Abstract
Background
Although many studies have attempted to clarify the association between hepatitis B virus (HBV) infection and fatty liver disease, no prior studies have emphasized the relationship of HBV and fatty liver regarding different demographics of age and body mass index (BMI).
Aim
To investigate the correlation of HBV and fatty liver in the different demographics of age and BMI.
Methods
We enrolled consecutive subjects who had received health check-up services at the Taipei Veterans General Hospital from 2002 to 2009 and ultrasonography was used to diagnose fatty liver according to the practice guidelines of the American Gastroenterological Association.
Results
Among the 33,439 subjects enrolled in this study, fatty liver was diagnosed in 43.9% of the population and 38.9% of patients with chronic HBV infection. Multivariate analysis showed that BMI, age, waist circumference, systolic blood pressure, fasting glucose, cholesterol, alanine aminotransferase (ALT) levels, and platelet counts were positively associated, while hepatitis B surface antigen (HBsAg) positivity was inversely associated with fatty liver, especially for subjects with BMI>22.4 kg/m2 and age>50 years. On the contrary, HBV infection was positively correlated with the presence of elevated serum ALT levels in subjects with fatty liver disease regardless of their age and BMI.
Conclusions
Metabolic factors are important determinants for the prevalence of fatty liver. Patients with HBV infection were inversely associated with fatty liver disease than the general population, especially in older and obese patients. Furthermore, metabolic factors and HBV infection were associated with elevated serum ALT levels in fatty liver disease.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of abnormal liver biochemistry tests in the world, both in high and low endemic countries for viral hepatitis [1]–[3]. With the different study populations and diagnosing modalities, the prevalence rate of NAFLD has been reported to be between 10–35% in the United States and it is correlated to metabolic factors, such as central obesity, insulin resistance, arterial hypertension, and hypertriglyceridemia [4].
With the increasing prevalence of the Western dietary pattern, the prevalence of NAFLD also continues to rise in Asian countries. A Korean study reported a NAFLD prevalence rate was 51% in 589 potential liver donors and the risk factors included older age, obesity and hypertriglyceridemia [5]. One population-based study from China revealed that the NAFLD is present in 23.3% of the study group and it is associated with higher serum alanine aminotransferase (ALT), triglyceride (TG) and fasting glucose levels [6]. However, the correlation between hepatitis B virus (HBV) infection and fatty liver disease has not been fully elucidated [7]–[13]. HBV X protein has been proven to influence lipogenic genes, such as sterol regulatory element binding protein-1c, fatty acid synthase, and peroxisome proliferator-activated receptor [14]. Nevertheless, several studies demonstrated chronic HBV infection was not associated with hepatic steatosis and insulin resistance, and hepatic steatosis in HBV infection is associated with metabolic but not viral factors [9], [12], [15]–[17].
Although many studies have tried to clarify the association between HBV infection and fatty liver disease [7]–[9], [11], [12], [16]–[18], no previous studies comprehensively emphasized on the relationship of HBV and fatty liver in the different demographics of age and body mass index (BMI). This large-scale cross-sectional study in Taiwan aimed to investigate the correlation of chronic hepatitis B virus infection and fatty liver in Taiwan.
Materials and Methods
Study Population
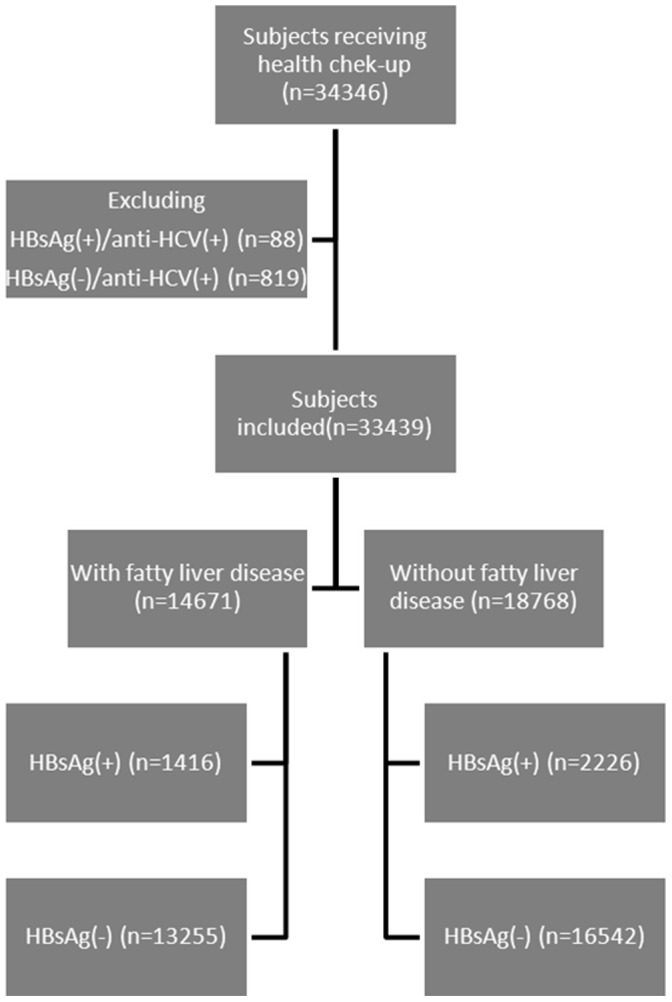
There were 34,346 subjects receiving health check-up services at the Taipei Veterans General Hospital from 2002 to 2009 [2], [19]. As shown in Figure 1 , those who had chronic HCV infection were excluded and the remaining 33,439 subjects were included in the analysis. All of them underwent complete clinical evaluation, laboratory examination and abdominal sonography. BMI was the result of the division of the body weight (in kilograms) by the square body height (in meters). We adopted a normal body mass index (BMI) was between 17.5 and 22.4 kg/m2, an overweight BMI between 22.5 and 24.9 kg/m2, and an obese BMI higher than 25 kg/m2 [20]. Blood pressure (BP) was measured after the subjects had been seated for more than 5 min. The means of three consecutive readings were recorded as systolic and diastolic BP with a difference in systolic BP<10 mmHg. Ultrasonography with Aloka SSD 4000 (Aloka, Tokyo, Japan), Aloka SSD 5000 (Aloka) or Philips HD 15 (Philips, Bothell, WA, USA) were used to diagnose fatty liver by the practice guideline of the American Gastroenterological Association [1]. The study followed the standards of the Declaration of Helsinki and has been approved by the Institutional Review Board of the Taipei Veterans General Hospital (2011-08-010IC). As the dataset used in this study is consisted of de-identified data from a retrospective cohort, the written informed consents from the subjects who receiving physical check-up services were waived by the approval of the IRB.
Figure 1. The flow of subjects in the study.
Biochemical and Serologic Markers
Venous blood samples were collected after an overnight fast. Radio-immunoassay (Abbott Laboratories, North Chicago, IL, USA) was used to test serum HBV surface antigen (HBsAg) and second-generation enzyme immunoassay (Abbott Laboratories) was used to test antibody to hepatitis C virus (anti-HCV). The Roche/Hitachi Modular Analytics System (Roche Diagnostics GmbH, Mannheim, Germany) was utilized to measure serum biochemical markers. The reference limits of these tests were as follows: ALT level, 40 IU/L; gamma-glutamyltransferase (GGT) level 51 IU/L; total cholesterol level, 200 mg/dL; high-density lipoprotein-cholesterol (HDL) level, 40 mg/dL for men and 50 mg/dL for women; low-density lipoprotein-cholesterol (LDL) level, 130 mg/dL; TG level, 150 mg/dL; fasting glucose level, 100 mg/dL; and 2-h post-load plasma glucose level, 150 mg/dL. Fatty liver index (FLI) was calculated as the following formula: FLI = (e 0.953*loge (TG) +0.139*BMI +0.718*loge (GGT) +0.053*waist circumference - 15.745)/(1+ e 0.953*loge (TG) +0.139*BMI +0.718*loge (GGT) +0.053*waist circumference - 15.745) * 100 [21].
Statistical Analysis
The population was analyzed by stratification of HBV infection and fatty liver disease. Subsequently, the different characteristics of subjects with and without fatty liver or HBV infection were analyzed, respectively. The population was then stratified with age, BMI and HBV status.
Pearson chi-squared and Student t-test analysis was performed to compare categorical and continuous variables with two samples, respectively. Variables with statistical significance (P<0.05) or proximate to it (P<0.1) in univariate analysis were included in multivariate analysis by logistic regression model with the forward stepwise selection procedure.
Results
Subjects’ Characteristics Stratified by HBV Infection and Fatty Liver
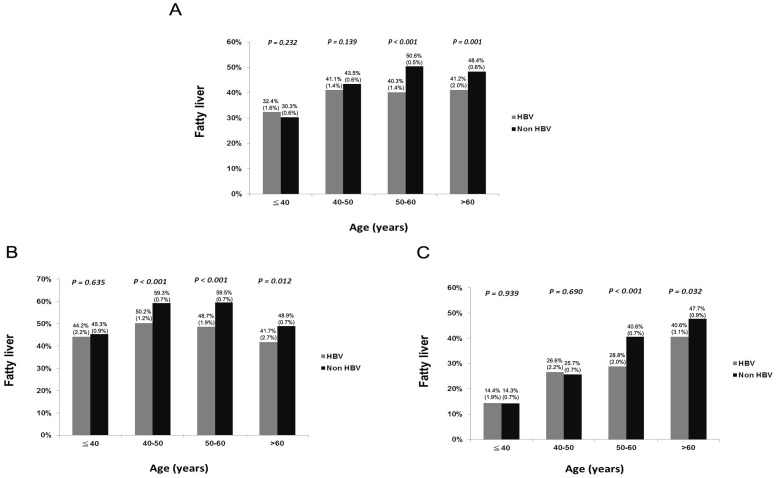
The demographic data of all subjects are shown in Table 1 . The mean age was 51.9 years, with a male prevalence of 54.6% and fatty liver was diagnosed in 43.9% of the whole population by ultrasonography. Patients with HBV infection were younger in age, moremales, with higher levels of ALT and AST levels, lower systolic blood pressure (SBP), lower levels of cholesterol, LDL, TG, glucose levels, and platelet counts (all of the P<0.001). Fatty liver presented in 38.9% of HBV patients, lower than the 44.5% in non-HBV subjects (P<0.001). ( Table 1 and Figure 2 ).
Table 1. Factors associated with HBV and non-HBV subjects.
| ALL (n = 33439) | HBV(+) (n = 3642) | HBV(−) (n = 29797) | P value | |
| BMI, kg/m2 * | 23.81±3.56 | 22.77±3.38 | 23.82±3.56 | 0.434 |
| Age, years * | 51.9±13.1 | 49.5±11.5 | 52.2±13.3 | <0.001 |
| Sex (M/F) (%) | 18257/15182 (54.6%/45.4%) | 2159/1483 (59.3%/40.7%) | 18681/15665 (54.4%/45.6%) | <0.001 |
| WC, cm * | 83.7±10.2 | 83.4±9.9 | 83.8±10.3 | 0.051 |
| SBP, mmHg * | 124.1±18.5 | 122.5±17.8 | 124.3±18.6 | <0.001 |
| DBP, mmHg * | 77.5±14.4 | 77.3±15.7 | 77.5±14.3 | 0.399 |
| Fasting glucose, mg/dL * | 95.4±24.6 | 94.2±23.4 | 95.5±24.8 | <0.001 |
| Cholesterol, mg/dL * | 198.6±37.0 | 193.5±36.8 | 199.2±37.0 | <0.001 |
| HDL, mg/dL * | 53.7±15.1 | 53.7±15.4 | 53.7±15.0 | 0.747 |
| LDL, mg/dL * | 124.9±32.9 | 121.5±32.7 | 125.3±32.9 | <0.001 |
| TG, mg/dL * | 128.9±87.4 | 116.6±80.2 | 130.4±88.1 | <0.001 |
| AST, IU/L * | 23.7±15.0 | 28.8±24.9 | 23.1±13.2 | <0.001 |
| ALT, IU/L * | 28.2±26.4 | 37.7±47.6 | 27.0±22.2 | <0.001 |
| GGT, IU/L * | 24.7±36.5 | 24.3±33.2 | 24.8±36.8 | 0.504 |
| Platelet, 1000/mm3 * | 247.6±60.7 | 229.1±60.6 | 249.8±60.3 | <0.001 |
| Fatty liver (yes/no) (%) | 14671/18768 (43.9/56.1) | 1416/2226 (38.9%/61.1%) | 13255/16542 (44.5%/55.5%) | <0.001 |
| FLI * | 26.96±24.03 | 24.72±22.67 | 27.24±14.18 | <0.001 |
HBV, hepatitis B virus; BMI, body mass index; SD, standard deviation; M, male; F, female; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; FLI, fatty liver index.
Expressed as mean ± standard deviation.
Figure 2. Age distribution of fatty liver stratified by HBV status (A) all subjects, (B) males and (C) females.
The prevalence rates of fatty liver in all subgroups are expressed as prevalence rate (standard error).
As shown in Table 2 , compared to subjects without fatty liver, those with fatty liver were older in age, more males, and had higher BMI, larger waist circumference (WC), higher SBP, higher levels of glucose, cholesterol, LDL, TG, ALT, GGT, platelet counts, FLI, but lower HDL levels (all of the P<0.001) by univariate analysis.
Table 2. Comparison of demographic characteristics between subjects with and without fatty liver.
| Without fatty liver (n = 18768 ) | With fatty liver (n = 14671) | P value | |
| BMI, kg/m2 * | 22.35±2.90 | 25.68±3.46 | <0.001 |
| Age, years * | 50.6±13.9 | 53.6±11.9 | <0.001 |
| Sex (M/F) (%) | 8531/10237(45.3%/54.7%) | 9726/4945(66.1%/33.9%) | <0.001 |
| WC, cm * | 79.6±8.9 | 89.1±9.3 | <0.001 |
| SBP, mmHg * | 121.0±18.4 | 128.0±18.0 | <0.001 |
| Fasting glucose, mg/dL * | 90.6±18.9 | 101.5±29.4 | <0.001 |
| Cholesterol, mg/dL * | 194.1±36.0 | 204.2±37.6 | <0.001 |
| HDL, mg/dL * | 58.1±15.5 | 48.0±12.4 | <0.001 |
| LDL, mg/dL * | 120.2±31.8 | 130.9±33.3 | <0.001 |
| TG, mg/dL * | 100.5±57.0 | 165.3±104.4 | <0.001 |
| ALT, IU/L * | 22.7±24.1 | 35.2±27.4 | <0.001 |
| GGT, IU/L * | 20.1±33.7 | 30.7±38.9 | <0.001 |
| Platelet, 1000/mm3 * | 245.1±61.5 | 250.8±60.7 | <0.001 |
| HBsAg (positive/negative) (%) | 2226/16542(11.9%/88.1%) | 1416/13255(10.7%/89.3%) | <0.001 |
| FLI * | 15.64±16.31 | 41.45±24.54 | <0.001 |
BMI, body mass index; SD, standard deviation; M, male; F, female; WC, waist circumference; SBP, systolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; HBsAg, hepatitis B surface antigen; FLI, fatty liver index.
Expressed as mean ± standard deviation.
For patients with HBV infections, the demographic characteristics between those with and without fatty liver were compared in Table 3 . Similarly, subjects with fatty liver were older in age, more males, and had higher BMI, larger WC, higher SBP, higher levels of fasting glucose, cholesterol, LDL, TG, ALT, GGT, platelet counts, FLI, but lower HDL levels by univariate analysis.
Table 3. Comparison of demographic characteristics between HBV subjects with and without fatty liver.
| Without fatty liver (n = 2226 ) | With fatty liver (n = 1416) | P value | |
| BMI, kg/m2 * | 22.57±2.86 | 25.65±3.28 | <0.001 |
| Age, years * | 49.0±11.8 | 50.3±10.9 | 0.001 |
| Sex (M/F) (%) | 1143/1083 (51.3%/48.7%) | 1016/400 (71.8%/28.2%) | <0.001 |
| WC, cm * | 79.9±8.8 | 89.0±9.0 | <0.001 |
| SBP, mmHg * | 120.0±17.4 | 126.3±17.8 | <0.001 |
| Fasting glucose, mg/dL * | 90.1±17.3 | 100.4±29.5 | <0.001 |
| Cholesterol, mg/dL * | 191.5±36.0 | 196.8±37.6 | <0.001 |
| HDL, mg/dL * | 57.7±15.6 | 47.5±12.9 | <0.001 |
| LDL, mg/dL * | 118.5±32.0 | 126.3±33.3 | <0.001 |
| TG, mg/dL * | 96.7±55.2 | 147.7±100.8 | <0.001 |
| ALT, IU/L * | 33.9±50.7 | 43.7±41.5 | <0.001 |
| GGT, IU/L * | 21.0±32.5 | 29.6±34.7 | <0.001 |
| Platelet, 1000/mm3 * | 226.0±62.6 | 234.1±57.1 | <0.001 |
| FLI * | 15.87±16.56 | 38.63±23.98 | <0.001 |
BMI, body mass index; SD, standard deviation; M, male; F, female; WC, waist circumference; SBP, systolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; HBsAg, hepatitis B surface antigen; FLI, fatty liver index.
Expressed as mean ± standard deviation.
Factors Associated with Fatty Liver Stratified by HBV, BMI and Age
By multivariate analysis, higher BMI, older age, higher WC, SBP, fasting glucose, cholesterol, and ALT levels, lower HDL levels, higher platelet count and HBsAg negativity were associated with fatty liver. (Table 4) For subjects with HBV infection, metabolic factors, including BMI, WC, fasting glucose, cholesterol, HDL, ALT and platelet count were correlated with fatty liver.
Table 4. Factors associated with fatty liver in different populations by multivariate analysis.
| Odds ratio | 95% confidence interval | P | |
| All subjects | |||
| BMI (per kg/m2) | 1.209 | 1.191–1.227 | <0.001 |
| Age (per years) | 1.004 | 1.002–1.006 | 0.001 |
| WC (per cm) | 1.036 | 1.031–1.042 | <0.001 |
| SBP (per mmHg) | 1.002 | 1.000–1.003 | 0.040 |
| Fasting glucose (per mg/dL) | 1.010 | 1.009–1.011 | <0.001 |
| Cholesterol (per mg/dL) | 1.008 | 1.007–1.009 | <0.001 |
| HDL (per mg/dL) | 0.969 | 0.967–0.971 | <0.001 |
| ALT (per U/L) | 1.018 | 1.016–1.020 | <0.001 |
| Platelet (per/mm3) | 1.002 | 1.002–1.003 | <0.001 |
| HBsAg | 0.700 | 0.642–0.764 | <0.001 |
| For subjects with HBV infection | |||
| BMI (per kg/m2) | 1.161 | 1.110–1.214 | <0.001 |
| WC (per cm) | 1.055 | 1.038–1.072 | <0.001 |
| Fasting glucose (per mg/dL) | 1.013 | 1.009–1.017 | <0.001 |
| Cholesterol (per mg/dL) | 1.006 | 1.003–1.008 | <0.001 |
| HDL (per mg/dL) | 0.973 | 0.967–0.979 | <0.001 |
| ALT (per IU/L) | 1.003 | 1.001–1.005 | 0.003 |
| Platelet (per/mm3) | 1.003 | 1.002–1.004 | <0.001 |
| For subjects with BMI≤22.4 kg/m2 | |||
| Age (per years) | 1.005 | 1.001–1.009 | 0.02 |
| WC (per cm) | 1.071 | 1.062–1.080 | <0.001 |
| Fasting glucose (per mg/dL) | 1.010 | 1.008–1.012 | <0.001 |
| Cholesterol (per mg/dL) | 1.006 | 1.005–1.008 | <0.001 |
| HDL (per mg/dL) | 0.977 | 0.973–0.981 | <0.001 |
| ALT (per IU/L) | 1.007 | 1.005–1.009 | <0.001 |
| Platelet (per/mm3) | 1.002 | 1.001–1.003 | 0.001 |
| For subjects with BMI>22.4 kg/m2 | |||
| Male gender | 1.193 | 1.111–1.282 | <0.001 |
| WC (per cm) | 1.068 | 1.063–1.073 | <0.001 |
| SBP (per mmHg) | 1.003 | 1.002–1.005 | <0.001 |
| Fasting glucose (per mg/dL) | 1.010 | 1.009–1.012 | <0.001 |
| Cholesterol (per mg/dL) | 1.008 | 1.007–1.009 | <0.001 |
| HDL (per mg/dL) | 0.964 | 0.961–0.966 | <0.001 |
| ALT (per IU/L) | 1.025 | 1.023–1.027 | <0.001 |
| Platelet (per/mm3) | 1.003 | 1.002–1.003 | <0.001 |
| HBsAg positivity | 0.655 | 0.593–0.723 | <0.001 |
| For subjects with age≦50 years | |||
| BMI (per kg/m2) | 1.176 | 1.147–1.204 | <0.001 |
| Male gender | 0.817 | 0.736–0.907 | <0.001 |
| WC (per cm) | 1.048 | 1.038–1.057 | <0.001 |
| SBP (per mmHg) | 1.004 | 1.001–1.007 | 0.009 |
| Fasting glucose (per mg/dL) | 1.013 | 1.010–1.016 | <0.001 |
| Cholesterol (per mg/dL) | 1.008 | 1.007–1.009 | <0.001 |
| HDL (per mg/dL) | 0.972 | 0.968–0.976 | <0.001 |
| ALT (per IU/L) | 1.011 | 1.009–1.014 | <0.001 |
| GGT (per IU/L) | 1.003 | 1.001–1.004 | <0.001 |
| Platelet (per/mm3) | 1.003 | 1.002–1.003 | <0.001 |
| For subjects with age >50 years | |||
| BMI (per kg/m2) | 1.225 | 1.202–1.249 | <0.001 |
| Male gender | 1.115 | 1.032–1.205 | 0.006 |
| WC (per cm) | 1.030 | 1.023–1.036 | <0.001 |
| Fasting glucose (per mg/dL) | 1.009 | 1.008–1.011 | <0.001 |
| Cholesterol (per mg/dL) | 1.007 | 1.006–1.008 | <0.001 |
| HDL (per mg/dL) | 0.967 | 0.964–0.970 | <0.001 |
| ALT (per IU/L) | 1.022 | 1.020–1.025 | <0.001 |
| Platelet (per/mm3) | 1.003 | 1.002–1.003 | <0.001 |
| HBsAg positivity | 0.624 | 0.553–0.703 | <0.001 |
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; HDL, high-density lipoprotein; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; HBsAg, hepatitis B surface antigen.
For the whole subjects stratified by BMI, those with BMI ≦22.4 kg/m2 in combination with HBV infections have a lower rate of fatty liver (14.7%), comparing to those with BMI ≦22.4 kg/m2 and without HBV infections (17.2%). (Figure 3) However, the difference was not significant by multivariate analysis. (Table 4) On the contrary, subjects with BMI >22.4 kg/m2 in combination with HBV infections have a significant lower rate of fatty liver (52.3%) in compared to those with BMI >22.4 kg/m2 but without HBV infections (59.8%) both in univariate and multivariate analyses.
Figure 3. Distribution of fatty liver disease stratified by HBV status and BMI.

The prevalence rates of fatty liver in all subgroups are expressed as prevalence rate (standard error). For subjects with BMI ≦22.4 kg/m2, the prevalence rate of fatty liver between those with and without HBV infection were insignificant by multivariate analysis though the P value was 0.021 by univariate analysis. For subjects with BMI >22.4 kg/m2 in combination with HBV infections have a significant lower rate of fatty liver (52.3%) in compared to those with BMI >22.4 kg/m2 but without HBV infections (59.8%).
Stratified by age, BMI, sex, WC, fasting glucose, cholesterol, HDL, ALT, platelet count were significantly related with fatty liver whether patients were older or younger than 50 years. Nevertheless, viral status was not related to fatty liver in younger patients (age ≦50 years). On the contrary, the diagnosis of fatty liver was 0.624 fold less in HBV patients than in non-HBV patients with older than 50 years. (Table 4 and Figure 2).
Factors Associated with Elevated Serum ALT Levels in Fatty Liver Stratified by HBV, BMI and Age
As shown in Table 5 , for subjects with fatty liver, multivariate analysis showed that larger BMI, older age, higher WC, fasting glucose, cholesterol, HDL, higher GGT levels and HBsAg positivity were the independent risk factors correlated with elevated serum ALT levels. For subjects with HBV infection and fatty liver, younger age, larger WC, lower HDL, higher GGT, and platelet count were associated with elevated serum ALT levels by multivariate analysis.
Table 5. Multivariate analysis of factors associated with elevated serum ALT levels in subjects with fatty liver.
| Odds Ratio | 95% Confidence interval | P | |
| All subjects | |||
| BMI (per kg/m2) | 1.062 | 1.040–1.084 | <0.001 |
| Age (per years) | 0.969 | 0.965–0.973 | <0.001 |
| WC (per cm) | 1.023 | 1.014–1.031 | <0.001 |
| Fasting glucose (per mg/dL) | 1.002 | 1.000–1.003 | 0.009 |
| Cholesterol (per mg/dL) | 1.004 | 1.002–1.005 | <0.001 |
| HDL (per mg/dL) | 0.976 | 0.972–0.980 | <0.001 |
| GGT (per IU/L) | 1.032 | 1.030–1.035 | <0.001 |
| HBsAg positivity | 1.782 | 1.563–2.031 | <0.001 |
| For subjects with HBV infection | |||
| Age (per years) | 0.969 | 0.957–0.980 | <0.001 |
| WC (per cm) | 1.022 | 1.007–1.037 | 0.005 |
| HDL (mg/dL) | 0.980 | 0.970–0.991 | <0.001 |
| GGT (IU/L) | 1.038 | 1.030–1.046 | <0.001 |
| Platelet (per 1000/mm3) | 0.980 | 0.970–0.991 | <0.001 |
| For subjects with BMI≤22.4 kg/m2 | |||
| Age (per years) | 0.979 | 0.966–0.993 | 0.002 |
| Cholesterol (per mg/dL) | 1.008 | 1.004–1.011 | <0.001 |
| HDL (per mg/dL) | 0.973 | 0.962–0.984 | <0.001 |
| GGT (per IU/L) | 1.024 | 1.018–1.029 | <0.001 |
| HBsAg positivity | 3.306 | 2.017–4.569 | <0.001 |
| For subjects with BMI>22.4 kg/m2 | |||
| Age (per years) | 0.966 | 0.962–0.970 | <0.001 |
| WC (per cm) | 1.036 | 1.030–1.042 | <0.001 |
| Fasting glucose (per mg/dL) | 1.003 | 1.001–1.004 | 0.002 |
| Cholesterol (per mg/dL) | 1.003 | 1.002–1.004 | <0.001 |
| HDL (per mg/dL) | 0.978 | 0.973–0.982 | <0.001 |
| GGT (per IU/L) | 1.033 | 1.031–1.036 | <0.001 |
| HBsAg positivity | 1.673 | 1.458–1.920 | <0.001 |
| For subjects with age≦50 years | |||
| BMI (per kg/m2) | 1.130 | 1.109–1.151 | <0.001 |
| Cholesterol (per mg/dL) | 1.004 | 1.002–1.006 | <0.001 |
| HDL (per mg/dL) | 0.978 | 0.971–0.984 | <0.001 |
| GGT (per IU/L) | 1.032 | 1.029–1.036 | <0.001 |
| HBsAg positivity | 1.681 | 1.400–2.017 | <0.001 |
| For subjects with age >50 years | |||
| BMI (per kg/m2) | 1.073 | 1.043–1.104 | <0.001 |
| WC (per cm) | 1.014 | 1.004±1.025 | 0.008 |
| SBP (per mmHg) | 0.997 | 0.993–1.000 | 0.031 |
| Fasting glucose (per mg/dL) | 1.002 | 1.000–1.004 | 0.030 |
| Cholesterol (per mg/dL) | 1.004 | 1.002–1.005 | <0.001 |
| HDL (per mg/dL) | 0.975 | 0.970–0.981 | <0.001 |
| GGT (per IU/L) | 1.033 | 1.030–1.036 | <0.001 |
| Platelet (per/mm3) | 0.998 | 0.997–0.999 | <0.001 |
| HBsAg positivity | 1.946 | 1.614–2.347 | <0.001 |
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; HDL, high-density lipoprotein; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; HBsAg, hepatitis B surface antigen.
For the whole subjects with fatty liver, HBsAg positivity, higher GGT levels, and presence of metabolic factors, such as higher cholesterol and lower HDL levels, were still associated with elevated serum ALT levels irrespective of age and BMI status. The present of elevated serum ALT levels were 3.306 fold, 1.673 fold, 1.681 fold and 1.946 fold more in HBV patients than in non-HBV patients with age ≦50 years, age >50 years, BMI≦22.4 kg/m2 and BMI >22.4 kg/m2, respectively. ( Table 5 ).
Discussion
The prevalence of NAFLD varies by the diagnostic modality and ethnicity and present in 23–51% in Asian populations [5], [6]. In the present study, fatty liver was diagnosed by ultrasonography in 43.9% of patients who received physical checkup in a single medical center. Of note, HBV infection was associated with lower prevalence of fatty liver, especially in subjects of overweight or obesity, or in those who were older than 50 years. However, the association between HBV and fatty liver was less apparent if patients were normal-weight or lean, or younger than 50 years. On the contrary, HBV infection was positively correlated with the presence of elevated liver biochemistries in subjects with fatty liver disease regardless of their age and BMI. It implies that viral factors might play some roles in the pathogenesis of fatty liver in subjects with fatty liver.
The most common cause of mortality in NAFLD patients is cardiovascular disease and the rate of cirrhosis development in patients with NAFLD is around 5–20% over 10 years [1], [22]. Identification and improvement of the risk factors of NAFLD may contribute to reduce these complications. The risk factors of fatty liver disease reported by previous studies include age, gender and metabolic factors, such as central obesity, higher BMI, elevated fasting blood glucose, TG, cholesterol and lower HDL levels [6], [23]. Our present study further validated that patients were at risk of fatty liver disease if they possessed these metabolic aberrances. While the evidence of all medications to improve NAFLD was inconclusive or conflicting, lifestyle interventions, such as diet modification, exercise programs and weight loss, and the treatment of the associated metabolic comorbidities such as obesity, hyperlipidemia, insulin resistance and type 2 diabetes mellitus are considered beneficial [1].
Fatty liver index, an algorithm based on BMI, waist circumference, triglycerides and GGT, was designed to assist the diagnosis of fatty liver and is used to predict patients at risk of type 2 diabetes mellitus and metabolic syndrome recently [24]. The present study demonstrated that FLI averaged 15.64 and 41.45 in non-fatty and fatty liver patients, respectively, with a significant difference.
The prevalence of fatty liver between HBV patients and general population was inconclusive. Nascimento et al. found that NAFLD presented in HBV patients from 10–76% of the time [25]. In Beijing, China, the frequency of fatty liver in HBV patients is higher than that for the general population, but lower than that in HCV patients [16]. On the contrary, the prevalence of NAFLD in HBV patients was less than that for the general population in Shanghai, China [26]. In our study, fatty liver was less in HBV patients (38.9%) than the general population (43.9%). Further stratification analysis showed that chronic HBV infection presented with no significant influence in the prevalence of fatty liver in patients younger than 50 years and the result was comparable with Wang et al.’s study whose mean age was 46.56 years [12]. Of note, the inverse relationships between the prevalence of fatty liver and positive HBsAg status was observed in patients older than 50 years. HBsAg seroclearance occurs more often after 50 years in patients with chronic hepatitis B [27]–[29], and Chu demonstrated that HBsAg carriers with moderate-to-severe hepatic steatosis were associated with more than threefold HBsAg seroclearance than those without hepatic steatosis [30]. These studies implied that there were inverse relationships between the prevalence of fatty liver and positive HBsAg status, especially in patients older than 50 years as demonstrated in our study [7], [15].
Higher BMI has been declared to be related with fatty liver in previous studies and it is consistent in the present study [1]. Further stratification analysis demonstrated that the presence of HBV infection was associated with less fatty liver in patients of BMI over 22.4 kg/m2. Obese patients with chronic HBV infection may be more concerned about their dietary habits and have less prevalence of fatty liver. Moreover, excessive BMI is involved in the transition from HBV carrier state to HCC and liver-related mortality [10], [31]. The reduced prevalence of fatty liver in HBV patients with higher BMI may also reflect the higher mortality in obese patients with chronic HBV infection [32]. As mentioned above, several studies have shown high HBsAg seroclearance in patients with hepatic steatosis [28]–[30]. Further stratification analysis in REVEAL-HBV study [18], [28], found that extreme obesity was associated with low HBV viral load and was a significant predictor of HBsAg seroclearance in chronic hepatitis B patients. This may be a reason why HBV patients with higher BMI had less fatty liver. Nevertheless, HBV infection was positively correlated with the elevated serum ALT levels in subjects with fatty liver disease regardless of their age and BMI, suggesting that liver inflammation induced by viral infection still played an important role for the formation and degree of NASH.
The strengths of this study are the large sample size and detailed biochemistry data. However, it has several limitations that are worth noting. First, the study population had a higher socioeconomic status and subjects could afford the expense of a physical check-up, so the results may not represent the general population. Second, some liver related diseases, such as alcohol consumption, medication use, autoimmune liver disease, and congenital liver disease, are not documented in the study. Nevertheless, the prevalence of elevated serum ALT levels due to alcohol consumption was low (0.8%) in a Taiwanese community study [33]. Drug-induced liver injury is rare and the incidence was 14 cases per 100,000 inhabitants per year in a French population-based prospective study [34], [35]. With available epidemiological data, autoimmune hepatitis, primary biliary cirrhosis, hematochromatosis and Wilson disease are all rare in Asia [36]–[38]. Those suggest that these factors may only play a small role and have little influence on the result. Third, smoking was not listed in the database though it has been noted as a risk for the progression of HBV and HCV infection [39]. Fourth, the gold standard for the diagnosis of fatty liver is liver biopsy. However, the invasiveness of that procedure is not justified for surveillance in the general population. In the present study, the diagnosis of fatty liver was made by ultrasonography and defined by at least two of three abnormal findings: diffusely increased echogenic liver greater than kidney or spleen, vascular blurring and deep attenuation of ultrasound signal with a sensitivity of 89% and specificity of 93% [40], [41]. Fifth, the rate of NAFLD progression to cirrhosis is about 5–20% over 10 years, which has a 10-year liver-related mortality of 25% [22]. However, our study is a cross-sectional design, so the prognosis of the population was unattainable. Sixth, severities of HBV infections, such as HBV viral load, hepatitis B e antigen status, HBV viral mutations, and the use of antiviral therapy, were not available in this study, which led to a difficulty of further analysis regarding these viral factors [42]–[44].
In conclusion, metabolic factors are imperative for the prevalence of fatty liver. Fatty liver disease is less common in HBV patients than in the general population, especially in older and obese patients. On the contrary, HBV infection was positively correlated with the presence of elevated liver biochemistries in subjects with fatty liver disease regardless of their age and BMI.
Funding Statement
These authors have no support or funding to report.
References
- 1. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142: 1592–1609. [DOI] [PubMed] [Google Scholar]
- 2. Wu WC, Wu CY, Wang YJ, Hung HH, Yang HI, et al. (2012) Updated thresholds for serum alanine aminotransferase level in a large-scale population study composed of 34 346 subjects. Aliment Pharmacol Ther 36: 560–568. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, et al. (2011) Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 9: 524–530. [DOI] [PubMed] [Google Scholar]
- 4. Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 5. Lee JY, Kim KM, Lee SG, Yu E, Lim YS, et al. (2007) Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 47: 239–244. [DOI] [PubMed] [Google Scholar]
- 6. Hou XH, Zhu YX, Lu HJ, Chen HF, Li Q, et al. (2011) Non-alcoholic fatty liver disease’s prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the Chinese. J Gastroenterol Hepatol 26: 722–730. [DOI] [PubMed] [Google Scholar]
- 7. Wong VW, Wong GL, Chu WC, Chim AM, Ong A, et al. (2012) Hepatitis B virus infection and fatty liver in the general population. J Hepatol 56: 533–540. [DOI] [PubMed] [Google Scholar]
- 8. Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, et al. (2012) Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat 19: e48–57. [DOI] [PubMed] [Google Scholar]
- 9. Machado MV, Oliveira AG, Cortez-Pinto H (2011) Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol 26: 1361–1367. [DOI] [PubMed] [Google Scholar]
- 10. Lee IC, Huang YH, Chan CC, Huo TI, Chu CJ, et al. (2011) Impact of body mass index and viral load on liver histology in hepatitis B e antigen-negative chronic hepatitis B. Clin Nutr. 30: 647–652. [DOI] [PubMed] [Google Scholar]
- 11. Yun JW, Cho YK, Park JH, Kim HJ, Park DI, et al. (2009) Hepatic steatosis and fibrosis in young men with treatment-naive chronic hepatitis B. Liver Int. 29: 878–883. [DOI] [PubMed] [Google Scholar]
- 12. Wang CC, Hsu CS, Liu CJ, Kao JH, Chen DS (2008) Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol 23: 779–782. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, et al. (2012) Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol 27: 1858–1864. [DOI] [PubMed] [Google Scholar]
- 14. Na TY, Shin YK, Roh KJ, Kang SA, Hong I, et al. (2009) Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology 49: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 15. Jan CF, Chen CJ, Chiu YH, Chen LS, Wu HM, et al. (2006) A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community-based Integrated Screening study No. 10). Int J Obes (Lond) 30: 794–799. [DOI] [PubMed] [Google Scholar]
- 16. Peng D, Han Y, Ding H, Wei L (2008) Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol 23: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 17. Shi JP, Fan JG, Wu R, Gao XQ, Zhang L, et al. (2008) Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J Gastroenterol Hepatol 23: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 18. Chiang CH, Yang HI, Jen CL, Lu SN, Wang LY, et al. (2013) Association between obesity, hypertriglyceridemia and low hepatitis B viral load. Int J Obes 37: 410–415. [DOI] [PubMed] [Google Scholar]
- 19.Huang KW, Leu HB, Wang YJ, Luo JC, Lin HC, et al.. (2013) Patients with non-alcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Dis (in press). [DOI] [PubMed]
- 20. Liu CJ (2012) Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol 27: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 21. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, et al. (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterology 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farrell GC, Larter CZ (2006) Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112. [DOI] [PubMed] [Google Scholar]
- 23. Koehler EM, Schouten JN, Hansen BE, van Rooij FJ, Hofman A, et al. (2012) Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: results from the Rotterdam study. J Hepatol 57: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 24.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, et al.. (2013) External Validation of the Fatty Liver Index for Identifying Nonalcoholic Fatty Liver Disease in a Population-based Study. Clin Gastroenterol Hepatol (in press). [DOI] [PubMed]
- 25. Nascimento AC, Maia DR, Neto SM, Lima EM, Twycross M, et al. (2012) Nonalcoholic Fatty liver disease in chronic hepatitis B and C patients from Western Amazon. Int J Hepatol 2012: 695950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan JG, Farrell GC (2009) Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol 50: 204–210. [DOI] [PubMed] [Google Scholar]
- 27. Yuen MF, Wong DK, Fung J, Ip P, But D, et al. (2008) HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 135: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Yang HI, Lee MH, Lu SN, Jen CL, et al. (2010) Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 139: 474–482. [DOI] [PubMed] [Google Scholar]
- 29. Tai DI, Tsay PK, Chen WT, Chu CM, Liaw YF (2010) Relative roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am J Gastroenterol 105: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 30. Chu CM, Lin DY, Liaw YF (2007) Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 31: 871–875. [DOI] [PubMed] [Google Scholar]
- 31. Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, et al. (2009) Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 58: 111–117. [DOI] [PubMed] [Google Scholar]
- 32. Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, et al. (2008) Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol 26: 5576–5582. [DOI] [PubMed] [Google Scholar]
- 33. Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, et al. (2007) Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J Gastroenterol Hepatol 22: 1482–1489. [DOI] [PubMed] [Google Scholar]
- 34. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, et al. (2002) Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36: 451–455. [DOI] [PubMed] [Google Scholar]
- 35.Kao WY, Su CW, Huang YS, Chou YC, Chen YC, et al.. (2013) Risk of Oral Anti-fungal Agent-Induced Liver Injury in Taiwanese. Brit J Clin Pharmacol (in press). [DOI] [PMC free article] [PubMed]
- 36. Hershko C (2009) Iron overload in Asia. Blood 114: 1–2. [DOI] [PubMed] [Google Scholar]
- 37. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, et al. (2010) Diagnosis and management of autoimmune hepatitis. Hepatology 51: 2193–2213. [DOI] [PubMed] [Google Scholar]
- 38. Su CW, Chan CC, Hung HH, Huo TI, Huang YH, et al. (2009) Predictive value of aspartate aminotransferase to alanine aminotransferase ratio for hepatic fibrosis and clinical adverse outcomes in patients with primary biliary cirrhosis. J Clin Gastroenterol 43: 876–883. [DOI] [PubMed] [Google Scholar]
- 39. Chuang SC, Lee YC, Hashibe M, Dai M, Zheng T, et al. (2010) Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 19: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD (1991) Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 43: 26–31. [DOI] [PubMed] [Google Scholar]
- 41. Haring R, Wallaschofski H, Nauck M, Dorr M, Baumeister SE, et al. (2009) Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology 50: 1403–1411. [DOI] [PubMed] [Google Scholar]
- 42.Su CW, Wu CY, Hung HH, Wu CH, Sheen IJ, et al.. (2013) Differential roles of serum HBV DNA and HBsAg level in predicting virological breakthrough in patients receiving lamivudine therapy. J Gastroenterol Hepatol (in press). [DOI] [PubMed]
- 43. Su CW, Chiou YW, Tsai YH, Teng RD, Chau GY, et al. (2013) The influence of hepatitis B viral load and pre-S deletion mutations on post-operative recurrence of hepatocellular carcinoma and the tertiary preventive effects by anti-viral therapy. PLoS ONE 8: e66457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin X, Chen YP, Yang YD, Li YM, Zheng L, et al. (2012) Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. PLoS ONE. 7: e34198. [DOI] [PMC free article] [PubMed] [Google Scholar]