Abstract
The interrelationship between muscle strength, motor unit (MU) number, and age is poorly understood, and in this study we sought to determine whether age-related differences in muscle strength are moderated by estimates of functioning MU number and size. Eighteen older adults (OA; 67±1.20 yrs) and 24 young adults (YA; 22±0.74 yrs) participated in this study. Maximum voluntary pinch grip strength of the nondominant hand was determined and estimates of MU number were obtained from the abductor pollicis brevis muscle using the noninvasive motor unit number index (MUNIX) technique. The MUNIX technique was also utilized to derive a motor unit size index (MUSIX). An analysis of covariance (Age Group X MUNIX or MUSIX) was used to test heterogeneity of regression slopes, with body mass and gender serving as covariates. We observed that the slope of pinch grip strength on the estimated number of MUs between YA and OA differed, indicated by an Age Group X MUNIX interaction (p=0.04). Specifically, after controlling for the effect of body mass and gender, the slope in OA was significantly positive (0.14±0.06 N/MUs, p=0.03), whereas no such relationship was found in YA (−0.08±0.09 N/MUs, p=0.35). A significant Age Group X MUSIX interaction was also observed for strength (p<0.01). In contrast to MUNIX, the slope in younger adults was significantly positive (0.48±0.11 N/μV, p<0.01), whereas no such relationship was found in older adults (−0.30±0.22 N/μV, p=0.18). These findings indicate that there is an interrelationship between muscle strength, MU numbers, and aging, which suggests that a portion of muscle weakness in seniors may be attributable to the loss of functioning motor units.
INTRODUCTION
In 2011, the U.S. Census estimated there were 41.4 million persons aged 65 and older, or 13% of the population. By 2030, the number of older persons is expected to increase to more than 72 million (20%) and continue to increase thru 2050. Aging is associated with a myriad of physiological and functional changes, with one dramatic change being a reduction in neuromuscular function (e.g., decreased muscle strength) (Manini & Clark, 2011). A number of cellular and systems level neuromuscular changes have been suggested to occur with advancing age (Clark & Taylor, 2011; Delbono, 2011; Deschenes, 2011; Fry & Rasmussen, 2011; Russ et al., 2011), with one of the most commonly cited changes being a reduction in the number of functioning motor units (MUs) with incomplete compensatory reinnervation (Brown 1972; Brown et al., 1988; Doherty et al., 1993; Galea, 1996; McNeil et al., 2005).
The loss of MUs with advancing age has previously been hypothesized to be functionally significant by directly contributing to muscle weakness commonly observed in elders (McNeil et al., 2005). A positive linear correlation was found when motor unit number was regressed over strength in a sample of physically active older adults (Doherty et al., 1993), indicating the role motor units play in modulating strength production in elders. However, other studies have reported that muscle weakness in aging is not necessarily related to reductions in motor unit number (Dalton et al., 2008; Power et al., 2010). Accordingly, the purpose of this experiment was to determine whether age-related differences in muscle strength are moderated by number of functioning motor units. We also examined the size of the motor units and their effects on muscle strength. We hypothesized that there was an interrelationship between muscle strength, motor unit number, and aging, such that weaker older adults exhibit a reduced number of motor units in the absence of differences in motor unit size. We utilized the motor unit number index (MUNIX) technique, which is a recently developed non-invasive electrophysiological technique thought to provide an index proportional to the number of motor units in a muscle (Ahn et al., 2010; Boekestein et al., 2012; Furtula et al., 2013; Li et al., 2012; Nandedkar et al., 2010; Nandedkar et al., 2004; Neurwirth et al., 2010; Sandberg et al., 2011).
METHODS
General Overview of the Study
Eighteen older adults (OA) and 24 young adults (YA) participated in this study. Following an orientation visit to the laboratory, subjects underwent maximum voluntary pinch grip strength testing of the nondominant hand and estimates of motor unit number were obtained from the abductor pollicis brevis muscle using the noninvasive MUNIX technique. The MUNIX technique is a recently developed noninvasive neurophysiological technique that has been suggested to provide an index proportional to the number of MUs in a muscle (Nandedkar et al., 2004, 2010). The MUNIX value is derived from mathematical derivations based on the area and power of the maximum compound muscle fiber action potential (CMAP) and voluntary surface electromyogram (EMG) recordings. An analysis of covariance (Age Group X MUNIX) was used to test heterogeneity of regression slopes (moderation analysis), with body mass and gender serving as covariates.
Study Participants
Community dwelling OA (67±1.20 years; 12 females and 6 males) and YA (22±0.74 years; 10 females and 14 males) participated in this study. To be eligible for the study, subjects had to have a body mass index <30 kg/m2 (to ensure high quality EMG recordings by excluding individuals with excessive amounts of subcutaneous adipose tissue). Individuals were excluded if they had any known neurological or orthopedic conditions. Additionally, individuals were excluded if they were participating in a resistance exercise-training regimen greater than one day per week. Subjects were asked to refrain from alcohol (24 hours) and caffeine, nicotine, and exercise (4 hours) before the testing sessions. This study was approved by the Ohio University IRB and all subjects gave written informed consent prior to study participation.
Electrical and Mechanical Recordings
EMG signals were recorded using surface electrodes (Kendall Soft-E H69PSurface Electrodes, Kendall Ltp, Mansfield, MA) arranged in a monopolar fashion on the palmar surface of the non-dominant hand. Specifically, the active recording electrode was placed over the belly of the abductor pollicis brevis (APB) muscle halfway between the metacarpo-phalangeal joint and the trapezium of the thumb, the reference electrode was placed on the ipsilateral wrist flexor tendon, and the ground electrode was placed on the bony portion of the dorsal aspect of the contralateral hand. The EMG signals were amplified (500–1,000×), bandpass-filtered (10–500 Hz), and sampled at 10,000 Hz (MP150, Biopac Systems, Goleta, CA).
Pinch-grip strength was quantified using a force transducer (TSD121C, Biopac Systems), and the signals were smoothed over a 5-msec running average (AcqKnowledge 4.2, Biopac Systems). Participants forearm and wrist were positioned in an anatomically neutral position at the level of the xiphoid process while seated at a table. During the pinch-grip task, participants pinched a 3.7 cm wide transducer with the pads of the thumb (1st finger) and index finger (2nd finger). The 3rd–5th fingers were flexed and secured to the hand using an elastic band (Fabrifoam, Exton, Pennsylvania). Subjects were given real-time visual feedback on a computer monitor located 0.5 meters in front of them (AcqKnowledge 4.2, Biopac Systems). An illustration of the experimental set-up is shown in Figure 1.
Figure 1.
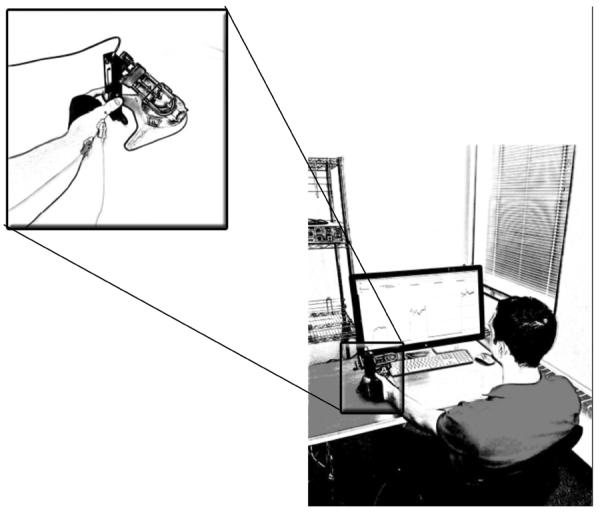
Experimental setup of subject performing graded contractions at percentage(s) of their maximal voluntary contraction force. Inset: Enlarged view of the force transducer and pinch-grip task protocol.
Pinch-Grip Strength
Maximal pinch-grip strength was assessed by having subjects perform a minimum of three maximal voluntary isometric contractions (MVCs) (Figure 1). A one to two minute rest period was given between trials. The MVC force was considered as the highest value recorded. If the participants' highest values were not within 5% of each other, additional trials were permitted. Verbal encouragement was given with each attempt and subjects were provided visual feedback of their force output on the computer monitor.
CMAP and Surface Interference Patterns
The CMAP (Figure 2, left trace) was evoked by stimulation of the median nerve with a constant current stimulator (200-microsecond pulse; Model DS7AH, Digitmer Ltd., England) in the area two centimeters proximal of the wrist crease. The maximum CMAP was determined by failure of the M-wave to increase in amplitude despite an increase in stimulator output.
Figure 2.
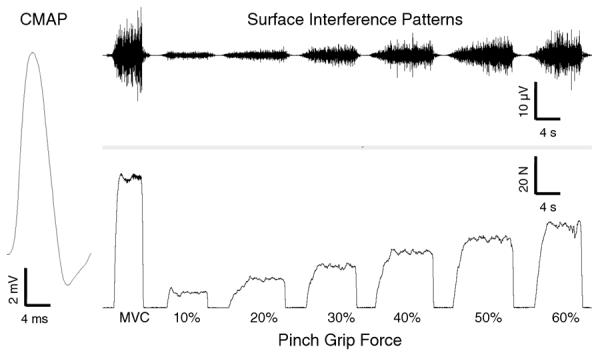
Representative example of an evoked compound muscle action potential (left trace), and the surface electrographic interference patterns (top right trace) during brief pinch-grip isometric force contraction tasks (bottom right trace).
Surface interference patterns (SIPs) (Figure 2, top trace) were recorded during brief (~ 5 second) pinch-grip submaximal contractions as well as during the MVCs. During the submaximal contractions, study participants performed a series of 12 pinch-grip contractions with the first six increasing in intensity (from 10% MVC to 60% MVC in 10% increments). Subsequently, participants performed the same series of submaximal contractions in reverse order (i.e., a brief contraction at 60% MVC followed by decreasing intensity contractions in 10% increments). During each of these contractions, a target line representing each specified contraction level was displayed on the computer monitor and subjects were asked to match this target line. At least 20-seconds rest was allowed between each contraction. Data from the first trial at each of the submaximal contraction intensities and the MVC, equaling 7 contractions, were used in the MUNIX calculation. If the first trial exhibited artifact or noise, the second trial was used for analysis (see below for further details).
MUNIX & MUSIX Calculations
The MUNIX value is based on a mathematical model that relies on evoked CMAPs and surface EMG data from the voluntary contractions (i.e., the SIP's) (Nandedkar et al. 2004, 2010). Specifically, the CMAP and SIP area and power were calculated, and applied to equation 1 to yield an ideal case motor unit count (ICMUC) for every contraction intensity: ICMUC = (CMAPpower * SIPAREA)/(CMAPAREA * SIPPOWER). The ICMUC assumes that all motor unit action potentials are the same and no phase cancellation occurs, giving an indication of the number of motor units in the muscle. Each ICMUC value was plotted over the SIP area, and the points were fit with a power regression. Where the line crosses 20-mVms on the x-axis the ICMUC value is reported as the MUNIX value, representing an estimate of the number of motor units in the muscle. The SIP area of 20-mVms is based on the premise that it reflects a low level of force output that elicits the firing of all low threshold motor units (Nandedkar et al., 2004, 2010).
To provide further insight about motor unit losses, the motor unit size index (MUSIX) was also calculated and used as an index of collateral reinnervation. Once MUNIX is calculated, MUSIX was computed based on equation 2: MUSIX = CMAPAmplitude/MUNIX. Prior to the analyses, the SIP signals were visually scrutinized to identify any artifact in the signal. If substantial noise was detected, the SIP epoch was rejected for analysis. For the acceptable SIP signals, a 1-sec epoch was used to calculate the SIP area and power. In order to standardize what epochs were accepted, the inclusion criteria previously described by Nandedkar et al. (2010) were utilized. The inclusion criteria are as follows: a) SIP area > 20 mVms, b) ICMUC < 100, c) SIP area/CMAP area > 1.
Statistical Analysis
Descriptive statistics between groups were compared using independent t- and χ2 tests. All values were reported in mean±standard error. Analysis of covariance procedures (Age Group X MUNIX or MUSIX) were used to test heterogeneity of regression slopes (moderation analysis), with body mass and biological gender serving as a covariates for both the MUNIX and MUSIX estimates. Continuous predictors were mean-centered so that the main effect of the age group would reflect the group difference at the mean of the predictors. Additionally, previous studies have reported an association between the CMAP amplitude and MUNIX in both healthy individuals as well as those with motor neuron disease or neurological disorders (Ahn et al., 2010; Nandedkar et al., 2010; Sandberg et al, 2010; Neuwirth et al., 2011; Boekestein et al., 2012; Li et al., 2012; Furula et al., 2013). As such, we examined this relationship by calculating a correlation coefficient between the respective measures. For all analyses, the R statistical language (version 2.15.0) was used, and a p-value ≤ 0.05 was required for statistical significance (2-tailed).
RESULTS
Descriptive Characteristics
The mean age of the OAs was 67±1.20 years, and the YAs were 22±0.74 years (p<0.01). The OAs had a similar body weight and height as the YAs (69.7±2.77 kg vs. 72.6±2.39 kg, p=0.44; 167.7±1.98 cm vs. 173.2±2.30 cm, p=0.09). After controlling for gender and body weight, OAs and YAs had similar MUNIX values (112±11 MUs vs. 115±10 MUs, p=0.80), while OAs had a 28% smaller MUSIX than YAs (52±5 μV vs. 72±4 μV, p<0.01). The OAs also exhibited a 30% lower CMAP amplitude in comparison to the YAs after controlling for gender and body weight (5.39±0.64 mV vs. 8.00±0.56 mV, p<0.01).
Pinch Grip Strength, MUNIX, & MUSIX
The slope of the abductor pollicis brevis MUNIX on pinch grip strength between the OAs and the YAs differed, as indicated by a Age Group X MUNIX interaction (p=0.04). Specifically, the model implied that after controlling for the effect of body mass and gender, the slope in OAs was significantly positive (0.14± 0.06 N of strength/MU, p=0.03; see Figure 3), whereas no such relationship was found in YAs (−0.08±0.09 N of strength/MU, p=0.35; see Figure 3). Reflecting this regression heterogeneity, the main effect of MUNIX was not significant (p = 0.35). No age group main effect was observed indicating that the OAs, on average, exhibited similar pinch-grip strength when compared to the YAs after controlling for MUNIX and the other factors (OA=44.45±3.02 N vs. YA = 36.52±3.50 N, p=0.10). Sex was significantly correlated with pinch grip strength (p=0.03) but body weight was not (p=0.72) when MUNIX was used as a predictor. We observed a modest association between CMAP amplitude and MUNIX, with CMAP amplitude explaining approximately 32% of the between subject variability in MUNIX (r=0.56, p<0.01).
Figure 3.
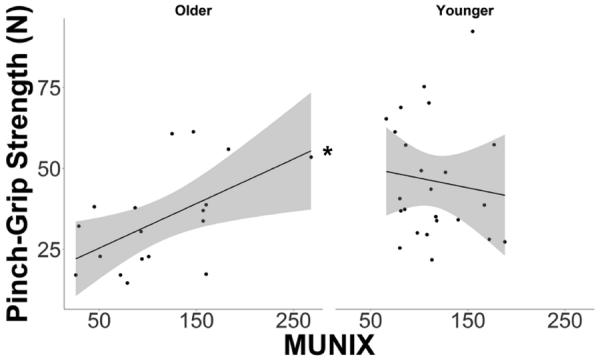
The relationship between pinch-grip strength and motor unit number index (MUNIX) in both young and older adults. A positive linear relationship between strength and MUNIX was found in the elderly (left panel). No relationship between strength and MUNIX was found in the young (right panel).
*Slope is statistically significant at p<0.05.
A significant Age Group X MUSIX interaction was also observed for strength (p<0.01; see Figure 5C). In contrast to MUNIX, the slope in YAs was significantly positive (0.48±0.11 N/μV, p<0.01; see Figure 4), whereas no such relationship was found in OAs (−0.30±0.22 N/μV, p=0.18; see Figure 4). While the main effect of MUSIX was again not significant (p=0.45), an age group main effect was observed indicating that the OAs exhibited 20% smaller pinch-grip strength than the YAs after controlling for MUSIX and the other factors (32.98±3.91 N vs. 40.50±2.78 N, p<0.01). Both gender nor body weight was correlated with pinch-grip strength with the effect of MUSIX and the other factors removed (p-values>0.06).
Figure 4.
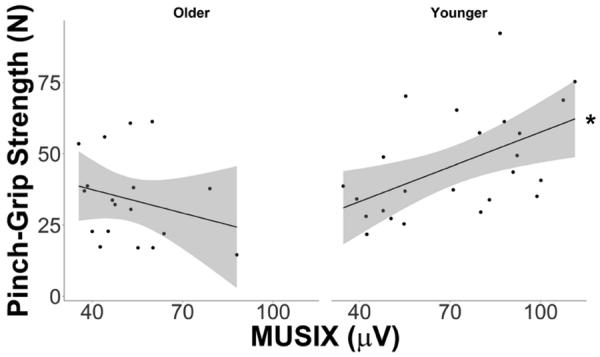
The relationship between pinch-grip strength and motor unit size index (MUSIX) in both young and older adults. No relationship was observed between strength and MUSIX in the elderly (left panel). A positive linear relationship between strength and MUSIX was found in the young (right panel).
*Slope is statistically significant at p<0.05.
DISCUSSION
This investigation utilized the MUNIX technique to obtain an index of functioning motor unit number and size in younger and older adults. It was hypothesized that there would be an interrelationship between muscle strength, motor unit number, and aging, such that weaker older adults would exhibit a reduced number of motor units in the absence of differences in motor unit size. Consistent with the hypothesis, the relationship between muscle strength and MUNIX amongst the older adult cohort indicated that the weaker individuals exhibited a smaller MUNIX value. No such relationship was observed for the older adult cohort as it related to MUSIX. Also consistent with the hypothesis, these relationships differed between the younger and older adult groups, indicating the age-dependent nature of these relationships. To our knowledge this work represents one of the first studies to report an interrelationship between muscle strength, motor unit number, and aging. Below these findings are discussed in the context of the mechanisms and contributors to muscle weakness as well as limitations of the current work.
Mechanisms and Contributors to Muscle Weakness
The finding that between-subject differences in muscle strength were mediated by indices of functioning motor unit number in an OA is in agreement with earlier findings by Doherty and colleagues (1993) when the spike-triggered averaging MUNE technique was used to estimate motor unit number. Here, a modest positive linear correlation (r=0.52) was found when estimates of motor unit number were regressed over maximal voluntary strength of the elbow flexor muscles in healthy, physically active older adults (> 60 years). Conversely, while there are numerous studies suggesting that there is generally a marked reduction in motor unit number in OA (Brown 1972; Brown et al., 1988; Doherty et al., 1993; Galea, 1996; McNeil et al., 2005), the majority of studies do not assess or evaluate strength, and in the ones that do, a concomitant reduction in voluntary strength is not always observed (McNeil et al., 2005). As such, this finding is somewhat in disagreement with some prior reports. For instance, McNeil et al. (2005) reported that motor unit number of the tibialis anterior muscle, which were quantified using the decomposition-enhanced spike-triggered averaging (DE-STA) MU number estimation technique, were reduced in men in their 60's (91±22 MUs) and men in their 80's (59±15 MU's) when compared to young adults (150±43 MUs); however, dorsiflexion muscle strength was only significantly reduced in the oldest cohort relative to the young adults. Similarly, Power and colleagues (2010) also used the DE-STA estimation technique and observed that older runners (64±3 years) exhibited 25% less dorsiflexion muscle strength when compared to young, recreationally active adults, but no differences in motor unit number were observed. Most recently, Drey and colleagues (2013) used the MUNIX technique to estimate motor unit number and size of the hypothenar muscle in 27 sarcopenic patients (i.e., individuals with low gender-specific skeletal muscle mass index and exhibiting impairments in physical function). While muscle strength was not assessed, they did observe that the sarcopenic patients MUNIX values, on average, were lower than those observed in healthy, young adults, and the authors even suggested that a subset of the patients' sarcopenia could be directly attributed to the loss of motor neurons.
Our findings help clarify some of the discrepancy in the literature by providing evidence that there is an interrelationship between muscle strength, estimates of MU number, and aging. At first glance, our results appeared to suggest no association between muscle strength and MU number as the OA and YA did not exhibit mean group differences in MUNIX or strength per se. However, closer inspection using a moderation analysis indicated that indeed an interrelationship between muscle strength, motor unit number, and aging exists with the weaker elders demonstrating fewer motor units. Interestingly, an interrelationship was also observed as it relates to estimates of motor unit size; however, this relationship was primarily driven by the young adult group as the older adult group did not demonstrate a significant relationship between MUSIX and muscle strength. The older adults' lack of relationship between MUSIX and muscle strength conceptually could be indicative of the weaker older adults experiencing a loss of motor units without collateral reinnervation. With regards to the positive association observed in YA, it raises the question of whether this provides evidence that the stronger YA have larger threshold motor units with higher innervation ratios, which have been shown to result in higher evoked muscle force (Kwa, Korfage, & Weijs, 1995).
In recent years our understanding of the mechanistic contributors to muscle weakness in the elderly has expanded. For the better part of the last half-century the prevailing thought attributed muscle weakness in the elderly to muscle wasting (i.e., atrophy) (Clark & Manini, 2008; Manini & Clark, 2012). However, recent longitudinal studies have indicated that the change in muscle mass with advancing age only explains a modest amount of the change in muscle strength (for review please see Cruz-Jentoft, 2012; Manini & Clark, 2012). Data from the last one to two decades now indicates that several physiological mechanisms are likely associated with the etiology of muscle weakness in seniors. For instance, a series of elegant studies examining the effects of aging on excitation-contraction coupling suggests that impairment in this process is likely one contributor to muscle weakness (Boncompagni et al., 2006; González et al., 2000; Renganathan et al., 1997; Renganathan & Delbono, 1998; Russ et al., 2011). Additionally, there is compelling evidence to suggest that changes in the nervous systems form and function also contribute to muscle weakness (for review see Clark & Taylor, 2011). For example, Harridge et al. (1999) reported that OA in their 80's and 90's were only able to voluntarily activate their quadriceps muscles at ~80% of its maximal activation capacity suggesting that if the nervous system were able to fully activate the musculature a nearly 20% increase in muscle strength would result. A neurological impairment in voluntary activation could be due to many factors, such as a reduction in descending drive from the supraspinal centers arising from alterations in intracortical excitability and/or neuroanatomical changes (Clark et al., 2010; Clark & Taylor, 2011; McGinley et al., 2012) as well as from alterations in alpha motor neuron properties that alter their discharge rate characteristics (Christie & Kamen, 2006; Kamen et al., 1995). Similarly, the data from the present work suggests that reductions in motor units without complete collateral reinnervation may also be a contributor to muscle weakness. It is difficult to know whether our observed association is indicative of an actual anatomical reduction in motor unit number per se or whether it is reflective of motor units being functionally unresponsive. Interestingly, prolonged disuse, which is a common experimental model to study muscle weakness as it is well known to induce dramatic reductions in muscle strength (Clark, 2009). Additionally, a 3-week immobilization model in felines has been shown to result in some MUs no longer producing measureable force or EMG activity in response to direct electrical stimulation of the MU (Robinson et al., 1991). Future work is needed to more thoroughly understand whether proportion of the weakness observed in aging results from functionally unresponsive MUs, and, if so, subsequently developing effective strategies to enhance functional responsivity.
Motor Unit Number Estimation Techniques and Limitations of the Current Work
There are several limitations of the current work that should be noted. First, while we have demonstrated moderate to moderately-high reliability of the MUNIX and MUSIX measures (Kaya et al., In Revision), it is critical to recognize that these techniques are still in their relative infancy and there are still questions surrounding the validity of the MUNIX technique to quantify motor unit number and size. The major concern previous investigations have cited is the inability of MUNIX to accurately reflect the true number of motor units in the studied muscle. MUNIX was designed as an “index,” a measurement that is not intended to be used as an “estimate,” therefore investigators should not interpret the MUNIX and MUSIX values as true reflections of anatomic MU number and size. There is a lack of studies comparing the MUNIX technique to other MU number estimation techniques, and the limited work that has examined this issue is inconclusive with a correlation between techniques noted in some populations but not others (Boekestein et al., 2013; Furtula et al., 2012). As such, further work is needed to better identify the strengths and limitations of the MUNIX technique. Additionally, reservation should be taken interpreting our findings until further work is done exploring the effect of atrophy of MUNIX. Recently, Li and colleagues conducted a computer-modeling simulation study where muscle atrophy was simulated by assigning a reduced amplitude of the motor unit action potentials, and observed that reducing the amplitude resulted in underestimating the MUNIX value (Li et al., 2012). Thus, it is possible that between-subject and between-group differences in MUNIX could be influenced by differences in muscle size; however, it should be noted that the association between fiber size and action potential amplitude is only modest to moderate (Hakansson, 1956) and further work is required to better understand how various factors influence MUNIX. Other limitations of the current work that should be noted are the cross-sectional study design and the relatively small sample size. Further, the findings of the present work should be delimited to the muscle group studied, as there is evidence that age-related changes in neuromuscular form and function varies depending on the muscle group studied (Candow & Chilibeck, 2005; Lanza et al., 2013).
CONCLUSIONS
In this study we sought to determine whether age-related differences in muscle strength are moderated by estimates of functioning MU number and size. We used the MUNIX technique to obtain indexes of MU number and size, and conducted a moderation analysis to examine the interrelationship between aging, strength, and MU properties. Our findings indicated that a positive association between muscle strength and MUNIX in OAs with no such relationship observed among the OA for MUSIX. These findings indicate that there is an interrelationship between muscle strength, MU numbers, and aging, which suggests that a portion of muscle weakness in seniors may be attributable to the loss of functioning motor units.
HIGHLIGHTS
Interrelationship between strength, motor unit (MU) characteristics, and aging.
Positive association noted between strength and MU number in seniors.
No association noted between strength and MU size in seniors.
Loss of functioning MU may be responsible for a portion of weakness in seniors.
FUNDING ACKNOLWEDGEMENT
This work was supported in part by grant R15HD065552 from the National Institutes of Health's Eunice Kennedy Shriver National Institute of Child Health and Human Development to B. C. Clark.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: B.C. Clark has received consulting fees from Regeneron Pharmaceuticals, Inc. and Abbott Laboratories. No other conflicts were reported.
REFERENCES
- Ahn SW, Kim SH, Kim JE, Kim SM, Kim SH, Park KS, Sung JJ, Lee KW, Hong YH. Reproducibility of the motor unit number index (MUNIX) in normal controls and amyotrophic lateral sclerosis patients. Muscle & Nerve. 2010;42:808. doi: 10.1002/mus.21765. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr. Accuracy of the college alumnus physical activity questionnaire. Journal of Clinical Epidemiology. 1993;46:1403–1411. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- Boekestein WA, Schelhaas HJ, van Putten MJ, Stegeman DF, Zwarts MJ, van Dijk JP. Motor unit number index (MUNIX) versus motor unit number estimation (MUNE): A direct comparison in a longitudinal study of ALS patients. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2012;123:1644–1649. doi: 10.1016/j.clinph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Boncompagni S, d'Amelio L, Fulle S, Fano G, Protasi F. Progressive disorganization of the excitation-contraction coupling apparatus in aging human skeletal muscle as revealed by electron microscopy: A possible role in the decline of muscle performance. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2006;61:995–1008. doi: 10.1093/gerona/61.10.995. [DOI] [PubMed] [Google Scholar]
- Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. Journal of Neurology, Neurosurgery, and Psychiatry. 1972;35:845–852. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle & Nerve. 1988;11:423–432. doi: 10.1002/mus.880110503. [DOI] [PubMed] [Google Scholar]
- Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2005;60:148–156. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. Journal of Neurophysiology. 2006;95:2787–2795. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- Clark BC. In vivo alterations in skeletal muscle form and function after disuse atrophy. Medicine and Science in Sports and Exercise. 2009;41:1869–1875. doi: 10.1249/MSS.0b013e3181a645a6. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Sarcopenia =/= dynapenia. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Current Aging Science. 2011;4:192–199. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle & Nerve. 2010;42:363–372. doi: 10.1002/mus.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AA, Morley JE, editors. Sarcopenia. Wiley-Blackwell; Oxford, UK: 2012. [Google Scholar]
- Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Current Aging Science. 2011;4:248–259. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Current Aging Science. 2011;4:209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. Journal of Applied Physiology (Bethesda, Md.: 1985) 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Drey M, Grosch C, Neuwirth C, Bauer JM, Sieber CC. The motor unit number index (MUNIX) in sarcopenic patients. Experimental Gerontology. 2013;48:381–384. doi: 10.1016/j.exger.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Fry CS, Rasmussen BB. Skeletal muscle protein balance and metabolism in the elderly. Current Aging Science. 2011;4:260–268. doi: 10.2174/1874609811104030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtula J, Johnsen B, Christensen PB, Pugdahl K, Bisgaard C, Christensen MK, Arentsen J, Frydenberg M, Fuglsang-Frederiksen A. MUNIX and incremental stimulation MUNE in ALS patients and control subjects. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2013;124:610–618. doi: 10.1016/j.clinph.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Galea V. Changes in motor unit estimates with aging. Journal of Clinical Neurophysiology : Official Publication of the American Electroencephalographic Society. 1996;13:253–260. doi: 10.1097/00004691-199605000-00010. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. The Journal of Membrane Biology. 2000;178:175–183. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- Kakansson CH. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiologica. 1956;37:14–34. doi: 10.1111/j.1748-1716.1956.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle & Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. Journal of the Neurological Sciences. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. Journal of Applied Physiology (Bethesda, Md.: 1985) 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- Kaya RD, Nakazawa M, Hoffman RL, Clark BC. In Revision. Reliability of the modified motor unit number index (MUNIX) technique. Journal of Electromyography and Kinesiology. doi: 10.1016/j.jelekin.2013.10.005. In Revision (Revisions requested 5/28/2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa SH, Korfage JA, Weijs WA. Function-dependent anatomical parameters of rabbit masseter motor units. Journal of Dental Research. 1995;74:1649–1657. doi: 10.1177/00220345950740100501. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. Journal of Applied Physiology (Bethesda, Md.: 1985) 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- Li X, Rymer WZ, Zhou P. A simulation-based analysis of motor unit number index (MUNIX) technique using motoneuron pool and surface electromyogram models. IEEE Transactions on Neural Systems and Rehabilitation Engineering : A Publication of the IEEE Engineering in Medicine and Biology Society. 2012;20:297–304. doi: 10.1109/TNSRE.2012.2194311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC. Dynapenia and aging: An update. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Experimental Gerontology. 2010;45:671–678. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle & Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Nandedkar SD, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX): Principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle & Nerve. 2010;42:798–807. doi: 10.1002/mus.21824. [DOI] [PubMed] [Google Scholar]
- Nandedkar SD, Nandedkar DS, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX) IEEE Transactions on Bio-Medical Engineering. 2004;51:2209–2211. doi: 10.1109/TBME.2004.834281. [DOI] [PubMed] [Google Scholar]
- Neuwirth C, Nandedkar S, Stålberg E, Weber M. Motor unit number index (MUNIX): A novel neurophysiological technique to follow disease progression in amyotrophic lateral sclerosis. Muscle & Nerve. 2010;42:379–384. doi: 10.1002/mus.21707. [DOI] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Behm DG, Vandervoort AA, Doherty TJ, Rice CL. Motor unit number estimates in masters runners: Use it or lose it? Medicine and Science in Sports and Exercise. 2010;42:1644–1650. doi: 10.1249/MSS.0b013e3181d6f9e9. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Delbono O. Caloric restriction prevents age-related decline in skeletal muscle dihydropyridine receptor and ryanodine receptor expression. FEBS Letters. 1998;434:346–350. doi: 10.1016/s0014-5793(98)01009-6. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. The Journal of Membrane Biology. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Enoka RM, Stuart DG. Immobilization-induced changes in motor unit force and fatigability in the cat. Muscle & Nerve. 1991;14:563–573. doi: 10.1002/mus.880140611. [DOI] [PubMed] [Google Scholar]
- Russ DW, Grandy JS, Toma K, Ward CW. Ageing, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiologica (Oxford, England) 2011;201:391–403. doi: 10.1111/j.1748-1716.2010.02191.x. [DOI] [PubMed] [Google Scholar]
- Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality”. Journal of Cachexia, Sarcopenia and Muscle. 2012;3:95–109. doi: 10.1007/s13539-011-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Lanza IR. The impact of old age on skeletal muscle energetics: Supply and demand. Current Aging Science. 2011;4:234–247. doi: 10.2174/1874609811104030234. [DOI] [PubMed] [Google Scholar]
- Sandberg A, Nandedkar SD, Stålberg E. Macro electromyography and motor unit number index in the tibialis anterior muscle: Differences and similarities in characterizing motor unit properties in prior polio. Muscle & Nerve. 2011;43:335–341. doi: 10.1002/mus.21878. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Administration on Aging A Profile of Older Americans: 2011 Future Growth [Graphic illustration of future growth of aging individuals February 10, 2012] 2012 Retrieved from http://www.aoa.gov/AoARoot/Aging_Statistics/Profile/2011/14.aspx.


