Abstract
Alginate overproduction, or mucoidy, plays an important role in the pathogenesis of P. aeruginosa lung infection in cystic fibrosis (CF). Mucoid strains with mucA mutations predominantly populate in chronically-infected patients. However, the mucoid strains can revert to nonmucoidy in vitro through suppressor mutations. We screened a mariner transposon library using CF149, a non-mucoid clinical isolate with a misssense mutation in algU (AlgUA61V). The wild type AlgU is a stress-related sigma factor that activates transcription of alginate biosynthesis. Three mucoid mutants were identified with transposon insertions that caused 1) an overexpression of AlgUA61V, 2) an overexpression of the stringent starvation protein A (SspA), and 3) a reduced expression of the major sigma factor RpoD (σ70). Induction of AlgUA61V in trans caused conversion to mucoidy in CF149 and PAO1DalgU, suggesting that AlgUA61V is functional in activating alginate production. Furthermore, the level of AlgUA61V was increased in all three mutants relative to CF149. However, compared to the wild type AlgU, AlgUA61V had a reduced activity in promoting alginate production in PAO1ΔalgU. SspA and three other anti-σ70 orthologues, P. aeruginosa AlgQ, E. coli Rsd, and T4 phage AsiA, all induced mucoidy, suggesting that reducing activity of RpoD is linked to mucoid conversion in CF149. Conversely, RpoD overexpression resulted in suppression of mucoidy in all mucoid strains tested, indicating that sigma factor competition can regulate mucoidy. Additionally, an RpoD-dependent promoter (PssrA) was more active in non-mucoid strains than in isogenic mucoid variants. Altogether, our results indicate that the anti-σ70 factors can induce conversion to mucoidy in P. aeruginosa CF149 with algU-suppressor mutation via modulation of RpoD.
Introduction
The Gram-negative bacterium P. aeruginosa is an important opportunistic pathogen in humans, and has the potential to proliferate in a wide range of niches. P. aeruginosa is one of the major etiological agents of hospital-acquired infections and ventilator-associated pneumonia [1]. More importantly, P. aeruginosa is the leading cause of morbidity and mortality in cystic fibrosis (CF) patients [2].
P. aeruginosa can produce a capsule-like polysaccharide called alginate. Overproduction of alginate is also known as mucoidy [3]. Mucoid conversion facilitates the establishment of persistent infection with P. aeruginosa in CF. The role of alginate in pathogenesis includes: increased resistance to antibiotics [2], increased resistance to phagocytic killing [4], [5] and evasion of the host’s immune response [4]. However, the mucoid phenotype observed in CF isolates is extremely unstable ex vivo [6], [7], [8]. Reversion to non-mucoidy is common in vitro in the absence of a selective pressure, and in vivo during the end-stage of CF disease [9]. Although, environmental signals such as high osmolarity, nitrogen or phosphate starvation, and ethanol-induced membrane perturbation can activate transcription of algD encoding the key enzyme for alginate biosynthesis [10], the selective pressure for mucoid conversion of P. aeruginosa in CF respiratory environment is not fully understood.
Several genes in P. aeruginosa are known to regulate alginate production. Specifically, AlgU (AlgT, σ22) is an alternative sigma factor that drives the transcription of algD [11]; MucA is a trans-membrane protein that negatively regulates alginate production by sequestering AlgU [12]; MucB and proteases AlgW, MucP and ClpXP affect alginate production by altering the stability of MucA [13]. Mutations in mucA are recognized as the primary reason for mucoid conversion in CF isolates [14], [15], [16], [17]. However, the reversion from a mucoid to a non-mucoid phenotype is still possible. Sautter et al. isolated 34 spontaneous non-mucoid variants from a MucA truncated mucoid stain PDO300 [18]. In another study, 70% of the non-mucoid CF isolates carried a mucA mutation [19].
The aim of this study was to better understand the alginate regulation by determining if there are upstream mutations that restore alginate overproduction to a clinical nonmucoid, algU mucA double mutant (CF149). To achieve this objective, we conjugated the mariner transposon plasmid pFAC [20] into the mucA mutant CF149 with an alginate-suppressing mutation [21], and screened for mucoid variants. Three genes were identified that regulated mucoidy in CF149. Mechanistic studies suggest that mucoid conversion in MucA truncated strains including CF149, is related to competition between the two sigma factors, the major house keeping sigma factor RpoD and AlgU for binding to the core RNA polymerase (RNAP). Additionally, we documented that anti-σ70 factors have the ability to induce mucoidy in the suppressed non-mucoid P. aeruginosa strain CF149.
Materials and Methods
Bacteria Strains, Plasmids, and Growth Conditions
Bacterial strains and plasmids used in this study are shown in Table S1. Escherichia coli strains were grown at 37°C in Lennox broth (LB) or LB agar. P. aeruginosa strains were grown at 37°C in LB or on Pseudomonas isolation agar (PIA) plates (Difco). When required, carbenicillin, tetracycline or gentamicin were added to the broth or plates. The concentrations of carbenicillin, tetracycline or gentamicin added in LB broth or plates were 100 µg ml−1, 20 µg ml−1 and 15 µg ml−1, respectively. The concentration of these antibiotics added to PIA plates were 300 µg ml−1, 150 µg ml−1 or 300 µg ml−1, respectively.
Phage Culture and Genomic DNA Extraction
E. coli BB was inoculated into 6 ml LB, and incubated at 37°C with shaking. At an OD600 of 0.6, 200 µl of T4 phage stock was added to the culture, and incubated overnight. Phage lysates were collected using a 0.2 µm filter. Phage genomic DNA was extracted using the standard procedure with phenol:chloroform:isoamyl alcohol, and precipitated with ethanol.
Transformation and Conjugation
The One Shot® TOP10 (Invitrogen) chemical transformation method was used according to the supplier’s instruction. The transfer of plasmids from E. coli to Pseudomonas was performed via triparental conjugations using the helper plasmid pRK2013 [22].
Transposon Mutagenesis
Biparental conjugations were carried out for transposon mutagenesis, using E. coli SM10 λpir carrying plasmid pFAC as the donor strain [20] and CF149 as the recipient strain. After incubation, bacteria were collected and streaked onto PIA plates supplemented with gentamicin (300 µg ml−1). Mucoid colonies were identified and subjected to further genetic analyses. The chromosomal DNA of mucoid mutants was isolated using the QIAamp genomic DNA Extraction kit (Qiagen). Approximately, 2 µg DNA was digested with SalI overnight at 37°C followed by purification and self-ligation using Fast-Link DNA ligase (Epicentre). The circular closed DNA was used as template for inverse PCR using GM3OUT and GM5OUT primers [23]. The PCR products were purified and sequenced. Finally, southern blot hybridization was used to monitor the copy number of transposon insertions using the GmR gene as the probe [24].
Protein Preparation, SDS-PAGE and Western Blotting
Bacteria were cultured on PIA plates for 24 hrs and then collected for cell lysis. Following sonication, the protein concentration of the resulting supernatant was measured using the Bio-Rad Dc protein assay reagents (Bio-Rad). Equal amounts protein were mixed with 2×sample loading buffer and separated on a pre-cast SDS-PAGE gels (Bio-Rad); Total proteins were transferred to PVDF membrane (GE) for immuno-detection. A primary monoclonal antibody of rat anti-HA (Roche) was used at a dilution of 1∶5000, while a goat anti-rat immunoglobulin G (heavy and light chains) conjugated with horseradish peroxidase (Pierce) (1∶5000) was used as the secondary antibody. The immunoreactive proteins were visualized using the Amersham ECL kit (GE).
Alginate Assay
P. aeruginosa strains were grown at 37°C on triplicate PIA plates for 24 hrs. The bacteria were collected and suspended in PBS. The OD600 of bacterial suspension in PBS, which corresponds to the bacterial density, was measured. The amount of uronic acid was analyzed in comparison with a standard curve made with D-mannuronic acid lactone (Sigma-Aldrich), as previously described [25].
β-galactosidase Activity Assay
Pseudomonas strains carrying the plasmid pLP170 containing the PssrA, PalgD and PalgW promoters fused with the promoterless lacZ were cultured on three PIA plates. After 24 hrs, bacterial cells were harvested and re-suspended in PBS. OD600 was measured and adjusted to approximately 0.3. Cells were then permeabilized using toluene, and the β-galactosidase activity was measured at OD420 and OD550. The results of Miller Units were calculated according to this formula: Miller Units = 1000×[OD420–(1.75×OD550)]/[Reaction time (minutes)×Volume (ml)×OD600] [26]. The reported values represent an average of three independent experiments with standard error.
RNA Isolation and Real-time PCR
Bacteria total RNA were extracted with a RNeasy Mini Kit (QIAGEN, USA) according to the manufacturer’s instructions. The real-time PCR assays were performed on ABI PRISM® 7000 (ABI, USA) with One Step SYBR® PrimeScript™ RT-PCR KIT II (TaKaRa, Japan) according to the manufacturer’s instructions. The rpoD gene was amplified using primers rpoD-RT-F (5′-AGA AGG ACG ACG AGG AAG A-3′) and rpoD-RT-R (5′-GCA CCA GCT TGA TCG GCA TGA -3′). The 16S rRNA gene was amplified using primers UniF340 (5′-ACT CCT ACG GGG AGG CAG CAG T-3′) and UniR514 (5′-ATT ACC GCG GCT GCT GGC-3′) [27]. The relative expression level of rpoD was normalized to 16S rRNA, and calculated according to the formula: fold change = 2−ΔΔct [28].
Measurement of Bacterial Growth
The Pseudomonas strains were grown in LB culture medium overnight at 37°C, then diluted to OD600 = 0.5 using PBS. Then 1 ml bacteria suspension was inoculated into a 250 ml flask containing 50 ml Pseudomonas Isolation Broth (PIB; Alpha Biosciences). To measure bacterial growth, OD600 was monitored every 2 hrs for 24 hrs. Triplicate of samples at each time point were measured, and the means with standard error were used to generate the growth curve.
Statistical Analysis
The student t test and one-way ANOVA were performed using the statistical software SPSS 13.0 (IBM, US) with P<0.05 considered as significant.
Results
Identification of Alginate Regulators in a P. aeruginosa Strain with a Suppressor Mutation
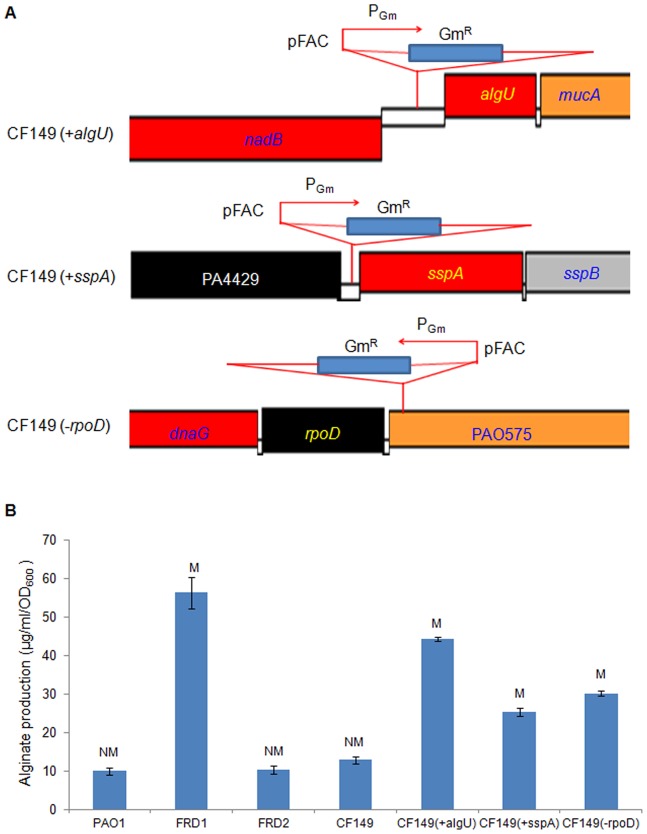
CF149 is a clinical isolate from a patient with CF [24]. CF149 displays a nonmucoid phenotype on PIA and PIA plus ammonium metavanadate (PIA-AMV) plates [29]. Previously, we reported CF149 have two mutations resulting in abrogation of an AlgU-dependent transcription of lipotoxin LptF [21]. First, a frameshift mutation in mucA is predicted to produce a truncated MucA protein with 128 amino acids in contrast to wild type MucA with 194 amino acids. Second, CF149 carries a missense mutation in algU predicted to result in a substitution of alanine for valine at position 61 of the primary amino acid sequence of AlgU (AlgUA61V) [21]. To determine whether the alginate suppressor strain CF149 still had the ability to restore mucoidy, a transposon library was constructed and screened. As seen in Figure 1A, we identified three insertions that promote alginate production [17]. Southern blot analysis showed that only one copy of the transposon was inserted on the chromosome in these mucoid strains (data not shown). We mapped the insertion site using inverse PCR as previously described [17], [23]. Two insertions were identified in intergenic regions between PA0762 (algU) and PA0761 (nadB), and PA4428 (sspA) and PA4429 (probable cytochrome c1 precursor) (Figure 1A). In these two mutants, the transposon was upstream of algU and sspA, and was oriented in the same direction as the previously-observed insertion causing an overexpression of mucE [17]. These two mutants likely had an overexpression of algU and sspA from read through of the gentamicin-resistance gene (aacC1) promoter (PGm) [30]. These strains were named CF149 (+algU) and CF149 (+sspA). The third mutant had an insertion site of 78 base pairs behind the stop codon of the rpoD gene (Figure 1A). The rpoD gene (PA0576) encodes the major housekeeping sigma factor (σ70). RpoD is essential for the growth and viability of cells, and no rpoD mutant has been reported in the P. aeruginosa mutant bank [31]. Because the orientation of the pFAC PGm is opposite to the direction of rpoD gene, the insertion is predicted to reduce the expression of rpoD. This strain was named CF149 (−rpoD). To verify the decreased transcription of rpoD in CF149 (−rpoD), we compared the transcript level by real-time PCR, and the activity of an RpoD-dependent promoter PssrA-lacZ in two isogenic strains. The results showed that the transcript level of rpoD in CF149 (−rpoD) was 60% of the value of CF149. The Miller assay results also showed that the PssrA-lacZ activity reduced by 68% in CF149 (−rpoD) compared to CF149. The alginate production by these mucoid strains was also measured and shown to be more than 2-fold higher than the parent strain (Figure 1B).
Figure 1. Increased alginate production in three mutants of CF149 with an algU-suppressor mutation.
(A) Schematic diagram showing the transposon insertions of CF149 (+algU), CF149 (+sspA), and CF149 (−rpoD), respectively. (B) Alginate production of CF149 (+algU), CF149 (+sspA), and CF149 (−rpoD) in comparison to other strains of P. aeruginosa. Three mucoid mutants were identified as a result of a transposon library screen. Alginate production was measured on PIA plates after incubation at 37°C for 24 hrs. Alginate production (µg/ml/OD600) was measured as described in Materials and Methods. M and NM, represent mucoidy and nonmucoidy, respectively.
SspA, not SspB, Induces Mucoidy in CF149
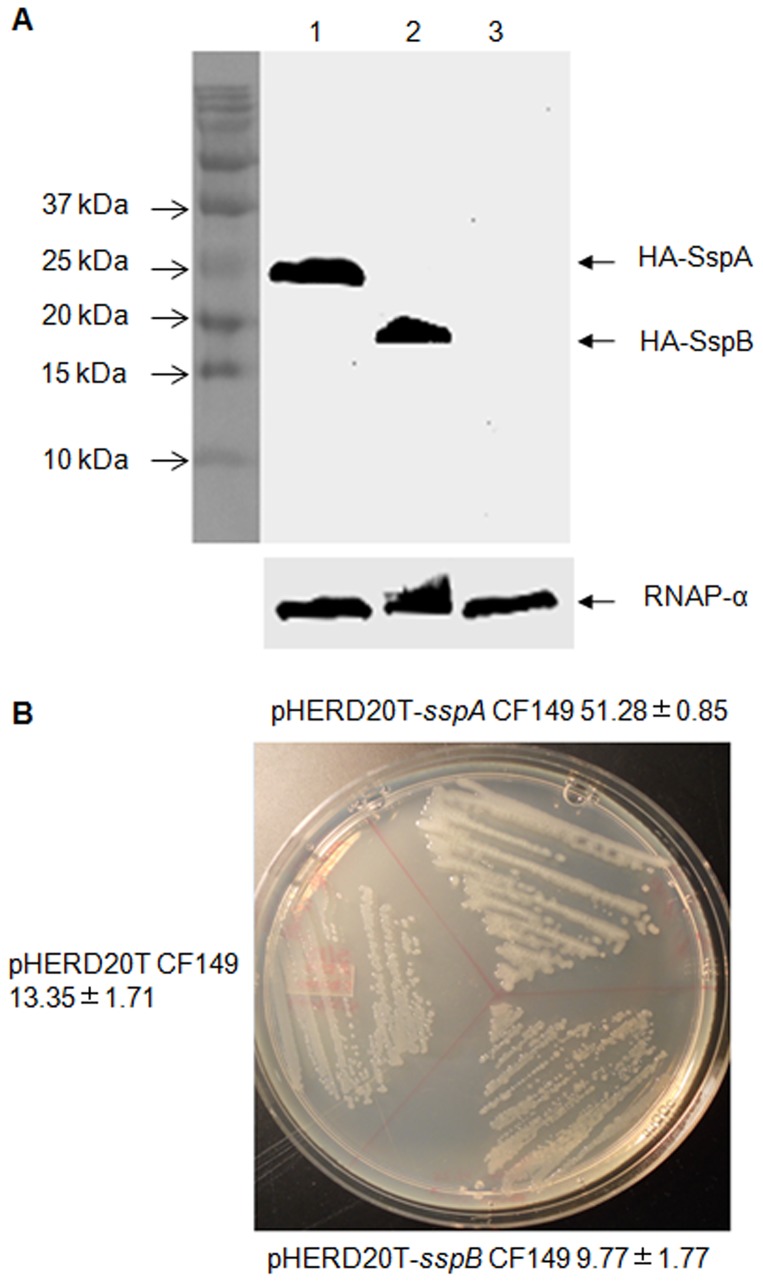
The operon of sspAB encodes the stringent starvation proteins A and B that function in response to amino acid starvation [32]. The sspA and sspB genes share the same promoter, and are co-expressed in E. coli [32]. SspB is also a specificity-enhancing factor for the protease ClpXP in E. coli [33]. ClpXP has been reported to regulate alginate production in P. aeruginosa [23]. In order to determine which gene is responsible for the mucoid conversion in CF149 (+sspA), P. aeruginosa sspA and sspB were cloned behind the PBAD promoter in the shuttle vector pHERD20T [34]. As seen in Figure 2A, we detected the expression of SspA and SspB in CF149 after induction with 0.1% L-arabinose (L-Ara). However, we observed a mucoid phenotype only with the overexpression of sspA (Figure 2B), indicating that SspA is an inducer of alginate production when overexpressed in CF149.
Figure 2. Over-expression of SspA induces mucoidy in CF149.
The sspA and sspB genes from P. aeruginosa were cloned behind the PBAD promoter in the pHERD20T vector, and conjugated into CF149. (A) Western blot analysis of SspA and SspB proteins using an anti-HA monoclonal antibody. Lanes 1, 2 and 3 represent total cellular proteins extracted from CF149 carrying pHERD20T-sspA, pHERD20T-sspB, and pHERD20T, respectively. (B) Morphology of CF149 containing pHERD20T-sspA, pHERD20T-sspB and pHERD20T. These strains were streaked on PIA plates supplemented with 300 µg/ml of carbenicillin, 0.1% L-Ara and incubated overnight at 37°C. Alginate production (µg/ml/OD600) was measured as described in Materials and Methods.
Anti-σ70 Factors Induce Conversion to Mucoidy in CF149
The mucoid phenotype expressed by CF149 (+sspA) and CF149 (−rpoD) suggests that RpoD may be involved in the alginate regulation. Schlictman et al. reported that E. coli sspA can complement the algQ mutation by restoring mucoidy in a CF clinical isolate [35]. AlgQ and Rsd belong to the family of the regulators of the major sigma factor RpoD (σ70) in Proteobacteria. Members of the anti-σ70 factors are thought to interact with the conserved region 4 of σ70 subunit of RNAP [36]. Similarly, AsiA, encoded in the T4 phage, also functions as an anti-σ70 factor [37]. Our hypothesis is that CF149 (+sspA) and CF149 (−rpoD) utilize the mechanism of modulating RpoD to become mucoid. To test this, we cloned the rsd gene from E.coli, algQ and sspA from P. aeruginosa, and asiA from T4 phage in pHERD20T. As seen in Table 1, overexpression of these genes caused conversion to mucoidy in CF149. However, the anti-σ70 factors had no effect on mucoid induction in other strains tested, even though CF4349 has a wild type algU and the same predicted length of MucA as CF149 (Table 1). However, mucoidy of CF149 (+sspA) could be due to a non-specific effect. To test this possibility, we examined the effect of anti-RpoF factor FlgM on mucoid conversion. As seen in Figure S1, FlgM had no effect on mucoid induction in PAO1 and CF149.
Table 1. The effect of anti-σ70 factors on mucoid induction in strains of P. aeruginosa.
| Strains | MucA length | AlgU length | rsd (TOP 10) | algQ b (PAO1) | sspA (PAO1) | asiA (T4 phage) |
| CF149 | 125+3 aa a | Ala61Val (193 aa) | M (29.04±2.44) | M (28.33±2.74) | M (51.28±0.85) | M (54.51±2.28) |
| CF4349 | 125+3 aa | WT (193 aa) | NM (2.35±0.18) | NM (9.49±1.26) | NM (8.53±2.06) | NM (5.60±2.07) |
| CF28 | 117 aa | Tyr29Cys (193 aa) (193aa) | NM (6.37±2.51) | NM (8.10±2.79) | NM (13.20±0.72) (13.20±0.72) (13.20±0.72) | NM (11.56±2.05) |
| CF17 | 143+3 aa | WT (193 aa) | NM (11.45±2.97) | NM (13.52±2.64 | NM (10.57±0.75) | NM (7.94±0.72) |
| FRD2 | 143+3 aa | Asp18Gly (193 aa) | NM (14.08±0.21) | NM (11.74±1.53) | NM (7.05±0.03) | NM (14.64±0.94) |
| PAO1 | WT (194 aa) | WT (193 aa) | NM (14.48±1.56) | NM (7.84±1.67) | NM (9.38±0.3) | NM (17.76±1.46) |
| PA14 | WT (194 aa) | WT (193 aa) | NM (9.63±1.25) | NM (8.95±1.15) | NM (8.75±0.24) | NM (14.47±2.59) |
| CF3715 | WT (194 aa) | WT (193 aa) | NM (13.05±3.60) | NM (6.10±0.67) | NM (6.20±0.19) | NM (15.59±1.28) |
| CF4009 | WT (194 aa) | WT (193 aa) | NM (9.21±0.49) | NM (7.24±2.31) | NM (8.69±0.11) | NM (15.64±2.52) |
M and NM represent a mucoid and a non-mucoid phenotype, respectively, after incubation on PIA plates supplemented with 300 µg/ml of carbenicillin and 0.1% L-Ara at 37°C for 24 hrs. The quantity of alginate production was measured (mean ± standard error, µg/ml/OD600) and listed in the brackets.
the frameshift mutation in mucA results in the fusion of a truncated MucA (125 amino acids of N-terminal MucA) with an additional 3 amino acids with no homology to the amino acid sequence of wild type MucA.
a concentration of 0.5% L-Ara was needed to induce the conversion to mucoidy in CF149. The alginate production of all AlgQ-overexpressing strains was measured on PIA plates containing 300 µg/ml of carbenicillin and 0.5% L-Ara.
Overexpression of rpoD Results in Suppression of Mucoidy
Mucoidy in CF149(−rpoD), CF149(+sspA) and CF149(+algU ) could be due to the competition between RpoD and AlgU for RNAP. To test this hypothesis, rpoD was cloned and overexpressed in various mucoid strains. The mucoid laboratory and clinical isolates were all suppressed by the overexpression of RpoD (Table 2). However PAO1-VE2, PAO1-VE19, PAO579 and PAO581 required a higher concentration of L-ara (0.5% vs. 0.1%) for a complete suppression of mucoidy (Table 2). To further test the hypothesis that sigma factor competition can reduce the activity of AlgU resulting in the suppression of alginate production, we induced RpoN (σ54), RpoS(σ38), and RpoF(σ28) in various mucoid mutants. As expected, overexpression of these sigma factors suppressed alginate production (Table S2). We also observed that overexpressed RpoD was unstable in P. aeruginosa and E.coli (Figure S2).
Table 2. Over-expression of RpoD suppresses mucoidy in laboratory and clinical strains of P. aeruginosa.
| Strains | Alginate production(µg/ml/OD600) | MucA length | AlgU length | Alginate regulator | PHERD 20T- HA-rpoD-His | |
| Laboratory strains | PAO1-VE2 | M (64.80±6.52) | WT (194 aa) | WT (193 aa) | Over-expression of MucE | NMa |
| PAO1-VE13 | M (20.93±1.05) | WT (194 aa) | WT (193 aa) | Inactivation of KinB | NM | |
| PAO1-VE19 | M (61.23±2.90) | WT (194 aa) | WT (193 aa) | Inactivation of MucD | NMa | |
| PAO581 | M (58.95±3.07) | 59 aa+35 aa | WT (193 aa) | MucA25 | NMa | |
| PAO579 | M (48.04±0.11) | WT (194 aa) | WT (193 aa) | PilA108 | NMa | |
| Clinical strains | CF149 (−rpoD) | M (30.16±0.61) | 125+3 aa | Ala61Val | Reduced-expression of rpoD | NM |
| CF149 (+algU) | M (44.20±0.49) | 125+3 aa | Ala61Val | Over-expression of algU | NMa | |
| CF149 (+sspA) | M (25.40±1.07) | 125+3 aa | Ala61Val | Over-expression of sspA | NM | |
| FRD1 | M (56.27±4.06) | 143+3 aa | WT | MucA22 | NM | |
| PDO300 | M (68.08±1.25) | 143+3 aa | WT | MucA22 | NM | |
| CF1003M | M (30.61±2.28) | 59 aa+35 aa | WT (193 aa) | Unknown | NM | |
| CF7447M | M (20.22±1.40) | WT (194 aa) | WT (193 aa) | Unknown | NM | |
NM and M represent non-mucoidy and mucoidy, respectively, after incubation on PIA plates supplemented with 300 µg/ml of carbenicillin and 0.1% L-Ara at 37°C for 24 hrs.
a concentration of 0.5% L-Ara was needed to completely suppress mucoidy. The values for alginate production represent an average of three independent experiments with standard error.
Missense Mutation in CF149 algU Results in Production of a Variant of AlgU with Reduced Function
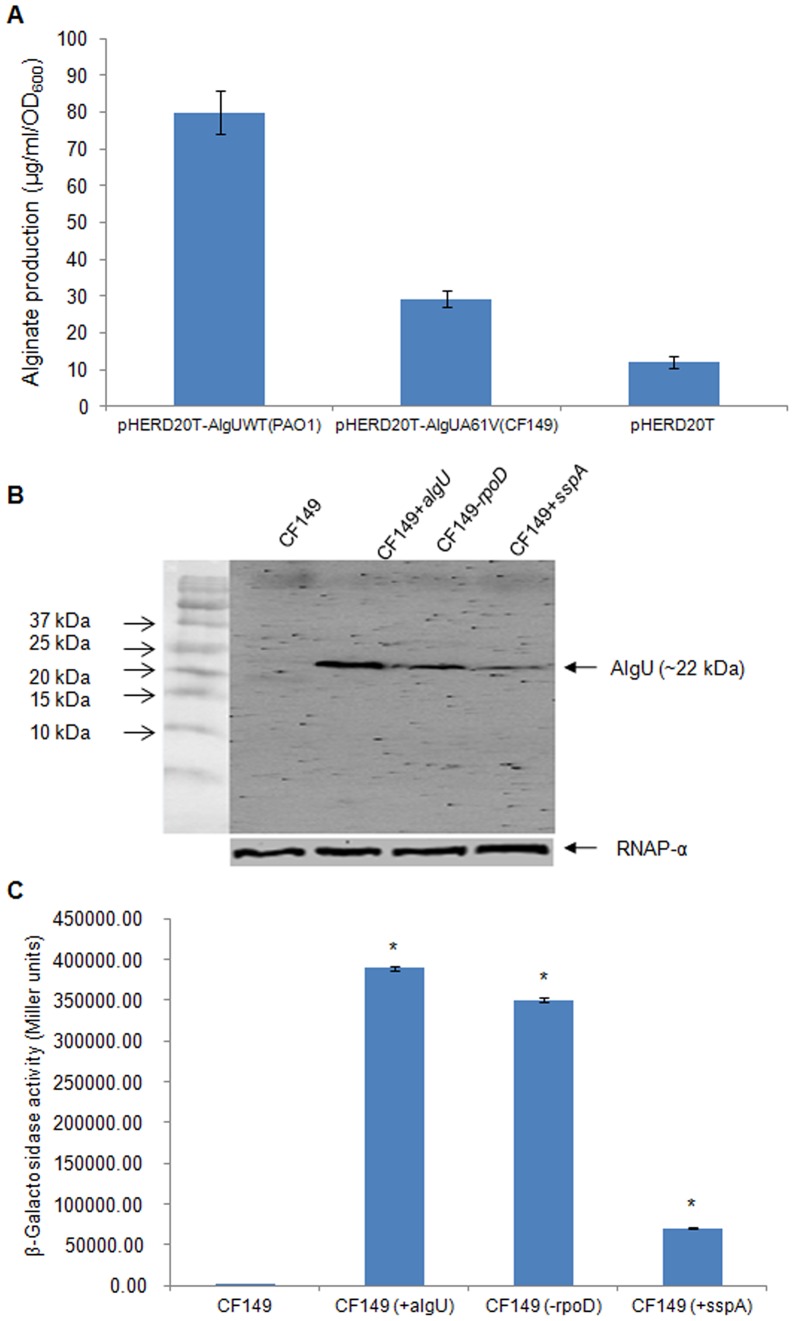
One explanation for mucoidy in CF149 (+algU) is that AlgUA61V retains some function despite the amino acid substitution. To test this, we compared the function of AlgUA61V vs. wild type AlgU by measuring the amount of alginate induced by two forms of AlgU in the PAO1ΔalgU strain. CF149 AlgUA61V kept the ability to induce mucoidy, albeit with a reduced amount of alginate (Figure 3A). To explain how the three mutants become mucoid, we next measured the level of AlgUA61V in the total cell lysates of CF149 (+algU), CF149 (−rpoD) and CF149 (+sspA) through Western blot. The AlgU protein level was increased in CF149 (+algU), CF149 (−rpoD) and CF149 (+sspA) compared to the parent CF149 (Figure 3B). The promoter activity of PalgD-lacZ also increased in these mucoid strains (Figure 3C).We also tested the hypothesis that the mucoidy in all three mutants was due to the increased expression of AlgUA61V. To do so, we introduced pHERD20T-AlgUA61V into CF149. As predicted, CF149 carrying pHERD20T-AlgUA61V displayed a mucoid phenotype (data not shown). Furthermore, the absence of AlgUA61V in Figure 3B is consistent with non-mucoidy of CF149 on PIA, which is probably due to the reduced auto-regulatory activity of AlgUA61V [38].
Figure 3. Alginate induction by CF149 AlgUA61V, the detection of AlgUA61V and the promoter activity of PalgD-lacZ in CF149 (+algU), CF149 (+sspA) and CF149 (− rpoD).
(A) AlgUA61V retained the function of inducing alginate production. The wild type algU gene of PAO1 and its variant of CF149 were cloned into pHERD20T, and conjugated into PAO1ΔalgU. Strains containing pHERD20T-algU WT (PAO1), pHERD20T-algU A61V (CF149), and pHERD20T were streaked on PIA plates supplemented with 300 µg/ml of carbenicillin and incubated overnight at 37°C. Alginate production (µg/ml/OD600) was measured as described in Materials and Methods. (B) The level of AlgU in CF149, CF149 (+algU), CF149 (−rpoD) and CF149 (+sspA) was detected using Western blot with anti-AlgU monoclonal antibody [16]. (C) Measurement of the activity of the algD promoter in the respective strains. The PalgD promoter was inserted into pLP170 vector containing the promoterless lacZ gene. The pLP170 PalgD-lacZ was transferred into the respective strains via triparental conjugation. The β-galactosidase activity was measured as described in Materials and Methods. *, represents a significant difference compared to CF149 (P<0.05).
Intramembrane Proteolysis has a Minimal Role in the Regulation of Mucoidy in CF149
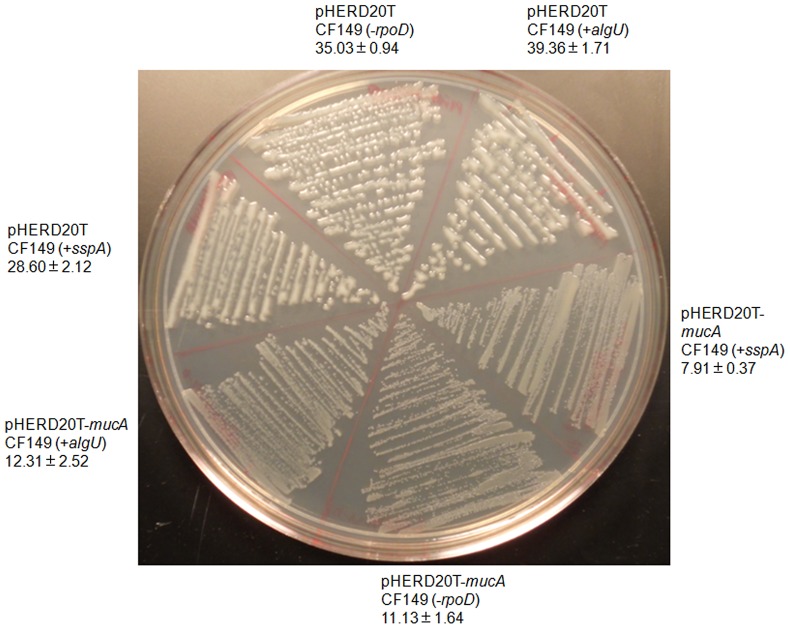
In wild type strain PAO1, AlgU can be sequestered by wild type MucA, thereby preventing it from activating the alginate biosynthetic operon [12], [38], [39]. Since all three mutants of CF149 have an increased level of AlgUA61V, we next investigated whether the wild type MucA can still exert an inhibitory effect on AlgUA61V. The wild type mucA gene was transferred into these mucoid strains: CF149 (+algU), CF149 (+sspA) and CF149 (−rpoD). Over-expression of mucA suppressed mucoidy (Figure 4) suggesting that the mucoidy of all three mutants is due to the activation of the AlgU pathway. But the mutant MucA in CF149 has 128 amino acid residues, and carries the intact trans-membrane domain of MucA84–104. We also noticed that the promoter activity of PalgW in CF149 (−rpoD) was increased compared to CF149 (Figure S3). To test if intramembrane proteolysis has a role in cleaving the periplasmic portion of MucA in CF149, we overexpressed proteases algW, mucP, clpX, clpP and clpP2 and found this had no effect on mucoid induction in CF149. Together, these results suggest that these proteases have a minimal role in regulating the mucoid conversion in CF149, or they may require a mechanism of activation which is absent in CF149.
Figure 4. The effect of mucA on the mucoid suppression in CF149 (+algU), CF149 (+sspA) and CF149 (− rpoD).
AlgUA61V–induced mucoidy was suppressed by the wild type mucA in trans. The mucA gene was over-expressed in trans in CF149(+algU), CF149(−rpoD) and CF149(+sspA) by adding 0.1% L-Ara in PIA supplemented with 300 µg/ml carbenicllin. The plate was incubated for 24 hrs at 37°C.
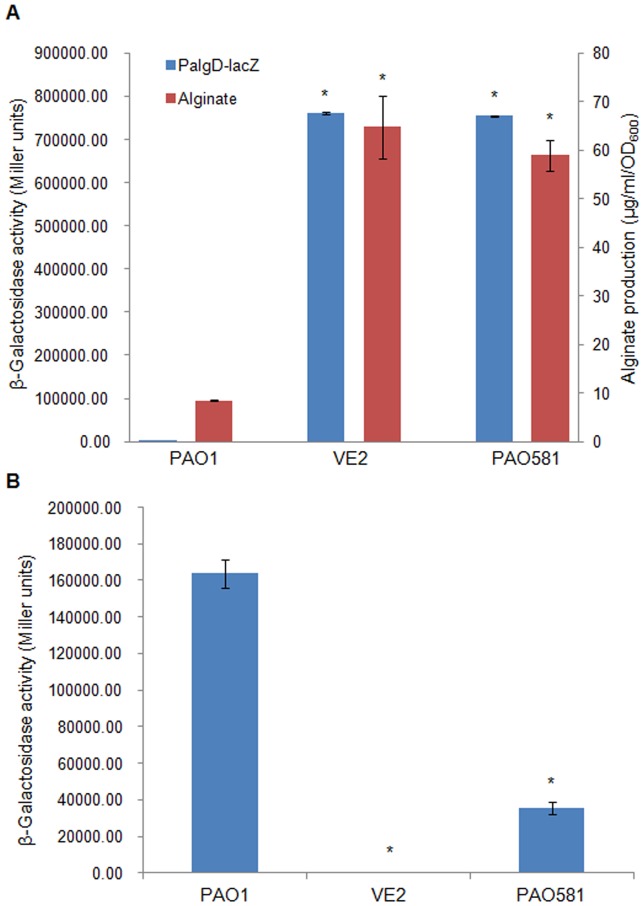
Competition between RpoD- and AlgU-dependent Promoters in Mucoid and Non-mucoid Strains
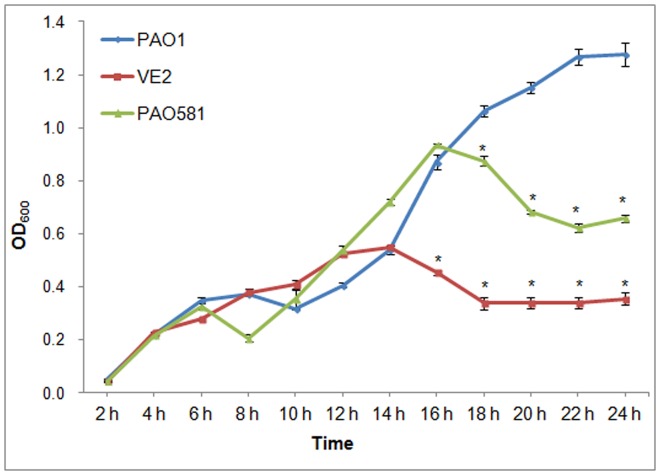
Since the transcription from two promoters, PalgD and PssrA is AlgU-dependent and RpoD-dependent, respectively, we cloned these two promoters into a lacZ fusion vector (pLP170) and the reporter β-galactosidase activity was measured [26]. As seen in Figure 5A, alginate production in the mucoid strains VE2 and PAO581 was higher than that in the non-mucoid strain PAO1. Similarly, the promoter activity of PalgD also was higher in these mucoid strains (Figure 5A). However, the promoter activity of PssrA was inversely related to PalgD activity and alginate production (Figure 5B), suggesting that the RpoD-dependent promoter was less active in mucoid cells than in the isogenic non-mucoid cells. We also measured the growth curve for mucoid and non mucoid strains. As seen in Figure 6, the growth rate for mucoid strains was reduced after 16 hrs growth for VE2 and PAO581 in comparison with nonmoucid strain PAO1.
Figure 5. Regulation of RpoD- and AlgU-dependent promoters in isogenic non-mucoid and mucoid strains of P. aeruginosa.
(A) The β-galactosidase activity of AlgU-dependent promoter PalgD-lacZ and alginate production (µg/ml/OD600) were measured in non-mucoid and mucoid strains. (B) The β-galactosidase activity of an RpoD-dependent promoter PssrA-lacZ was measured in non-mucoid and mucoid strains. *, represents a significant difference compared to PAO1 (P<0.05).
Figure 6. Mucoid mutants of P. aeruginosa display a reduced growth rate compared to the isogenic nonmucoid strain PAO1.
The growth curves were created by growth of PAO1 (nonmucoid), VE2 (mucoid) and PAO581 (mucoid) in PIB. The horizontal axis represents time in hrs, while the vertical axis is the optical density at 600 nm. *, represents a significant difference compared to PAO1 (P<0.05).
Discussion
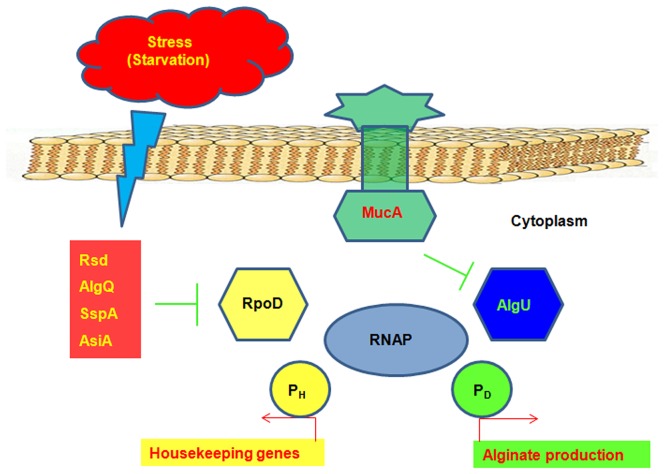
Individuals with CF are thought to acquire initial colonization of P. aeruginosa from environmental sources [9]. These early colonizing strains display a non-mucoid phenotype with a wild-type MucA [17]. Due to strong selective pressure in CF lungs, mucoid mucA mutants eventually become a dominant population [14], [40]. However, secondary mutations that suppress alginate overproduction have been reported [8], [19]. One presumed advantage with non-mucoid suppressors is the loss of mucoid status is coupled with the presence of the flagella, which may promote the colonization of new niches in the lungs [41], [42]. Through screening a transposon library, we found that overexpression of sspA and CF149 algU, and reduced expression of rpoD, are functionally equivalent in causing mucoid conversion in the non-mucoid clinical isolate CF149. This mucoid phenotype can be suppressed by overexpression of the anti-sigma factor MucA [12]. We propose that the mechanism for mucoid conversion mediated by AlgU, SspA and RpoD in CF149 may be related to the competition between sigma factors RpoD and AlgU for the core RNAP binding site (Figure 7). Because of the differential binding ability among sigma factors for core RNAP [43], σ factor competition exists within a cell at any given time [44]. We investigated whether this competition is also present in CF149 and CF149(−rpoD), by measuring the promoter activity of Palgw whose activation depends on RpoN [45], and PalgD which is driven by AlgU [11]. As seen in Figures 3C and S3, activities of both PalgD and PalgW were increased in CF149 (−rpoD). Furthermore, the mucoid suppression resulting from the overexpression of RpoD can be attributed to the competition between two sigma factors (Table 2). Table S2 illustrates that sigma factors besides RpoD can also exert the same effect on mucoid suppression in mucA plus and minus mucoid strains. Thus, any major shift in the intracellular level of sigma factors can potentially affect mucoid conversion, because the pool of core RNAP, which is made up of five different subunits, must be in a limiting amount inside bacterial cells. However, data in Figure S1 demonstrate that induction of FlgM, which is the anti-sigma factor for RpoF responsible for the transcription initiation of flagella biosynthesis [46], failed to induce mucoidy in PAO1 and CF149. This may be due to the fact that the impact on the pool of RNAP is somewhat different between a minor sigma factor RpoF and a major sigma factor RpoD. Therefore not all anti-sigma factors are functionally equal in terms of alginate induction.
Figure 7. Schematic diagram for mucoid conversion in CF149 caused by competition between two sigma factors, RpoD and AlgU.
Stress or starvation can affect the expression of anti-σ70 factors resulting in a decrease in the activity of RpoD, which in turn increases the chances for the alternative sigma factor AlgU to bind to the core RNAP to initiate transcription for alginate biosynthesis. MucA is an anti-sigma factor that has the ability to inhibit the activity of AlgU in alginate production. Competition for RNAP between RpoD and AlgU can determine which promoter is activated. RpoD binds to core RNAP to promote the transcription of housekeeping genes, while AlgU binds to core RNAP at the PalgD promoter to activate alginate production.
Anti-sigma factors are proteins that bind to cognate sigma factors, thereby inhibiting their transcriptional activity [47]. The E.coli protein Rsd associates specifically with σ70 to inhibit the σ70-dependent transcription [48]. The P. aeruginosa transcriptional regulator AlgQ/AlgR2, shares 55% identity at the amino acid sequence level with Rsd [48], and has been proposed as an anti-σ70 factor that interacts with σ70 to affect the transcriptional activity of algD [49]. The T4 phage anti-σ70 factor AsiA also has a similar function as Rsd and AlgQ. Specifically, AsiA can regulate the transcriptional activity of rpoD and other promoters [37], [50], [51], [52]. Although the amino acid sequence similarity is low (<10%) between P. aeruginosa SspA to anti-σ70 factors Rsd, AsiA and AlgQ, the similarity at amino acid level between E. coli SspA to P. aeruginosa SspA is higher than 50%. Hansen et al reported that E. coli SspA is an RNAP-associated protein, and can down-regulate expression from the σ70-dependent promoters [53]. More importantly, E. coli SspA can functionally replace P. aeruginosa anti-sigma factor AlgQ [35]. The sequence diversity in anti-sigma factors suggests that they may have different binding sites on RpoD [36]. Their inhibitory activity to the σ70-driven transcription is therefore different. Compared with the strong inhibition of AsiA, Rsd shows only a modest effect on σ70 transcription in vitro and in vivo [48], [54], [55]. This may explain why overexpression of Rsd and AsiA can induce mucoidy in CF149 when the growth media are supplemented with 0.1% L-Ara, while AlgQ-induced mucoidy in this strain requires a higher concentration of 0.5% L-Ara (Table 1). Also, compared with Rsd and AsiA, AlgQ does not have the ability to significantly inhibit the growth of E. coli in vivo [54].
In the current study, anti-σ70 factors were found to have an effect on mucoid induction in suppressed nonmucoid strain CF149. This induction is not directly through the interaction with the core RNAP, rather it is through the reduction of RpoD activity. CF149 may be a rare alginate suppressor mutant. The amino acid substitution in AlgUA61V in CF149 was mapped to the conserved region of Sigma 70_r2 Superfamily [NCBI CDD cl08419]. This region contains both the −10 promoter recognition helix and the primary core RNAP binding determinant. However, the change from alanine to valine is of conservative nature (non-polar to non-polar), which may explain why this mutation renders AlgUA61V partially defective. Therefore, it is not surprising to see that not all clinical isolates respond to the induction by anti-σ70 factors in the same manner (Table 1). For example, CF4349 is not inducible, even though it has a wild type AlgU and the mucA genotype is the same as CF149. This suggests that there could be another unknown suppressor mutation, which nullifies the effect of anti-σ70 induction in CF4349, or the mutation in CF149 AlgUA61V somehow amplifies the effect of anti-σ70 induction.
Earlier work showed that the growth condition that promotes the fast growth rate of P. aeruginosa PAO1 does not select for the mucoid variants, but the condition that caused a slow growth rate does [56]. In Salmonella, the expression of RpoE, the AlgU homolog induced the stationary phase of growth (slow growth rate) [57]. In the current study, overexpression of anti-RpoD factors can induce mucoidy in CF149 and overexrpression of RpoD can inhibit mucoidy in all tested strains. Furthermore, there is a competition between RpoD- and AlgU-dependent promoters (Figure 5), and the growth rate of both mucoid strain VE2 and PAO581 were slower than that of the isogenic nonmucoid strain PAO1 (Figure 6). These data provide genetic evidence for the sigma factor competiton in the regulation of alginate production by P. aeruginosa.
Mucoid conversion in P. aeruginosa can be achieved through two known mechanisms. Intra-membrane proteolysis acts as a primary mechanism to initiate the degradation of MucA in P. aeruginosa with wild type mucA [13]. Mutation of the mucA gene is another mechanism to become mucoid and is prevalent in isolates from chronic lung infections in CF [2]. In the current study, we showed that a missense mutation in algU causes the loss of mucoidy in the mucA mutant, but augmentation of anti-σ70 factors such as SspA leads to increased amounts of AlgU, causing mucoid conversion in the AlgU-suppressed strain. Williams et al reported that carbon, amino acid, nitrogen and phosphate starvation can induce the expression of SspA [32], and Roychoudhury et al found that starvation caused by nitrogen and phosphate limitation is one of the signals leading to AlgQ-mediated activation of the algD promoter in P. aeruginosa [58]. Therefore, stress signals may activate the expression of anti-RpoD factors, thus causing mucoid conversion in the suppressed strains.
In summary, we found three genes algU, sspA and rpoD that regulate the conversion to mucoidy in the MucA-truncated, non-mucoid P. aeruginosa strain CF149, which contains an AlgU-suppressor mutation. Interestingly, our data indicate the missense mutation in CF149 AlgU only reduces, but does not completely abolish the function as the sigma factor that drives alginate biosynthetic operon. The mechanism by which these genes cause mucoidy may be due to competition between the sigma factors AlgU and RpoD. Also, anti-σ70 factors AsiA, Rsd, AlgQ and SspA can induce mucoidy in strain CF149.
Acknowledgments
We thank Richard M. Niles for assistance in revising this manuscript, and Gary Schultz from the Department of Biology at Marshall University for providing the T4 bacteriophage and its host E. coli BB.
Supporting Information
FlgM, an anti-sigma factor for RpoF, fails to induce mucoidy in CF149 and PAO1. pHERD20T-flgM was conjugated into CF149 and PAO1, respectively. Strains carrying pHERD20T-flgM, pHERD20T-sspA, and pHERD20T were incubated on PIA plates supplemented with 300 µg/ml carbenicillin, 0.1% L-ara and incubated at 37°C for 24 hrs. Alginate was harvested and measured as described in Materials and Methods. *, represents a significant difference between each group (P<0.05).
(TIF)
The stability of over-expressed RpoD in P. aeruginosa and E.coli. RpoD was expressed in P. aeruginosa PAO581 (A) and E. coli TOP10 cells (B) carrying pHERD20T-HA-rpoD-His under the induction with different concentration of L-Ara. PAO581 carried pHERD20T-HA-rpoD-His was cultured on PIA plates supplemented with 300 µg/ml carbenicllin and different concentrations of L-Ara for 24 hrs and the cells were then collected for cell lysis. TOP10 carried pHERD20T-HA-rpoD-His was cultured on LB plates supplemented with 100 µg/ml carbenicllin and L-Ara for 24 hours. Following sonication, 50 µg protein of total cell lysate from each sample was used for SDS-PAGE and Western blotting analysis. Lane 1: the protein molecular mass standards; Lane 2: cells no L-Ara; Lane 3: cells induced by adding 0.1% L-Ara; Lane 4: cells induced by adding 0.5% L-Ara; Lane 5: control with the empty vector.
(TIF)
Reduced expression of RpoD in CF149( −rpoD ) is correlated with increased promoter activity of P algW . RpoN dependent promoter pLP170-PalgW was conjugated into CF149 and CF149 (−rpoD), respectively. The Miller assay was used to detect the activation of PalgW in these strains. *, represents the difference of the β-galactosidase activity between these strains is significant (P<0.05).
(TIF)
Strains and plasmids used in this study.
(DOC)
Inhibitory effect of sigma factors RpoN, RpoS and RpoF on P. aeruginosa mucoidy.
(DOC)
Funding Statement
This work was supported by the National Aeronautics and Space Administration West Virginia Space Grant Consortium (NASA WVSGC) and the Cystic Fibrosis Foundation (CFF-YU11G0). H.D.Y. was supported by National Institutes of Health P20RR016477 and P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence. T.R.W. was supported through the NASA WVSGC Graduate Research Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bodey GP, Bolivar R, Fainstein V, Jadeja L (1983) Infections caused by Pseudomonas aeruginosa . Rev Infect Dis 5: 279–313. [DOI] [PubMed] [Google Scholar]
- 2. Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia . Microbiol Rev 60: 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans LR, Linker A (1973) Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa . J Bacteriol 116: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, et al. (2005) The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol 175: 7512–7518. [DOI] [PubMed] [Google Scholar]
- 5. Pier GB, Coleman F, Grout M, Franklin M, Ohman DE (2001) Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun 69: 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacGeorge J, Korolik V, Morgan AF, Asche V, Holloway BW (1986) Transfer of a chromosomal locus responsible for mucoid colony morphology in Pseudomonas aeruginosa isolated from cystic fibrosis patients to P. aeruginosa PAO. J Med Microbiol 21: 331–336. [DOI] [PubMed] [Google Scholar]
- 7. Ohman DE, Chakrabarty AM (1981) Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun 33: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schurr MJ, Martin DW, Mudd MH, Deretic V (1994) Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J Bacteriol 176: 3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogardt M, Heesemann J (2010) Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 300: 557–562. [DOI] [PubMed] [Google Scholar]
- 10. May TB, Shinabarger D, Maharaj R, Kato J, Chu L, et al. (1991) Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev 4: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin DW, Holloway BW, Deretic V (1993) Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J Bacteriol 175: 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie ZD, Hershberger CD, Shankar S, Ye RW, Chakrabarty AM (1996) Sigma factor-anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol 178: 4990–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damron FH, Goldberg JB (2012) Proteolytic regulation of alginate overproduction in Pseudomonas aeruginosa . Mol Microbiol 84: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boucher JC, Yu H, Mudd MH, Deretic V (1997) Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun 65: 3838–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathee K, McPherson CJ, Ohman DE (1997) Posttranslational control of the algT (algU)-encoded sigma22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). J Bacteriol 179: 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schurr MJ, Yu H, Martinez-Salazar JM, Boucher JC, Deretic V (1996) Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol 178: 4997–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiu D, Eisinger VM, Rowen DW, Yu HD (2007) Regulated proteolysis controls mucoid conversion in Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 104: 8107–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sautter R, Ramos D, Schneper L, Ciofu O, Wassermann T, et al. (2012) A complex multilevel attack on Pseudomonas aeruginosa algT/U expression and algT/U activity results in the loss of alginate production. Gene 498: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ciofu O, Mandsberg LF, Bjarnsholt T, Wassermann T, Hoiby N (2010) Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology 156: 1108–1119. [DOI] [PubMed] [Google Scholar]
- 20. Wong SM, Mekalanos JJ (2000) Genetic footprinting with mariner-based transposition in Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 97: 10191–10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damron FH, Napper J, Teter MA, Yu HD (2009) Lipotoxin F of Pseudomonas aeruginosa is an AlgU-dependent and alginate-independent outer membrane protein involved in resistance to oxidative stress and adhesion to A549 human lung epithelia. Microbiology 155: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76: 1648–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu D, Eisinger VM, Head NE, Pier GB, Yu HD (2008) ClpXP proteases positively regulate alginate overexpression and mucoid conversion in Pseudomonas aeruginosa . Microbiology 154: 2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Head NE, Yu H (2004) Cross-sectional analysis of clinical and environmental isolates of Pseudomonas aeruginosa: biofilm formation, virulence, and genome diversity. Infect Immun 72: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damron FH, Qiu D, Yu HD (2009) The Pseudomonas aeruginosa sensor kinase KinB negatively controls alginate production through AlgW-dependent MucA proteolysis. J Bacteriol 191: 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller JH (1972) beta-galactosidase assay. In: Miller JH, editor. Experiments in molecular genetics. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory. 352–355.
- 27. Barman M, Unold D, Shifley K, Amir E, Hung K, et al. (2008) Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 76: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Damron FH, Davis MR Jr, Withers TR, Ernst RK, Goldberg JB, et al. (2011) Vanadate and triclosan synergistically induce alginate production by Pseudomonas aeruginosa strain PAO1. Mol Microbiol 81: 554–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubin EJ, Akerley BJ, Novik VN, Lampe DJ, Husson RN, et al. (1999) In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc Natl Acad Sci U S A 96: 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, et al. (2003) Comprehensive transposon mutant library of Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 100: 14339–14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams MD, Ouyang TX, Flickinger MC (1994) Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coli protein synthesis and survival during growth and prolonged starvation. Mol Microbiol 11: 1029–1043. [DOI] [PubMed] [Google Scholar]
- 33. Levchenko I, Seidel M, Sauer RT, Baker TA (2000) A specificity-enhancing factor for the ClpXP degradation machine. Science 289: 2354–2356. [DOI] [PubMed] [Google Scholar]
- 34. Qiu D, Damron FH, Mima T, Schweizer HP, Yu HD (2008) PBAD-based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl Environ Microbiol 74: 7422–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schlictman D, Shankar S, Chakrabarty AM (1995) The Escherichia coli genes sspA and rnk can functionally replace the Pseudomonas aeruginosa alginate regulatory gene algR2 . Mol Microbiol 16: 309–320. [DOI] [PubMed] [Google Scholar]
- 36. Yuan AH, Gregory BD, Sharp JS, McCleary KD, Dove SL, et al. (2008) Rsd family proteins make simultaneous interactions with regions 2 and 4 of the primary sigma factor. Mol Microbiol 70: 1136–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orsini G, Ouhammouch M, Le Caer JP, Brody EN (1993) The asiA gene of bacteriophage T4 codes for the anti-sigma 70 protein. J Bacteriol 175: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hershberger CD, Ye RW, Parsek MR, Xie ZD, Chakrabarty AM (1995) The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative sigma factor (sigma E). Proc Natl Acad Sci U S A 92: 7941–7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potvin E, Sanschagrin F, Levesque RC (2008) Sigma factors in Pseudomonas aeruginosa . FEMS Microbiol Rev 32: 38–55. [DOI] [PubMed] [Google Scholar]
- 40. Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, et al. (2006) Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest 116: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garrett ES, Perlegas D, Wozniak DJ (1999) Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU). J Bacteriol 181: 7401–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tart AH, Blanks MJ, Wozniak DJ (2006) The AlgT-dependent transcriptional regulator AmrZ (AlgZ) inhibits flagellum biosynthesis in mucoid, nonmotile Pseudomonas aeruginosa cystic fibrosis isolates. J Bacteriol 188: 6483–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maeda H, Fujita N, Ishihama A (2000) Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res 28: 3497–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ishihama A (2000) Functional modulation of Escherichia coli RNA polymerase. Annu Rev Microbiol 54: 499–518. [DOI] [PubMed] [Google Scholar]
- 45. Damron FH, Owings JP, Okkotsu Y, Varga JJ, Schurr JR, et al. (2012) Analysis of the Pseudomonas aeruginosa regulon controlled by the sensor kinase KinB and sigma factor RpoN. J Bacteriol 194: 1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Starnbach MN, Lory S (1992) The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol 6: 459–469. [DOI] [PubMed] [Google Scholar]
- 47. Hughes KT, Mathee K (1998) The anti-sigma factors. Annu Rev Microbiol 52: 231–286. [DOI] [PubMed] [Google Scholar]
- 48. Jishage M, Ishihama A (1998) A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc Natl Acad Sci U S A 95: 4953–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dove SL, Hochschild A (2001) Bacterial two-hybrid analysis of interactions between region 4 of the sigma(70) subunit of RNA polymerase and the transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa . J Bacteriol 183: 6413–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ouhammouch M, Adelman K, Harvey SR, Orsini G, Brody EN (1995) Bacteriophage T4 MotA and AsiA proteins suffice to direct Escherichia coli RNA polymerase to initiate transcription at T4 middle promoters. Proc Natl Acad Sci U S A 92: 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adelman K, Brody EN, Buckle M (1998) Stimulation of bacteriophage T4 middle transcription by the T4 proteins MotA and AsiA occurs at two distinct steps in the transcription cycle. Proc Natl Acad Sci U S A 95: 15247–15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hinton DM, March-Amegadzie R, Gerber JS, Sharma M (1996) Bacteriophage T4 middle transcription system: T4-modified RNA polymerase; AsiA, a sigma 70 binding protein; and transcriptional activator MotA. Methods Enzymol 274: 43–57. [DOI] [PubMed] [Google Scholar]
- 53. Hansen AM, Lehnherr H, Wang X, Mobley V, Jin DJ (2003) Escherichia coli SspA is a transcription activator for bacteriophage P1 late genes. Mol Microbiol 48: 1621–1631. [DOI] [PubMed] [Google Scholar]
- 54. Pineda M, Gregory BD, Szczypinski B, Baxter KR, Hochschild A, et al. (2004) A family of anti-sigma70 proteins in T4-type phages and bacteria that are similar to AsiA, a Transcription inhibitor and co-activator of bacteriophage T4. J Mol Biol 344: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 55. Sharma UK, Chatterji D (2008) Differential mechanisms of binding of anti-sigma factors Escherichia coli Rsd and bacteriophage T4 AsiA to E. coli RNA polymerase lead to diverse physiological consequences. J Bacteriol 190: 3434–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Terry JM, Pina SE, Mattingly SJ (1991) Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun 59: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, et al. (2002) The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol 43: 771–782. [DOI] [PubMed] [Google Scholar]
- 58. Roychoudhury S, Sakai K, Chakrabarty AM (1992) AlgR2 is an ATP/GTP-dependent protein kinase involved in alginate synthesis by Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 89: 2659–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FlgM, an anti-sigma factor for RpoF, fails to induce mucoidy in CF149 and PAO1. pHERD20T-flgM was conjugated into CF149 and PAO1, respectively. Strains carrying pHERD20T-flgM, pHERD20T-sspA, and pHERD20T were incubated on PIA plates supplemented with 300 µg/ml carbenicillin, 0.1% L-ara and incubated at 37°C for 24 hrs. Alginate was harvested and measured as described in Materials and Methods. *, represents a significant difference between each group (P<0.05).
(TIF)
The stability of over-expressed RpoD in P. aeruginosa and E.coli. RpoD was expressed in P. aeruginosa PAO581 (A) and E. coli TOP10 cells (B) carrying pHERD20T-HA-rpoD-His under the induction with different concentration of L-Ara. PAO581 carried pHERD20T-HA-rpoD-His was cultured on PIA plates supplemented with 300 µg/ml carbenicllin and different concentrations of L-Ara for 24 hrs and the cells were then collected for cell lysis. TOP10 carried pHERD20T-HA-rpoD-His was cultured on LB plates supplemented with 100 µg/ml carbenicllin and L-Ara for 24 hours. Following sonication, 50 µg protein of total cell lysate from each sample was used for SDS-PAGE and Western blotting analysis. Lane 1: the protein molecular mass standards; Lane 2: cells no L-Ara; Lane 3: cells induced by adding 0.1% L-Ara; Lane 4: cells induced by adding 0.5% L-Ara; Lane 5: control with the empty vector.
(TIF)
Reduced expression of RpoD in CF149( −rpoD ) is correlated with increased promoter activity of P algW . RpoN dependent promoter pLP170-PalgW was conjugated into CF149 and CF149 (−rpoD), respectively. The Miller assay was used to detect the activation of PalgW in these strains. *, represents the difference of the β-galactosidase activity between these strains is significant (P<0.05).
(TIF)
Strains and plasmids used in this study.
(DOC)
Inhibitory effect of sigma factors RpoN, RpoS and RpoF on P. aeruginosa mucoidy.
(DOC)