Abstract
Oxidatively-induced DNA damage was measured in the DNA of WBC from two groups of women: carriers of a BRCA mutation, but asymptomatic for disease, and healthy controls. Two oxidatively induced lesions were measured: a formamide remnant of pyrimidine base and the glycol modification of thymine. These lesions, employed previously in studies of the effects of smoking, antioxidant usage and ovarian cancer, are proving valuable indicators of oxidative stress. The BRCA carriers of mutations, with no overt sign of cancer, nevertheless had significantly higher levels of DNA damage than the controls. The level measured for the formamide lesion was 5.9 ± 1.0 (femtomoles/μg of DNA ± SEM) compared with 2.4 ± 0.3 in controls. The level of the glycol lesion was 2.9 ± 0.4 compared with 1.8 ± 0.2 in controls. The experimental design utilized DNA from WBC and employed LC-MS/MS to detect the lesions.
1. INTRODUCTION
There are persistent reports that oxidatively induced DNA damage is significantly higher in cancer patients compared with healthy controls. The base modification 8-oxo-7,8-dihydroguanine, a biomarker for oxidative stress, was found to be elevated in the DNA of WBC from patients with cancer at a variety of sites: lung [1,2], lymphocytic leukemia [3], colorectum [4], bladder [5], breast [6] and esophagus [7]. Somewhat disconcerting, mean values from healthy controls reported by these various laboratories varied by more than two orders of magnitude. Moreover, all of the reported control values exceeded the maximum value (4.3 dGh/106dG) considered credible by the European Standards Committee for Oxidative DNA Damage [8]. Higher values are presumed caused mainly by inadvertent oxidation of guanine during the preparation of the DNA sample. A variety of mechanisms may generate 8-oxo-7,8-dihydroguanine including hydroxyl radical reactions, one-electron oxidations and singlet oxygen reactions [9]. Concerns over inadvertent loss of 8-oxo-7,8-dihydroguanine, which has an even lower redox potential than guanine, contribute to the uncertainty of 8-oxy-7,8-dihydroguanine measurements [10,11]. Pyrimidine bases, on the other hand, have a higher redox potential than purines and are less prone to artifactual oxidation [12].
The problems associated with measurements of oxidative damage can largely be avoided using a different biomarker for oxidative stress. We previously proposed two pyrimidine oxidation products as preferable biomarkers of oxidative stress, namely a glycol modification of thymine and a formamido remnant of the pyrimidine bases. The lesions are shown in Fig. 1 in the dimer form in which they were measured. The assay has been applied in a study of women with ovarian cancer [13]. These lesions, derived from the DNA of WBC of women with ovarian cancer, were found to have significantly higher levels compared with controls.
Fig. 1.
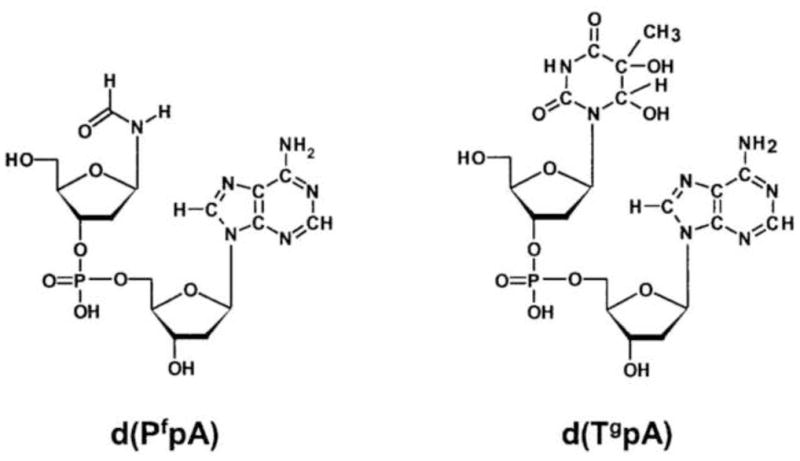
The formamide and thymine glycol lesions shown incorporated in dinucleoside monophosphates, the form in which they are actually measured.
The variety of risk factors associated with higher levels of the formamide and thymine glycol biomarkers continues to grow. In addition to demonstrating higher levels of oxidative DNA damage in women with ovarian cancer, it has been shown that former smokers have a lower level of these lesions after quitting [14]. Also, antioxidant users were shown to have significantly lower levels of formamide and thymine glycol damage than non-users [15]. Furthermore, antioxidants further reduced DNA damage in former smokers independently of the decrease due to quitting (unpublished results). Women harboring a BRCA mutation, but asymptomatic for disease, have significantly higher levels of pyrimidine lesions than healthy controls as reported herein.
In the present investigation two groups of women were studied: (a) women carrying either a BRCA1 or a BRCA2 mutation, but having no overt signs of cancer, and (b) healthy adult female controls. The BRCA group, composed of women having no overt signs of disease, had a significantly higher level of damage than the healthy control group. This finding is consistent with BRCA1’s growing reputation as the “master regulator” for maintaining the integrity of the genome. Perhaps it should be expected that women with a defective BRCA1 gene would exhibit higher levels of damage prior to manifesting cancer (see Discussion). It may be appropriate to consider whether a high level of oxidatively induced DNA damage could serve as an early clinical indicator of cancer susceptibility.
2. MATERIALS AND METHODS
Blood donors were recruited under an approved protocol (RPCI I-03007). Donors harboring a BRCA1 mutation, but asymptomatic for cancer, were recruited from the Gynecology Clinic at RPCI. These women did not have ovarian, primary peritoneal or fallopian tube cancer. The mutational status of the BRCA genes was determined by Myriad Genetics (Overland, WS ) [16]. The analysis by Myriad consisted of a full sequence analysis of BRCA1 and BRCA2 in both forward and reverse directions of, respectively, 22 coding exons (approximately 5000 base pairs and 750 adjacent base pairs) and 26 coding exons (approximately 10,200 base pairs and 900 adjacent base pairs).
DNA was extracted from WBC of whole blood samples using a ZeptoMetrix kit that is based on the chaotropic method of sample preparation. Sample prepration methods, important in measurements oxidative DNA damage, have been thoroughly investigated by Ames and coworkers (17). The chaotropic method was strongly endorsed. Also, the practice of adding desferal to the DNA sample as a precaution against artifactual oxidation, typically at 0.1 mM concentration, has been recommended (17,18). Adhering to these advisements the extracted DNA was denatured and subjected to enzynmatic hydrolysis at 37°C using nuclease P1 and alkaline phosphatase as described previously (14). The lesions of interest inhibit the hydrolysis by nuclease P1 of the phosphoester bond 3′ to the modified nucleoside [19]. Consequently, the lesions tend to accumulate in a nuclease P1-alkaline phosphatase hydrolysate of DNA as modified dinucleoside monophoshates. A significant advantage accrues from LC-MS/MS measurements made at the dimer level. Due to the presence of the phosphodiester bond, detection sensitivity is better. For example, the thymine glycol lesion is detected seven fold more sensitively at the dimer level than as a monomer [20]. An internal standard in the form of a labeled modified oligomer, d(GAAPfA*) and d(GTgA*C) where A* stands for universally 15N-labeled deoxyadenosine, is added to the DNA sample prior to digest in order to be able to account for any loss of modified dinucleoside monophosphate during digestion. The standard sample amount was 100 μg, a substantial amount, advantageous for guarding against inaccuracies that become more troublesome in small samples (17).
There were some differences between methods used in this study and the methods employed in our earlier study (13). Sample preparation was simplified by elimination of the HPLC step after hydrolysis of the DNA and prior to LC-MS/MS analysis. The HPLC step was used to remove normal 2′-deoxyribonucleosides, the bulk of the DNA sample, before LC-MS/MS analysis. It was found that the entire hydrolyzed sample could be injected into the spectrometer without serious compromise of detection sensitivity. Another difference from the previous study was the instrumentation. An AB Sciex Qtrap 5500 LC-MS/MS was employed in this study. Internal standards were added to the DNA sample prior to enzymatic digest. The internal standards and the DNA were hydrolyzed concomitantly.
3. RESULTS
The observed levels of d(PfpA) and d(TgpA) are plotted as a function of age of the donor in Figs 2 and 3 respectively for BRCA mutation carriers and for healthy controls. The specific mutations identified by Myriad are listed in Table 1.
Fig. 2.
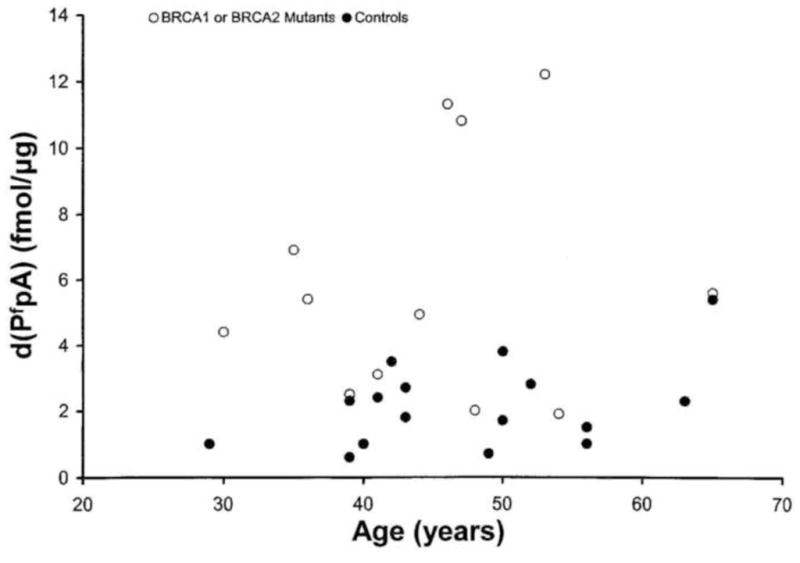
Levels of d(PfpA) (fmol/ug DNA) versus age in carriers of BRCA mutations (empty circles) and healthy controls (filled circles).
Fig. 3.
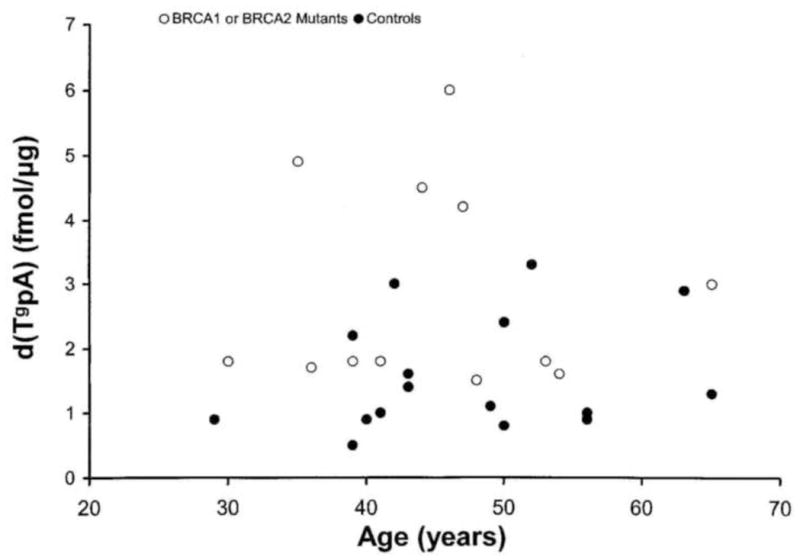
Levels of d(TgpA) (fmol/ug DNA) versus age in carriers of BRCA mutations (empty circles) and healthy controls (filled circles).
Table 1.
Mutations in BRCA 1 and BRCA 2 genes, as determined by Myriad Genetic Laboratories, in women with no overt indication of disease. For mutation nomenclature see Beaudet and Tsui [11].
| Sample # | Gene Analyzed | Mutation |
|---|---|---|
| 100821906 | BRCA1 | 387del4 |
| 102982219 | BRCA1 | C61G (300T>G) |
| 102982177 | BRCA1 | C61G (300T>G) |
| 111570319 | BRCA1 | exon 13 ins 6kb |
| 113251661 | BRCA2 | F1870X |
| 113111361 | BRCA2 | 4206ins4 |
| 113080701 | BRCA1 | K679X (2154A>T) |
| 102180275 | BRCA2 | 7218del5 |
| 093132179 | BRCA2 | N31241 (9599A>T) |
| 071791068 | BRCA2 | G1529R |
| 071501608 | BRCA1 | K679X |
| 113461439 | BRCA2 | G1529R |
| 113461401 | BRCA2 | 5385insC |
The scatter plots in Figs. 2 and 3 indicate some dependence of DNA damage levels on age but over and above any age dependence a risk factor effect is clearly discernible. The mean values of the formamide and thymine glycol lesions measured in the BRCA carriers and healthy control groups are listed in Table 2. The levels observed in the carrier group, composed of women having no overt signs of the disease, were 5.9 × 10−15 mol d(PfpA)/μg of DNA and 2.9 × 10−15 mol d(TgpA)/μg of DNA. These levels are significantly higher than the levels observed in the healthy control group, namely 2.4 × 10−15 mol d(PfpA)/μg DNA and 1.8 × 10−15 mol d(TgpA)/μg DNA. The p values for the significance of the differences in the mean damage levels between carriers and controls are <.01.
Table 2.
Levels of pyrimidine base damage are listed in terms of femtomoles of lesion per microgram of DNA ± SEM. The number in parenthesis is the number of participants in the group.*
| d(PfpA) | d(TgpA) | |
|---|---|---|
| BRCA mutation carriers (14) | 5.9 ±1.0 | 2.9 ± 0.4 |
| Healthy controls (18) | 2.4 ± 0.3 | 1.8 ± 0.2 |
The results may be stated in terms of DNA lesions per 106 nucleotides. For example, the value 5.9 × 10−15 mol d(PfpA)/μg of DNA converts to 6.0 mol dPf/106 nucleotides. The conversion is based on the expectation that the molar abundaces of d(PfpA), d(PfpC), d(PfpT) and d(PfpG) in the sample are in the ratios 3/2/3/2. The value for the healthy control group is 2.5 dPf/106 nucleotides.
In our previous study the d(PfpA) and d(TgpA) lesions were measured in ovarian cancer patients versus healthy controls using a somewhat different methodology and technology as noted above in Methods. For comparison purposes those results are reproduced in Table 3.
Table 3.
Levels of pyrimidine base damage are listed in terms of femtomoles of lesion per microgram of DNA ± SEM. The number in parenthesis is the number of participants in the group.
| Ovarian Cancer Patients (19) | 6.3 ± 0.8 | 2.8 ± 0.5 |
| Controls (16) | 5.5 ± 0.8 | 2.2± 0.4 |
That study did not include any known carriers of BRCA mutations. The present study is constituted of a completely separate set of measurements. Our conclusion is that BRCA mutations in the absence of disease raise the level of oxidative DNA damage to approximately the same level as actual cancer.
4. DISCUSSION
Oxidative stress and oxidatively induced DNA damage are recurrent themes in cancer research [21]. BRCA mutations are the focus for many of these investigations. It was recognized early that cells containing mutated BRCA1 are hypersensitive to radiation [22]. The purview accorded BRCA1 has continued to expand as new functions are discovered. The mechanism of BRCA1 control is, at least in part, transcription coupled. New insights have come from the realization that BRCA1 forms multiple complexes containing a BARDI component [23]. The BRCA1/BARDI complex is a participant in larger protein complexes that are involved in checkpoint control or DNA repair [24]. Thus, BRCA1 may be regarded as the “master regulator” of genome integrity [25,26]. It is well known that the glycosylase endo III excises the formamide and thymine glycol lesions from DNA [27]. More recently it has been shown that NTH1, a homolog of endonuclease III, is stimulated by BRCA1 [26]. Thus, a connection between BRCA1 function and the lesions observed in this study is clearly indicated.
Carriers of a BRCA1 or a BRCA2 gene have a lifetime risk of breast cancer of up to 80% as well as an increased risk of ovarian cancer (28 ). It was found in the present study that women who carry a BRCA mutation, but who are asymptomatic for cancer, have, on average, higher levels of DNA damage than healthy controls. This observation suggests that the oxidative DNA damage is not consequent to the disease but rather is precursor to the disease. Since the genomic defect is inherited, affected women are probably under lifelong oxidative stress. The higher incidence of cancer among these women can logically be attributed to mutations consequent to BRCA deficiency. One may speculate whether DNA damage measurements could become useful clinically and whether the prospects of affected women could be improved by taking antioxidants.
Multiple studies of the 8-oxo-7.8-dihydroguanine lesion (1–7, 13) have reported higher levels of oxidative DNA damage in WBC of cancer patients. The present study is unique in associating elevated levels of oxidative DNA damage with a high probability of developing the disease. BRCA1 and BRCA2 are tumor suppressor genes. Since their gene products participate in multiple cell functions including maintenance of genome integrity and DNA repair, it should not be surprising that, even in the absence of cancer, germ line carriers of a BRCA mutation exhibit higher levels of oxidative DNA damage. Presumably, the higher level of damage is due to one allele being dysfunctional. Both alleles must be dysfunctional for cancer to develop [29].
The equally famous mutated p53 cancer susceptibility gene produces effects similar to mutated BRCA genes. The p53 tumor suppressor also possesses antioxidant functions [30, 31] Studies of oxidative DNA damage in germ line carriers of p53 mutations have been few and focused mainly on the 8-oxo-7,8-dihydroguanine lesion. It would seem worthwhile to investigate more thoroughly the effect of germ line mutations in relation to oxidative damage and cancer.
Higher levels of the formamide and thymine glycol lesions have recently been associated with other risk factors for cancer. In addition to carriers of BRCA mutations, higher levels of formamide and thymine glycol lesions have also been associated with smoking [14] and poor antioxidant status [15].
This study is similar to that of Dziaman et al [32] who measured oxidative DNA damage in three groups of women with the differences from our study in that the control group was composed of relatives of BRCA1 carriers and the cancer patients were also carriers. These investigators measured the 8-oxo-7,8-dihydroguanine biomarker. An observation of these investigators was, also, that BRCA carriers have a higher level of oxidative damage than healthy controls.
Acknowledgments
The work was supported by grant RO3 CA139513 from the National Cancer Institute. Mass spectrometer facilities are shared resources supported by NCI grant CA 016056.
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vulimiri SV, Wu X, Baer-Dubowska W, de Andrade M, Detry M, Spitz MR, DiGiovanni J. Analysis of aromatic DNA adducts and 7,8-dihydro-8-oxo-2′-deoxyguanosine in lymphocyte DNA from a case-control study of lung cancer involving minority populations. Mol Carcinogenesis. 2000;27:34–46. doi: 10.1002/(sici)1098-2744(200001)27:1<34::aid-mc6>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Gackowski D, Speina E, Zielinska M, Kowalewski J, Rozalski R, Siomek A, Paciorek T, Tudek B, Olinski R. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003;63:4899–4902. [PubMed] [Google Scholar]
- 3.Oltra AM, Carbonell F, Tormos C, Iradi A, Saez GT. Antioxidant enzyme activities and the production of MDA and 8-oxo-DG in chronic lymphocytic leukemia. Free Radic Biol Med. 2001;30:1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 4.Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer. 2002;101:395–397. doi: 10.1002/ijc.10610. [DOI] [PubMed] [Google Scholar]
- 5.Akcay T, Saygili I, Andican G, Yalcin V. Increased formation of 8-hydroxy-2′-deoxyguanosine in peripheral blood leukocytes in bladder cancer. Urol Int. 2003;71:271–274. doi: 10.1159/000072677. [DOI] [PubMed] [Google Scholar]
- 6.Soliman AS, Vulimiri SV, Kleiner HE, Shen J, Eissa S, Morad M, Taha H, Lukmanji F, Li D, Johnston DA, Lo H-H, Lau S, Digiovanni J, Bondy ML. High levels of oxidative DNA damage in lymphocyte DNA of premenopausal breast cancer patients in Egypt. Inter J Environ Health Res. 2004;14:121–134. doi: 10.1080/0960312042000209534. [DOI] [PubMed] [Google Scholar]
- 7.Breton J, Sichel F, Pottier D, Prevost V. Measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in peripheral blood mononuclear cells: optimization and application to samples from a case-contol study on cancers of the oesophagus and cardia. Free Radic Res. 2005;39:21–30. doi: 10.1080/10715760400023523. [DOI] [PubMed] [Google Scholar]
- 8.Collins AR, Cadet J, Moller L, Poulsen HE, Vina J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch Biochem Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Cadet J, Douki T, Ravanat J-L. Oxidatively generated base damage to cellular DNA. Free Rad Biol Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Leipold MD, Muller JG, Burrows CJ, David SS. Removal of hydantoin products of 8-oxoguanine oxidation by the Escherichia coli DNA repair enzyme FPG. Biochem. 2000;39:14984–14992. doi: 10.1021/bi0017982. [DOI] [PubMed] [Google Scholar]
- 11.Fleming AM, Muller JG, Dlouhy AC, Burrows CJ. Structural context effects in oxidation of 8-oxo-7,8-dihydro-2′–deoxyguanosine to hydantoin products: Electrostatics, base stacking, and base pairing. J Am Chem Soc. 2012;134:15091–15102. doi: 10.1021/ja306077b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crespo-Hernandez CE, Close D, Gorb L, Leszczynski J. Determination of redox potentials for Watson-Crick base pairs, DNA nucleosides, and relevant nucleocide analogues. J Phys Chem. 2007;B111:5386–5395. doi: 10.1021/jp0684224. [DOI] [PubMed] [Google Scholar]
- 13.Iijima H, Patrzyc HB, Budzinski EE, Freund HG, Dawidzik JB, Rodabaugh KJ, Box HC. A study of pyrimidine base damage in relation to oxidative stress and cancer. Brit J Cancer. 2009;101:152–156. doi: 10.1038/sj.bjc.6605176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Box HC, O’Connor RJ, Patrzyc HB, Iijima H, Dawidzik JB, Freund HG, Budzinski EE, Cummings KM, Mahoney MC. Reduction of oxidatively-induced DNA damage following smoking cessation. Tob Ind Diseases. 2011;9:5. doi: 10.1186/1617-9625-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Box HC, Dawidzik JB, Freund HG, Patrzyc HB, Budzinski EE, Zeitouni N, Mahoney MC. Profiling oxidative DNA damage: Effects of antioxidants. Cancer Sci. 2012;103:2002–2006. doi: 10.1111/j.1349-7006.2012.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaudet AL, Tsui LCA. A suggested nomenclature for designating mutations. Hum Mutat. 1993;2:245–248. doi: 10.1002/humu.1380020402. [DOI] [PubMed] [Google Scholar]
- 17.Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN. DNA oxidation matters: The HPLC-electrochemical detection of 8-oxo-deoxyguanosine and 8-oixo-guanine. Proc Nat Acad ScI USA. 1998;95:288–293. doi: 10.1073/pnas.95.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouget JP, Douki T, Richard MJ, Cadet J. DNA Damage induced in cells by γ and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and comet assay. Chem Res Toxicol. 2000;13:541–549. doi: 10.1021/tx000020e. [DOI] [PubMed] [Google Scholar]
- 19.Falcone JM, Box HC. Selective hydrolysis of damaged DNA by nuclease P1. Biochim Biophys. 1997;1337:267–275. doi: 10.1016/s0167-4838(96)00172-0. [DOI] [PubMed] [Google Scholar]
- 20.Greene KF, Budzinski EE, Iijima H, Dawidzik JB, DeFredericis HC, Patrzyc HB, Evans MS, Bailey DT, Freund HG, Box HC. Assessment of DNA damage at the dimer level: measuurement of the formamide lesion. Rad Res. 2007;167:146–151. doi: 10.1667/rr0693.1. [DOI] [PubMed] [Google Scholar]
- 21.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Abbott DW, Thompson ME, Robinson-Benion C, Tomlinson G, Jensen RA, Holt JT. BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Biol Chem. 1999;274:18808–18812. doi: 10.1074/jbc.274.26.18808. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg RA, Sobhian B, Pathania S, Cantor SB, Nakatani Y, Livingston DM. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes & Development. 2006;20:34–46. doi: 10.1101/gad.1381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha T, Keun Rih J, Roy R, Ballai R, Rosen EM. Transcriptional regulation of the base excision repair pathway by BRCA1. J Biol Chem. 2010;285(25):19092–19105. doi: 10.1074/jbc.M110.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huen MSVY, Sy SMH, Chen JJ. BRCA1 and its toolbox for the maintenance of genome integrity. Mol Cell Biol. 2010;138:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weberpals, Clark-Knowles KV, Vanderhyden BC. Sporadic epithelial ovarian cancer: Clinical relevance of BRCA1 inhibition in DNA damage and repair pathway. J Clin, Oncol. 2008;26:3208–3267. doi: 10.1200/JCO.2007.11.3902. [DOI] [PubMed] [Google Scholar]
- 27.Cadet J, Bourdat AG, D’Ham C, Duarte V, Gasparutto D, Romieu A, Ravanat J-L. Oxidative base damage to DNA: specificity of base excision repair enzymes. Mutation Res. 2000;B462:121–128. doi: 10.1016/s1383-5742(00)00022-3. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez H, Jaruga P, Leber D, Nyaga SG, Evans MK, Dizdaroglu M. Lymphoblasts of women with BRCA1 mutations are deficient in cellular repair of 8,5-cyclopurine-2′-deoxynucleosides and 8-hydroxy-2′-deoxyguanosine. Biochem. 2007;46:2488–2496. doi: 10.1021/bi062022p. [DOI] [PubMed] [Google Scholar]
- 29.Welcsh L, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Gen. 2001;10:705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- 30.Sablina AA, Budanov AV, IIyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nature Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman A-R, Ford JM. BRCA1 Induces DNA damage recognition factors and enhances nucleotide excision repair. Nature Genetics. 2002;32:180–184. doi: 10.1038/ng953. [DOI] [PubMed] [Google Scholar]
- 32.Dziaman T, Hurzarski T, Gackowski D, Rozalski R, Siomek A, Szpila A, Guz J, Lubinski J, Olinski R. Elevated level of 8-oxo-7,8-2′-deoxyguanine in leukocytes of BRCA1 mutation carriers compared to healthy controls. Int J Cancer. 2009;125:2209–2213. doi: 10.1002/ijc.24600. [DOI] [PubMed] [Google Scholar]


