Abstract
Isodon rubescens, a Chinese herb, has been used as a folk, botanical medicine in China for inflammatory diseases and cancer treatment for many years. Recently, we isolated a new ent-kaurene diterpenoid, named Jaridonin, from Isodon rubescens. The chemical structure of Jaridonin was verified by Infrared (IR), Nuclear magnetic resonance (NMR), and Mass spectrum (MS) data as well as X-ray spectra. Jaridonin potently reduced viabilities of several esophageal cancer cell lines, including EC109, EC9706 and EC1. Jaridonin treatment resulted in typical apoptotic morphological characteristics, increased the number of annexin V-positive staining cells, as well as caused a G2/M arrest in cell cycle progression. Furthermore, Jaridonin resulted in a significant loss of mitochondrial membrane potential, release of cytochrome c into the cytosol, and then activation of Caspase-9 and -3, leading to activation of the mitochondria mediated apoptosis. Furthermore, these effects of Jaridonin were accompanied by marked reactive oxygen species (ROS) production and increased expression of p53, p21waf1/Cip1 and Bax, whereas two ROS scavengers, N-acetyl-L-cysteine (L-NAC) and Vitamin C, significantly attenuated the effects of Jaridonin on the mitochondrial membrane potential, DNA damage, expression of p53 and p21waf1/Cip1 and reduction of cell viabilities. Taken together, our results suggest that a natural ent-kaurenoid diterpenoid, Jaridonin, is a novel apoptosis inducer and deserves further investigation as a new chemotherapeutic strategy for patients with esophageal cancer.
Keywords: Apoptosis, ent-kaurene diterpenoid, esophageal cancer, mitochondria pathway, rabdosia rubescens, reactive oxygen species
INTRODUCTION
Esophageal cancer is a relatively rare form of cancer with remarkable geographic variation in incidence [1]. Some areas in the world have a much higher incidence than others [2, 3]. For example, Linzhou (formerly Linxian) and its nearby cities in Henan Province, China were reported to be areas with the highest incidence rates of esophageal squamous cell cancer (ESCC) in the world, which were estimated to be about 161 and 103 per 100,000 in men and women, respectively [4–7]. Until now, primary modalities for treatment of ESCC have included surgery alone and chemotherapy plus radiation therapy. However, the overall 5-year survival rate in ESCC patients undergoing definitive treatments remains very low, only ranging from 5% to 30% [8, 9]. New agents for treatment of patients with ESCC are thus urgently needed.
Since the natural world has much more chemical diversity than synthetic compounds, natural products (e.g. plant products and herbs) are rich resources for identifying new lead compounds for anti-cancer agents [10]. Isodon rubescens (“Donglingcao” in Chinese) is a perennial herb of the Isodon genus belonging to the Labiatae family. The herb has been used as a folk, botanical medicine for the treatment of cancer (including esophageal cancer) and inflammatory disease for many years in China [11]. In fact, the standard extract of this herb has become a drug product since 1977 for clinical use in alleviating symptoms of sore throats and inflammation in China [12]. The major constituents of Isodon rubescens are diterpenoids with a very unique chemical backbone (i.e. the α–methylene cyclopentanone system, Fig. 1A) and a variety of bioactivities. So far, most studies on Isodon rubescens have been focused on isolation of new ent-kaurane diterpernoids from this plant. However, the underlying mechanism by which ent-kaurane diterpernoids inhibit cancer cell growth remains controversial. Multiple pathways, including telomerase [13], DNA binding and cleavage [14, 15], p53 [16, 17], phosphatidylinositol 3-kinases (PI3K)/ protein kinase B (AKT) [18, 19], epidermal growth factor receptor (EGFR) signaling [20], AMP-activated protein kinase [21] and nuclear factor-kappa B (NF-κB) [22, 23], have been implicated in anti-cancer activities of this class of diterpenoids.
Fig. 1. Effects of Jaridonin on the growth of esophageal cancer cells and human normal liver cells.
A. Chemical structures of Jaridonin (1) and Oridonin (2). B–D. 8×103 EC109, EC9706 and EC1 cells were plated in 96-well culture plates. 24 hours later, the cells were changed with fresh medium and treated with Jaridonin or Oridonin at the indicated concentrations for 24 or 48 hours, respectively. Culture medium with 0.1% DMSO was used as controls. E. Normal human liver cells HL-7702, as well as EC109, EC9706 and EC1 cells were plated and treated under the same conditions with Jaridonin at the indicated concentrations for 24 hours. Cell viability was measured by MTT assay. Data are Means ± SD. Each sample was counted in duplicate. IC50s were estimated by dose-response curves and calculated by Graphpad prism software.
In this study, we have isolated and characterized several novel ent-kaurane diterpenoids from Isodon rubescens in Jiyuan, Henan Province, China. Of the diterpenoids, Jaridonin is abundant and consists of up to 1.1% of the contents of the Jiyuan Isodon rubescens extract, whereas oridonin, the first ent-kaurane diterpenoid isolated from Isodon rubescens in other areas, was almost undetectable in the Jiyuan Isodon rubescens extracts. The chemical structures of Jaridonin (Fig. 1A) and its analogs have been fully characterized and patented by us [24]. We showed that Jaridonin exhibited strong anti-proliferative and pro-apoptotic effects in human EC cell lines. The anti-proliferative and apoptotic effects of Jaridonin were associated with activation of the mitochondria mediated apoptotic pathway, induction of G2/M arrest, as well as increased expression of p53, p21Waf1/Cip1. Finally, we are the first to show that the production of reactive oxygen species (ROS) by Jaridonin is partly necessary for growth inhibition, DNA damage and induction of p53 and p21Waf1/Cip1 expression. Therefore, we have identified a potentially new mechanism of action for Jaridonin.
MATERIALS AND METHODS
Reagents
Antibodies against p53, p21 Waf1/Cip1, Bax, cytochrome c, Caspase-9, and Caspase-3 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibodies against β-actin and GAPDH were from Abgent Biotech (Suzhou, China) and Good HERE Biotech Inc. (Hangzhou, China), respectively. The horseradish peroxidase-conjugated secondary antibodies were obtained from Zhongshan Golden Bridge Biotech Inc. (Beijing, China). Fluorescein isothiocyanate (FITC)-Annexin V/ Propidium iodide (PI) apoptosis assays kit was from Biovision, Inc. (Palo Alto, CA). Enhanced chemiluminescence Western blot detection reagents were from Pierce Biotechnology, Inc. (Rockford, IL). The ROS detection kit, Caspase-3/9 activity kit, N-acetyl-L-cysteine (L-NAC), and 5,5′,6,6′-tetrachloro-1,1′, 3,3′-tetraethyl- benzimidazolocarbocyanine iodide (JC-1) were purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Caspase-3 inhibitor (z-DEVD-fmk), Caspase-9 inhibitor (z-LEHD-fmk), Caspase inhibitor (z-VAD-fmk) and p53 inhibitor pifithrin-α (PFTα) were from Calbiochem, Inc. (La Jolla, CA). Vitamin C (Vit C), Hoechst 33258, PI and 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were from Sigma (St. Louis, USA).
Cell Culture Conditions and Compounds
Human esophageal cancer cell lines EC9706, EC109, EC1 and primary normal human liver cells (HL-7702) were purchased from China Center for Type Culture Collection (CCTCC, Shanghai, China). The CCTCC has authenticated all these cell lines; and all cell lines were used here within their 20 passages. The human esophageal carcinoma EC9706 cell line has been proven to be poorly-differentiated squamous cell carcinoma and esophageal carcinoma of the fungating type [25–27]. EC1 cell line was derived from EC9706 [28]; EC109 cell line is well-differentiated [25, 26]. Culture media for the three human esophageal cell lines and HL-7702 cells were RPMI 1640 medium plus 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin and DMEM medium with 20% FBS. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. Pure Jaridonin and oridonin were isolated from Isodon rubescens in our laboratory. 99.9% purity Jaridonin was used. The chemical structures are shown in Fig. (1A) and were confirmed by NMR, MS and IR data as well as X-ray spectra. Purities were determined by HPLC and were all above 98%. Jaridonin and oridonin were solubilized in dimethyl sulfoxide (DMSO) and stored at −80 °C. The concentration of DMSO in culture medium was used under 0.1% (v/v) without any effect on cell proliferation on its own.
Isolation of Jaridonin
The air-dried and powdered leaves of Isodon Rubescens (6.0 kg) were extracted two times with 75% Me2CO (90,000 ml×2) at 45 °C for 3 hours. After applying vacuum-rotary evaporation procedure to remove about 95% of the solvent, the residue was partitioned in water and extracted three times with EtOAc (5,000 ml×3). The EtOAc extract (240g) was loaded to silica gel column chromatography (CC), and then CHCl3, CHCl3-Me2CO (10:1, 5:1, 3:1, 1:1) and Me2CO was used to elute the CC in turn. Compound Jaridonin was obtained fraction from the CHCl3-Me2CO (3:1) after repeated silica gel CC separations and recrystallized in EtOAc.
MTT Assay
8×103/well cells in culture media were seeded into 96-well plates. 24 hours later, the media were changed to fresh media and cells were treated with vehicle control or with indicated concentrations of compounds. After twenty-four or forty-eight hours later, the wells were added with 1mg/mL MTT of and incubated at 37 °C for 4 hours. The cell densities were determined by reading the absorbance at 490 nm.
Nuclear Staining by Hoechst 33258
Cells were seeded on a 6-well plate and treated with Jaridonin at the indicated concentrations for 24 hours. The cells were then fixed with immunostaining fix solution (Beyotime Institute of Biotechnology, Haimen, China) for 10 minutes and then 5μg/mL Hoechst 33258 were added. Thirty minutes later, the cells were carefully washed twice with phosphate buffered saline (PBS). Apoptotic cells were examined and identified according to the condensation and fragmentation of their nuclei by fluorescence microscopy.
Apoptosis and Cell Cycle Analysis by Flow Cytometry
FITC- annexin V (green) and PI (red) were used to stain and identify subpopulations of cells with early stage apoptosis and necrotic or late apoptotic cells, respectively [29, 30]. Cells at 70% to 80% confluence were treated with 10, 20 and 40 μM Jaridonin for 24 hours. Cells were stained with FITC-conjugated Annexin V and PI according to the manufacturer’s protocol (Biovision). Cellular debris and aggregated cells were excluded from all analyses by using appropriate scatter gates. Ten thousand events for each sample were counted and analyzed by either Beckman Coulter Epics XL Cytometer (Fullerton, CA) or Accuri C6 flow cytometer (Becton, Dickinson & Co.; Franklin Lakes, NJ). The early and late apoptotic cells were also examined by fluorescence microscopy and identified by the localization of Annexin V and PI.
For cell cycle analysis, cells were treated with the indicated concentrations of Jaridonin or vehicle control for 24 hours. After the treatment, cells were fixed in ice cold 70% ethanol overnight. The fixed cells were incubated in hypotonic buffer with 0.5 mg/ml RNase at 37 °C for 30 minutes, and then stained with PI (100μg/ml) at room temperature for 30 minutes. DNA contents/cells were analyzed in Beckman Coulter Epics XL Cytometer (Fullerton, CA), with a total of 10,000 cells tested. The histograms of DNA distribution were shown as a sum of cell populations at G0/G1, G2/M or S phase, using FlowJo software.
Measurement of ROS Levels
The redox-sensitive probes 2′, 7′-dichlorodihydro- fluorescein diacetate (DCFH-DA) or dihydroethidium (DHE) were used to measure intracellular levels of hydrogen peroxide or superoxide) [31, 32]. Control and treated cells were incubated with 10μM DCFH-DA or DHE for 30 minutes at 37 °C. After extracellular fluorescent dye was removed by washing cells with serum free RPMI1640 medium for three times. The fluorescence signal was observed by florescence microscopy and the intensity was determined by a flow cytometer.
Measurement of Mitochondrial Membrane Potential (ΔΨm)
Cells were exposed to Jaridonin at indicated concentrations alone or to a combination with L-NAC (5mM) for 24 hours. At the end of the treatments, the cells were stained with 10μg/mL JC-1 for 30 minutes at 37 °C. The fluorescence intensity was examined by a fluorescence microscopy under an excitation at 488 nm and an emission at 530 nm..
Measurement of Cytochrome c Release from the Mitochondria
EC9706 cells were treated with 20 and 40μM of Jaridonin for 24 hours. The mitochondria and cytosol were separated using the cell mitochondria isolation kit (Beyotime Institute of Biotechnology, China) following the kit instruction. Briefly, cells were incubated in 200μL ice cold mitochondrial lyses buffer for 10 minutes, and homogenized in a glass homogenizer and by stroking 30 times with a tight pestle on ice. Nuclei and unbroken cells were removed by centrifuging at 600g for 10 minutes. Then the supernatant was further centrifuged again at 12,000g for 30 minutes at 4 °C to separate the cytosol (supernatant) and the mitochondria (pellet) fractions. The levels of cytochrome C in the cytosol and the mitochondria were determined by Western blot analysis.
Measurement of Caspase 3/9 Activity
The enzymatic activity of Caspase-3/9 was measured, using Caspase-3/9 activity kit (Beyotime Institute of Biotechnology, Haimen, China) following the kit’s instructions. Briefly, cells were lysed in lysis buffer for 15 minutes. The lysates were incubated with Caspase-3 and -9 substrate peptides: Ac-DEVD- p-nitroanilide (pNA) and Ac-LEHD-pNA, and the release of pNA was quantified as values of the absorbance at 405 nm for Caspase-3 and-9 activities.
Comet Assay
EC9706 cells were treated with Jaridonin alone at the indicated concentrations or in combination with L-NAC (5mM) for 4 hours. After treatment, cells were suspended in 0.6% low melting agarose at 37 °C and placed on a 1% normal melting agarose precoated slide. After the slides solidify at 4 °C, the slides were first incubated with lysis solution (2.5M NaCl, 100 mM EDTA-Na2, 10 mM Tris with 1% Triton X-100, 10% DMSO freshly added, PH 10) in the dark for an hour, and then with electrophoresis buffer (1 mM EDTA-Na2 and 300 mM NaOH, pH 13) for 25 minutes, followed by and electrophoresis. The slides were then washed twice with ice-cold neutralizing buffer (0.4 M Tris, pH 7.5) and stained with 20 μg/mL ethidium bromide. Comets were photographed under a fluorescent microscope at 200× magnification. The olive tail moment (OTM) of each comet was calculated using CASP analyzed software. OTM equals percentage of DNA in the tail × distance between the center of gravity of DNA in the tail and the center of gravity of DNA in the head [33]. The OTM was expressed as a arbitrary unit by integrating both the distance and intensity of each comet.
Western Blotting Analysis
Cells were lysed in cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). Clarified protein lysates (30–80μg) were electrophoretically resolved on denaturing SDS-PAGE gel (8–12%), and then transferred to nitro- cellulose membranes. The membranes were probed with appropriated primary and secondary antibodies. An enhanced ECL kit and a film were used to visualize protein bands that are reacted with antibodies, Protein levels were semi-quantified by using the Image-Pro Plus software and adjusted by a loading control beta-actin or GAPDH.
Statistics
Data are expressed as means ± standard deviation (SD). The significance of the difference between different groups was determined with analysis of variance (ANOVA) and Student t test. The differences were considered significant at P<0.05.
RESULTS
Jaridonin Reduced Viabilities of Human Esophageal Cancer Cells
Chemical structures of Jaridonin and a representative ent-kaurane diterpernoid from Isodon rubescens, Oridonin, are shown in Fig. (1A). IR, NMR, MS data of Jaridonin are shown as supplementary data in Fig. (1) and 1H-NMR and X-ray spectra are shown as supplementary in Fig. (2).
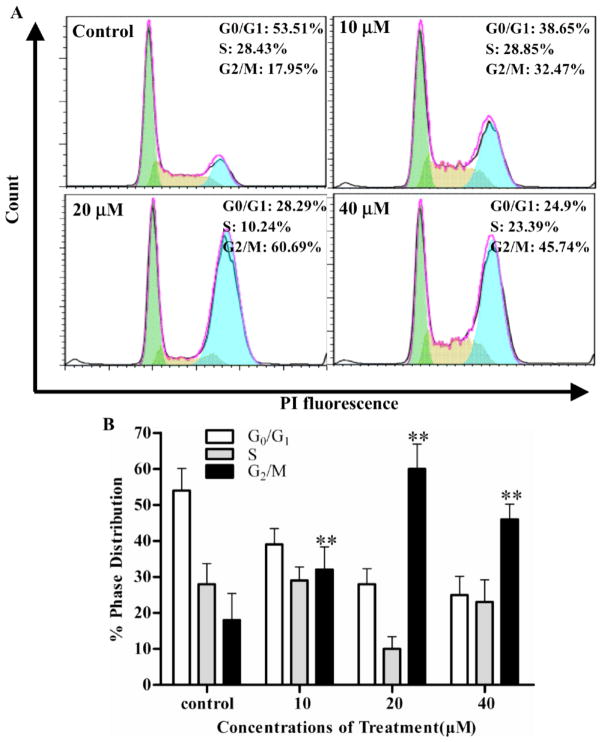
Fig. 2. Jaridonin induces accumulation of G2/M cell population in EC9706 cells.
EC9706 Cells were seeded in 6-well plate for 24 hours, and then treated with Jaridonin at the indicated concentrations. After exposure to Jaridonin for 24 hours, cells were washed with PBS and fixed in 70% ethanol overnight at 4 °C. The cells were then treated with RNAse A solution (0.5g/ml) at 37°C for 30 minutes and stained by PI (100μg/ml). A. Flow cytometric determination of DNA contents was analyzed by Beckman Coulter Epics XL Cytometer (Fullerton, CA). B. Percentages of cell cycle distribution among different treatment groups are presented as a histogram graph. The experiments were repeated three times and the data are shown as means ± SD. * P < 0.05 and ** P < 0.01.
Figs. (1B–D) show that Jaridonin significantly inhibits the growth of EC109, EC9706 and EC1 cells in a both dose- and time-dependent manner. Compared to control treatments, Jaridonin treatment at a concentration of 20μM for 24 and 48 hours resulted in 38% and 74% growth inhibition in EC109 cells, 52% and 65% in EC9706 cells, and 54% and 68% in EC1 cells, respectively. However, Jaridonin treatment for 24 hours at the same concentration inhibited the growth of primary normal human liver cells by about 8.45% (Fig. 1E). The IC50 values of Jaridonin treatment for 48 hours were estimated to be 12.0μM in EC109 cells, 11.2μM in EC9706 cells and 4.6μM in EC1 cells, respectively. The IC50 values for oridonin treatment of EC109, EC9706, and EC1 cells for 48 hours were 19.7, 31.3 and 25.8μM, respectively. These results indicate that Jaridonin is more effective in inhibition of the growth of ESCC cell lines than that of human normal liver cells, and that Jaridonin is a more potent anti-proliferative agent than oridonin.
Jaridonin Induces G2/M Phase Arrest and Apoptosis of EC Cells
We next examined whether the growth inhibitory effect of Jaridonin was due to the induction of cell cycle arrest and apoptosis. Figs. (2A and B) show that Jaridonin treatment of EC9706 cells at 10, 20 and 40 μM concentrations for 24 hours significantly increased G2/M population up to 32.47%, 60.69% and 45.74%, respectively, whereas 17.95% of the control treated cell population was at G2/M phase (Ps<0.01). The increase in G2/M population by Jaridonin is at the expense of a decrease in G1 and S population (Figs. 2A and B).
In addition, EC9706 cells treated with Jaridonin exhibits apoptosis-related morphologies, such as cell shrinkage and rounding up, cell membrane blebbing, and nuclear fragmentation and condensation (Figs. 3A and B). Similar results were obtained when other ESCC cell lines EC109 and EC1 were treated with Jaridonin (data not shown). The percentages of apoptotic cells with control and Jaridonin treatments were further determined by flow cytometric analysis, following FITC-annexin V/PI staining. 10, 20 and 40μM Jaridonin treatments of EC9706 cells for 24 hours resulted in a significant increase of FITC-Annexin V positive /PI negative (early apoptosis) population up to 7.4%, 26.5% and 45.9%, respectively, compared to control treated cells (3.6%) (Figs. 4A, B and D; Ps<0.01). Similarly, Jaridonin treatment of EC109 cells also significantly increased the percentage of the apoptotic population up to 16.6, 39.0 and 50.4%, respectively, compared to control treated cells (0.4%) (Figs. 4C and E; Ps<0.01). These data indicate that the growth inhibitory effect of Jaridonin is associated with induction of both G2/M arrest and apoptosis.
Fig. 3. EC9706 cells treated by Jaridonin show typical apoptotic morphologies.

A. Morphology changes in EC9706 cells treated with Jaridonin at the indicated concentrations. After exposure to Jaridonin for 24 hours, cells were photographed using inverted microscope. 0.1% DMSO was used as a control (magnification 200×). B. Effects of Jaridonin on the nuclear morphology of EC9706 cells were assayed by Hoechst 33258 staining. After treatment with Jaridonin for 24 hours, EC9706 cells were stained with Hoechst 33258 and photographed under a fluorescence microscope (magnification 200×).
Fig. 4. Apoptosis analyzed by florescence microscopy and flow cytometry.
A. EC9706 cells were treated by Jaridonin at indicated concentrations for 24 hours. Then the cells were stained with FITC-annexinV/PI for 5 minutes at room temperature according to the manufacture’s protocol (Biovision). Apoptotic cells were photographed under florescence microscopy (magnification 200×). B and C. After EC9706 or EC109 cells were treated with 0.1% DMSO or Jaridonin at the indicated concentrations for 24 hours, the cells were stained with FITC-AnnexinV/PI and then analyzed by flow cytometry. D and E. Percentages of cells with apoptosis were summarized with histogram graphs. The experiments were repeated three times and the results were presented as means ± SD. * P < 0.05 and ** P < 0.01.
Jaridonin Increases Protein Expressions of p53 and its Downstream Targets: p21 and Bax
Since p53 is a major tumor suppressor, we examined the role of p53 and its target genes, p21 and Bax, in Jaridonin mediated growth inhibition. Figs. (5A–D) show that Jaridonin treatment resulted in an increase of protein levels of p53, p21 and Bax in both EC9706 and EC109 cells. To further examine whether p53 activity is required for Jaridonin induced death, we co-treated EC9706 cells with 20 μM Jaridonin and a p53 inhibitor, pifithrin-α (PFTα), for 24 hours to assess their effect on cell viability. Fig. (6E) shows that PFTα significantly attenuated the Jaridonin-induced cell death by 21.8% (P<0.05). This result suggests that p53 activity is required, at least in part, for the Jaridonin-mediated growth inhibitory effect.
Fig. 5. Jaridonin increases the expression of p53, P21 and Bax protein.
A and C. EC9706 or EC109 cells were treated with Jaridonin at indicated concentrations. Protein expression of p53, p21 and Bax was examined by Western blot analysis. GAPDH and beta-actin served as loading controls. A representative picture is shown. B and D. Relative levels of p53, p21 and Bax protein were quantified by densitometry measurement after adjusting levels of loading controls. All data are means ± SD. * P < 0.05 and **P<0.01.
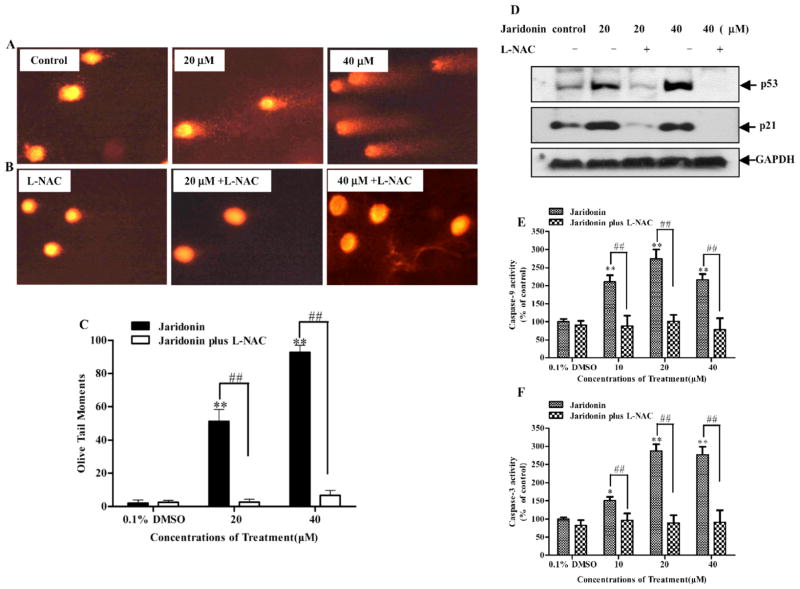
Fig. 6. Jaridonin induces release of cytochrome c and the cleavage of Caspase-9/3.
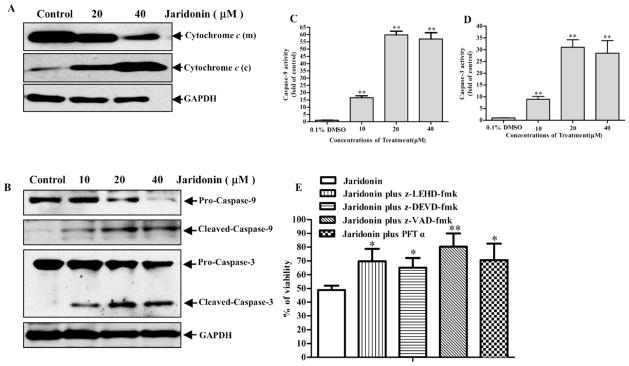
EC9706 cells were treated with either 0.1% DMSO (control) or Jaridonin for 24 hours. Whole cell lysates from the indicated treatments were prepared and Western blots were performed. A. The mitochondria (m) and the cytosolic (c) fractions were prepared and Western blotting analysis was performed for cytochrome C levels. B. Protein levels of cleaved Caspase-9/-3 were determined by Western blot and quantified by densitometry measurement (C and D). GAPDH was used as a loading control. E. EC9706 cells were plated in 96-well plates. At 70% to 80% confluence in the presence of 10% FBS condition, cells were pretreated with Caspase-9 inhibitor (z-LEHD-fmk), Caspase-3 inhibitor (z-DEVD-fmk), Caspase inhibitor (z-VAD-fmk) or p53 inhibitor PFTα each at 40 μM for 1 hour followed by 20μM Jaridonin for an additional 24 hours. Viability of cells was measured by MTT assays. Columns, means of three independent plates; All data are means ± SD. * P < 0.05; **P < 0.01 for Jaridonin versus Jaridonin + inhibitors.
Jaridonin Induces the Release of Cytochrome c and then Activates Caspase 3/9
Bax is a key proapoptotic molecule in the mitochondria-mediated apoptotic pathway and is responsible for the pore opening of the mitochondria to release cytochrome c to activate the Caspase cleavage cascade [34]. Therefore, we examined the effect of Jaridonin on the release of cytochrome c and Caspase 9/3 activation. Fig. (6) shows that Jaridonin significantly enhanced cytochrome c release from the mitochondria to the cytosol, leading to an increased expression of cleaved Caspases 3/9 and a decreased expression of their proenzymes. Pretreatment with a Caspase-3 specific inhibitor (z-DEVD-fmk), a Caspase-9 specific inhibitor (z-LEHD-fmk), and a broad-spectrum Caspase inhibitor (z-VAD-fmk) attenuated the ability of Jaridonin in reducing cell viability in EC9706 cells (Fig. 6E). In particular, the reduction of cell viability by Jaridonin was more effectively blocked by adding the z-VAD-fmk. These Caspase inhibitors alone at the concentrations used above did not significantly affect cell viabilities (data not shown). These results strongly suggest that activation of the Caspase cleavage cascade is required for Jaridonin-induced apoptosis.
Jaridonin Significantly Induces Intracellular ROS Accumulation
ROS is an upstream initiator for p53 and Bax activation and has been proven to be involved in the mitochondrial apoptotic pathway [35]. In addition, the induction of ROS production by natural products has been considered to be one of major mechanisms for killing cancer cells while sparing normal cells [36]. To investigate whether Jaridonin stimulates ROS generation in EC9706 cells, we used fluorescence microscopy and flow cytometry to measure hydrogen peroxide and superoxide in cells that were stained by ROS-detecting fluorescence dyes, DCFH-DA and DHE, respectively. When EC9706 cells were exposed to 10 μM Jaridonin for 16 hours, a moderate increase was observed in the number of cells generating hydrogen peroxide under fluorescence microscope, and the cellular level of hydrogen peroxide was further increased, when treated with 20 and 40μM Jaridonin (Fig. 7A). Similarly, by flow cytometry analysis, 20 and 40μM Jaridonin treatment for 16 hours resulted in a significant increase the percentage of DCF-positive cells to 31.2% and 43.8%, respectively, compared to 8.66% in controls (Figs. 7A, B and F), whereas the level of superoxide was barely changed (Figs. 7D, E and G), demonstrating that hydrogen peroxide is a major component of ROS induced by Jaridonin.
Fig. 7. Jaridonin increases ROS generation in EC9706 cells.
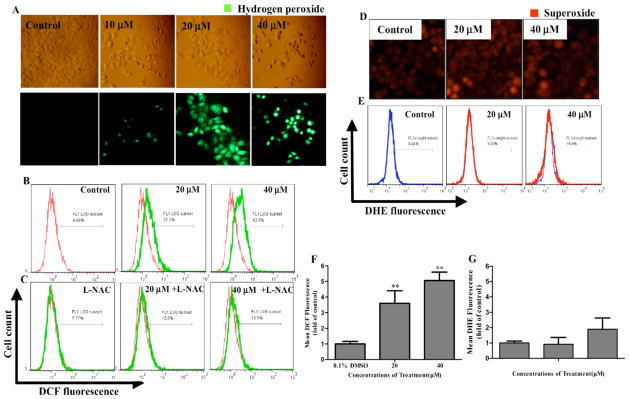
Cells were treated with Jaridonin at indicated concentrations for 16 hours, followed by incubation with 10μM DCFH-DA or DHE for 30 minutes at 37 °C. The intracellular levels of ROS were determined by fluorescent microscope (magnification 200×) and flow cytometer. A and B. Representative fluorescent images and percentages of DCF positive cells were analyzed by flow cytometry. C. Flow cytometry analysis of percentages of DCF positive cells that were co-treated with Jaridonin and L-NAC. D and E. Representative fluorescent images and percentages of DHE positive cells detected by flow cytometry. F and G. Bar graphs for percentages of cell producing ROS. Columns, means of three independent experiments; bars, SD. ** P < 0.01 for Jaridonin versus control.
To further confirm this finding, we used 5 mM ROS scavenger L-NAC, a dose capable of completely inhibiting ROS generation in cells as previously reported by others [37], to pre-treat EC9706 cells for 2 hours, followed by Jaridonin treatment. Fig. (7C) shows that L-NAC reduced the cellular levels of the Jaridonin induced hydrogen peroxide by about 21.2 to 33.2% (Ps<0.05). The 5mM L-NAC alone did not have any significant effect on intracellular ROS.
Effect of Antioxidants L-NAC and Vitamin C on Jaridonin-Induced Cell Death
Since the loss of the mitochondrial membrane potential (MMP) is a key event for the mitochondria dependent apoptotic pathway [38], we next examined whether Jaridonin affected the MMP and whether L-NAC can reverse Jaridonin’s effect on the MMP and cell viability. After EC9706 cells were treated with 10, 20 and 40μM Jaridonin for 24 hours, a clear shift in JC-1 staining of the mitochondria from red to green fluorescence was observed in a dose-dependent manner (Fig. 8A). This result indicates that Jaridonin caused loss of the MMP in EC9706 cells. However, pretreatment with L-NAC almost completely blocked the Jaridonin-induced loss of the MMP (Fig. 8B). Furthermore, both 5mM L-NAC and Vit.C, two structurally unrelated antioxidants, significantly attenuated the inhibitory effect of Jaridonin on viabilities of EC9706 and EC109 cells (Figs. 8C and D). Taken together, our data strongly support the notion that ROS production is required for Jaridonin’s effects on inducing loss of MMP and inhibiting the growth of ESCC cells.
Fig. 8. Jaridonin reduced the MMP of EC9706 cells and antioxidants reversed the effect of Jaridonin on the MMP and cell growth.
A and B. EC9706 Cells were pretreated with or without 5mM L-NAC for 2 hours, and then incubated with Jaridonin for an additional 24 hours. The cells were stained with JC-1 and imaged by fluorescent microscopy. C and D. EC9706 or EC109 Cells were pretreated with or without 5mM L-NAC or Vit C for 2 hours, and then incubated with the indicated concentrations of Jaridonin for 24 hours. Cell viabilities were evaluated by MTT assay. **P<0.01 for Jaridonin versus control; ## P<0.01 for Jaridonin versus Jaridonin + antioxidants. Data are expressed as means ± SD. Each data point is an average of three independent experiments.
ROS Scavenger L-NAC Attenuates the Effect of Jaridonin on DNA Damage, Expression of p53 and p21, and Caspase-3/9 Activities
ROS generation is often accompanied by DNA damage and apoptosis [39]. Using the comet assay, we examined the effect of Jaridonin alone or in combination with L-NAC on the formation of DNA fragmentation in EC9706 cells. Fig. (9A) shows that Jaridonin induced DNA damage in a dose-dependent fashion. However, there was a significant decrease in comet formation when the cells were co-incubated with 5 mM L-NAC and the indicated concentrations of Jaridonin for 4 hours (Fig. 9B). The OTMs, calculated by CASP analyzed software, were significantly increased after 20 and 40μM Jaridonin treatment; however, 5 mM L-NAC co-incubation completely blocked the effects of Jaridonin on DNA damage (Fig. 9C). This result indicates that the Jaridonin-induced DNA damage is mainly due to increased ROS production in EC9706 cells. Moreover, pretreatment with 5mM L-NAC significantly impaired the increased protein levels of p53 and p21 and the increased activity of Caspase-3/9 by Jaridonin (Figs. 9D, E and F), further supporting the hypothesis that ROS is indeed involved in the Jaridonin-induced apoptosis.
Fig. 9. Effect of L-NAC on Jaridonin induced DNA damage and changes in p53 and p21 expression and Caspase-3/9 activity in EC9706 cells.
A. Cells were treated with various concentrations of Jaridonin for 4 hours, and then DNA fragmentation was measured by a comet assay. Intact cells with undamaged DNA (without comet tail) are shown in control group. Cells with minor DNA damage (shorter comet tails) and with major DNA damage (longer comet tails) are shown in 20μM and 40μM Jaridonin treatment groups, respectively. B. 5mM L-NAC almost completely inhibited the Jaridonin-induced DNA damage. C. The Olive Tail Moments (OTM) of the comet were measured using CASP analysis software in each cell. Columns, means obtained from at least 50 cells in each treatment group. Bars are SD. ** P < 0.01 represents a significant increase in OTM for Jaridonin versus control. ## P < 0.01 represents a significant decrease in OTM for antioxidant L-NAC plus Jaridonin versus Jaridonin treatment alone. D. Western blot analysis of p53 and p21 in EC9706 cells pretreated with or without 5mM L-NAC for 2 hours, and then incubated with the indicated concentrations of Jaridonin for 24 hours. E and F. EC9706 cells were treated with Jaridonin at indicated concentrations in the presence or absence of 5mM L-NAC for 24 hours. Caspase-9 and Caspase-3 activation were determined, respectively. All data represent means ± SD of three independent experiments. * P < 0.05; ** P < 0.01, Jaridonin versus control; ## P < 0.01, Jaridonin versus Jaridonin + L-NAC.
DISCUSSION
In the current study, a novel ent-kaurene deterpenoid, Jaridonin, was isolated from extracts of Jiyuan Isodon rubescens. There is significant geographical variation in the contents of ent-kaurene diterpenoids in Isodon rubescens extracts. Jaridonin is abundant in the Jiyuan Isodon rubescens extracts, whereas oridonin is barely found in this extract. Jaridonin induced apoptosis in human ESCC cell lines through the ROS-dependent and mitochondria-mediated apoptotic pathway. This is the first report regarding a cellular pro-apoptotic mechanism of Jaridonin in EC cell lines. Our preliminary data have shown no or low in vivo toxicity of Jaridonin in animals (data not shown). Further experiments are ongoing to evaluate the in vivo anti-ESCC efficacy of this compound using xenograft models in nude mice. These results may provide insight for further developing this novel ent-kaurene diterpenoid compound as a new chemotherapeutic agent for treatment of ESCC.
Apoptosis is regulated at multiple levels through extrinsic and intrinsic pathways. The extrinsic pathway involves death receptors on the cell surface and can directly activate Caspase-8. The intrinsic pathway centers on the mitochondria and is associated with the loss of the MMP, the release of cytochrome c into the cytosol, and the activation of Caspase-9/3 [38]. Oxidative stress and elevated ROS levels have been suggested to play a causative role in the mitochondria mediated apoptosis induced by numerous anticancer chemotherapeutic agents and naturally-occurring compounds [36, 40, 41]. Jaridonin treatment resulted in high levels of ROS in EC9706 cells, and two ROS scavengers, L-NAC and Vit C, blocked the inhibitory effects of Jaridonin on MMP and cell growth. These results confirmed that elevated ROS was necessary for Jaridonin-induced apoptosis in ESCC cells and acted as an upstream event to initiate cell death. However, at present, it is still unknown as to whether the apoptotic effect of Jaridonin is limited only to the intrinsic apoptotic pathway. Therefore, the effect of Jaridonin on the extrinsic apoptosis pathway via death receptors also needs further investigation.
The induction of apoptosis by DNA damaging agents is an important protective mechanism against neoplastic transformation [42]. Consistent with previous reports that other ent-kaurene diterpenoids resulted in DNA damage in various types of cells [43–45], our results showed that the Jaridonin induced DNA damage occurred before the obvious apoptosis was observed, and was caused by the Jaridonin induced ROS generation. It has been reported that DNA damage directly increases p53 expression levels [46, 47]. Therefore, up-regulation of p53 expression by Jaridonin may be associated with DNA damage. The disruption of p53 activation by a p53 inhibitor pifithrin-α led to a decrease in the Jaridonin-induced cytotoxic activity. This result indicated that p53 participates in Jaridonin-induced EC cell apoptosis as a down-stream event of ROS production and DNA damage. Futhermore, p21 and Bax are putative p53 target genes [34, 48]. Consistent with this finding, the increased p53 expression by Jaridonin resulted in elevated levels of p21 and Bax proteins. Up-regulation of p21Cip1 would lead to cell cycle arrest, while the elevated Bax protein would accelerate the opening of the mitochondrial pore for release of cytochrome c and induction of apoptosis. Nevertheless, since about 50% of cancers have mutant p53, it is important to know whether Jaridonin can also induce apoptosis in p53 mutant ESCC cells. Studies are therefore in progress to compare the apoptotic effects of Jaridonin between p53 wild-type, mutant and knockout cell lines for determining the requirements of p53. In addition, sustained levels of excess ROS are known to induce apoptosis through a variety of other mechanisms and pathways [49]. For examples, ROS modulates ASK-1 kinase function, resulting in proapoptotic JNK and p38 activation [50], or regulates the phosphorylation and ubiquitination of Bcl-2 family members leading to apoptosis [51]. In the event that Jaridonin induces apoptosis in p53 defective cells, these alternative mechanisms of Jaridonin’s action would be examined in future experiments.
Based on our present results, we have proposed an outline of Jaridonin induced apoptotic signaling pathways in Fig. (10). Jaridonin induces generation of ROS that causes mitochondrial damage to ESCC cells, leading to mitochondrial permeability, cytochrome c release, and Caspase activation for induction of apoptosis. In addition, DNA damage induced by Jaridonin activates p53, which in turn up-regulates p21 and Bax, leading to cycle arrest and the loss of mitochondrial membrane potential for induction of apoptosis. Jaridonin merits further study as a novel apoptosis inducer of ESCC cell lines and as a potential option for the treatment of esophageal malignancy.
Fig. 10. Proposed mechanisms for Jaridonin induced apoptosis and cell cycle arrest in ESCC cells.
Jaridonin induces generation of ROS that causes mitochondrial damage, leading to the loss of mitochondrial membrane potential, cytochrome c release, and Caspase activation for induction of apoptosis. In addition, Jaridonin causes DNA damage, which activates p53 and up-regulates p21 and Bax expression leading to cell cycle arrest and the disruption of mitochondrial membranes.
Supplementary Material
Acknowledgments
We thank Ms. Amy Le from the Wharton School of the University of Pennsylvania for English editing help. This work was financially supported by the Ministry of Science and Technology (no. 2009ZX09102-154).
ABBREVIATIONS
- ROS
reactive oxygen species
- L-NAC
N-acetyl-L-cysteine
- Vit C
Vitamin C
- JC-1
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl- benzimidazolocarbocyanine iodide
- DHE
dihydroethidium
- DCFH-DA
2′, 7′-dichlorodihydrofluorescein diacetate
- Bax
Bcl-2-associated X protein
- Caspase
cysteine aspartic proteases
- DMSO
dimethylsulfoxide
- ESCC
esophageal squamous cell cancer
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2-5-diphen- yltetrazoliumbromide
- PBS
phosphate buffered saline
- PI
propidium iodide
- OTM
olive tail moment
- RPMI1640
Roswell park memorial institute medium 1640
- DMEM
Dulbecco’s modified eagle medium
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
Supplementary material is available on the publisher’s web site along with the published article.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Wei WQ, Abnet CC, Lu N. Risk factors for esophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut. 2005;54:759–763. doi: 10.1136/gut.2004.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li LD, Lu FZ, Zhang SW, Mu R, Sun XD, Huangpu XM, Sun J. Epidemiological study of Chinese malignancies from 1990 to 1992. Chin J Oncol. 1996;18:403–407. [Google Scholar]
- 4.Wang LD, Zhou Q, Yang CS. Esophageal and gastric cardia epithelial cell proliferation in Northern Chinese subjects living in a high incidence area. J Cell Biochem. 1997;28–29(Suppl):159–165. [PubMed] [Google Scholar]
- 5.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 6.Lu JB, Yang WX, Zu SK, Chang QL, Sun XB, Lu WQ, Quan PL, Qin YM. Cancer mortality and mortality trends in Henan, China, 1974–1985. Cancer Detect Prev. 1988;13:167–173. [PubMed] [Google Scholar]
- 7.Li MH, Li P, Li PJ. Recent progress in research on esophageal cancer in China. Adv Cancer Res. 1980;33:173–249. [PubMed] [Google Scholar]
- 8.Gonzaga IM, Soares-Lima SC, de Santos PT, Blanco TC, de Reis BS, Quintella DC, de Oliveira IM, de Faria PA, Kruel CD, Andreollo NA, de Simão TA, Pinto LF. Alterations in epidermal growth factor receptors 1 and 2 in esophageal squamous cell carcinomas. BMC Cancer. 2012;12:569. doi: 10.1186/1471-2407-12-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Li YY, Tsao SW, Cheung AM. Targeting NF-κB signaling pathway suppresses tumor growth, angiogenesis, and metastasis of human esophageal cancer. Mol Cancer Ther. 2009;8:2635–2644. doi: 10.1158/1535-7163.MCT-09-0162. [DOI] [PubMed] [Google Scholar]
- 10.Normile D. Asian medicine: the new face of traditional Chinese medicine. Science. 2003;299:188–190. doi: 10.1126/science.299.5604.188. [DOI] [PubMed] [Google Scholar]
- 11.Sun HD, Huang SX, Han QB. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006;23:673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 12.Zou J, Pan LT, Li QJ, Zhao JH, Pu JX, Yao P, Gong NB, Lu Y, Kondratyuk TP, Pezzuto JM, Fong HHS, Zhang HJ, Sun HD. Rubesanolides A and B: Diterpenoids from Isodon rubescens. Org Lett. 2011;13:1406–1409. doi: 10.1021/ol200086k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Ye Y, Pan SY, Zhu GY, Li YW, Fong DWF, Yu ZL. Proteomic identification of proteins involved in the anticancer activities of oridonin in HepG2 cells. Phytomedicine. 2011;18:163–169. doi: 10.1016/j.phymed.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Leung CH, Grill SP, Lam W, Gao WL, Sun HD, Cheng YC. Eriocalyxin B inhibits nuclear factor-κB activation by interfering with the binding of both p65 and p50 to the response element in a noncompetitive manner. Mol Pharmacol. 2006;70:1946–1955. doi: 10.1124/mol.106.028480. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Ke Y, Zhang Q, Qi XY, Liu HM. DNA binding and cleavage properties of ponicidin. Tumori. 2009;95:348–351. doi: 10.1177/030089160909500313. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Yue GG, Lau CB, Sun H, Fung KP, Leung PC, Han Q, Leung PS. Eriocalyxin B induces apoptosis and cell cycle arrest in pancreatic adenocarcinoma cells through caspase- and p53-dependent pathways. Toxicol Appl Pharmacol. 2012;262:80–90. doi: 10.1016/j.taap.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Wu LJ, Tashiro SI, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370– 379. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- 18.Deng R, Tang J, Xia LP, Li DD, Zhou WJ, Wang LL, Feng GK, Zeng YX, Gao YH, Zhu XF. Excisanin A, a diterpenoid compound purified from Isodon Macrocalyxin D, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of its signal pathway. Mol Cancer Ther. 2009;8:873–882. doi: 10.1158/1535-7163.MCT-08-1080. [DOI] [PubMed] [Google Scholar]
- 19.Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z, Li XM, Shen HM, Zeng HT. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol Sin. 2007;28:1819–1826. doi: 10.1111/j.1745-7254.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 20.Kang N, Zhang JH, Qiu F, Tashiro SI, Onodera S, Ikejima T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Letters. 2010;294:147–158. doi: 10.1016/j.canlet.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Sul YH, Lee MS, Cha EY, Thuong PT, Khoi NM, Song IS. An ent-kaurane diterpenoid from Croton tonkinensis induces apoptosis by regulating AMP-activated protein kinase in SK-HEP1 human hepatocellular carcinoma cells. Biol Pharm Bull. 2013;36:158–64. doi: 10.1248/bpb.b12-00873. [DOI] [PubMed] [Google Scholar]
- 22.Ban JO, Oh JH, Hwang BY, Moon DC, Jeong HS, Lee S, Kim S, Lee H, Kim KB, Han SB, Hong JT. Inflexinol inhibits colon cancer cell growth through inhibition of nuclear factor-κB activity via direct interaction with p50. Mol Cancer Ther. 2009;8:1613–1624. doi: 10.1158/1535-7163.MCT-08-0694. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Chen B, Song JH, Zhen T, Wang BY, Li X, Liu P, Yang X, Zhang QL, Xi XD, Chen SD, Zuo JP, Chen Z, Chen SJ. Eriocalyxin B ameliorates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells. Proc Natl Acad Sci USA. 2013;10:2258–2263. doi: 10.1073/pnas.1222426110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu HM, Zhu CG, Wang QD, Ke Y, Liu ZZ, Yan XB, Zhang JY, Qu HL. U.S. Patent 8,084,430. Novel ent-kaurene diterpene compound and its derivatives, their preparation and their use. 2011 Dec 27;
- 25.Hou GQ, Xue LX, Lu ZM, Fan TL, Tian F, Xue YL. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against Mtor. Cancer Letters. 2007;253:236–248. doi: 10.1016/j.canlet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Zhang ST, Yu ZL, Wu YD, Liu X, Xu CM, Cho CH. Effects of cyclooxygenase-2 non-selective and selective inhibitors on proliferation inhibition and apoptosis induction of esophageal squamous carcinoma cells. Diseases of the Esophagus. 2009;22:21–31. doi: 10.1111/j.1442-2050.2008.00836.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang XX, Liu R, Shun QJ, Fan FY, Zhan QM. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Research. 2006;16:356–366. doi: 10.1038/sj.cr.7310046. [DOI] [PubMed] [Google Scholar]
- 28.Han YL, Feng YB, Luo ML, Xu X, Cai Y, Wang MR. Isolation and comparative study of clones from human esophageal carcinoma cell line EC9706. Hereditas. 2007;29:1331–1335. doi: 10.1360/yc-007-1331. [DOI] [PubMed] [Google Scholar]
- 29.Vermes I, Haanen C, Steffens NH, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 30.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 31.Fan J, Cai H, Tan WS. Role of the plasma membrane ROS-generating NADPH oxidase in CD34+ progenitor cells preservation by hypoxia. J Biotechnol. 2007;130:455–462. doi: 10.1016/j.jbiotec.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Goffe LC, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandhakumar S, Parasuraman S, Shanmugam MM, Ramachandra RK, Parkash C, Vishnu BB. Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay) Journal of Pharmacology and Pharmacotherapeutics. 2011;2:107–111. doi: 10.4103/0976-500X.81903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chipuk JE, Kuwana T, Hayes LB, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 35.Li ZS, Shi KJ, Guan LY, Cao TM, Jiang Q, Yang Y, Xu CM. ROS leads to MnSOD upregulation through ERK2 translocation and p53 activation in selenite-induced apoptosis of NB4 cells. FEBS Letters. 2010;584:2291–2297. doi: 10.1016/j.febslet.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354, 1–12. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessica R, Kirshner SH, Vishwasenani B. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 39.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabello CM, Bair LWB, Wondrak GT. Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–1037. [PubMed] [Google Scholar]
- 41.Lin Y, Shi RX, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Current Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sitailo LA, Tibudan SS, Denning MF. Activation of Caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- 43.Thirugnanasampandan R, Jayakumar R. Cytotoxic and anti- carcinogenic activity of the ent-kaurene diterpenoid, melissoidesin, from Isodon wightii (Bentham) H. Hara Natural Product Research. 2009;23:1499–1506. doi: 10.1080/14786410802632812. [DOI] [PubMed] [Google Scholar]
- 44.Ding L, Zhou QY, Wang L, Wang W, Zhang SD, Liu B. Comparison of cytotoxicity and DNA damage potential induced by ent-kaurene diterpenoids from Isodon plant. Int J Mol Sci. 2008;9:2265–2277. doi: 10.1080/14786410802267734. [DOI] [PubMed] [Google Scholar]
- 45.Ding L, Hou Q, Zhou QY, Zhang Q, Hou TD, Liu GA. Structure-activity relationships of eight ent-kaurene diterpenoids from three Isodon plants. Research on Chemical Intermediates. 2010;36:443–452. [Google Scholar]
- 46.Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 47.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nature. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 48.Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21: p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Research in veterinary science. 2009:8791–8796. doi: 10.1016/j.rvsc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 50.Davis WJ, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- 51.Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;95:644–650. doi: 10.1111/j.1349-7006.2004.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.