Abstract
The blood pressure response to exercise is exaggerated in hypertension. Recent evidence suggests that an overactive skeletal muscle mechanoreflex contributes significantly to this augmented circulatory responsiveness. Sensory information from the mechanoreflex is processed within the nucleus tractus solitarii (NTS) of the medulla oblongata. Normally, endogenously produced nitric oxide (NO) within the NTS attenuates the increase in mean arterial pressure (MAP) induced by mechanoreflex stimulation. Thus, it has been suggested that decreases in NTS-NO production underlie the generation of mechanoreflex dysfunction in hypertension. Supporting this postulate, it has been shown that blocking NO production within the NTS of normotensive rats reproduces the exaggerated pressor response elicited by mechanoreflex activation in hypertensive animals. What is not known is whether increasing NO production within the NTS of hypertensive rats mitigates mechanoreflex overactivity. In this study, the mechanoreflex was selectively activated by passively stretching hindlimb muscle before and after the dialysis of 1 and 10 μM L-arginine (a NO precursor) within the NTS of decerebrate normotensive Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Stretch induced larger elevations in MAP in SHR compared to WKY. In both groups, dialysis of 1 μM L-arginine significantly attenuated the pressor response to stretch. However, at the 10 μM dose, L-arginine had no effect on the MAP response to stretch in WKY while it enhanced the response in SHR. The data demonstrate that increasing NO availability within the NTS using lower doses of L-arginine partially normalizes mechanoreflex dysfunction in hypertension whereas higher doses do not. The findings could prove valuable in the development of treatment options for mechanoreflex overactivity in this disease.
Keywords: blood pressure, heart rate, exercise
INTRODUCTION
The cardiovascular response to physical activity in hypertensive patients is often characterized by larger than normal sympathetically mediated increases in heart rate (HR) and mean arterial pressure (MAP) (Aoki et al., 1983; Pickering, 1987; Seguro et al., 1991; Delaney et al., 2010; Vongpatanasin et al., 2011). It has been suggested that these augmented elevations in circulatory hemodynamics enhance the risk of stroke, arrhythmogenesis and myocardial infarction during or immediately after a bout of exercise (Hoberg et al., 1990; Mittleman et al., 1993; Kokkinos et al., 2002). As such, the safety of exercise prescription is limited, to some extent, in this patient population. From the standpoint of treatment, this is unfortunate as participation in regular exercise is known to elicit many benefits including reductions in baseline blood pressure and improvements in lipid and glucose metabolism (Dengel et al., 1998). Given the potential benefits exercise training could have on the health and quality of life of hypertensive individuals, understanding the mechanisms that largely contraindicate their participation in such activities is important. To do so may lead to the development of novel therapeutic strategies targeted at reducing the risks inherent to exercise in this disease.
The exercise pressor reflex (a reflex originating in contracting skeletal muscle) profoundly influences cardiovascular regulation during physical activity (McCloskey & Mitchell, 1972) and has recently been implicated as a significant contributor to the generation of the abnormal hemodynamic response to exercise in hypertension (Smith et al., 2006; Sausen et al., 2009). Further, it has been demonstrated in a rat model of human hypertension that the mechanically sensitive component of the reflex (i.e. the muscle mechanoreflex) drives, at least in part, the exercise pressor reflex overactivity manifest in this disease (Leal et al., 2008; Mizuno et al., 2011). The muscle mechanoreflex increases MAP and HR at the onset of muscle contraction primarily via activation of mechanically sensitive afferent neurons (McCloskey & Mitchell, 1972; Kaufman et al., 1983; Andres et al., 1985). These small-diameter sensory neurons are predominantly group III (A-δ) fibers whose associated receptors respond to mechanical distortion of their receptive fields via stretch or pressure (Stebbins et al., 1988; Nobrega et al., 1994). Once activated, signals from these fibers are processed within the nucleus tractus solitarii (NTS) of the medullary brainstem (Kalia et al., 1981; Toney & Mifflin, 1994; Gamboa-Esteves et al., 2001; Potts et al., 2002). Recent evidence from our laboratory suggests that alterations in the manner in which the signals are processed within the NTS partly mediate the mechanoreflex dysfunction that develops in hypertension (Leal et al., 2012).
Within the NTS, it has been shown in animal models that nitric oxide (NO) maintains the potential to modulate muscle reflex activity (Li & Potts, 2001; Smith et al., 2005; Leal et al., 2012). NO is produced when L-arginine is oxidized via an enzymatic reaction with nitric oxide synthase (NOS) (Moncada & Higgs, 1993). Importantly, our laboratory has shown that blocking NO production within the NTS of healthy normotensive rats reproduces the mechanoreflex overactivity demonstrated in hypertensive animals (Leal et al., 2012). This finding suggests that a decrease in NO production within the NTS may contribute to the generation of the exaggerated cardiovascular response to mechanoreflex activation in hypertension. This finding further suggests that restoring or increasing NO production within the NTS maintains the potential to abrogate mechanoreflex overactivity in hypertension. To date, however, this postulate remains untested.
Given these recent findings, the purpose of the current investigation was to determine the effect of increasing NO production within the NTS on mechanoreflex function in normotensive and hypertensive rats. It was hypothesized that pharmacologically increasing NO production in the NTS of hypertensive rats mitigates mechanoreflex overactivity. In order to test this hypothesis, microdialysis techniques were used to deliver the NO precursor L-arginine to the NTS of normotensive and hypertensive animals while simultaneously activating mechanically sensitive afferent fibers in hindlimb skeletal muscle. The findings of this study may prove valuable in the development of viable treatment options for individuals with hypertension that allow for the safe prescription of exercise.
METHODS
Ethical Approval
Experiments were performed in 50 age-matched (14-20 weeks old), male spontaneously hypertensive (SHR; n=24) and normotensive Wistar-Kyoto (WKY; n=26) rats (Harlan, Indianapolis, IN). Animals were provided food and water ad libitum and housed in standard rodent cages on 12-h light-dark cycles. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas at Southwestern Medical Center. In addition, all studies were conducted in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals.
General Surgical Procedures
Animals were anesthetized with isoflurane gas (2-3% in pure oxygen), intubated, and mechanically ventilated (Harvard Apparatus). To minimize edema, 0.15 mg dexamethasone was given intramuscularly in the left hindlimb (Tian & Duffin, 1996). Each carotid artery and the right jugular vein were cannulated (PE-50, polyethylene tubing) to allow the measurement of blood pressure and the administration of fluids, respectively. Arterial blood gases and pH were measured periodically throughout experimentation using an automated blood gas analyzer (50 μL blood samples; Model ABL 5, Radiometer) to ensure these variables were maintained within physiological ranges. As needed, a 1 M NaHCO3, 5% dextrose Ringers solution was infused intravenously at a rate of 2 mL/hr for the maintenance of fluid balance and animal stabilization (Quintin et al., 1989). Body temperature was maintained between 36.5 and 38.0°C by an isothermal pad (Deltaphase). At the completion of acute surgical procedures, the rats were placed in a stereotaxic head unit (Kopf Instruments) and a pre-collicular decerebration performed as described previously (Smith et al., 2001). Briefly, burr holes were drilled into the parietal skull and the bone superior to the central sagittal sinus removed. The cerebrum was aspirated until the superior and inferior colliculi were visible. Subsequently, the brain tissue was sectioned pre-collicularly and the remaining forebrain aspirated rendering the animal insentient. To minimize cranial bleeding, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface and the cranial cavity was packed with cotton. Isoflurane anesthesia was immediately discontinued at the conclusion of the decerebrate procedure.
Microdialysis Procedures
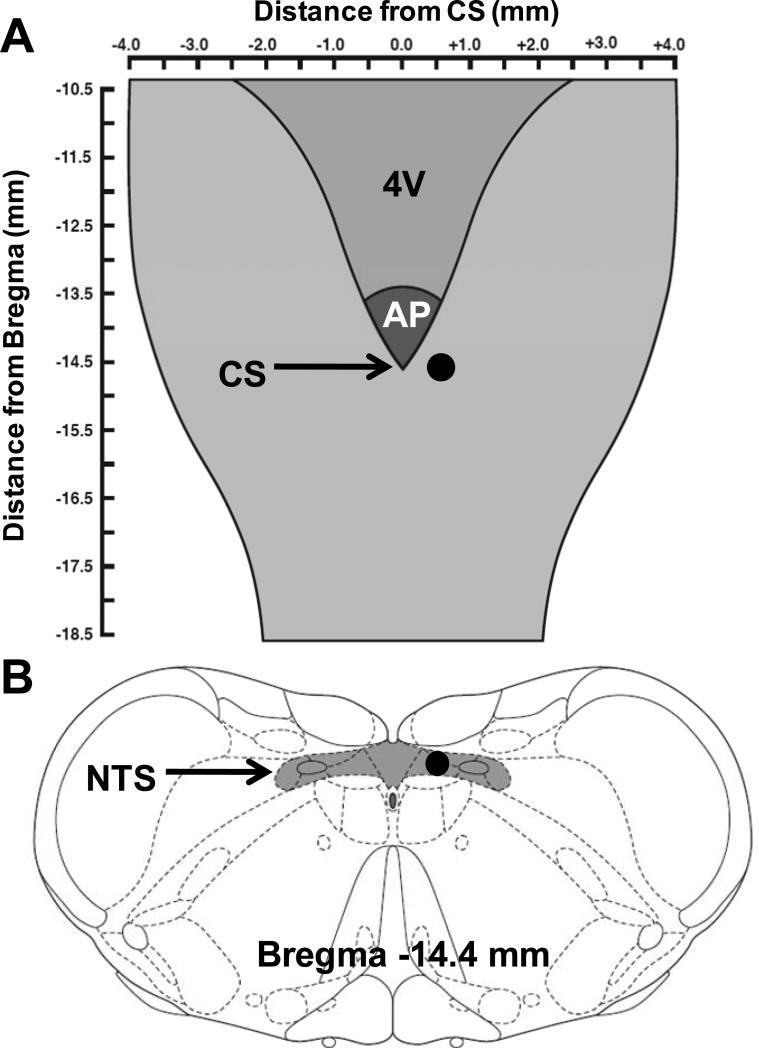
After decerebration, a limited occipital craniotomy was performed to expose the dorsal surface of the brainstem. Microdialysis probes (Bioanalytical Systems, model CMA 11, 0.24 mm outer diameter, 1 mm membrane tip) were stereotaxically positioned unilaterally within the NTS in areas known to receive projections from skeletal muscle reflex afferent fibers in rats (coordinates: 0.0 rostro-caudal to the calamus scriptorius, 0.5 mm lateral to the calamus scriptorius and 0.5 mm below the dorsal medullary surface) (Potts et al., 2002; Murphy et al., 2013). A schematic demonstrating the location of dialysis probe placement is presented in Figure 1. Once positioned, the probe was continuously perfused at a rate of 2.5 μl/min with artificial cerebral spinal fluid (aCSF) buffered to a pH of 7.4. The aCSF contained 0.2 % bovine serum albumin, 0.1 % bacitracin, and the following ions (in mM): 6.2 K+, 134 Cl-, 2.4 Ca2+, 150 Na+, 1.3 P-, 13 HCO3- and 1.3 Mg2+. After decerebration and probe insertion, the animal was allowed to stabilize for a minimum of one hour before beginning experimental procedures.
Figure 1. Microdialysis probe placement within the medullary brain stem.
A, schematic diagram adapted from Dhruava et al ((Dhruva et al., 1998) displaying the dorsal surface of the brainstem. The darkened circle marks the location at which the dialysis probe was positioned. B, a schematic coronal section of the medulla modified from Paxinos and Watson (Paxinos & Watson, 2007) depicts the position of the tip of the dialysis probe (darkened circle) within the NTS. 4V, fourth ventricle; AP, area postrema; CS, calamus scriptorius; NTS, nucleus tractus solitarius.
Skeletal Muscle Mechanoreflex Activation
Rats were secured in a customized spinal frame that utilized steel posts to stabilize the pelvis. The right hindlimb was fixed in position by clamping the tibial bone. The soleus and gastrocnemius muscles were surgically isolated and the calcaneal bone cut. The cut end of the Achilles’ tendon was connected to a force transducer (Grass Instruments, FT10) allowing for the measurement of muscle tension. To selectively activate mechanically sensitive afferent fibers associated with the skeletal muscle mechanoreflex, the soleus and gastrocnemius muscles of the hindlimb were passively stretched. Use of this technique to preferentially activate mechanically sensitive afferent fibers in skeletal muscle has been validated and is commonly used for this purpose (Stebbins et al., 1988).
Experimental Protocol
Using a calibrated 9.5 mm rack and pinion system (Harvard Apparatus), preferential activation of the skeletal muscle mechanoreflex was induced by passively stretching the soleus and gastrocnemius muscles for 30 seconds. The amount of tension developed in the muscles during passive stretch was matched to that previously reported during maximal hindlimb contractions in WKY and SHR rats (approximately 1200 g) (Smith et al., 2006). Before all stretch maneuvers, hindlimb muscles were preloaded by stretching to 70-100 g of tension.
The cardiovascular response to mechanoreflex activation was quantified during the dialysis of aCSF (control) within the NTS as well as during the dialysis of 1 μM L-arginine (WKY, n=9; SHR, n=8). In a separate group of animals, the response to stretch was assessed during the dialysis of aCSF and 10 μM L-arginine (WKY, n=9; SHR, n=8). In all experiments, the microdialysis probe was placed ipsilateral to the hindlimb muscles being stretched. aCSF and L-arginine were dialyzed a minimum of 45 minutes prior to mechanoreflex activation ensuring sufficient time for chemical delivery (Li & Potts, 2001; Smith et al., 2005; Leal et al., 2012). During dialysis of each substance, two reproducible responses to stretch were obtained with a minimum of 15 minutes between maneuvers. The total volume dialyzed by performing these procedures was approximately 300 μl. As a control, mechanoreflex function was assessed during the dialysis of aCSF and 1 μM D-arginine; the inactive enantiomer of L-arginine (WKY, n=4; SHR, n=4). A similar control study (data not shown) was performed in a separate group of animals in which aCSF and 10 μM D-arginine were dialyzed (WKY, n=4; SHR, n=4).
Morphological Measurements and Validation of Probe Placement
After completing experimental testing, insentient decerebrated animals were killed via an intravenous injection of saturated potassium chloride (4M, 2 ml/kg). Use of this procedure adheres to the guidelines established by the Panel on Euthanasia of the American Veterinary Medical Association. The heart and lungs were harvested and wet weights obtained. Tibial length was also obtained to assess heart mass/tibial length ratios. Additionally, the brainstem was excised and fixed in 10 % phosphate buffered formalin and stored at 4°C. Medullary tissue was blocked, and 40 μm sections were cut serially using a cryostat (Cambridge Instruments). Sections were placed on coated slides and examined to confirm that the probe was placed within the NTS. In a subset of animals (n=5), Evans blue dye was administered through the dialysis probe at the conclusion of the experiment to assess the perfusion area of the probe.
Data Acquisition
In all physiologic experiments, baseline as well as reflex changes in MAP, HR, and tension were measured and recorded. Baseline values for each variable were obtained over a 30 second period prior to reflex activation. The greatest change in each variable from this baseline in response to muscle stretch was taken as the peak value.
Statistical Analyses
All cardiovascular and contractile force data were acquired, recorded, and analyzed using data acquisition software (Spike 2, version 3, Cambridge Electronic Design, Ltd) for the CED micro 1401 system (Cambridge Electronic Design Ltd). Data were analyzed by means of paired t-tests as well as analysis of variance (ANOVA) as appropriate. With regard to the latter, statistical analyses between and within animal groups were performed using a Two-Way Mixed Design ANOVA. Student Newman-Keuls multiple comparison tests were utilized to identify differences between specific group means when ANOVA was found to be significant. Results are presented as means ± S.E.M. The significance level was set at P<0.05. All statistical analyses were performed using Sigma Stat for Windows (SPSS Inc.)
RESULTS
Characterization of Hypertensive Model
Morphometric and hemodynamic baseline data for WKY and SHR animals are presented in Table 1. Ratios of heart weight to both body weight and tibial length were significantly greater in SHR compared to WKY. Lung weight to body weight ratios were not different between groups. Baseline MAP was significantly higher in SHR than WKY whereas baseline HR was not different between the two groups.
Table 1.
Morphometric characteristics and baseline hemodynamics
| WKY | SHR | |
|---|---|---|
| n | 26 | 24 |
| Body weight, g | 338±6 | 370±6* |
| Heart weight/body weight, mg/g | 2.9±0.1 | 3.3±0.1* |
| Lung weight/body weight, mg/g | 6.8±0.3 | 7.3±0.3 |
| Heart weight/tibial length, mg/mm | 25.6±0.7 | 31.4±0.6* |
| MAP, mmHg | 95±5 | 134±6* |
| HR, beats/min | 432±9 | 424±11 |
Data are means ± S.E.M. MAP, mean arterial pressure; HR, heart rate.
Significantly different from WKY. P<0.05.
Dialysis of L-arginine in the NTS Alters the Cardiovascular Response to Mechanoreflex Activation
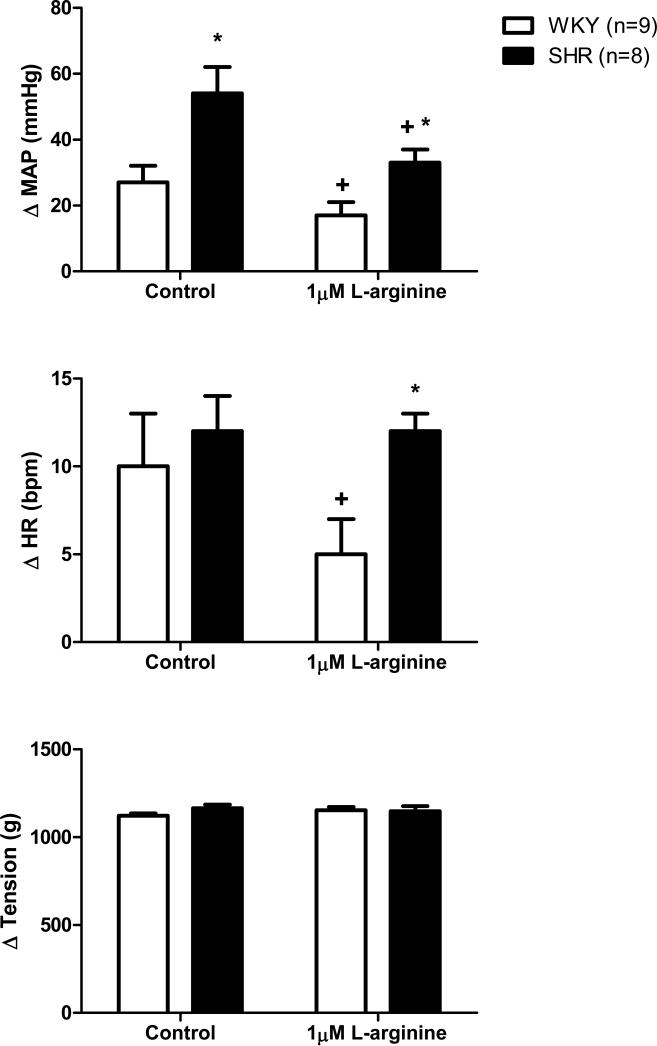
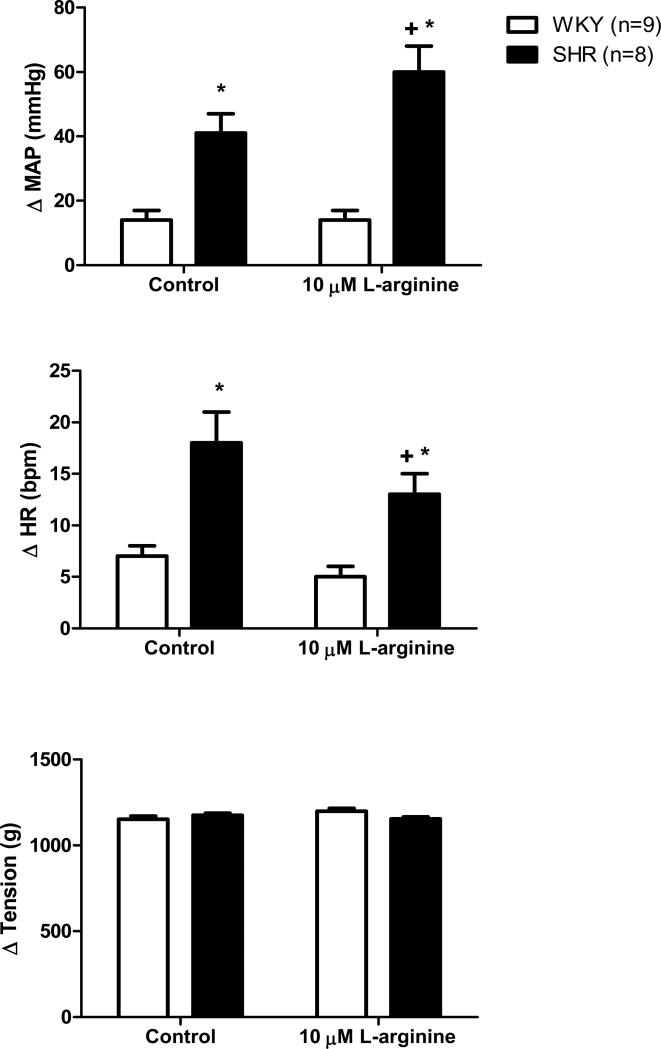
In agreement with previous reports (Leal et al., 2008; Mizuno et al., 2011; Leal et al., 2012), activation of mechanically sensitive afferent fibers by stretching hindlimb skeletal muscle induced significantly greater increases in MAP (Figures 2 and 3) and HR (Figure 3) in SHR compared to WKY under control conditions. In SHR, microdialysis of 1 μM L-arginine, a NO precursor, within the NTS significantly attenuated the exaggerated pressor response to mechanoreflex activation (Figure 2). For example, in response to muscle stretch, MAP increased by 54 ± 8 mmHg before the dialysis of L-arginine and by only 33 ± 4 mmHg after. It should be noted that 1 μM L-arginine reduced the pressor response to stretch in all SHR animals tested with one exception in which L-arginine induced an increase in MAP. Additionally, increasing NO production in the NTS of normotensive WKY also had an inhibitory affect on the cardiovascular response to muscle stretch (Figure 2). Before the dialysis of 1 μM L-arginine, the MAP and HR responses to stretch were 27 ± 5 mmHg and 10 ± 3 bpm, respectively. After L-arginine dialysis, the stretch-induced pressor and tachycardic responses were decreased to 17 ± 4 mmHg and 5 ± 2 bpm, respectively. With regard to MAP, it should likewise be noted that the percent reduction in the pressor response to stretch evoked by the dialysis of 1 μM L-arginine was similar between WKY (37 ± 14%) and SHR (39 ± 15%). Interestingly, dialyzing a larger concentration of L-arginine (10 μM) into the NTS produced variable effects on the hemodynamic response to stimulation of mechanically sensitive afferent fibers in skeletal muscle (Figure 3). In WKY, the dialysis of 10 μM L-arginine had no effect on either the MAP or HR response to passive stretch. In SHR, the dialysis of 10 μM L-arginine surprisingly elicited a significant increase in MAP while inducing a significant reduction in HR. For example, in response to stretch, MAP increased by 41 ± 6 mmHg before the dialysis of L-arginine and by 60 ± 8 mmHg after. In contrast, the HR response was decreased being 18 ± 2 bpm before and 13 ± 2 bpm after the dialysis of L-arginine. In all trials, the muscle tension developed was not different.
Figure 2. Cardiovascular responses to activation of mechanically sensitive afferent fibers in skeletal muscle before and during dialysis of 1 μM L-arginine in the NTS.
Passive stretch of hindlimb skeletal muscle induced larger increases in MAP, from baseline, in SHR as compared to WKY. The dialysis of 1 μM L-arginine into the NTS significantly reduced the pressor response to hindlimb stretch in both WKY and SHR as well as the tachycardic response to stretch in WKY compared to control trials. * Significantly different from WKY. † Significantly different from control response in which aCSF was dialyzed. P<0.05.
Figure 3. Cardiovascular responses to muscle stretch before and during dialysis of 10 μM L-arginine in the NTS.
Passive stretch of hindlimb skeletal muscle induced larger increases in MAP and HR, from baseline, in SHR as compared to WKY. Compared to control trials, passive stretch of hindlimb skeletal muscle during dialysis of 10 μM L-arginine caused significant increases and decreases in MAP and HR, respectively, in SHR. The 10 μM dose of L-arginine had no effect on the pressor and tachycardic responses to stretch in WKY. * Significantly different from WKY. † Significantly different from control response in which aCSF was dialyzed. P<0.05.
Dialysis of D-arginine in the NTS Does Not Alter Mechanoreflex Function
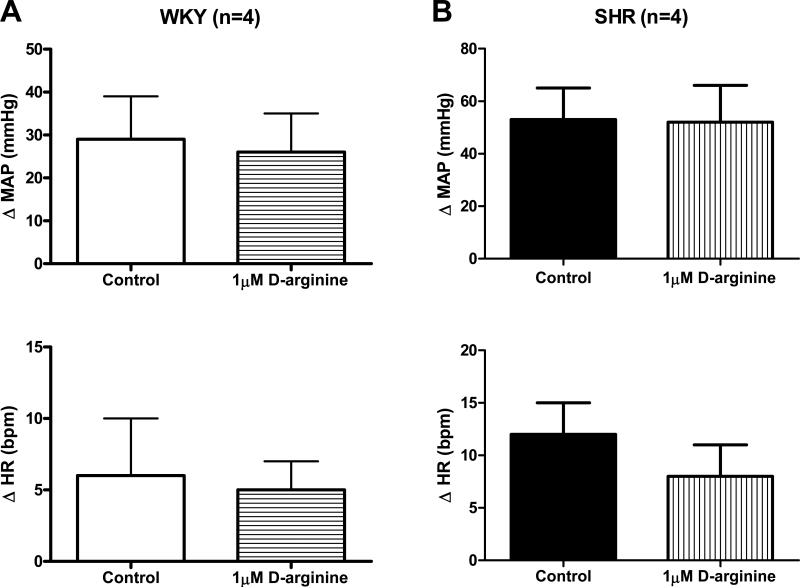
As a control, the inactive enantiomer D-arginine (1 μM) was dialyzed into the NTS of WKY and SHR animals and mechanoreflex function assessed. Microdialysis of 1 μM D-arginine within the NTS had no effect on the circulatory response to muscle stretch in either group of animals. Specifically, in WKY, stretch induced increases in MAP and HR of 29 ± 10 mmHg and 6 ± 4 bpm, respectively, before the dialysis of D-arginine and 26 ± 9 mmHg and 5 ± 2 bpm, respectively, after (Figure 4A). In SHR, stimulation of mechanically sensitive afferent fibers produced elevations in MAP and HR of 53 ± 12 mmHg and 12 ± 3 bpm, respectively, before and 52 ± 14 mmHg and 8 ± 3 bpm after D-arginine dialysis (Figure 4B). Similar results were produced in both groups of animals during the dialysis of 10 μM D-arginine (data not shown). The muscle tension developed was similar in all trials.
Figure 4. Cardiovascular responses to activation of mechanically sensitive afferent fibers in skeletal muscle before and during the dialysis of 1 μM D-arginine in the NTS of WKY and SHR.
Dialysis of the inactive enantiomer D-arginine had no effect on the MAP and HR responses to passive stretch of hindlimb skeletal muscle in WKY (A) or SHR (B). aCSF was dialyzed during control trials.
Verification of Probe Placement
To verify probe placement, the brainstem was excised, fixed and sliced into 40 μm sections. All probes were placed ipsilateral to the stretched hindlimb and were found to be located within portions of the NTS known to receive projections from skeletal muscle afferent fibers (Gamboa-Esteves et al., 2001; Potts et al., 2002; Murphy et al., 2013). In a subset of 5 animals, Evans blue dye was additionally dialyzed to assess the perfusion area of the dialysis probe. The spread of dye was similar to that shown by our laboratory using the same coordinates in a previous investigation (Leal et al., 2012). The dye was found to be distributed primarily in the solitary tract as well as several NTS subnuclei. However, although relatively limited, the spread of dye was also found to partially perfuse structures in close proximity to the borders of the NTS outside the nucleus.
DISCUSSION
The significant findings from the current investigation were: i) the dialysis of lower doses (1 μM) of the NO-precursor L-arginine within the NTS significantly attenuated the increase in MAP induced by passive muscle stretch in both normotensive and hypertensive rats; ii) the dialysis of lower doses (1 μM) of L-arginine effectively reduced the pressor response to stretch in SHR (33 ± 4 mmHg) to relatively the same absolute level as that elicited during mechanoreflex activation in control WKY (27 ± 5 mmHg); and iii) the dialysis of a higher dose of L-arginine (10 μM) produced variable responses having no effect in normotensive animals while significantly increasing and decreasing the MAP and HR responses to stretch, respectively, in hypertensive rats. Collectively, these findings suggest that increasing NO production within the NTS using lower doses of L-arginine can partially normalize mechanoreflex overactivity in hypertensive rats whereas the effects of larger doses are equivocal .
Previous investigations have reported that NO within the NTS maintains the ability to modulate skeletal muscle mechanoreflex activity. For example, it has been recently demonstrated that pharmacologically blocking endogenous NTS-NO production with the non-specific NOS inhibitor L-NAME in normotensive rats augments the MAP response to stimulation of the muscle mechanoreflex (Leal et al., 2012). Conversely, it has been shown in normal healthy cats that L-arginine dialysis within the NTS significantly attenuates the pressor response to muscle reflex activation (Li & Potts, 2001; Smith et al., 2005). In agreement, increasing NTS-NO production in normotensive rats via lower doses of L-arginine elicited reductions in the blood pressure response to stretch in the current study. These findings provide strong support for the tenet that, within the NTS, NO acts to modify muscle reflex function. Moreover, the data suggest that NO acts as a functional buffer partially restraining muscle reflex-induced increases in cardiovascular hemodynamics.
As shown in this investigation and others, the cardiovascular response to activation of the muscle mechanoreflex is exaggerated in hypertension (Leal et al., 2008; Leal et al., 2012). Given the demonstrated buffering action of NO within the NTS of normotensive animals, it is our contention that the enhanced mechanoreflex-induced cardiovascular response in hypertension is due, in part, to a reduction in the availability of NO within the NTS. In support of this postulate, increasing/restoring NO production via lower doses of L-arginine in hypertensive rats partially corrected the aberrant MAP response to passive muscle stretch in the current investigation. As a parallel line evidence, blocking NO production within the NTS of healthy rats has previously been shown to reproduce the augmented cardiovascular response to passive stretch observed in hypertensive animals (Leal et al., 2012). Combined, these studies suggest that a reduction in the availability of NO within the NTS may contribute importantly to the generation of mechanoreflex overactivity in hypertension. However, when interpreting the findings of each of these investigations, alternative explanations should also be considered. For example, when analyzed as a percent decrease, the reductions in MAP in response to stretch during L-arginine (1μM) dialysis were similar between WKY and SHR in the current study being approximately 35-40% in each group. This could be interpreted to mean that the effects of L-arginine are comparable between normotensive and hypertensive animals and/or the sensitivity to NO remains unchanged in SHR. Similarly, in the previous study cited (Leal et al., 2012), the percent increases in MAP evoked by mechanoreflex activation after NOS inhibition were shown to be comparable in WKY and SHR. This finding could be interpreted to suggest that similar levels of NO are endogenously produced in both groups of animals. That being stated, Murphy et al (Murphy et al., 2013) have recently demonstrated that the expression of the neuronal isoform of NOS is reduced within the NTS of hypertensive compared to normotensive rats rendering the latter possibility more unlikely. Despite the validity of these alternative explanations, it is significant that treatment of SHR with 1μM L-arginine decreased the absolute MAP response to stretch to a level similar to that of control WKY. Arguably, it is the peak absolute elevation in MAP that presents the greatest safety concern during exercise. Therefore, methods that decrease the absolute augmentation in blood pressure during mechanoreflex activation may prove to be the most promising in reducing the risks associated with physical activity in hypertension.
How NO within the NTS operationally affects mechanoreflex function remains a point of speculation. That being stated, the cardiovascular response to muscle reflex activation is known to be predominately mediated by the sympathetic nervous system (McCloskey & Mitchell, 1972; Mitchell et al., 1983; Mark et al., 1985). As such, it is likely that NO functions within the NTS to buffer reflex-induced increases in sympathetic outflow to the heart and vasculature. If, in hypertension, NO availability is in fact reduced, the capacity to buffer elevations in sympathetic activity would be compromised. Supporting this concept, we recently demonstrated that the exaggerated cardiovascular response to mechanoreflex activation in hypertension is mediated by abnormally large elevations in sympathetic nerve activity (Mizuno et al., 2011).
The question remains as to what could cause a reduction in the availability of NO within the NTS in hypertension. Since NOS is required to enzymatically convert L-arginine to NO (Moncada & Higgs, 1993), one possibility is a decrease in the expression or activity of any of the isoforms of NOS (i.e. endothelial, neuronal or inducible NOS) known to be present within the brainstem (Dawson et al., 1991). As stated previously, it has recently been demonstrated that the expression of at least one of these isoforms (i.e. neuronal NOS) is reduced within the NTS of SHR (Murphy et al., 2013). However likely a candidate, it should be noted that some NOS continues to be expressed and remains active within the NTS of hypertensive rats as evidenced by the finding that L-arginine dialysis was able to affect mechanoreflex function in this study. Independent of NOS, other factors may impact NO availability within the NTS. It is well known that reactive oxygen species (ROS), such as superoxide anion, react with NO to form peroxynitrite (Thomas et al., 2001; Infanger et al., 2006). The main source of ROS in the brain is the multi-subunit enzyme nicotinamide-adenine dinucleotide phosphate (NAD(P)H) oxidase which catalyzes the production of superoxide from molecular oxygen (Babior, 2004; Infanger et al., 2006). Angiotensin II (Ang-II), a peptide that regulates sympathetic outflow, increases the activity of NAD(P)H oxidase and enhances superoxide production within the central nervous system (Zimmerman et al., 2002; Gao et al., 2004). Therefore, the availability of NO within the NTS may be reduced by the generation of superoxide via an AngII/NAD(P)H oxidase mechanism in hypertension. Most likely, each of these factors work in concert to negatively impact NO production/availability within the NTS in this disease. Future research in this area is warranted.
Interestingly, it is of note that only the lower concentration (1 μM) of L-arginine dialyzed into the NTS in both hypertensive and normotensive animals had a consistent inhibitory effect on the pressor response to mechanoreflex activation. Perplexingly, the higher dose (10 μM) of L-arginine had no effect on the pressor response to stretch in WKY while augmenting the response in SHR. Moreover, this higher dose of L-arginine significantly reduced the HR response to stretch in SHR; again with no effect in WKY. The reason for this discrepancy is not readily apparent. That being stated, such a concentration-dependant phenomena is not uncommon and has been shown to occur in various tissues such as bone, nerve, and myocardium where lower doses of NO are efficacious while higher concentrations are either less effective or their effectiveness reversed (Baggetta et al., 1992; Lipton et al., 1993; Przegalinski et al., 1994; Mohan et al., 1996; Calabrese, 2001). Moreover, these dose-dependent regulatory influences of NO have been shown to occur regardless of the NO donor or precursor used. For example, within the central nervous system it has been shown that, depending on the concentration used, NO donors can both decrease and increase basal levels of excitatory amino acids as well as their release in the ventral hippocampus of Wistar rats (Segieth et al., 1995). As another example, central-acting NO has been demonstrated to be both neuroprotective as well as neurotoxic when examining convulsant behaviors (Baggetta et al., 1992; Lipton et al., 1993; Przegalinski et al., 1994). Based on these examples, it seems likely that NO has the ability to mediate both excitatory and inhibitory functions depending on its concentration and location within brainstem nuclei. A similar phenomenon may account for the dose-related discrepancy in the effectiveness of L-arginine within the current study.
The dialysis of the lower dose of L-arginine within the NTS was able to only partially, but not completely, normalize mechanoreflex overactivity in hypertensive rats. Given that the larger dose of L-arginine administered actually evoked an increase in the pressor response to stretch rather than a decrease, the inability of the lower dose of L-arginine to fully abrogate mechanoreflex dysfunction does not appear to be dose related. More likely, other factors independent of alterations in central processing may be at play. For example, increases in the expression or sensitivity of mechanoreceptors in peripheral skeletal muscle could contribute significantly to muscle reflex overactivity in hypertension (Mizuno et al., 2011). In addition, it is plausible that the responsiveness of skeletal muscle afferents, sympathetic efferents and end-organ targets (e.g. heart, vasculature) to any given mechanical stimuli is enhanced in hypertension (Esler, 2000). While increasing NO production in the NTS with lower doses of L-arginine may mitigate abnormal behavior by any one of these components of the muscle reflex arc, it is likely to do so incompletely.
Limitations
A few limitations to the current study are acknowledged. To begin, L-arginine concentrations were not assessed within the NTS either at baseline or after dialysis. In addition, NO production within the NTS during the dialysis of L-arginine was not directly quantified nor were the by-products of its production (e.g. citrulline) or metabolism (e.g. nitrates and nitrites). While techniques to measure NO exist, they are difficult to implement in vivo due to the molecule's labile nature which allows it to be rapidly oxidized (6-10 seconds in biological systems) (Beckman et al., 1990; Stamler et al., 1992). In addition, common methods of NO detection such as the quantification of nitrates and nitrites as well as L-citrulline are thought to be better indicators of NOS activity rather than NO production (Nagano, 1999; Tarpey et al., 2003). This limitation should be kept in mind when interpreting the results of the present study. Second, as indicated by the spread of Evans blue dye, portions of the medulla outside the NTS were also perfused with L-arginine. As such, neuronal activity from these areas may have influenced the cardiovascular responses to stretch observed. Contributions from such neurons is likely minimal as the spread of dye outside the NTS region was limited. Third, the method used to activate mechanically sensitive skeletal muscle afferent fibers (i.e. passive muscle stretch) may not do so in exactly the same manner as in exercise. In cats, it has been demonstrated that static contraction and passive stretch of hindlimb skeletal muscle can, to some extent, activate different populations of group III afferent fibers (Hayes et al., 2005). On the other hand, a percentage of these group III neurons (25-75%) are activated by both stimuli. However, both contraction and stretch produce similar effects on afferent neuronal discharge rates and changes in cardiovascular parameters (Hayes & Kaufman, 2001; Hayes et al., 2005). In addition, stretch has been shown to increase discharge activity in the same efferent neurons responsive to contraction (Hayes & Kaufman, 2001). As such, passive stretch is a commonly accepted technique for the activation of the muscle mechanoreflex. Finally, it is of note that many studies supporting NO as a modulatory neurotransmitter within the brainstem often report alterations in baseline hemodynamics in response to changes in NO levels (Zanzinger et al., 1995; Tseng et al., 1996). In our experiments, however, baseline MAP and HR were not significantly altered by the dialysis of either concentration of L-arginine within the NTS of normotensive or hypertensive rats. Such discrepancies between studies may be due to the method and duration of chemical delivery within the NTS (e.g. microinjection vs. microdialysis) or the mechanism of action of the chemical (e.g. NO precursor vs. NO donor).
Other Considerations
Additional considerations are relevant to the interpretation of the current study. The cardiovascular response to exercise is mediated, neurally, by the integration of input from three primary sources: central command, the exercise pressor reflex and the arterial baroreflex (Goodwin et al., 1972; McCloskey & Mitchell, 1972; Mancia & Mark, 1983; Raven et al., 1997). Evidence suggests that each of these inputs maintains the ability to modify one another functionally. For example, it has been demonstrated that the baroreflex buffers muscle reflex activity in both cats and rats (Waldrop & Mitchell, 1985; Smith et al., 2006). Further, sensory fibers of the baroreflex have been shown to project to several areas within the NTS where their activity is modulated by NO (Paton et al., 2001; Smith et al., 2005). Thus, it is possible within this study that the dialysis of L-arginine in the NTS altered baroreflex sensitivity which in turn could have modified its interactive relationship with the muscle mechanoreflex. That being stated, it has been shown that increasing NO production via dialysis of L-arginine decreases baroreflex sensitivity (Smith et al., 2005). Congruent with such a decrease would be a reduction in the ability of the baroreflex to buffer mechanoreflex input. This could potentially result in an elevation in the response to muscle stretch. Such a finding did not manifest during the dialysis of the 1 μM dose of L-arginine. On the contrary, the MAP response to muscle reflex activation was blunted in both WKY and SHR. Conversely, it may partially explain the surprisingly enhanced pressor response to muscle stretch in SHR during the dialysis of 10 μM of L-arginine. With regard to central command, one of the advantages of using the decerebrate procedure is that it removes the areas of the brain from which central command putatively originates (Williamson et al., 2006). This eliminates input from central command that could potentially influence the cardiovascular responses evoked by muscle stretch. Decerebration also obviates the need for inhalant anesthetics which have been shown to blunt autonomic control of MAP and HR in rats (Smith et al., 2001). However, use of the decerebrate technique also removes tonic modulation of neurons within the NTS by all higher brain centers that would normally be operative in a conscious animal or human. As such, although abolishing input from central command is a distinct advantage in the surgical preparation used in this study, it must be acknowledged that experiments in decerebrate animals are not necessarily directly comparable to those performed in the conscious state.
Relevance
Increasing evidence in rat models of human hypertension suggests that the skeletal muscle mechanoreflex contributes importantly to the exaggerated cardiovascular response to exercise in hypertension (Leal et al., 2008; Mizuno et al., 2011; Leal et al., 2012). Given that this abnormally large hemodynamic responsiveness increases the risk for stroke, arrhythmia and myocardial infarction during or immediately after exercise (Hoberg et al., 1990; Mittleman et al., 1993; Kokkinos et al., 2002), defining potential targets for the treatment of mechanoreflex dysfunction is a logical, potentially clinically important endeavor. To this end, the findings of the current study suggest that increasing NO production/availability via lower doses of L-arginine within the NTS maintains the potential to partially correct mechanoreflex dysfunction in hypertensive rats. Whether this finding is directly applicable to human hypertensive patients remains unknown. Studies in normotensive humans have demonstrated that activation of the muscle mechanoreflex inhibits cardiac vagal activity (in some reports by modifying cardiac baroreflex control) increasing HR (Gladwell & Coote, 2002; Gladwell et al., 2005; Vorluni & Volianitis, 2008). Others have shown that passive muscle stretch in humans transiently increases sympathetic nerve activity and MAP (Cui et al., 2006). However, in each study the changes reported were small. These minimal responses were likely due to the technical difficulties in isolating and substantially stimulating the mechanoreflex in subjects but, nonetheless, brings into question the relative impact of the reflex on cardiovascular control in humans. Clearly, further investigation is needed to delineate the role of this reflex in humans and whether it is altered in hypertensive patients. If mechanoreflex function is found to be altered in human hypertension, the results of the current study may aid in the development of therapeutic treatments that allow patients to participate in regular exercise of sufficient intensity and duration to elicit a clinically relevant physiological benefit with a reduced risk for the occurrence of an adverse cardiovascular event.
What is the central question of this study?
Does increasing nitric oxide (NO) production within the nucleus tractus solitarii (NTS) effect mechanoreflex function in normotensive and hypertensive rats?
What is the main finding and what is its importance?
Dialysis of 1 μM L-arginine, a NO-precursor, within the NTS significantly attenuated the pressor response to muscle stretch in normotensive and hypertensive rats. In contrast, 10 μM L-arginine had no effect in normotensive animals while increasing and decreasing the pressor and tachycardic responses to stretch, respectively, in hypertensive rats. This suggests that increasing NO within the NTS using lower doses of L-arginine can partially normalize mechanoreflex overactivity in hypertensive rats whereas the effects of larger doses are equivocal.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (HL-094075 to A.K. Leal and HL-088422 to S.A. Smith) and the Lawson & Rogers Lacy Research Fund in Cardiovascular Diseases (to J.H. Mitchell). The authors thank Martha Romero, Julius Lamar, Jr. and Ryan Downey for their expert technical assistance.
REFERENCES
- Andres KH, During Mv, Schmidt RF. Sensory innervation of the Achilles tendon by group III and IV afferent fibers. Anat Embryol. 1985;172:145–156. doi: 10.1007/BF00319597. [DOI] [PubMed] [Google Scholar]
- Aoki K, Sato K, Kondo S, Pyon C, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J. 1983;47:802–809. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immun. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Baggetta G, Iannone M, Scorsa AM, Nistic G. Tacrine-induced seizures and brain damage in LiCl-treated rats can be prevented by Nw-nitro-L-arginine methyl ester. Eur J Pharmacol. 1992;213:301–304. doi: 10.1016/0014-2999(92)90695-z. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production of peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Nat Acad Sci. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Nitric Oxide: biphasic dose response. Crit Rev Toxicol. 2001;31:489–501. doi: 10.1080/20014091111776. [DOI] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identified in brain and peripheral tissues. Proc Nat Acad Sci. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney E, Greaney J, Edwards D, Rose W, Fadel P, Farquhar W. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol. 2010;299:H1318–H1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengel DR, Hagberg JM, Pratley RE, Rogus EM, Goldberg AP. Improvements in blood pressure, glucose metabolism and lipoprotein lipids after aerobic exercise plus weigt loss in obese hypertensive middle-aged men. Metabolism. 1998;47:1075–1082. doi: 10.1016/s0026-0495(98)90281-5. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Bhatnagar T, Sapru HN. Cardiovascular responses to microinjections of glutamate into the nucleus tractus solitarii of unesthetized supracollicular decerebrate rats. Brain Res. 1998;801:88–100. doi: 10.1016/s0006-8993(98)00550-2. [DOI] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Gamboa-Esteves FO, Tavares I, Almeida A, Batten TFC, McWilliam PN, Lima D. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res. 2001;921:195–205. doi: 10.1016/s0006-8993(01)03118-3. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540.3:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567.2:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol. 2001;280:2153–2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mauther HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation and function. Antiox Redox Sign. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circ Res. 1981;48:I48–I62. [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehab. 2002;22:178–183. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Leal A, Murphy M, Iwamoto G, Mitchell J, Smith S. A role for nitric oxide within the nucleus tractus solitarii in the development of muscle mechanoreflex dysfunction in hypertension. Exp Physiol. 2012;97:1292–1304. doi: 10.1113/expphysiol.2012.065433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol. 2008;295:H1429–H1438. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Potts JT. NO formation in nucleus tractus solitarii attenuates pressor response evoked by skeletal muscle afferents. Am J Physiol. 2001;280:H2371–H2379. doi: 10.1152/ajpheart.2001.280.5.H2371. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HSV, Sucher SJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Mancia G, Mark AL. Handbook of Physiology The Cardiovascular System Peripheral Circulation and Organ Blood Flow. Am. Physiol. Soc.; Bethesda, MD: 1983. Arterial baroreflexes in humans. pp. 755–793. [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol. 2011;300:H968–H977. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan P, Brutsaert DL, Paulus WJ, Sys SU. Myocardial contractile response to nitric oxide and cGMP. Circ Res. 1996;93:1223–1229. doi: 10.1161/01.cir.93.6.1223. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. New Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Murphy MN, Mizuno M, Squiers JJ, Squiers KE, Smith SA. Neuronal nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle reflexes in hypertensive rats. Am J Physiol. 2013 doi: 10.1152/ajpheart.00235.2012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–290. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Williamson JW, Friedman DB, Araujo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc. 1994;26:709–714. doi: 10.1249/00005768-199406000-00009. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press Elsevier Inc; London, UK: 2007. [Google Scholar]
- Pickering TG. Pathophysiology of exercise hypertension. Herz. 1987;12:119–124. [PubMed] [Google Scholar]
- Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Auto Neurosci. 2002;98:64–69. doi: 10.1016/s1566-0702(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Baran L, Siwanowicz J. The role of nitric oxide in the kainate-induced seizures of mice. Neurosci Lett. 1994;170:74–76. doi: 10.1016/0304-3940(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Quintin L, Gillon JY, Saunier CF, Ghignone M. Continuous volume infusion improves circulatory stability in anesthetized rats. J Neurosci Meth. 1989;30:77–83. doi: 10.1016/0165-0270(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise. Exerc Sport Sci Rev. 1997;25:365–389. [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol. 2009;105:351–356. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- Segieth J, Getting SJ, Biggs CS, Whitton PS. Nitric oxide regulates excitatory amino acid release in a biphasic manner in freely moving rats. Neurosci Lett. 1995;84:427–434. doi: 10.1016/0304-3940(95)12088-l. [DOI] [PubMed] [Google Scholar]
- Seguro C, Sau F, Zedda N, Scano G, Cherchi A. Arterial blood pressure behavior during progressive muscular exercise in subjects with stable arterial hypertension. Cardiologia. 1991;36:867–877. [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Li J. Independent modification of baroreceptor and exercise pressor reflex function by nitric oxide in nucleus tractus solitarius. Am J Physiol. 2005;288:2068–2076. doi: 10.1152/ajpheart.00919.2003. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effects of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol. 2003;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Nitric oxide deficiency as a cause of clinical hypertension. J Med Am Assoc. 2001;285:2055–2057. doi: 10.1001/jama.285.16.2055. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent inputs to nucleus tractus solitarius. J Neurophysiol. 1994;72:63–71. doi: 10.1152/jn.1994.72.1.63. [DOI] [PubMed] [Google Scholar]
- Tseng C-J, Liu H-Y, Lin H-C, Ger L-P, Tung C-S, Yen M-H. Cardiovascular effects of nitric oxide in the brain stem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Admas-Huet B, Mitchell J, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorluni L, Volianitis S. Baroreflex control of sinus node during dynamic exercise in humans: effect of muscle mechanoreflex. Acta Physiol. 2008;192:351–357. doi: 10.1111/j.1748-1716.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to muscle contraction. Am J Physiol. 1985;249:H710–H714. doi: 10.1152/ajpheart.1985.249.4.H710. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Exp Physiol. 2006;91:51–58. doi: 10.1113/expphysiol.2005.032037. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Effects of nitric oxide on sympathetic baroreflex transmission in the nucleus tractus solitarii and caudal ventrolateral medulla in cats. Neurosci Lett. 1995;197:199–202. doi: 10.1016/0304-3940(95)11929-q. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazatigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circulation. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]