Abstract
This study focuses on determining whether and how the novel PI3 kinase inhibitor NVP-BKM120 (BKM120) induces apoptosis and enhances TRAIL-induced apoptosis in human lung cancer cells. We found that BKM120 reduced Mcl-1 levels across the tested cell lines along with induction of apoptosis and enhancement of TRAIL-induced apoptosis. Enforced expression of ectopic Mcl-1 significantly attenuated the effects of BKM120 alone or in combination with TRAIL on induction of apoptosis. Thus Mcl-1 downregulation contributes to BKM120-induced apoptosis or enhancement of TRAIL-induced apoptosis. Moreover, we have demonstrated that BMK120 decreases Mcl-1 levels through facilitating its degradation involving a GSK3/FBXW7-dependent mechanism.
Keywords: BKM120, PI3 kinase, TRAIL, apoptosis, lung cancer
1. Introduction
NVP-BKM120 is a novel, potent and highly selective pan-class I phosphatidylinositol-3 kinase (PI3K) inhibitor and is currently being investigated in phase I clinical trials. In preclinical studies, it has been shown to be active in suppressing proliferation and inducing apoptosis of cancer cell lines and in inhibiting the growth of human tumors (xenografts) in mice at tolerated doses [1; 2; 3]. When combined with dexamethasone, synergistic cytotoxicity in dexamethasone-sensitive multiple myeloma cells was also observed [2]. Clinically, BKM120 at the maximum-tolerated dose has been shown to be safe with a favorable pharmacokinetic profile, clear evidence of target inhibition and preliminary antitumor activity in a recently completed phase I trial [4].
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a potential cancer therapeutic cytokine with the ability to preferentially induce apoptosis in transformed or malignant cells [5]. Currently, recombinant human TRAIL is being tested in phase I clinical trials [6; 7]. However, some cancer cell lines are intrinsically insensitive to TRAIL; this issue has thus become a major obstacle in TRAIL-based cancer therapy [8; 9]. Hence additional sensitization is needed to potentiate the killing effect of TRAIL in these insensitive cancer cells. PI3K/Akt signaling has been considered to be one possible mechanism accounting for cancer cell resistance to TRAIL. Accordingly, inhibition of this signaling with both small molecule inhibitors or small interfering RNA (siRNA) sensitizes cancer cells to TRAIL-induced apoptosis in some types of cancer cells [10; 11; 12; 13; 14; 15]. Whether BKM120 sensitizes cancer cells to TRAIL-induced apoptosis has not been reported.
It is well known that cells can die of apoptosis primarily through the extrinsic death receptor-induced pathway and/or the intrinsic mitochondria-mediated pathway: the extrinsic pathway is characterized by the oligomerization of cell surface death receptors and activation of caspase-8, while the intrinsic pathway involves in the disruption of mitochondrial membranes, the release of cytochrome c and the activation of caspase-9. Cross-talk between these two pathways is mediated by the truncated proapoptotic protein Bid [16]. A central step in the execution of apoptosis is the activation of caspases (e.g., caspase-8 and caspase-9), which are widely expressed as inactive forms [16]. Thus, this step is negatively regulated by multiple anti-apoptotic proteins such as c-FLIP (for caspase-8) and anti-apoptotic Bcl-2 family members (e.g., Mcl-1, Bcl-2 and Bcl-XL) (for caspase-9) to prevent unnecessary activation of these caspases. In general, downregulation of these anti-apoptotic proteins induces apoptosis or enhances TRAIL-induced apoptosis [8; 17; 18].
In this study, we focused on determining whether BKM120 induced apoptosis and enhanced TRAIL-induced apoptosis in human non-small cell lung cancer (NSCLC) cells. Moreover, we studied possible mechanisms by which BKM induces apoptosis and sensitizes NSCLC cells to TRAIL-induced apoptosis.
2. Materials and Methods
2.1, Reagents
BKM120 was supplied by Novartis Pharmaceuticals Corporation (East Hanover, NJ), dissolved in DMSO and stored at −80°C. Soluble recombinant human TRAIL was purchased from PeproTech, Inc. (Rocky Hill, NJ). The proteasome inhibitor MG132, the protein synthesis inhibitor cycloheximide (CHX) and the GSK3 inhibitor SB216763 was purchased from Sigma Chemical Co. (St. Louis, MO). The PI3K inhibitor LY294002 was purchased from LC Laboratories (Woburn, MA). The pan caspase inhibitor Z-VAD-fmk was purchased from Bachem (Torrance, CA). Mouse monoclonal caspase-8, polyclonal caspase-9 and PARP antibodies were purchased from Cell Signaling Technology (Danvers, MA). Mouse monoclonal caspase-3 antibody was purchased from Imgenex (San Diego, CA). Rabbit polyclonal Mcl-1 and Bcl-XL and mouse monoclonal Bcl-2 antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). GSK3 antibody was purchased from Upstate/Millipore (Billerica, MA). Both polyclonal and monoclonal actin antibodies were purchased from Sigma Chemical Co.
2.2, Cell lines and cell culture
The human NSCLC cell lines used in this study were either provided by Dr. R. Lotan (M.D. Anderson Cancer Center, Houston, TX) [19] or purchased from the American Type Culture Collection ATCC (Manassas, VA). H157 and A549 cells were recently authenticated by Genetica DNA Laboratories, Inc. (Cincinnati, OH) through analyzing short tandem repeat DNA profile; other cell lines have not been authenticated. H460 cell lines that stably express Bcl-2 were described previously [20]. HCT116-FBXW7+/+ and HCT116-FBXW7−/− cell lines were kindly provided by Dr. B. Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MA). These cell lines were grown in monolayer culture in RPMI 1640 medium or McCoy’s medium supplemented with glutamine and 5% fetal bovine serum (FBS) at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
2.3, Lentiviral Mcl-1 expression
Full length Mcl-1 cDNA was amplified by PCR from pcDNA-Mcl-1 plasmid and sub-cloned into the lentiviral expression vector, pFUW [21] to generate pFUW-Mcl-1. Lentiviruses carrying pFUW-Mcl-1 or pFUW were produced by co-transfecting the HIV-1 packaging vector Delta 8.9 and the VSV-G envelope glycoprotein into 293T cells. After 48 h, the supernatant was harvested, filtered, and polybrene was added for the further viral infection of cancer cells. To establish stable lines that express ectoptic Mcl-1, the given cell lines were infected for 48 h followed with exposure to zeocin (500 µg/ml) for 7 days. The surviving cells were pooled as the stable cell lines used in the study. Mcl-1 expression was verified with Western blotting.
2.4, Cell survival and apoptosis assays
Cells were seeded in 96-well cell culture plates and treated the next day with the given agents. Viable cell numbers were determined using sulforhodamine B (SRB) assay as described previously [19]. Combination index (CI) for drug interaction (e.g., synergy) was calculated using the CompuSyn software (ComboSyn, Inc.; Paramus, NJ). Apoptosis was evaluated with an annexin V-PE apoptosis detection kit (BD Biosciences; San Jose, CA) or with a Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's instructions. The percent positive cells in the upper right and lower right quadrants in the annexin V assay represent the total apoptotic cell population. We also detected caspases and PARP cleavage by Western blot analysis as described below as additional indicators of apoptosis.
2.5, Western Blot Analysis
Preparation of whole-cell protein lysates and Western blot analysis were described previously [22; 23].
2.6, Detection of Mcl-1 ubiquitination
The H1299/Mcl-1 stable line was transfected with HA-ubiquitin plasmid using X-tremeGENE 9 DNA transfection reagent (Roche, Indianapolis, IN) based on the manufacturer’s instructions. After 24 h, the cells were treated with BKM120 or BKM120 plus MG132 for 6 h. Cells were collected and lysed for immunoprecipitation of Mcl-1 using Mcl-1 antibody (Santa Cruz Biotechnology, Inc.) followed by detection of ubiquitinated Mcl-1 with Western blot analysis using anti-HA antibody (Abgent, San Diego, CA).
2.7, siRNA and transfection
GSK3α/β siRNA (#6301) was purchased from Cell Signaling Technology. FBXW7 siRNA that targets the sequence of 5’-AACACAAAGCTGGTGTGTGCA-3’ [24] was synthesized from Qiagen (Valencia, CA). siRNA transfection was performed with HiPerFect transfection reagent (Qiagen) following the manufacturer’s instructions.
3. Results
3.2, BKM120 induces apoptosis in human NSCLC cells
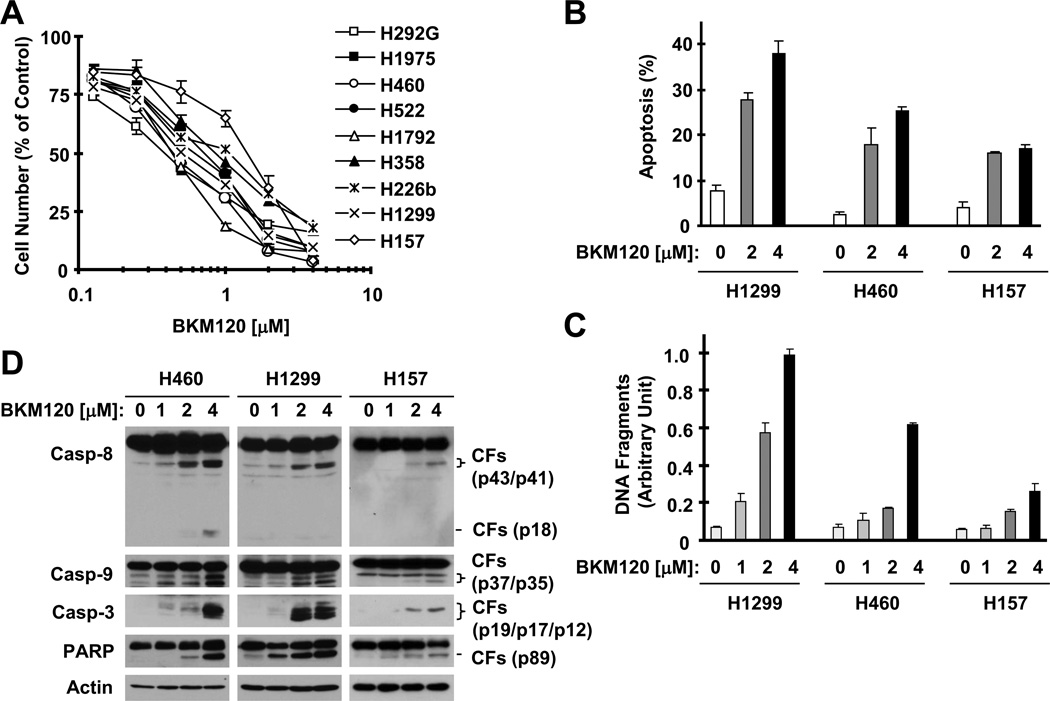
We conducted a 3-day cell survival assay to test the effects of BKM120 on the growth of NSCLC cells. All NSCLC cell lines tested were sensitive to BKM120 (Fig. 1A) with IC50s ranging from 0.4 to 2 µM. In apoptotic assays, we found that BKM120 at 1 µM or higher concentrations (up to 4 µM) clearly increased the population of annexin V-positive cells (Fig. 1B), DNA fragments (Fig. 1C) and the cleavage of caspase-8, caspase-9, caspase-3 and PARP (Fig. 1D) in all of the tested cell lines (H1299, H460 and H157) even after a 24 h incubation, indicating that BKM120 induces apoptosis in NSCLC cells.
Fig. 1. BKM120 effectively decreases the survival ( A) and induces apoptosis (B–D) of human NSCLC cells.
A, A panel of human NSCLC cell lines as indicated were seeded in 96-well plates and then treated with different concentrations of BKM120 ranging from 0.125 to 4 µM as indicated on the second day. After 3 days, cell numbers were estimated using SRB assay. Points, means of triplicate determinations; bars ± SD. B–D, The given cell lines were treated with the indicated concentrations of BKM120 for 24 h and then harvested for analysis of annexin V-positive cells with flow cytometry (B), for measurement of histone-associated DNA-fragments with a cell death ELISA kit (C) and for detection of caspase cleavage with Western blotting (D). Columns, means of duplicate (D) or triplicate (E) determinations; bars ± SD. Pro-casp, pro-caspase; CF, cleaved form.
3.2, BKM120 synergizes with TRAIL to induce apoptosis
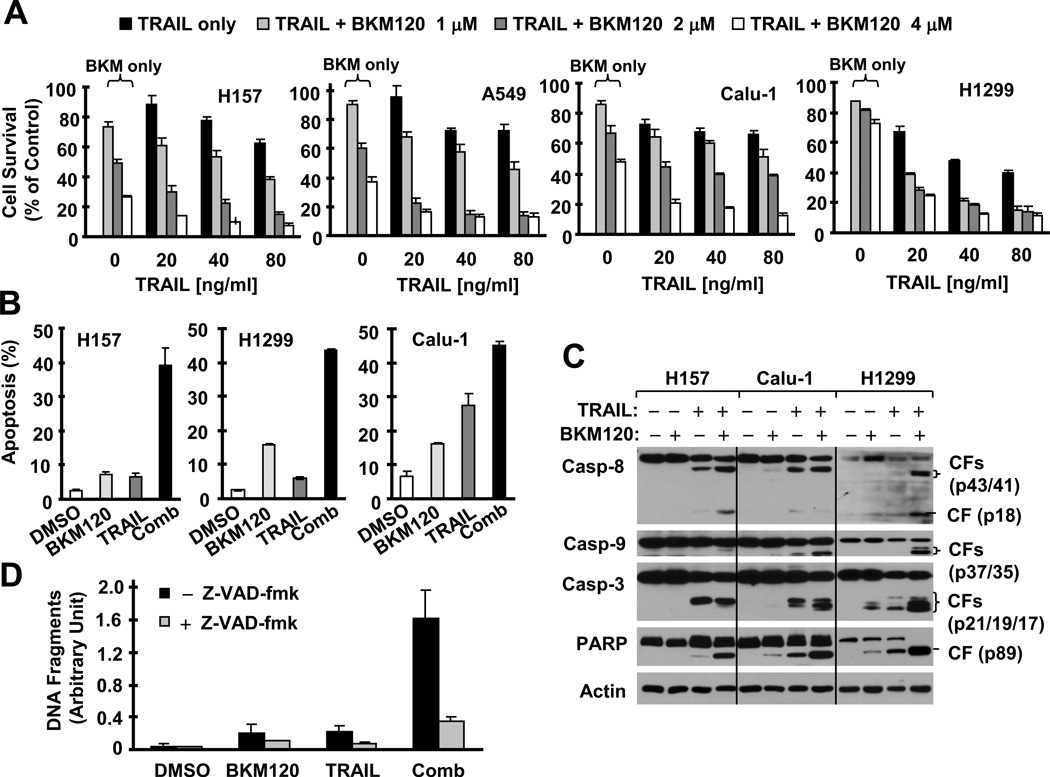
We next determined whether BKM120 combined with TRAIL exerts enhanced apoptosis-inducing effects in NSCLC cells. Under the tested concentration ranges, BKM120 (1–4 µM) alone or TRAIL (20–80 ng/ml) alone in part decreased the survival of the tested cell lines; however their combination exhibited much greater potency than either agent alone in decreasing cell survival (Fig. 2A). The CIs for the different combinations were far lower than 1 in every tested cell line (supplemental Fig. S1), indicating that the combination of BKM120 and TRAIL exerts synergistic effects on decreasing the survival of NSCLC cells. In agreement, the combination of BKM120 and TRAIL was also much more effective than either agent alone in increasing the population of annexin V-positive cells (Fig. 2B) and cleavage of caspases (including caspase-8, caspase-9 and caspase-3) and PARP (Fig. 2C). For example, in H1299 cells, the BKM120 and TRAIL combination caused about 45% apoptosis, whereas BKM120 and TRAIL alone induced 16% and 6% apoptosis, respectively (Fig. 2B). Thus, it is clear that the combination of BKM120 and TRAIL synergistically induces apoptosis of NSCLC cells. In the presence of the pan caspase inhibitor Z-VAD-fmk, the ability of the BKM120 and TRAIL combination to enhance apoptosis was substantially inhibited (Fig. 2D), indicating that the combination of BKM120 and TRAIL induces caspase-dependent apoptosis.
Fig. 2. BKM120 combined with TRAIL synergistically decreases the survival (A) and induces caspase-dependent apoptosis (B–D) of human NSCLC cells.
A, The indicated cell lines were seeded in 96-well plates and treated the next day with different concentrations of TRAIL alone, BKM120 alone and their respective combinations as indicated. After 24 h, cell numbers were estimated using the SRB assay. Columns, means of four replicate determinations; bars, ± SD. B and C, The given cell lines were treated with 2 µM BKM120, 20 ng/ml TRAIL or their combination for 24 h and then harvested for detection of annexin V-positive cells with flow cytometry (B) and caspase cleavage with Western blotting (C). Columns, means of duplicate determinations; bars, ± SD. Pro-casp, pro-caspase; CF, cleaved form. D, H1299 cells were pre-treated with 20 µM Z-VAD-fmk for 30 min and then co-treated with DMSO, 2 µM BKM120, 20 ng/ml TRAIL or the combination of BKM120 and TRAIL. After 16 h, the cells were assayed for histone-associated DNA fragments with a cell death ELISA kit. Columns, means of triplicate determinations; bars, ± SD. Comb, combination.
3.3, BKM120 reduces the levels of Bcl-2 family members, Mcl-1, Bcl-2 and Bcl-XL, and blocks TRAIL-induced Mcl-1 upregulation
To understand the possible mechanisms by which BKM120 induces apoptosis and enhances TRAIL-induced apoptosis, we asked whether BKM120 altered the levels of proteins known to be involved in regulation of the extrinsic or intrinsic apoptotic pathway (i.e., death receptors, c-FLIP, Bcl-2 family members and survivin). To this end, we treated a few of NSCLC cell lines with different concentrations of BKM120 for 16 h and then detected these proteins. In all tested cell lines, the levels of DR5, DR4, Bid, Bim, Bad and Bax were not increased. Interestingly, we found that DR4 levels were even decreased in H1299 and H460 cells. The levels of FLIPS were slightly decreased in H157 cells, whereas FLIPL levels were not reduced in any of the tested cell lines. Survivin levels were reduced in H460 cells, but not in H157, Calu-1 or H1299 cells (Fig. S2). Thus, we believe that these proteins (DR5, DR4, Bid, Bim, Bad, Bax, FLIPL, and survivin) are unlikely to be important in mediating apoptosis induced by BKM120 or BKM120 plus TRAIL.
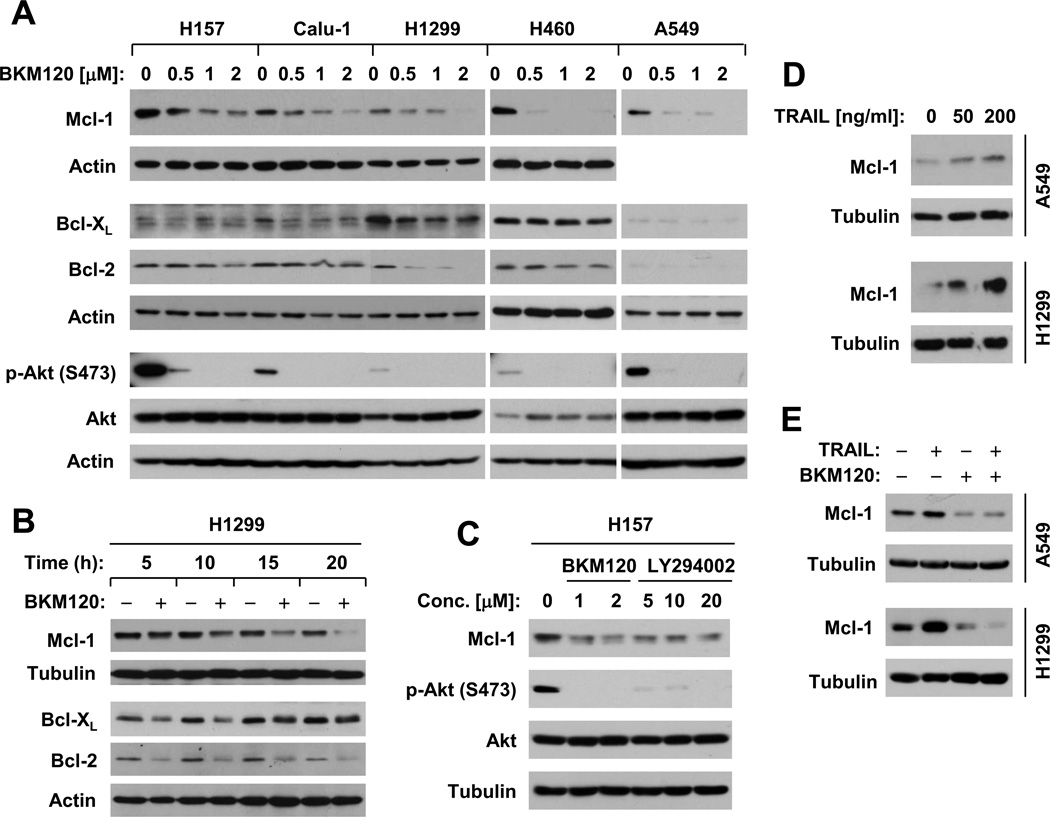
In contrast, we found that the levels of Bcl-2 and particularly Mcl-1 were reduced by BKM120 in a dose-dependent manner in most tested cell line. Bcl-XL levels were reduced in some of the tested cell lines (e.g, Calu-1 and H1299; Fig. 3A). By analyzing time-dependent effects of BKM120 on expression of these proteins in H1299 cells, we found that BKM120 reduced the levels of these proteins early at 5 h post treatment; these effects were sustained up to 20 h. We noted that the reduction of both Mcl-1 and Bcl-2 was maintained or enhanced as the incubation time increased, whereas the reduced levels of Bcl-XL were time-dependently recovered starting at 15 h post treatment (Fig. 3B). Together, these data imply that downregulation of these proteins, particularly Mcl-1, is likely critical for BKM120 to induce apoptosis and enhance TRAIL-induced apoptosis.
Fig. 3. BKM120 decreases the levels of Mcl-1, Bcl-2, and Bcl-XL in dose- (A) and time- (B) dependent fashions along with suppression of Akt phosphorylation (A and C), whereas TRAIL increases Mcl-1 expression (D), which can be abolished by the presence of BKM120 (E).
A, The given cell lines were treated with the indicated concentrations of BKM120 for 12 h. B, H1299 cells were treated with 2 µM BKM120 for different times as indicated. C, H157 cells were treated with the indicated concentrations of BKM120 or LY294002 for 8 h. D, The given cell lines were treated with the indicated concentrations of TRAIL for 6 h. E, The given cell lines were treated with 2 µM BKM120 alone, 50 ng/ml TRAIL alone and BKM120 plus TRAIL for 6 h. After these treatments, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis to detect the indicated proteins.
We found that BKM120, at the tested concentration ranges, effectively reduced the levels of p-Akt (Fig. 3A). Moreover, we compared the effects of BKM120 with LY294002, another well known pan-class I PI3K inhibitor, on Mcl-1 expression and found that LY294002 also decreased the levels of Mcl-1 as BKM120 did (Fig. 3C).
TRAIL has been shown to upregulate Mcl-1 expression, which is associated with TRAIL resistance [25; 26; 27; 28]. In our cell systems, TRAIL also increased Mcl-1 levels (Fig. 3D). In the presence of BKM120, TRAIL-induced Mcl-1 elevation was blocked (Fig. 3E). These results further support the notion that downregulation of Mcl-1 is a critical mechanism by which BKM120 enhances TRAIL-induced apoptosis. Therefore, we concentrated our study on Mcl-1 as well as Bcl-2 in the following experiments.
3.4, Enforced expression of Mcl-1 or Bcl-2 attenuates the ability of BKM120 to induce apoptosis
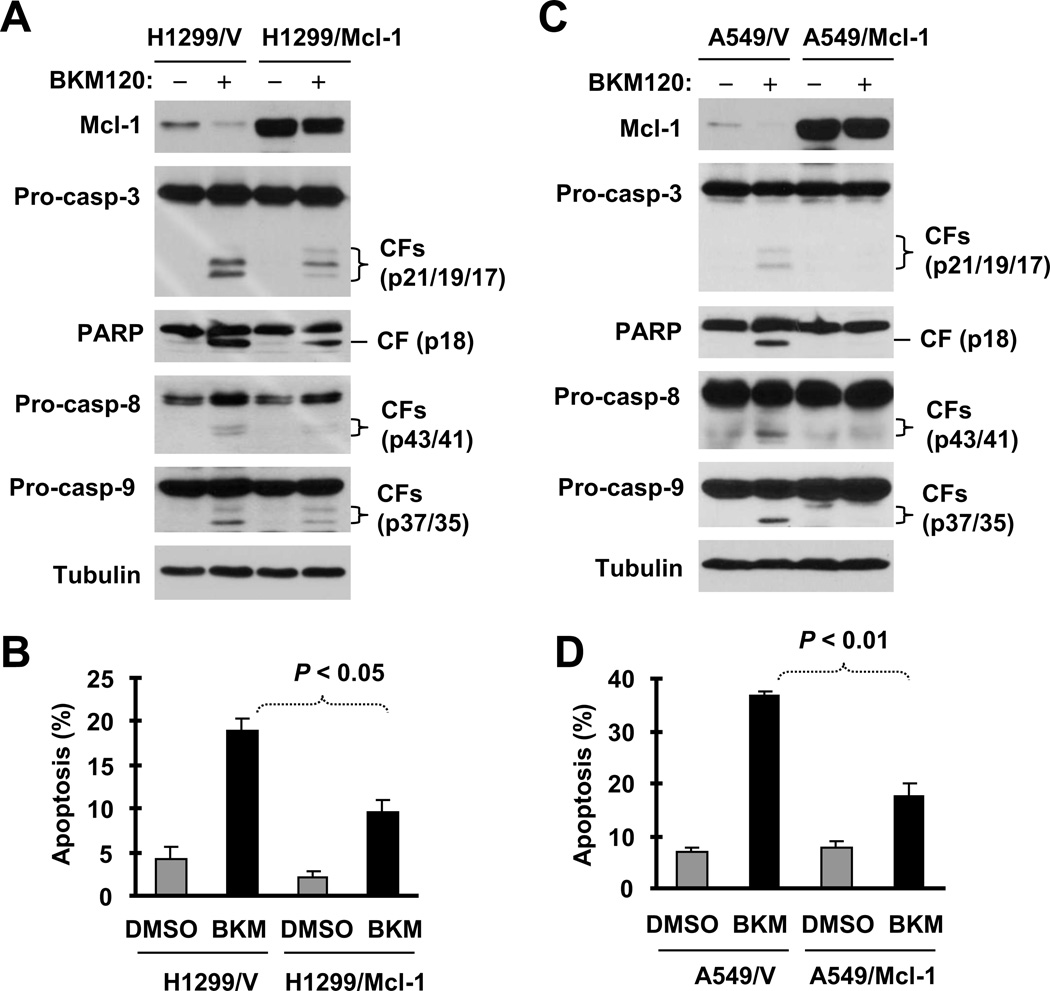
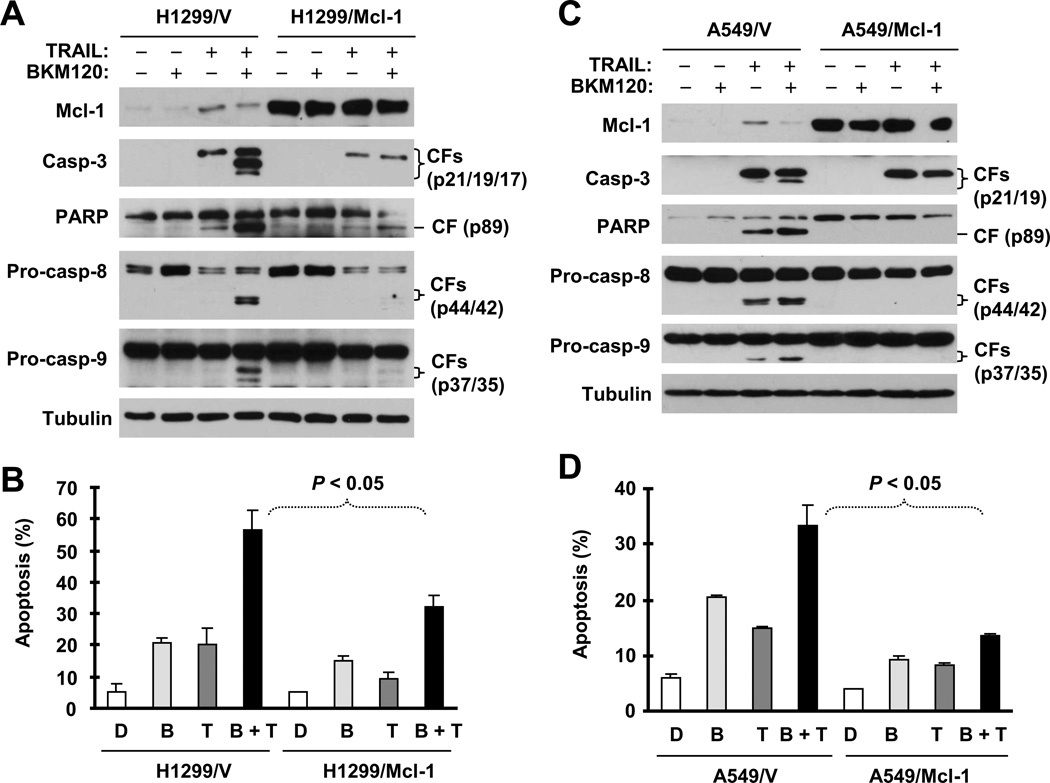
We then asked whether downregulation of Mcl-1 or Bcl-2 is indeed critical for induction of apoptosis by BKM120 or BKM120 plus TRAIL. To address this question, we determined whether enforced ectopic expression of these proteins protects cells from induction of apoptosis by BKM120 or BKM120 and TRAIL combination. We detected reduced levels of cleaved caspase-3 and PARP and fewer annexin V-positive cells in H1299/Mcl-1 cells compared with parental H1299/V cells (Figs. 4A and 4B). Similar results were also generated in A549 cells stably expressing ectopic Mcl-1 (Figs. 4C and 4D). This phenomenon was also observed in cells stably expressing Bcl-2 (Fig. S3). For example, we detected much lower levels of the cleaved forms of caspase-3 and PARP and fewer annexin V-positive cells in both H460 transfectants with ectopic Bcl-2 expression (Bcl-2#6 and Bcl-2#8) than in H460 control cells (Figs. S3A and S3B). Thus, it is clear that enforced expression of ectopic Mcl-1 or Bcl-2 attenuates the ability of BKM120 to induce apoptosis.
Fig. 4. Enforced expression of ectopic Mcl-1 in H1299 (A and B) or A549 cells (C and D) protects cells from BKM120-induced apoptosis.
The indicated cell lines were exposed to 4 µM BKM120 for 24 h and then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis to detect caspase cleavage (A and C) and for detection of apoptosis (i.e., annexin V-positive cells) with flow cytometry (B and D). Pro-casp, pro-caspase; CF, cleaved from.
3.5, Enforced expression of Mcl-1 or Bcl-2 confers resistance to induction of apoptosis by BKM120 and TRAIL combination
If the downregulation of Mcl-1 and Bcl-2 is important for BKM120 to enhance TRAIL-induced apoptosis, we anticipated that enforced expression of ectopic Mcl-1 or Bcl-2 would also protect cells from induction of apoptosis by BKM120 plus TRAIL. Indeed, the combination of BKM120 and TRAIL was much more potent than either single agent in inducing cleavage of caspase-9, caspase-3 and PARP and in increasing annexin-V cell numbers in different control cells (e.g., H1299 and H460); however, these enhanced effects were either abolished or substantially reduced in cells expressing ectopic Mcl-1 (Fig. 5) or Bcl-2 (Fig. S4). Thus, it is clear that enforced expression of these proteins confers resistance of cancer cells to augmented induction of apoptosis by BKM120 and TRAIL.
Fig. 5. Enforced expression of ectopic Mcl-1 in H1299 (A and B) or in A549 cells (C and D) protects cells from apoptosis induced by the BKM120 and TRAIL combination.
The indicated stable transfectants were treated with 2 µM BKM120 alone, 20 ng/ml TRAIL alone or their combination. After 8 h (A and C) or 24 h (B and D), the cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis to detect caspase cleavage (A and C) and for detection of apoptosis (i.e., annexin V-positive cells) with flow cytometry (B and D). Pro-casp, pro-caspase; CF, cleaved from.
In H460 cells that stably express Bcl-2 (Bcl-2#6 and Bcl-2#8), augmented cleavage of caspase-8 induced by the combination of BKM120 and TRAIL was still detected to the same degree as observed in H460 vector control cells (Fig. S4). In H1299 or A549 cells that stably express Mcl-1, this augmented cleavage of caspase-8 was abolished or reduced in comparison with that in their corresponding control cells (Figs. 5A and 5C). Thus, Mcl-1 and bcl-2 have different effects on augmented caspase-8 activation induced by the BKM120 and TRAIL combination.
3.6, BKM120 reduces Mcl-1 levels through facilitating its degradation in part via a GSK3-dependent and FBXW7-mediated mechanism
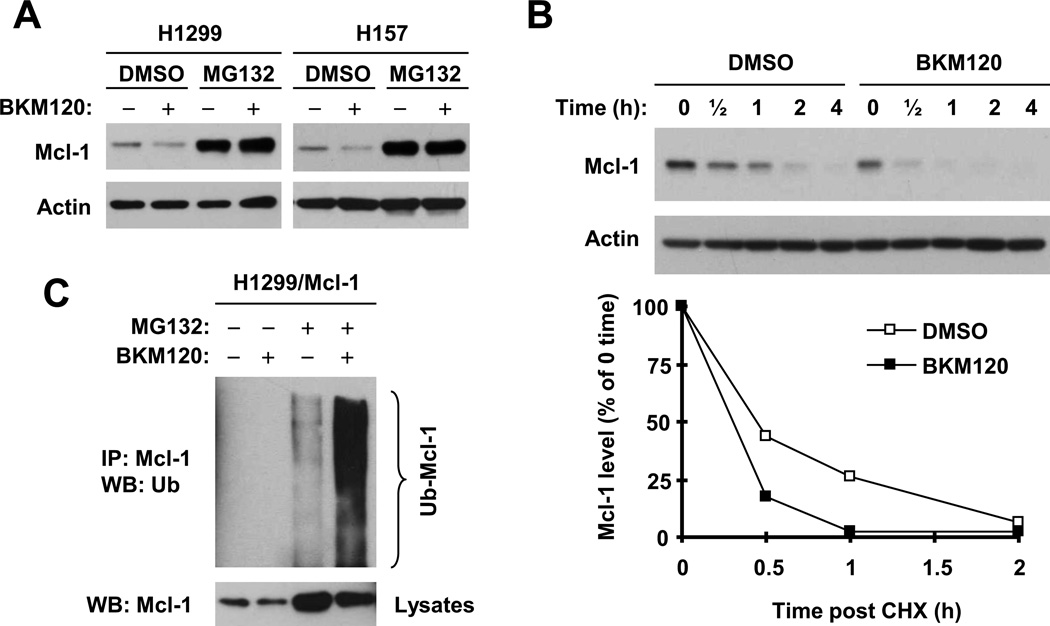
Finally, we determined the mechanisms by which BKM120 reduces the levels of Mcl-1, a protein that is substantially downregulated by BKM120 across the tested cell lines. It has been recently demonstrated that Mcl-1 levels are regulated by a GSK3/FBXW7-dependent degradation mechanism [29; 30]. Given that Akt inactivates GSK3 through phosphorylation, we wondered whether BKM120, as a PI3K inhibitor, reduces Mcl-1 levels through promoting its degradation by activating GSK3 via suppression of Akt. To test this hypothesis, we compared the effects of BKM120 on Mcl-1 levels in the absence and presence of the proteasome inhibitor MG132. As presented in Fig. 6A, the presence of MG132 not only elevated basal levels of Mcl-1, but also prevented Mcl-1 reduction induced by BKM120 in both H1299 and H157 cells. Moreover, we compared the stabilities of Mcl-1 between cells exposed to DMSO or BKM120. The half-life of Mc1-1 in BKM120-treated cells (~ 20 min) was shorter than that (~ 30 min) in DMSO-treated cells (Fig. 6B), indicating that BKM120 enhances Mcl-1 degradation. By detecting Mcl-1 ubiquitination with IP-Western blotting, we found that BKM120 also increased the levels of ubiquitinated Mcl-1 (Fig. 6C; lane 4 vs. lane 3), further supporting the notion that BKM120 enhances proteasome-mediated Mcl-1 degradation.
Fig. 6. BKM120 induces proteasome-mediated Mcl-1 degradation (A), decreases Mcl-1 stability (B) and increases Mcl-1 ubiquitination (C).
A, Both H157 and H1299 cells were pre-treated with 20 µM MG132 for 30 minutes prior to the addition of 2 µM BKM120. After co-treatment for an additional 6 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. B, H1299 cells were treated with 2 µM BKM120 for 6 h. The cells were then washed with PBS 3 times and refed with fresh medium containing 10 µg/ml cycloheximide (CHX). At the indicated times, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. Protein levels were quantified with NIH Image J software (Bethesda, MA) and were normalized to actin. The results were plotted as the relative Mcl-1 levels compared to those at the time 0 of CHX treatment (bottom panel). C, H1299/Mcl-1 cells were transfected with HA-ubiquitin expression plasmid using X-tremeGENE 9 DNA transfection reagent, After 24 h, the cells were pre-treated with 20 µM MG132 for 30 min and then co-treated with 2 µM BKM120 for another 4 h. Whole-cell protein lysates were then prepared for IP using anti-Mcl-1 antibody followed by Western blotting (WB) using anti-HA antibody for detection of ubiquitinated Mcl-1 (Ub-Mcl-1).
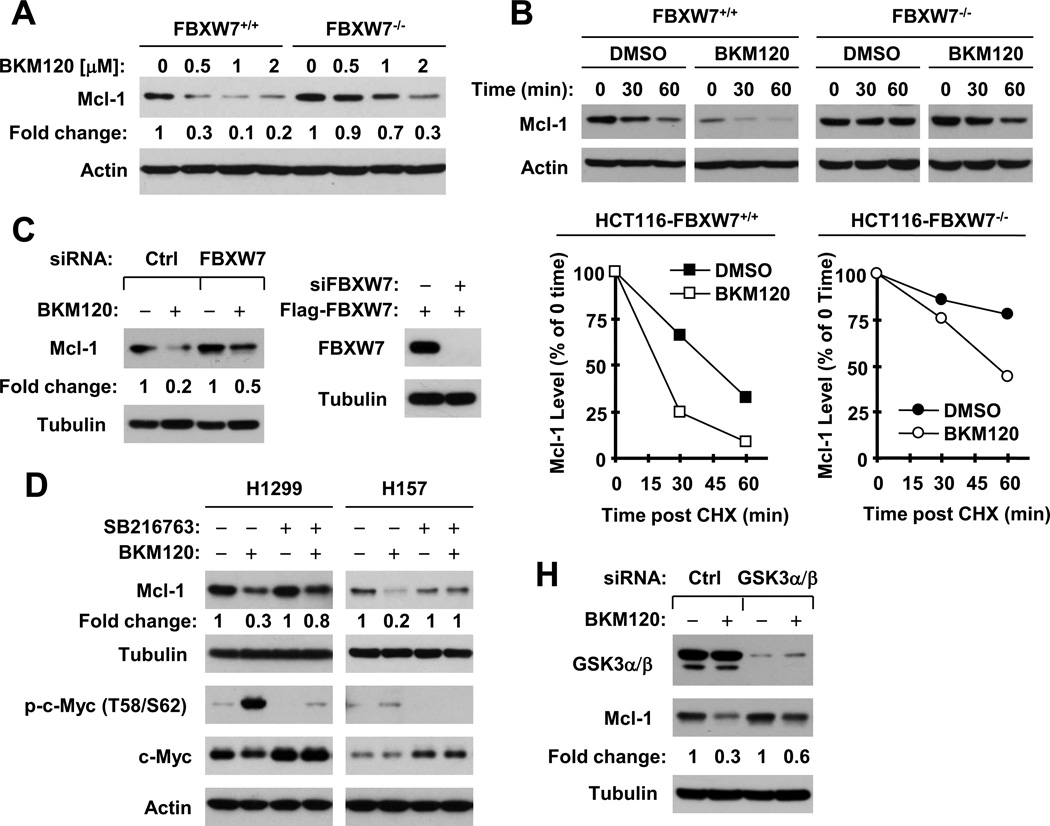
We next asked whether FBXW7 is involved in mediating BKM120-induced Mcl-1 degradation. To this end, we compared the effects of BKM120 on the reduction of Mcl-1 between cell lines with (FBXW7+/+) and without (FBXW7−/−) the FBXW7 gene. As shown in Fig. 7A, BKM120 dose-dependently reduced Mcl-1 levels in both cell lines. However, BKM120 exhibited a weaker effect on decreasing Mcl-1 in FBXW7−/− cells than in FBXW7+/+ cells, indicating that the knockout or deficiency of FBXW7 attenuates the effect of BKM120 on reduction of Mcl-1. Moreover, we compared the stabilities of Mcl-1 between these cell lines in the absence and presence of BKM120. Mcl-1 had a much longer half-life in FBXW7−/− cells than in FBXW7+/+ cells (> 60 min vs. ~ 45 min, respectively), indicating that FBXW7 indeed regulates Mcl-1 degradation. Upon treatment with BKM120, the half-life of Mcl-1 was shortened in both cell lines albeit with varied degrees: ~ 20 min in FBXW7+/+ cells and ~ 50 min in FBXW7−/− cells (Fig. 7B). These data indicate that deficiency of FBXW7 indeed delays Mcl-1 degradation induced by BKM120. Besides, we knocked down FBXW7 with FBXW7 siRNA in H1299 cells and examined its impact on BKM120-induced Mcl-1 reduction. We found that knockdown of FBXW7 greatly impaired the ability of BKM120 to decrease Mcl-1 levels (Fig. 7C, left panel). Due to lack of a good antibody for detection of endogenous FBXW7, we tested the knockdown efficiency of the FBXW7 siRNA by co-transfection of a Flag-FBXW7 as others did [31] and found that this FBXW7 siRNA effectively silenced the expression of ectopic FBXW7 (Fig. 7C, right panel). Taking all data together, it is clear that BKM120 induces FBXW7-dependent Mcl-1 degradation.
Fig. 7. Deficiency (A and B) or knockdown (C) of FBXW7 or inhibition of GSK3 (D and E) impairs the ability of BKM120 to induce Mcl-1 degradation.
A, The given cell lines were treated with different concentrations of BKM120 as indicated for 12 h and then harvested for Western blot analysis. B, The given cell lines were treated with 2 µM BKM120 for 6 h. The cells were then washed with PBS 3 times and refed with fresh medium containing 10 µg/ml cycloheximide (CHX). At the indicated times, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. C, H1299 cells were transfected with control (Ctrl) or FBXW7 siRNA (siFBXW7) for 48 h and then exposed to DMSO or 2 µM BKM120 for additional 8 h. Moreover, H1299 cells were further transfected for 48 h with Flag-FBXW7β plasmid 24 h after transfection of control or FBXW7 siRNA (right panel). D, The indicated cell lines were pretreated with DMSO or 20 µM SB216763 for 30 min and then co-treated with 2 µM BKM120 for an additional 10 h. E, H1299 cells were transfected with control (Ctrl) or GSK3 siRNA for 48 h and then exposed to DMSO or 2 µM BKM120 for additional 8 h. After the aforementioned treatments (C–E), the cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blotting. Protein levels were quantified with NIH Image J software (Bethesda, MA) and were normalized to actin. The results in B were plotted as the relative Mcl-1 levels compared to those at the time 0 of CHX treatment (bottom panel).
If BKM120-induced Mcl-1 degradation is GSK3-dependent, we anticipated that inhibition of GSK3 should block Mcl-1 degradation. Indeed, the presence of the GSK3 inhibitor, SB216763 prevented Mcl-1 from reduction or degradation induced by BKM120 in both H1299 and H157 cells (Fig. 7D). Here we used inhibition of c-Myc phosphorylation and stabilization of c-Myc as indications of GSK3 activity inhibition because GSK3 is known to phosphorylate c-Myc and promote its degradation [32; 33]. In agreement, siRNA-mediated knockdown of GSK3 also substantially rescued Mcl-1 reduction induced by BKM120 (Fig. 7H). Thus, it is likely that BKM120 induces a GSK3-dependent Mcl-1 degradation.
4. Discussion
There are several previous studies showing that inhibition of the PI3K/Akt signaling with either small molecule inhibitors (e.g., LY294002 and PI-103) or siRNA (e.g., p85 or PIK3CA siRNA) sensitizes certain types of cancer cells (e.g., neuroblastoma) to TRAIL-induced apoptosis [10; 11; 13; 15; 34]. In agreement with these studies, the present study further shows that the clinical tested novel PI3K inhibitor, BKM120, induces apoptosis and synergizes with TRAIL to induce apoptosis in NSCLC cells. Our findings provide further support for targeting the PI3K/Akt signaling as an effective strategy to enhance TRAIL-induced apoptosis and also warrant further investigation of the BKM120 and TRAIL combination as a potential cancer therapeutic regimen.
BKM120 clearly induces apoptosis when used at a relatively high concentration (e.g., ≥ 1 µM) as demonstrated in our current study and in other studies [2; 3]. However the underlying mechanisms are unknown. In our study, BKM120 activated both caspase-8 and caspase-9, suggesting that both the extrinsic and intrinsic apoptotic pathways may be activated. However, BKM120 did not increase the expression of either DR5 or DR4, nor reduce the levels of c-FLIP across the tested cell lines; rather it reduced the levels of the anti-apoptotic Bcl-2 family members, Mcl-1, Bcl-2 and Bcl-XL, although it did not alter the levels of pro-apoptotic Bcl-2 family member proteins (e.g., Bax, Bid, Bim and Bad). In another study with neuroblastoma cells, Mcl-1 levels were also reduced by PI-103 [15]. Although inhibition of PI3K with LY294002 or with expression of a dominant-negative p85 mutant downregulated survivin expression in neuroblastoma cell [11], we did not find that BKM120 could reduce survivin levels in our cell systems. Thus, it is likely that downregulation of some anti-apoptotic Bcl-2 family members (e.g., Mcl-1 and Bcl-2) is a critical event that mediates BKM120-induced apoptosis in NSCLC cells. Indeed, enforced expression of ectopic Mcl-1 or Bcl-2 attenuated the ability of BKM120 to induce apoptosis including caspase activation. Thus, it is reasonable to suggest that the downregulation of these anti-apoptotic Bcl-2 family proteins alters the ratios of pro-apoptotic Bcl-2 family members (e.g., Bax, Bak and Bad) over these anti-apoptotic Bcl-2 family proteins, leading to induction of apoptosis. We noted that enforced expression of ectopic Mcl-1 or Bcl-2 prevented or attenuated caspase-8 cleavage or activation induced by BKM120 (Figs. 4 and S3). These data imply that caspase-8 activation caused by BKM120 is secondary to activation of the intrinsic apoptotic pathway. This may be in line with our observation that BKM120 did not increase the expression of DR5 or DR4, nor reduce the levels of c-FLIP (Fig. S2). Given that caspase-8 can be activated by caspase-9 or caspase-3 [35; 36], our finding in this regard is not surprising.
Mcl-1 and/or Bcl-2 have been implicated in the regulation of cancer cell response to TRAIL-induced apoptosis [8; 9]. Accordingly, BH3 mimetics such as ABT-737 enhance TRAIL-induced apoptosis [37; 38; 39; 40; 41]. Although modulation of DR4, DR5 and/or c-FLIP is a common mechanism underlying the enhancement of TRAIL-induced apoptosis [42; 43], it is unlikely to contribute to the BKM120-mediated enhancement of TRAIL-induced apoptosis observed in this study since BKM120 neither increases the expression of DR4 and DR5, nor reduces the levels of c-FLIP (Fig. S2). In this study, enforced expression of ectopic Mcl-1 or Bcl-2 abrogated or attenuated the synergistic induction of apoptosis, including caspase activation, by the BKM120 and TRAIL combination (Figs. 5 and S4). Thus, we conclude that downregulation of these anti-apoptotic Bcl-2 family proteins is also responsible for sensitization of cancer cells to TRAIL-induced apoptosis by BKM120. It is well known that Mcl-1 or Bcl-2 negatively regulates the mitochondrial apoptotic signaling, which can be activated by the death receptor-mediated apoptotic pathway via caspase-8-mediated cleavage of Bid [44]. Thus, downregulation of these Bcl-2 family proteins (e.g., by BKM120) will release their suppressive effects on mitochondrial apoptotic signaling, leading to amplification of TRAIL/death receptor-induced apoptosis.
We noted that enforced expression of Bcl-2 did not affect augmented caspase-8 cleavage or activation while it blocked cleavage of caspase-9, caspase-3 and PARP (Fig. S4). However, enforced expression of Mcl-1 reduced the levels of cleaved caspase-8 induced by the combination of BKM120 and TRAIL (Figs. 5). These data suggest that BKM120 downregulates Mcl-1, thereby reversing the inhibition of intrinsic apoptotic signaling and eventually enhancing apoptosis. During this process, caspase-8 can be further activated by caspase-9/caspase-3, resulting in further amplification of apoptotic signaling. It is also possible that Mcl-1 may have an uncovered role in direct suppression of the extrinsic apoptotic pathway, which deserves a further investigation.
It has been reported that TRAIL increases Mcl-1 levels, which may result in a protective effect on TRAIL-mediated cell killing or the development of TRAIL resistance [25; 26; 27; 28]. Thus, prevention of TRAIL-induced Mcl-1 elevation may also contribute to the enhancement of TRAIL-induced apoptosis [26; 27]. In our cell systems, TRAIL indeed increased Mcl-1 levels. When combined with BKM120, Mcl-1 elevation was prevented (Fig. 3). Thus we believe that prevention of TRAIL-induced Mcl-1 increase by BKM120 may also contribute to BKM120 enhancement of TRAIL-induced apoptosis.
BKM120 effectively inhibited the PI3K/Akt signaling at the tested concentration ranges that decrease Mcl-1 evidenced by reducing p-Akt levels. Another pan-class I PI3K inhibitor LY294002 had a similar effect on reducing Mcl-1 levels (Fig. 3). Given that Mcl-1 is a PI3K–regulated protein [45], it is likely that BKM120-induced downregulation of Mcl-1 is secondary to inhibition of the PI3K/Akt signaling. It has been reported recently that BKM120 at concentrations equal or higher than 2 µM in cells displays off-target or PI3K inhibition-independent activities [46]. Therefore, we cannot exclude other mechanisms contributing to BKM120-induced Mcl-1 downregulation. Further studies in this regard are needed.
Mcl-1 has been recently demonstrated to be regulated by GSK3/FBXW7-mediated protein degradation [29; 30]. As a PI3K inhibitor, it is plausible to speculate that BKM120 downregulates Mcl-1 levels through enhancing GSK3/FBXW7-dependent Mcl-1 degradation by activating GSK3. In our study, BKM120 decreased Mcl-1 stability and increased Mcl-1 ubiquitination. BKM120-induced Mcl-1 reduction could be prevented by co-treatment with a proteasome inhibitor (Fig. 6). Knockout or knockdown of FBXW7 substantially attenuated the ability of BKM120 to decrease Mcl-1 levels, including decreasing Mcl-1 stability (Fig. 7). Moreover, inhibition of GSK3 with both small molecule GSK3 inhibitors and GSK3 siRNA in part prevented Mcl-1 from reduction induced by BKM120 (Fig. 7). Hence, it is convincingly concluded that a GSK3-dependent and FBXW7-mediated degradation mechanism accounts for BKM120-induced proteasomal degradation of Mcl-1. In this study, we found that knockout or knockdown of FBXW7 attenuated, but did not abolish, the ability of BKM120 to reduce Mcl-1 levels. So did the GSK3 inhibition (Fig. 7). Thus we suggest that additional degradation mechanism(s) may be involved in BKM120-induced Mcl-1 degradation.
In this study, we have not demonstrated the mechanisms by which BKM120 decreases the levels of Bcl-2 and Bcl-XL. BKM120 clearly exerted more striking suppressive effect on Mcl-1 than on Bcl-2 and Bcl-XL (Fig. 3). Hence it is unlikely that BKM120 reduces the levels of Bcl-2 and Bcl-XL through the same mechanism as it does on induction of Mcl-1 degradation. Nonetheless it is important to conduct further study in the future to understate the mechanisms by which BKM120 reduces the levels of Bcl-2 and Bcl-XL.
In summary, the current study has demonstrated that BKM120 induces apoptosis and enhances TRAIL-induced apoptosis in NSCLC cells. Downregulation of Mcl-1, Bcl-2 and Bcl-XL by BKM120 is likely to account for these processes. Moreover, the reduction of Mcl-1 by BKM120 is largely due to activation of a GSK3/FBXW7-dependent mechanism. Our findings warrant further investigation of the types of cancers in vivo. therapeutic potential of the BKM120 and TRAIL combination against NSCLC and other types of cancers in vivo.
Supplementary Material
Acknowledgements
We thank Dr. A. Hammond in our department for editing the manuscript and Dr. B. Vogelstein for providing FBXW7-deficient cells. This study is supported by the Georgia Cancer Coalition Distinguished Cancer Scholar award and NIH R01 CA118450 (S–Y Sun), R01 CA160522 (S–Y Sun) and P01 CA116676 (Project 1 to FR Khuri and S-Y Sun). TK Owonikoko, FR Khuri and S-Y Sun are Georgia Cancer Coalition Distinguished Cancer Scholars.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chene P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, Garcia-Echeverria C, Sellers WR, Voliva CF. Identification and Characterization of NVP-BKM120, an Orally Available Pan-Class I PI3-Kinase Inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Yang J, Qian J, Zhang L, Lu Y, Li H, Lin H, Lan Y, Liu Z, He J, Hong S, Thomas S, Shah J, Baladandayuthapani V, Kwak LW, Yi Q. Novel phosphatidylinositol 3-kinase inhibitor NVP-BKM120 induces apoptosis in myeloma cells and shows synergistic anti-myeloma activity with dexamethasone. J Mol Med (Berl) 2011 doi: 10.1007/s00109-011-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koul D, Fu J, Shen R, LaFortune TA, Wang S, Tiao N, Kim YW, Liu JL, Ramnarian D, Yuan Y, Garcia-Echevrria C, Maira SM, Yung WK. Antitumor activity of NVP-BKM120--a selective pan class I PI3 kinase inhibitor showed differential forms of cell death based on p53 status of glioma cells. Clin Cancer Res. 2012;18:184–195. doi: 10.1158/1078-0432.CCR-11-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, Goldbrunner M, Baselga J. Phase I, Dose-Escalation Study of BKM120, an Oral Pan-Class I PI3K Inhibitor, in Patients With Advanced Solid Tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 5.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 9.Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin Ther Targets. 2010;14:1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 10.Opel D, Westhoff MA, Bender A, Braun V, Debatin KM, Fulda S. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Kang J, Qiao J, Thomas RP, Evers BM, Chung DH. Phosphatidylinositol 3-kinase inhibition down-regulates survivin and facilitates TRAIL-mediated apoptosis in neuroblastomas. J Pediatr Surg. 2004;39:516–521. doi: 10.1016/j.jpedsurg.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K, Valony G, Eychene A, Auberger P, Ortonne JP, Ballotti R, Bertolotto C. PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death Differ. 2004;11:1084–1091. doi: 10.1038/sj.cdd.4401475. [DOI] [PubMed] [Google Scholar]
- 13.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL) Surgery. 2005;138:391–397. doi: 10.1016/j.surg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Oka N, Tanimoto S, Taue R, Nakatsuji H, Kishimoto T, Izaki H, Fukumori T, Takahashi M, Nishitani M, Kanayama HO. Role of phosphatidylinositol-3 kinase/Akt pathway in bladder cancer cell apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2006;97:1093–1098. doi: 10.1111/j.1349-7006.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opel D, Naumann I, Schneider M, Bertele D, Debatin KM, Fulda S. Targeting aberrant PI3K/Akt activation by PI103 restores sensitivity to TRAIL-induced apoptosis in neuroblastoma. Clin Cancer Res. 2011;17:3233–3247. doi: 10.1158/1078-0432.CCR-10-2530. [DOI] [PubMed] [Google Scholar]
- 16.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 17.Shirley S, Micheau O. Targeting c-FLIP in cancer. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, Heyman RA, Teng M, Chandraratna RA, Shudo K, Hong WK, Lotan R. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–4939. [PubMed] [Google Scholar]
- 20.Sun SY, Yue P, Zhou JY, Wang Y, Choi Kim HR, Lotan R, Wu GS. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun. 2001;280:788–797. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 21.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 23.Sun SY, Yue P, Wu GS, El-Deiry WS, Shroot B, Hong WK, Lotan R. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene. 1999;18:2357–2365. doi: 10.1038/sj.onc.1202543. [DOI] [PubMed] [Google Scholar]
- 24.Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, Brown K, Bryson S, Balmain A. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–779. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Chen W, Zeng W, Bai L, Tesfaigzi Y, Belinsky SA, Lin Y. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther. 2008;7:1156–1163. doi: 10.1158/1535-7163.MCT-07-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquemin G, Granci V, Gallouet AS, Lalaoui N, Morle A, Iessi E, Morizot A, Garrido C, Guillaudeux T, Micheau O. Quercetin-mediated Mcl-1 and survivin downregulation restores TRAIL-induced apoptosis in non-Hodgkin&s lymphoma B cells. Haematologica. 2012;97:38–46. doi: 10.3324/haematol.2011.046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Son JK, Varadarajan S, Bratton SB. TRAIL-activated stress kinases suppress apoptosis through transcriptional upregulation of MCL-1. Cell Death Differ. 2010;17:1288–1301. doi: 10.1038/cdd.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, Xiao Y, Christie AL, Aster J, Settleman J, Gygi SP, Kung AL, Look T, Nakayama KI, DePinho RA, Wei W. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O’Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ, Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC, Dixit VM. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 31.Kanei-Ishii C, Nomura T, Takagi T, Watanabe N, Nakayama KI, Ishii S. Fbxw7 acts as an E3 ubiquitin ligase that targets c-Myb for nemo-like kinase (NLK)-induced degradation. J Biol Chem. 2008;283:30540–30548. doi: 10.1074/jbc.M804340200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 33.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101:9085–9090. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 35.Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira KS, Kreutz C, Macnelly S, Neubert K, Haber A, Bogyo M, Timmer J, Borner C. Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas signaling in primary mouse hepatocytes. Apoptosis. 2012;17:503–515. doi: 10.1007/s10495-011-0691-0. [DOI] [PubMed] [Google Scholar]
- 37.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther. 2008;7:2339–2347. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeow WS, Baras A, Chua A, Nguyen DM, Sehgal SS, Schrump DS. Gossypol, a phytochemical with BH3-mimetic property, sensitizes cultured thoracic cancer cells to Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg. 2006;132:1356–1362. doi: 10.1016/j.jtcvs.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 41.Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis. 2005;10:1411–1418. doi: 10.1007/s10495-005-2490-y. [DOI] [PubMed] [Google Scholar]
- 42.Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther. 2007;7:163–173. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- 43.Sun SY. Chemopreventive agent-induced modulation of death receptors. Apoptosis. 2005;10:1203–1210. doi: 10.1007/s10495-005-2274-4. [DOI] [PubMed] [Google Scholar]
- 44.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 45.Chan G, Nogalski MT, Bentz GL, Smith MS, Parmater A, Yurochko AD. PI3K–dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J Immunol. 2010;184:3213–3222. doi: 10.4049/jimmunol.0903025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJ, Kazic-Legueux M, Laborde L, Hattenberger M, Stauffer F, Vaxelaire J, Romanet V, Henry C, Murakami M, Guthy DA, Sterker D, Bergling S, Wilson C, Brummendorf T, Fritsch C, Garcia-Echeverria C, Sellers WR, Hofmann F, Maira SM. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Mol Cancer Ther. 2012;11:1747–1757. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.