Abstract
Many tumor cells escape anti-tumor immunity through their expression of Programmed Death Ligand 1 (PDL1 or B7-H1), which interacts with T cell-expressed PD1 and results in T cell apoptosis. We previously reported that transfection of human tumor cells with a membrane-bound form of the human costimulatory molecule CD80 prevented PD1 binding and restored T cell activation. We now report that a membrane-bound form of murine CD80 similarly reduces PDL1-PD1-mediated suppression by mouse tumor cells, and that a soluble protein consisting of the extracellular domains of human or mouse CD80 fused to the Fc domain of IgG1 (CD80-Fc) overcomes PDL1-mediated suppression by human and mouse tumor cells, respectively. T cell activation experiments with human and mouse tumor cells indicate that CD80-Fc facilitates T cell activation by binding to PDL1 to inhibit PDL1-PD1 interactions and by costimulating through CD28. CD80-Fc is more effective in preventing PD1-PDL1-mediated suppression and restoring T cell activation compared to treatment with mAb to either PD1 or PDL1. These studies identify CD80-Fc as an alternative and potentially more efficacious therapeutic agent for overcoming PDL1-induced immune suppression and facilitating tumor-specific immunity.
Keywords: Tumor immunity, T cell activation, T cells
Introduction
Tumor-induced immune suppression is a major obstacle for therapies aimed at activating an individual’s immune system to eliminate autologous cancer cells. Various mechanisms contribute to this immune suppression. A major contributor is the co-inhibitory molecule Programmed Death Ligand 1 (PDL1; also known as B7 homolog 1 (B7-H1) or CD274). PDL1 obstructs anti-tumor immunity by (i) tolerizing tumor-reactive T cells by binding to its receptor PD1 (CD279) on activated T cells (1, 2); (ii) rendering tumor cells resistant to CD8+ T cell and FasL-mediated lysis by PD1 signaling through tumor cell-expressed PDL1 (3); (iii) tolerizing T cells by reverse signaling through T cell-expressed CD80 (4, 5); and (iv) promoting the development and maintenance of induced T regulatory cells (6).
Most malignant cells constitutively express or are induced by IFNγ to express PDL1 (1, 3, 7, 8), and the loss of tumor suppressor genes can increase tumor cell expression of PDL1 (9). Expression of PDL1 by human cancer cells is associated with decreased patient survival time and a significant increase in overall patient mortality (10, 11). Therefore, PDL1 is a major obstacle to natural anti-tumor immunity and to cancer immunotherapies requiring activation of host T cell-mediated anti-tumor immunity. This concept is supported by animal studies demonstrating that antibody blocking of PDL1-PD1 interactions improves T cell activation and reduces tumor progression (7, 12–16), and that antibody blocking of PDL1 reverse signaling through T cell-expressed CD80 prevents T cell anergy (5). Phase I/II clinical trials have indicated that PDL1-PD1 interactions are a major immune suppressive mechanism in cancer patients. Treatment with mAb to PD1 delays tumor progression in 18% of non-small-cell lung cancer patients, 28% of melanoma patients, and 27% of renal-cell cancer patients, while treatment with mAb to PDL1 delays tumor progression in 10% of advanced non-small-cell lung cancer patients, 17% of melanoma patients, 12% of renal-cell cancer patients, and 6% of ovarian cancer patients (17–19). However, a majority of patients do not benefit from antibody therapy leading us to explore alternative strategies for inhibiting PDL1-PD1 interactions. Based on earlier reports that PDL1 also binds to CD80 (4, 20), we hypothesized that co-expression of CD80 on PDL1+ tumor cells may prevent PDL1-PD1-mediated immune suppression (21). We tested this hypothesis using PDL1+ human melanoma, breast cancer, and lung cancer cells transfected with membrane-bound human CD80, and observed that co-expression of CD80 prevented PD1 binding and restored T cell activation. Because in vivo transfection of cancer cells with membrane-bound CD80 is not clinically feasible, we have tested the ability of a soluble form of CD80 to restore T cell activation in the presence of PDL1+ human tumor cells. We now report that a soluble fusion protein consisting of human or mouse CD80 fused to the Fc region of human IgG1 (CD80-Fc) reverses PDL1-PD1-mediated immune suppression by PDL1+ human or mouse tumor cells, respectively, and results in activation of CD4+ and CD8+ T lymphocytes. Comparison of CD80-Fc with antibodies to PDL1 or PD1 suggests that CD80-Fc may be more effective than antibody therapy and that CD80-Fc mediates its effects by the dual mechanisms of inhibiting PDL1-PD1 interactions and by costimulating through CD28.
Materials and Methods
Cell Lines, plasmids, and transfections
Human tumor cell lines MEL1011 and C8161 (cutaneous melanomas), MCF10CA1 (mammary carcinoma, hereafter called MCF10), H358 (bronchioloalveolar adenocarcinoma), H292 (squamous cell carcinoma), and murine tumor cell lines 4T1 (BALB/c-derived mammary carcinoma) and B16 MELF10 (C57BL/6-derived melanoma) were cultured as described (21–24). C8161/CD80, 4T1/CD80, and MELF10/CD80 transfectants were generated and maintained as described (21, 23, 25, 26). COS cells were cultured in DMEM, supplemented with 10% heat-inactivated FCS (Hyclone, Logan, UT), 1% penicillin/streptomycin (BioSource, Rockville, MD), 2mM Glutamax (BRL/Life Sciences, Grand Island, NY), 0.1% gentamicin (BioSource), and 5mg/ml Prophylactic Plasmocin (InvivoGen, San Diego, CA) (27). COS/PDL1/CD80 cells were made by transfecting COS cells with the pSNAPf vector (New England Biolabs, Ipswich, MA) containing PDL1, selecting for transfectants by culturing in 2mg/ml G418, followed by transfection with pLHCX/CD80 (28). The resulting COS/PDL1/CD80 cells were selected and maintained in media supplemented and 400μg/ml hygromycin (Calbiochem).
Western Blots
Western blots were performed as described (28) except cellular extracts of plasma membrane fractions were prepared as follows: Tumor cells were washed twice with sterile PBS, resuspended at 1×107 cells/800μl PBS, homogenized using a GentleMACS Dissociator in GentleMACS M tubes (Miltenyi Biotec, Auburn, CA) using program Protein_01.01, and centrifuged at 13,690 × g (Jouan, Winchester, VA) at 4°C for 30min. Supernatants containing the cytoplasmic proteins were discarded, 150μl ice-cold lysis buffer was added to the pellets and the mixture incubated at 4°C for 30min on an Eppendorf Mixer (Eppendorf Mixer 5432). The resulting lysates were centrifuged at 13,690 × g at 4°C for 30min, and the supernatants containing the membrane proteins were saved for subsequent electrophoresis. 40μg of the membrane proteins were mixed with 5μl sample buffer (0.5M Tris ph 6.8, Glycerol, 20% SDS, H20, and bromophenol blue) for 30min on an Eppendorf Mixer, centrifuged at 13,690 × g at 4°C for 30min, and electrophoresed on 12% SDS-PAGE gels. The proteins were transferred to polyvinylidene difluoride membranes (Amersham, Piscataway, NJ) using a Bio-Rad PowerPac HC (100V for 70min) and the membranes blocked with 5% nonfat dry milk in Tris-buffered saline and tween 20 (TBST). PDL1 and β-actin were detected using 1μg/ml PDL1 mAb (clone 5H1 (1)) and 0.05μg/ml β-actin mAb, respectively, followed by 1:5000 dilution of sheep anti-mouse HRP (GE Healthcare Life Sciences, Pittsburgh, PA).
Mice
Breeding stock for BALB/c, BALB/c T cell receptor transgenic DO11.10 (I-Ad-restricted, OVA peptide 323-339-specific), C57BL/6, C57BL/6 T cell receptor transgenic OT-1 (H-2Kb-restricted, OVA peptide 257-264-specific), and CD28−/− C57BL/6 mice were from The Jackson Laboratory. Mice were bred and maintained in the UMBC animal facility. All animal procedures were approved by the UMBC Institutional Animal Care and Use Committee.
Antibodies, reagents, and flow cytometry
Human CD80-FITC (clone L307.4), mouse CD80-FITC (clone 16-10A1), mouse PDL1-PE (clone MIH5), human CTLA4-PE (clone BNI3), mouse IgG1-FITC (clone X40), rat IgG2a-PE (clone R35-95), and hamster IgG-FITC (clone UC8-4B3) monoclonal antibodies (mAb) were from BD Biosciences. Human PDL1-APC (clone 29E.2A3), mouse PDL1-PE (clone 10F.9G2), human CD28-FITC (clone CD28.2), and rat IgG2a-Alexa Fluor 647 (clone RTK2758) were from BioLegend (San Diego, CA). Mouse PDL1 (clone MIH6), mouse IgG2b-APC (clone eBMG2b), and rat IgG2b-PE (clone TER-119) were from AbD Serotec (Raleigh, NC), eBioscience (San Diego, CA), and Caltag (Burlingame, CA), respectively. Ionomycin was from EMD Millipore (Billerica, MA). Phorbol-12-myristate-13-acetate (PMA) was from Sigma-Aldrich (St. Louis, MO). OVA323-339 (ISQAVHAAHAEINEAGR) and OVA 257-264 (SIINFEKL) peptides were synthesized in the Biopolymer Core Facility at the University of Maryland, Baltimore. Live cells were stained for cell surface expression and subjected to flow cytometry as described (28, 29), and analyzed using a Beckman Coulter Cyan ADP flow cytometer and Summit V4.3.02 software.
Human PBMC activation
Cryopreserved PBMC were obtained from healthy human donors as described (30). PBMC (6×104) and tumor cells (50Gy-irradiated, 3×104) were co-cultured with 5μg/ml PHA (Sigma-Aldrich) at 37°C, 5% CO2 for 72hrs in a total volume of 200μl/well T cell media (IMDM, human AB serum, glutamax, penicillin/streptomycin, gentamycin, sodium pyruvate, 10mM hepes buffer, β-mercaptoethanol, and prophylactic plasmocin) in 96 well plates. Soluble recombinant human CD80, CD86, PD1, and TROY (TNF receptor superfamily member), each fused to the Fc region of human IgG1 (CD80-Fc, CD86-Fc, PD1-Fc, and TROY-Fc, respectively; (R&D Systems, Minneapolis, MN)), blocking mAbs PDL1 (clone 29E.2A3) and PD1 (clone EH12.2H7) (BioLegend), blocking mAb PDL1 (clone MIH1), PD1 (clone EH12.1), and PD1 (clone PD1.3.1.3) (eBioscience, Miltenyi, and BD Biosciences, respectively), and functional grade mouse IgG2b (clone eBMG2b) and IgG1 (clone P3.6.2.8.1) (eBioscience) were added to some wells at 10μg/ml. Anti-CD28 (clone CD28.2, BioLegend) was used at 1–4μg/ml. PBMC were depleted of CD4+ and/or CD8+ T cells using CD4 and CD8 MicroBeads (Miltenyi) according to the manufacturer’s directions. Molar equivalence of anti-CD28 and CD80-Fc: 0.01μM anti-CD28 = 1.5μg/ml; 0.01μM CD80-Fc = 0.26μg/ml. IFNγ production was measured by ELISA (30).
IFN-γ treatment and PD1-Fc binding
4T1 and 4T1/CD80 cells were incubated at 37°C for 48hrs in their culture medium supplemented with 100U/ml recombinant mouse IFN-γ (Pierce-Endogen, Rockford, IL), and washed with excess culture medium. Tumor cells were subsequently incubated in the presence or absence of mouse PDL1 (clone 43H12 (5)), followed by incubation with mouse PD1-Fc and anti-mouse mAb PD1-APC (BioLegend). C8161 and C8161/CD80 cells were incubated in the presence of human PD1-Fc, followed by anti-human PD1-APC as described (21).
Mouse splenocyte activation
Splenocytes from DO11.10 or OT-1 mice were depleted of red blood cells, and incubated at 1×105 cells/well with 2μg/well OVA323-339 or OVA257-264 peptide, respectively, in the presence of 2×105 irradiated (100Gy) 4T1 or 4T1/CD80 cells, or irradiated (50Gy) MELF10 or MELF10/CD80 cells, respectively, at 37°C, 5% CO2 for 48hrs in a total volume of 200μl/well. Mouse CD80-Fc or mouse TROY-Fc was included in some wells at 10μg/ml. IFNγ production was measured by ELISA (BioLegend). Splenocytes from CD28-deficient mice were depleted of red blood cells and cultured at 37°C, 5% CO2 in 96-well plates at 1×105 cells/well with 20ng/ml PMA plus 1μg/well ionomycin in the presence of irradiated MELF10 cells plus mouse TROY-Fc, CD80-Fc, or CD86-Fc. IFNγ production was measured by ELISA (BioLegend).
Microscopy
Tumor cells were cultured in cover-glass slides containing 8 chambers (Thermo Scientific, Waltham, MA), washed with excess PBS containing 10% FCS, and labeled sequentially with mAb 5H1, anti-mouse IgG-Alexa Fluor 488 (Invitrogen, Grand Island, NY), and CD80-Alexa Fluor 647 (BioLegend, clone 2D10) (30min each step, with PBS/FCS washes after each antibody). Following labeling, cells were fixed with formaldehyde, mounted in ProLong (Invitrogen), and imaged with a Leica TCS SP5 confocal microscope using Leica Application Suite Advanced Fluorescence software (Leica Microsystems, Buffalo Grove, IL).
Procedures with human materials were approved by the UMBC Institutional Review Board.
Statistical Analysis
Standard deviation and Student’s t test were performed using Excel version 2008. Mann-Whitney test was performed using http://faculty.vassar.edu/lowry/VassarStats.html.
Results
CD80 prevents binding of some PDL1 mAb to CD80+PDL1+ human tumor cells
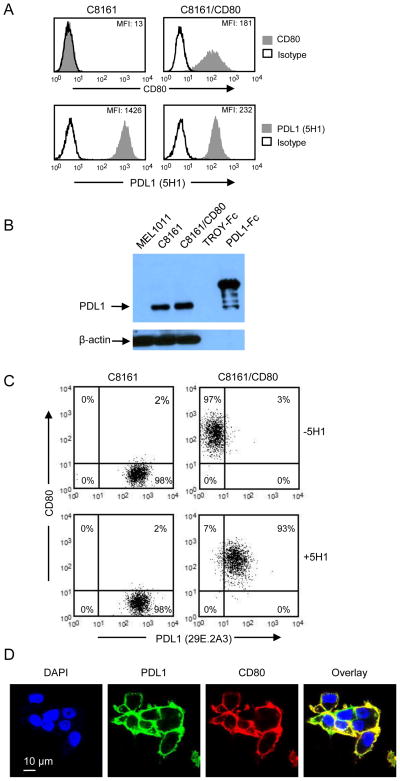
We previously reported that PDL1 was not detected on the cell surface of CD80-transfected PDL1+ human tumor cells (21). These studies used three anti-PDL1 mAbs (29E.2A3, MIH1, and 27A2) and raised the question of whether CD80 prevents expression or obstructs detection of cell surface PDL1. To resolve this issue, we used an additional anti-PDL1 mAb. C8161 and CD80-transfected C8161 (C8161/CD80) cells were stained for CD80 and PDL1 using the mAb 5H1 (1) and analyzed by flow cytometry (figure 1A). In contrast to previous findings with the 29E.2A3, MIH1, and 27A2 mAbs, 5H1 mAb stained C8161/CD80 cells, indicating that CD80 does not inhibit cell surface expression of PDL1. To confirm that CD80+ cells contain cell surface PDL1, C8161 and C8161/CD80 cells were lysed and the lysates enriched for cell membranes and subjected to western blotting with mAb 5H1 (figure 1B). PDL1-Fc and C8161 cells served as positive controls. MEL1011, a PDL1− cell line, and TROY-Fc, an irrelevant soluble protein, were negative controls. C8161 and C8161/CD80 cells contain similar levels of membrane PDL1, confirming that CD80 does not obstruct cell surface expression of PDL1.
Figure 1. CD80-transfected human tumor cells co-express CD80 and PDL1.
(A) PDL1 mAb 5H1 detects PDL1 on CD80+ tumor cells. Cutaneous melanoma C8161 and C8161/CD80 cells were stained for cell surface CD80 and PDL1 (mAb 5H1) and analyzed by flow cytometry. (B) Plasma membrane fractions of human tumor cells contain PDL1 and CD80. Plasma membrane fractions of lysates of PDL1− MEL1011, C8161, and C8161/CD80 cells, and recombinant proteins (human TROY-Fc and PDL1-Fc) were electrophoresed by SDS-PAGE and western blotted with mAb to PDL1 (5H1) or β-actin. (C) Treatment with PDL1 mAb 5H1 allows binding of PDL1 mAb 29E.2A3. C8161 and C8161/CD80 cells were incubated with unlabeled PDL1 mAb 5H1, followed by staining with PDL1 mAb 29E.2A3 plus CD80 and analysis by flow cytometry. (D) COS/PDL1/CD80 cells were plated in slides containing 8 individual chambers, stained with 5H1 + anti-mouse IgG-Alexa Fluor 488 and CD80-Alexa Fluor 647 (mAb 2D10), and analyzed by confocal microscopy. PDL1 is false colored green and CD80 is false colored red. Data are representative of 3, 2, 3, and 2 independent experiments for (A), (B), (C), and (D), respectively.
mAbs 29E.2A3, MIH1, and 27A2 may have failed to detect PDL1 because they recognized epitopes that were sterically blocked by CD80. Tamada and colleagues have generated a rat anti-mouse PDL1 mAb (43H12) that prevents the binding of mouse CD80 to mouse PDL1 (5). Since 5H1 detects PDL1 on PDL1+CD80+ human cells, it may dissociate CD80-PDL1 interactions on human cells similarly to 43H12-mediated dissociation of PDL1 and CD80 on mouse cells. To test this possibility C8161 and C8161/CD80 cells were either untreated or pre-treated with 5H1 mAb prior to labeling with 29E.2A3 mAb (figure 1C). As previously observed, 29E.2A3 did not detect PDL1 on C8161/CD80 cells. However, if C8161/CD80 cells were pre-treated with 5H1 mAb, then PDL1 was detected by 29E.A3 mAb. In contrast, pre-treatment with 5H1 did not result in subsequent binding of anti-PDL1 mAbs MIH1 or 27A2 (data not shown). To further examine CD80 and PDL1 co-expression, COS cells modified to stably express human PDL1 and human CD80 (COS/PDL1/CD80) were stained with mabs 5H1 and CD80, and analyzed by confocal microscopy (figure 1D).
COS/PDL1/CD80 co-expressed CD80 and PDL1 on the cell surface. These results demonstrate that PDL1 is present on the cell surface of CD80+ human tumor cells and that some mAbs do not detect it because their binding sites are sterically obstructed by CD80.
Soluble CD80 restores activation of CD4+ and CD8+ T lymphocytes
Membrane-bound CD80 prevents PDL1-PD1-mediated immune suppression by human tumor cells (21). However, introduction of membrane-bound CD80 into PDL1+ tumor cells is not an in vivo therapeutically feasible strategy. A soluble version of CD80 may have the same PDL1 binding properties as the membrane-bound form and therefore may also overcome PDL1 suppressive function. We first determined that activated human T cells express PD1 and are therefore susceptible to PDL1-mediated suppression, and then tested if the two extracellular domains of human CD80 fused to the Fc domain of human IgG1 (CD80-Fc) restored T cell activation in the presence of PDL1+ tumor cells.
To confirm that PHA activation induces PD1 expression, human PBMC were cultured with PHA and tested for IFNγ production and expression of PD1 at 24, 48, and 72hrs (supplemental figure 1). Activated CD3+CD4+ and CD3+CD8+ T cells were identified by their expression of the activation marker CD45RO and by production of IFNγ. PD1 expression on activated T cells increased with time and in proportion to IFNγ production. These results indicate that PHA-activated T cells express PD1 and therefore should be susceptible to PDL1-mediated suppression.
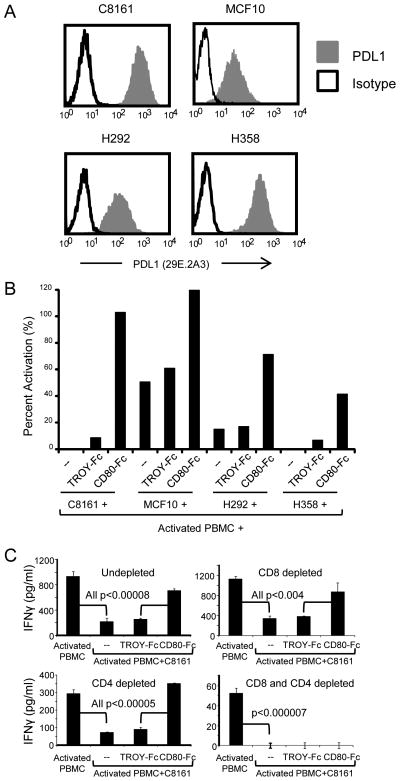
To determine if CD80-Fc restores T cell activation, PDL1+ C8161, MCF10, H292, and H358 cells (figure 2A) were co-cultured with allogeneic human PBMC plus PHA in the presence or absence of CD80-Fc or irrelevant TROY-Fc fusion protein. PBMC suppression was assessed by measuring IFNγ production (figure 2B). PDL1+ C8161, MCF10, H292, and H358 cells suppressed IFNγ production by PHA-activated PBMC and inclusion of CD80-Fc, but not TROY-Fc, restored IFNγ production.
Figure 2. Human CD80-Fc restores activation of human CD4+ and CD8+ T lymphocytes.
(A) Human C8161 cutaneous melanoma, MCF10 mammary carcinoma, H292 bronchioloalveolar adenocarcinoma, and H358 squamous cell carcinoma cells were cell surface stained with PDL1 mAb 29E.2A3 and analyzed by flow cytometry. (B) C8161, MCF10, H292, and H358 cells were cultured with PHA-activated PBMC from healthy donors in the presence of CD80-Fc or irrelevant fusion protein TROY-Fc (10μg/ml). Percent activation= (pg/ml of co-culture/pg/ml of activated PBMC)×100%. (C) Healthy donor PBMC were undepleted, CD8-depleted, CD4-depleted, or CD8 and CD4-depleted prior to PHA activation and subsequent incubation with C8161 human melanoma cells as in (B). Data are representative of 3, 3, and 2 independent experiments for (A), (B), and (C), respectively. Levels of IFNγ production for activated T cells ranged from 52 to 1135pg/ml.
To ascertain that the IFNγ-producing cells are T cells, PBMC were depleted for CD4+, CD8+, or CD4+ plus CD8+ T cells prior to co-culture with PDL1+ C8161 cells, PHA, and soluble fusion proteins (figure 2C). CD80-Fc restored IFNγ production in undepleted cultures. Depletion of either CD8+ or CD4+ T cells reduced IFNγ production, and concomitant depletion of CD4+ plus CD8+ T cells eliminated IFNγ production. These results demonstrate that CD80-Fc restores activation of both CD4+ and CD8+ T cells.
Co-expression of CD80 and PDL1 on mouse tumor cells prevents PD1 binding
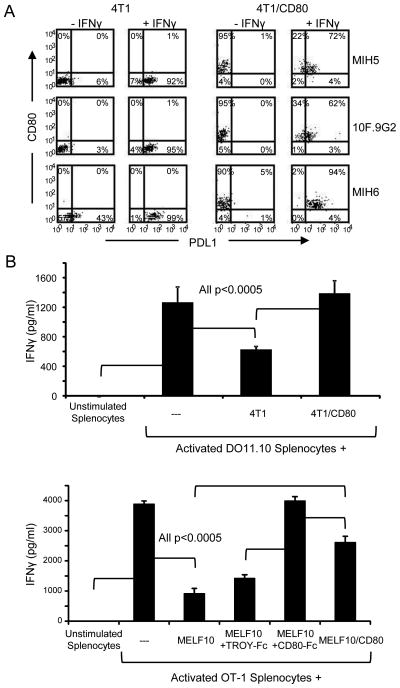
Our previous studies established that co-expression of human CD80 on PDL1+ human tumor cells prevented PD1 binding and PDL1-mediated immune suppression (21). To determine if the same phenomenon occurred with murine cells, we used CD80− 4T1 mammary carcinoma cells and B16 MELF10 cells and their CD80 transfectants (4T1/CD80 and MELF10/CD80). As is the case for human tumor cells, some murine tumor cells constitutively express PDL1 and treatment with IFNγ increases PDL1 expression (31). Mouse tumor cells were cultured with or without IFNγ, then stained and analyzed by flow cytometry for CD80 and PDL1 using mouse mAb (MIH5, 10F.9G2, and/or MIH6) (figure 3A and supplemental figure 2). IFNγ up-regulated cell surface PDL1 expression on all cells and all mAbs detected PDL1 on the CD80 transfectants. These results indicate that unlike human tumor cells, mAb to mouse PDL1 predominantly reacts with epitopes outside of the mouse CD80-PDL1 binding region.
Figure 3. Co-expression of membrane-bound CD80 on PDL1+ mouse tumor cells prevents binding of PD1.
(A) CD80+ mouse tumor cells co-express PDL1. Mouse 4T1 and CD80-transfected 4T1 (4T1/CD80) mammary carcinoma cells were cultured ± IFNγ for 48hrs and stained for PDL1 (mAb MIH5, 10F.9G2, and MIH6) and CD80, and analyzed by flow cytometry. (B) CD80-transfected PDL1+ murine tumor cells restore activation of antigen-activated T cells. Splenocytes were isolated from DO11.10 or OT-1 transgenic mice and activated with their respective OVA peptide in the presence of 4T1 or 4T1/CD80, or MELF10 or MELF10/CD80 cells, respectively, with or without CD80-Fc or TROY-Fc. IFNγ production was measured by ELISA. Data are representative of 3 and 2 independent experiments for (A) and (B) respectively.
To determine if co-expression of mouse CD80 restores T cell activation in the presence of PDL1+ mouse tumor cells, 4T1 and 4T1/CD80 tumor cells, or MELF10 and MELF10/CD80 cells were co-cultured with peptide-activated DO11.10 or OT-1 transgenic splenocytes, respectively, in the presence or absence of CD80-Fc or TROY-Fc (figure 3B). PDL1+ parental 4T1 and MELF10 cells suppressed T cell activation and 4T1/CD80 cells, MELF10/CD80 cells, and CD80-Fc restored T cell activation. These results demonstrate that similar to human PDL1+ tumors, CD80 restores T cell activation in the presence of PDL1+ murine tumor cells.
CD80 restores activation more effectively than mAb against PD1 or PDL1
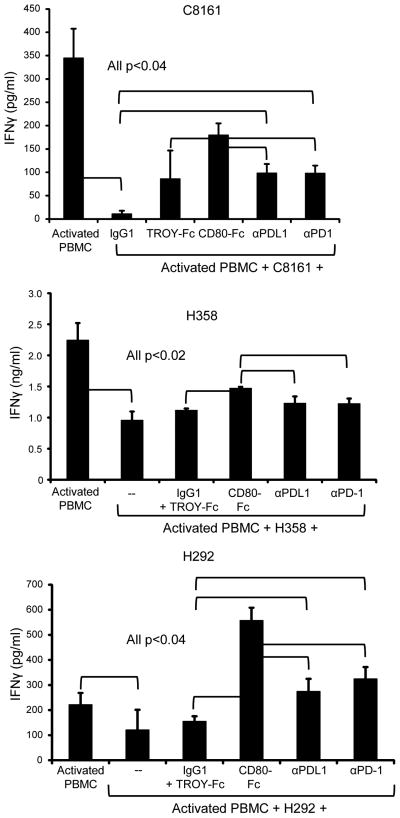
Antibodies to PDL1 and PD1 delayed tumor progression and improved anti-tumor immunity in mice (32, 33), and have shown clinical efficacy in extending survival time of some patients with melanoma, non-small cell lung cancer, and renal cell carcinoma (18, 19). To assess the relative activity of antibodies versus CD80-Fc, human PBMC were co-cultured with PHA and C8161, H358, or H292 cells in the presence or absence of human CD80-Fc, TROY-Fc, or antibody to human PDL1 (mAb 29E.2A3) or human PD1 (mAb EH12.2H7) (figure 4). As observed previously (figure 2B), PDL1+ tumor cells suppressed T cell activation. CD80-Fc reversed the suppression and was more effective in reversing suppression than antibodies to PDL1 or PD1. CD80-Fc was similarly more effective in reversing suppression as compared to other commonly used mAbs to PDL1 (MIH1 and 5H1), mAbs to PD1 (PD1.3.1.3 and EH12.1) (supplemental figure 3A), or human PD1-Fc (supplemental figure 3B).
Figure 4. CD80-Fc restores PBMC activation more efficiently than antibodies to PDL1 or PD1.
PHA-activated PBMC from healthy human donors were co-cultured with human C8161 melanoma, H358 squamous cell carcinoma, or H292 bronchioloalveolar adenocarcinoma cells in the presence of CD80-Fc, antibodies to PDL1 or PD1, or control IgG1 and/or irrelevant fusion protein TROY-Fc. IFNγ production was measured by ELISA. Data are representative of 3 independent experiments for each cell line.
CD80-Fc restores PBMC activation by both neutralizing PDL1-PD1 suppression and by costimulating
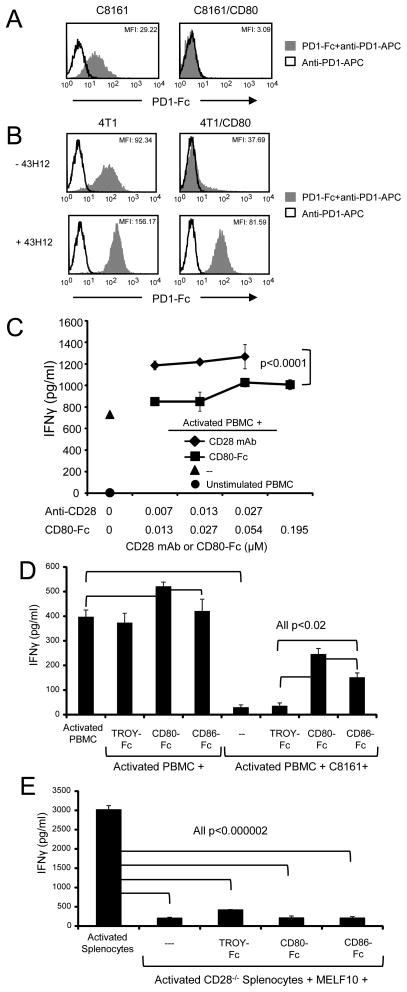
To determine if co-expression of CD80 and PDL1 by human and mouse tumor cells prevents binding of PD1, human C8161 and C8161/CD80 cells were incubated with or without PD1-Fc followed by anti-PD1-APC (figure 5A), or mouse 4T1 and 4T1/CD80 cells were treated with IFNγ and subsequently incubated with or without PD1-Fc followed by staining with anti-PD1-APC (figure 5B, top histograms). Neither human (C8161/CD80) nor mouse (4T1/CD80) cells bound PD1-Fc, whereas the parental C8161 and 4T1 cells bound PD1-Fc, demonstrating that co-expression of CD80 prevents PD1 binding.
Figure 5. CD80-Fc restores PBMC activation by neutralizing PDL1-PD1 suppression and by costimulating through CD28.
(A) Expression of CD80 prevents binding of PD1 to PDL1+ tumor cells. C8161 and C8161/CD80 human tumor cells were incubated with or without recombinant human PD1-Fc fusion protein followed by staining with mAb to PD1 and analyzed by flow cytometry (top panel). (B) 4T1 and 4T1/CD80 mouse tumor cells were cultured with IFNγ for 48hrs, followed by incubation ± unlabeled PDL1 mAb 43H12, which disrupts PDL1-CD80 interactions. Tumor cells were subsequently incubated with recombinant mouse PD1-Fc followed by staining with mAb to PD1, and analyzed by flow cytometry (bottom panels). (C) CD80-Fc has less costimulatory activity than antibodies to CD28. Healthy donor PBMC were activated with PHA and cultured with CD80-Fc or antibodies to CD28. IFNγ production was measured by ELISA. Molar concentration for antibody was ½ the molar concentration of CD80-Fc to compensate for each antibody molecule binding two molecules of CD28 vs. each CD80-Fc molecule binding only one molecule of CD28. (D) CD80-Fc restores PBMC activation more effectively than CD86-Fc. PHA-activated PBMC from healthy donors were co-cultured with or without C8161 human tumor cells in the presence or absence of CD80-Fc, CD86-Fc, or TROY-Fc. IFNγ production was measured by ELISA. (E) Splenocytes from CD28−/− mice were activated with PMA and ionomycin and co-cultured with MELF10 mouse tumor cells ± CD80-Fc, CD86-Fc, or TROY-Fc. Data are representative of 3, 3, 3, and 3 independent experiments for (A), (B), (C), and (D) respectively.
To confirm that mouse CD80 prevents mouse PD1 binding by interacting with PDL1, we used the PDL1 mAb 43H12, which dissociates mouse CD80-PDL1 complexes (5). 4T1 and 4T1/CD80 cells were treated with IFNγ, followed by incubation with mAb 43H12, and subsequent staining with PD1-Fc and anti-PD1-APC (figure 5B, bottom histograms). Cells treated with 43H12 bind PD1-Fc regardless of whether they express CD80, demonstrating that mouse CD80 prevents PD1-PDL1 interactions by binding to PDL1.
Our previous studies (21) and figures 5A and 5B establish that CD80 prevents PD1 binding to PDL1. However, CD80 may also promote T cell activation by delivering a costimulatory signal to T cells through CD28 (34, 35). PHA-activated human T cells express CD28 (supplemental figure 4A), so they have the potential to be costimulated. To determine whether CD80-Fc also costimulates, we compared the ability of CD80-Fc vs. antibodies to CD28 to boost the response of PHA-activated human PBMC in the absence of tumor cells. A single anti-CD28 antibody binds two CD28 molecules, whereas one CD80-Fc molecule binds only one CD28 molecule. To normalize binding capacity we compared the activity of two molar equivalents of CD80-Fc vs. one molar equivalent of anti-CD28 mAb (figure 5C). Antibodies to CD28 increased IFNγ production of PHA-activated human PBMC by 38–42%, whereas equal binding units of CD80-Fc increased IFNγ production by only 14–28% (p< 0.0001). These results suggest that although CD80-Fc has some costimulatory activity, it is significantly less than anti-CD28 antibodies, and its costimulatory activity is not sufficient to account for all of the restoration of T cell activation seen in figures 2–4. Therefore, CD80 appears to restore T cell activation by concomitantly preventing PDL1-PD1 interactions and by costimulating.
To further examine the possibility that costimulation is involved, we compared T cell activation in the presence of CD80-Fc vs. CD86-Fc since CD86 also costimulates via CD28 (36) and is 3–5 times more effective at costimulating than CD80 (37, 38), but does not bind PDL1 (4). If costimulation is involved, then CD86-Fc will also increase T cell activation. However, if CD80-Fc disrupts PDL1-PD1 interactions and costimulates, then CD80-Fc will be more effective than CD86-Fc in activating T cells. PHA-activated human PBMC were cultured with or without human C8161 tumor cells in the presence of CD80-Fc, CD86-Fc, or control TROY-Fc. (figure 5D). CD80-Fc treatment produced more IFNγ as compared to CD86-Fc indicating that CD80-Fc both costimulates and inhibits PDL1-PD1 interactions.
The results of figures 2–5 demonstrate that CD80-Fc mediates its effects by preventing PDL1-PD1 binding and by costimulating. However, these data do not determine if costimulation is obligatory for the PDL1-PD1 inhibitory function of CD80-Fc. To assess this possibility PMA plus ionomycin-activated splenocytes from MHC syngeneic CD28 knockout mice were co-cultured with murine MELF10 tumor cells in the presence or absence of CD80-Fc, CD86-Fc, or control TROY-Fc (figure 5E). CD28−/− splenocytes were suppressed by MELF10 cells and the suppression was not reversed by either CD80-Fc or CD86-Fc, indicating that costimulation through CD28 is integral to the ability of CD80-Fc to block PDL1-PD1 interactions.
CD80-Fc may also restore T cell activation by binding to T cell-expressed PDL1
A potential drawback to using CD80-Fc as a reagent to inhibit PDL1-PD1 interactions is its potential to suppress T cell activation by binding to T cell-expressed CTLA4 (39, 40). In the experiments presented here, CD80-Fc increases, rather than decreases, T cell production of IFNγ (see figure 2), making it unlikely that CD80-Fc is interacting with CTLA4. However, the lack of suppression in our experiments could be due to the absence of CTLA4 on PHA-activated T cells. To test this possibility, human PBMC were activated for 72hrs with PHA and subsequently stained for CD3 and CTLA4 (supplemental figure 4B). Thirty percent of PHA-activated PBMC were CD3+CTLA4+. Therefore, PHA-activated T cells are potentially susceptible to CTLA4-mediated immune suppression; however, CD80-Fc does not appear to induce suppression via CTLA4.
Sixty-five percent of PHA-activated PBMC express PDL1 (supplemental figure 4C), giving rise to the possibility that CD80-Fc may not only inhibit tumor cell PDL1-mediated suppression, but also neutralize PDL1-PD1 suppression caused by activated T cells.
Discussion
We previously reported that co-expression of membrane-bound CD80 on PDL1+ human tumor cells prevents PDL1-PD1-mediated immune suppression and restores activation of human T cells (21). The present report extends these findings to murine tumor cells, and importantly demonstrates that a soluble form of the extracellular domains of CD80 (CD80-Fc) similarly counteracts PDL1-PD1-mediated T cell apoptosis. CD80-Fc appears to be more effective than antibodies to either PDL1 or PD1 in preventing PDL1-mediated immune suppression, thus suggesting that CD80-Fc is a potentially useful therapeutic agent for enhancing anti-tumor immunity in cancer patients.
In addition to binding to PDL1, CD80 also binds to CTLA4 (40, 41). Therefore, soluble CD80 has the potential to cause T cell anergy by interacting with T cell-expressed CTLA4 (42). Although PHA-activated T cells express CTLA4, it is unlikely that soluble CD80 suppresses via CTLA4 because of the robust T cell activation observed. The absence of CTLA4-mediated suppression could be because PDL1 and CTLA4 share a common binding region on CD80 so that CD80 binding to PDL1 sterically prevents CTLA4 binding. This possibility is supported by cross-linking studies indicating that murine PDL1 and CTLA4 interact with CD80 at overlapping sites (4). However, we have generated CD80 mutants that do not bind CTLA4 but retain the ability to bind PDL1 (unpublished data), suggesting that PDL1 and CTLA4 do not share a common binding site on CD80. It is also unlikely that the lack of suppression via CTLA4 is due to preferential binding of CD80 to PDL1 vs. CTLA4 because human CD80 and CTLA4 interact more strongly (KD = 0.4 μM) than human CD80 and PDL1 (KD = ~1.4 μM) (4).
CD80-Fc maintains T cell activation in the presence of PDL1+ tumor cells by simultaneously preventing PDL1-PD1 interactions and by providing costimulation. PD1 binding studies demonstrate that CD80 prevents PD1 binding to both mouse and human PDL1+ tumor cells, while experiments with anti-CD28 antibodies and CD28-deficient mice demonstrate that costimulation also occurs. It is unlikely that this costimulatory activity will result in autoimmunity to self antigens since human CD80 binds PDL1 more tightly than it binds CD28 (KD = ~1.4 μM vs. 4 μM, respectively) (4). Additionally, we have observed that 65% of PHA-activated PBMC express PDL1 raising the possibility that CD80-Fc could also prevent PDL1-PD1 interactions between PDL1+ and PD1+ PBMC.
In our previous studies we did not detect PDL1 on CD80+ human tumor cells using three anti-PDL1 mAbs (21). Two of these antibodies (29E.2A3 and MIH1) were previously reported to recognize epitopes in the PDL1-CD80 binding region (20), while the binding site of the third antibody (27A2) was unknown. However, pretreatment with mAb 5H1, which dissociates PDL1-CD80 interactions (5) allows binding of anti-PDL1 mAb 29E.2A3, but not anti-PDL1 mAb MIH1 or 27A2. These observations confirm that mAb 29E.2A3 reacts with an epitope on PDL1 within the PDL1-CD80 binding region that is revealed by CD80-PDL1 dissociation. The absence of binding of mAb MIH1 and 27A2 following treatment with mAb 5H1 suggests that mAb 5H1 only partially dissociates CD80-PDL1 interactions and that the epitopes detected by mAb MIH1 and 27A2 remain blocked by CD80.
It is surprising that CD80-Fc is more effective than mAb to PDL1 or PD1 in preventing PDL1-PD1-mediated suppression. Given that the binding constants of antibodies for PDL1 or PD1 are typically several orders of magnitude stronger than that of soluble CD80 to PDL1, one would expect antibodies to be more effective in blocking PDL1-PD1 interactions. Structural studies (4, 20) and our results are consistent with the concept that soluble CD80 prevents PDL1-PD1 interactions by steric blocking, and antibodies to PDL1 and PD1 are thought to act in a similar fashion. It is likely that CD80-Fc is more effective than antibodies because CD80-Fc costimulates in addition to perturbing PDL1-PD1 binding. Therefore, CD80-Fc may be a more efficacious therapeutic than mAbs to PDL1 and/or PD1. If CD80-Fc and antibodies restore T cell activation via independent or partially overlapping mechanisms, then these two reagents may be additive or synergistic and their combined application could be more efficacious than either reagent alone. In either case, CD80-Fc may provide a new approach for reversing immune suppression and facilitating anti-tumor immunity in individuals with cancer.
Supplementary Material
Acknowledgments
We thank Dr. Eugene Kwon for providing mAb 5H1.
Abbreviations used in this paper
- PD1
Programmed Death 1
- PDL1
Programmed Death Ligand 1
- CD80-Fc
Fusion protein of the extracellular domains of CD80 and the Fc region of IgG1
- PD1-Fc
Fusion protein of the extracellular domains of PD1 and the Fc region of IgG1
- CD86-Fc
Fusion protein of the extracellular domains of CD86 and the Fc region of IgG1
- TROY-Fc
Fusion protein of the extracellular domains of Tumor Necrosis Factor Receptor Superfamily Member 19 (TROY) and the Fc region of IgG1
Footnotes
These studies were supported by NIH RO1CA115880 and NIH RO1CA84232. STH was partially supported by NIH T32 GM066706.
References
- 1.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 2.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco LM, V, Salinas H, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 8.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 9.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Medical oncology. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 12.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 13.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 14.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 15.Sakthivel P, Gereke M, Bruder D. Therapeutic intervention in cancer and chronic viral infections: Antibody mediated manipulation of PD-1/PD-L1 interaction. Rev Recent Clin Trials. 2011 doi: 10.2174/157488712799363262. [DOI] [PubMed] [Google Scholar]
- 16.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature medicine. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haile ST, Bosch JJ, Agu NI, Zeender AM, Somasundaram P, Srivastava MK, Britting S, Wolf JB, Ksander BR, Ostrand-Rosenberg S. Tumor cell programmed death ligand 1-mediated T cell suppression is overcome by coexpression of CD80. J Immunol. 2011;186:6822–6829. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava MK, Bosch JJ, Wilson AL, Edelman MJ, Ostrand-Rosenberg S. MHC II lung cancer vaccines prime and boost tumor-specific CD4+ T cells that cross-react with multiple histologic subtypes of nonsmall cell lung cancer cells. Int J Cancer. 2010;127:2612–2621. doi: 10.1002/ijc.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 24.Fidler IJ, I, Hart R. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S, Baskar S, Patterson N, Clements VK. Expression of MHC Class II and B7-1 and B7-2 costimulatory molecules accompanies tumor rejection and reduces the metastatic potential of tumor cells. Tissue Antigens. 1996;47:414–421. doi: 10.1111/j.1399-0039.1996.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 27.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 28.Dissanayake SK, Thompson JA, Bosch JJ, Clements VK, Chen PW, Ksander BR, Ostrand-Rosenberg S. Activation of tumor-specific CD4(+) T lymphocytes by major histocompatibility complex class II tumor cell vaccines: a novel cell-based immunotherapy. Cancer Res. 2004;64:1867–1874. doi: 10.1158/0008-5472.can-03-2634. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol Immunother. 2008;57:389–398. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch JJ, Thompson JA, Srivastava MK, Iheagwara UK, Murray TG, Lotem M, Ksander BR, Ostrand-Rosenberg S. MHC class II-transduced tumor cells originating in the immune-privileged eye prime and boost CD4(+) T lymphocytes that cross-react with primary and metastatic uveal melanoma cells. Cancer Res. 2007;67:4499–4506. doi: 10.1158/0008-5472.CAN-06-3770. [DOI] [PubMed] [Google Scholar]
- 31.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, Hwu P. PD-1 BLOCKADE ENHANCES T CELL MIGRATION TO TUMORS BY ELEVATING IFN-gamma INDUCIBLE CHEMOKINES. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins M, Taylor P, Norton S, Urdahl K. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2467. [PubMed] [Google Scholar]
- 35.Norton S, Zuckerman L, Urdahl K, Shefner R, Miller J, Jenkins M. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992;149:15506–1617. [PubMed] [Google Scholar]
- 36.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 37.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 38.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hunig T, Sorensen P, Stuart DI, Davis SJ. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 39.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peach RJ, Bajorath J, Naemura J, Leytze G, Greene J, Aruffo A, Linsley PS. Both extracellular immunoglobin-like domains of CD80 contain residues critical for binding T cell surface receptors CTLA-4 and CD28. The Journal of biological chemistry. 1995;270:21181–21187. doi: 10.1074/jbc.270.36.21181. [DOI] [PubMed] [Google Scholar]
- 41.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 42.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annual review of immunology. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.