphering antihormone-induced compensatory mechanisms in breast cancer and their therapeutic implications. Endocr Relat Cancer 13 Suppl 1: S77-S88. doi:10.1677/erc.1.01274. PubMed: 17259561. [DOI] [PubMed] [Google Scholar]
- 30. Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI et al. (2008) Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456: 663-666. doi:10.1038/nature07483. PubMed: 19005469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newman SP, Bates NP, Vernimmen D, Parker MG, Hurst HC (2000) Cofactor competition between the ligand-bound oestrogen receptor and an intron 1 enhancer leads to oestrogen repression of ERBB2 expression in breast cancer. Oncogene 19: 490-497. doi:10.1038/sj.onc.1203416. PubMed: 10698518. [DOI] [PubMed] [Google Scholar]
- 32. Yarden RI, Wilson MA, Chrysogelos SA (2001) Estrogen suppression of EGFR expression in breast cancer cells: a possible mechanism to modulate growth. J Cell Biochem Supplement Suppl 36: 232-246. PubMed: 11455588. [DOI] [PubMed] [Google Scholar]
- 33. Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E et al. (2009) Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 114: 263-275. doi:10.1007/s10549-008-0011-8. PubMed: 18409071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghayad SE, Vendrell JA, Ben Larbi S, Dumontet C, Bieche I et al. (2010) Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer 126: 545-562. doi:10.1002/ijc.24750. PubMed: 19609946. [DOI] [PubMed] [Google Scholar]
- 35. Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G et al. (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28: 1124-1130. doi:10.1200/JCO.2008.21.4437. PubMed: 20124187. [DOI] [PubMed] [Google Scholar]
- 36. Baselga J, Cortés J, Kim SB, Im SA, Hegg R et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366: 109-119. doi:10.1056/NEJMoa1113216. PubMed: 22149875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I et al. (2010) Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res 16: 1904-1914. doi:10.1158/1078-0432.CCR-09-2282. PubMed: 20215537. [DOI] [PubMed] [Google Scholar]
- 38. Dowsett M, Leary A, Evans A, A’Hern R, Bliss J et al. (2012) Prediction of antiproliferative response to lapatinib by HER3 in an exploratory analysis of HER2-non-amplified (HER2-) breast cancer in the MAPLE presurgical study (CRUK E/06/039). San Antonio, Texas; US. [Google Scholar]
- 39. Koutras AK, Fountzilas G, Kalogeras KT, Starakis I, Iconomou G et al. (2010) The upgraded role of HER3 and HER4 receptors in breast cancer. Crit Rev Oncol/Hematol 74: 73-78. doi:10.1016/j.critrevonc.2009.04.011. PubMed: 19481955. [DOI] [PubMed] [Google Scholar]
- 40. Keshamouni VG, Mattingly RR, Reddy KB (2002) Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta. J Biol Chem 277: 22558-22565. doi:10.1074/jbc.M202351200. PubMed: 11960991. [DOI] [PubMed] [Google Scholar]
- 41. Liu B, Ordonez-Ercan D, Fan Z, Huang X, Edgerton SM et al. (2009) Estrogenic promotion of ErbB2 tyrosine kinase activity in mammary tumor cells requires activation of ErbB3 signaling. Mol Cancer Res 7: 1882-1892. PubMed: 19861407. [DOI] [PubMed] [Google Scholar]
- 42. Revillion F, Pawlowski V, Lhotellier V, Louchez MM, Peyrat JP (2003) mRNA expression of the type I growth factor receptors in the human breast cancer cells MCF-7: regulation by estradiol and tamoxifen. Anticancer Res 23: 1455-1460. PubMed: 12820409. [PubMed] [Google Scholar]
- 43. Russell KS, Hung MC (1992) Transcriptional repression of the neu protooncogene by estrogen stimulated estrogen receptor. Cancer Res 52: 6624-6629. PubMed: 1358436. [PubMed] [Google Scholar]
- 44. Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ et al. (2003) Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol Endocrinol 17: 818-830. doi:10.1210/me.2002-0330. PubMed: 12554767. [DOI] [PubMed] [Google Scholar]
- 45. Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK et al. (2006) Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res 66: 7991-7998. doi:10.1158/0008-5472.CAN-05-4397. PubMed: 16912174. [DOI] [PubMed] [Google Scholar]
- 46. Citri A, Skaria KB, Yarden Y (2003) The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 284: 54-65. doi:10.1016/S0014-4827(02)00101-5. PubMed: 12648465. [DOI] [PubMed] [Google Scholar]
- 47. Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X et al. (2008) A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 68: 5878-5887. doi:10.1158/0008-5472.CAN-08-0380. PubMed: 18632642. [DOI] [PubMed] [Google Scholar]
- 48. Koutras AK, Kalogeras KT, Dimopoulos MA, Wirtz RM, Dafni U et al. (2008) Evaluation of the prognostic and predictive value of HER family mRNA expression in high-risk early breast cancer: a Hellenic Cooperative Oncology Group (HeCOG) study. Br J Cancer 99: 1775-1785. doi:10.1038/sj.bjc.6604769. PubMed: 18985033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sassen A, Rochon J, Wild P, Hartmann A, Hofstaedter F et al. (2008) Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Res 10: R2. doi:10.1186/bcr1886. PubMed: 18182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM (2003) Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 200: 290-297. doi:10.1002/path.1370. PubMed: 12845624. [DOI] [PubMed] [Google Scholar]
- 51. Chiu CG, Masoudi H, Leung S, Voduc DK, Gilks B et al. (2010) HER-3 overexpression is prognostic of reduced breast cancer survival: a study of 4046 patients. Ann Surg 251: 1107-1116. doi:10.1097/SLA.0b013e3181dbb77e. PubMed: 20485140. [DOI] [PubMed] [Google Scholar]
- 52. Sheng Q, Liu X, Fleming E, Yuan K, Piao H et al. (2010) An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 17: 298-310. doi:10.1016/j.ccr.2009.12.047. PubMed: 20227043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson TR, Lee DY, Berry L, Shames DS, Settleman J (2011) Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 20: 158-172. doi:10.1016/j.ccr.2011.07.011. PubMed: 21840482. [DOI] [PubMed] [Google Scholar]
- 54. Chua YL, Ito Y, Pole JC, Newman S, Chin SF et al. (2009) The NRG1 gene is frequently silenced by methylation in breast cancers and is a strong candidate for the 8p tumour suppressor gene. Oncogene 28: 4041-4052. doi:10.1038/onc.2009.259. PubMed: 19802002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. deFazio A, Chiew YE, Sini RL, Janes PW, Sutherland RL (2000) Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 87: 487-498. doi:10.1002/1097-0215(20000815)87:4. PubMed: 10918187. [PubMed] [Google Scholar]
- 56. Salvatori L, Caporuscio F, Coroniti G, Starace G, Frati L et al. (2009) Down-regulation of epidermal growth factor receptor induced by estrogens and phytoestrogens promotes the differentiation of U2OS human osteosarcoma cells. J Cell Physiol 220: 35-44. doi:10.1002/jcp.21724. PubMed: 19347870. [DOI] [PubMed] [Google Scholar]
- 57. Koenders PG, Beex LV, Geurts-Moespot A, Heuvel JJ, Kienhuis CB et al. (1991) Epidermal growth factor receptor-negative tumors are predominantly confined to the subgroup of estradiol receptor-positive human primary breast cancers. Cancer Res 51: 4544-4548. PubMed: 1873798. [PubMed] [Google Scholar]
- 58. Salvatori L, Ravenna L, Felli MP, Cardillo MR, Russo MA et al. (2000) Identification of an estrogen-mediated deoxyribonucleic acid-binding independent transactivation pathway on the epidermal growth factor receptor gene promoter. Endocrinology 141: 2266-2274. doi:10.1210/en.141.6.2266. PubMed: 10830317. [DOI] [PubMed] [Google Scholar]
- 59. Wilson MA, Chrysogelos SA (2002) Identification and characterization of a negative regulatory element within the epidermal growth factor receptor gene first intron in hormone-dependent breast cancer cells. J Cell Biochem 85: 601-614. doi:10.1002/jcb.10168. PubMed: 11968000. [DOI] [PubMed] [Google Scholar]
- 60. Bhargava R, Gerald WL, Li AR, Pan Q, Lal P et al. (2005) EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol 18: 1027-1033. doi:10.1038/modpathol.3800438. PubMed: 15920544. [DOI] [PubMed] [Google Scholar]
- 61. Press MF, Finn RS, Cameron D, Di Leo A, Geyer CE et al. (2008) HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 14: 7861-7870. doi:10.1158/1078-0432.CCR-08-1056. PubMed: 19047115. [DOI] [PubMed] [Google Scholar]