Abstract
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) have been on the increase during the past decade, due to the steady growth of the elderly and immunocompromised patients, and the emergence of multi-drug-resistant (MDR) bacterial strains. Although, only a limited number of anti-MRSA drugs are available, a number of different combination antimicrobial drug regimens have been used to treat serious MRSA infections. Thus, addition of several new antistaphylococcal drugs into clinical practice should broaden therapeutic options. Because MRSA is one of the most common and problematic bacteria associated with increasing antimicrobial resistance, continuous efforts on discovery of lead compounds as well as development of alternative therapies and faster diagnostics to ensure effective antistaphylococcal therapy are required.
Areas covered
This article summarizes the FDA approved drugs to treat MRSA infections, the drugs in clinical trials, and the drug leads for MRSA and related Gram-positive bacterial infections. In addition, the mode of action of antistaphylococcal molecules and resistant mechanisms of some molecules are briefly discussed.
Expert opinion
The number of pipeline drugs presently undergoing clinical trials is not particularly encouraging. There are limited and rather expensive therapeutic options for the infections by MRSA in the critically ill. This review article provides an update on antistaphylococcal drugs in clinical trials and antibacterial molecules effective against Gram-positive bacteria including MRSA. The structural and biological information of antibacterials summarized here are very useful for designing drug leads to develop into new anti-MRSA drugs.
Keywords: methicillin-resistant Staphylococcus aureus (MRSA), antibiotics, antibacterial agents, Gram-positive pathogens, RNA-polymerase inhibitor, dihydropteroate synthase inhibitor, dihydrofolate reductase inhibitor, β5 -lactamase inhibitor, FAS II inhibitor, peptidoglycan biosynthesis inhibitor, electron transport inhibitor, menaquinone biosynthesis inhibitor, PolIIC inhibitor, acyl t-RNA synthase inhibitor, elongation factor G (EF-G) inhibitor, peptide deformylase (PDF) inhibitor, two-component system inhibitor, Clp protease inhibitor, phage therapy, antibacterial vaccine, antimicrobial peptides, vancomycin, daptomycin, linezolid, combination therapy
1. Introduction
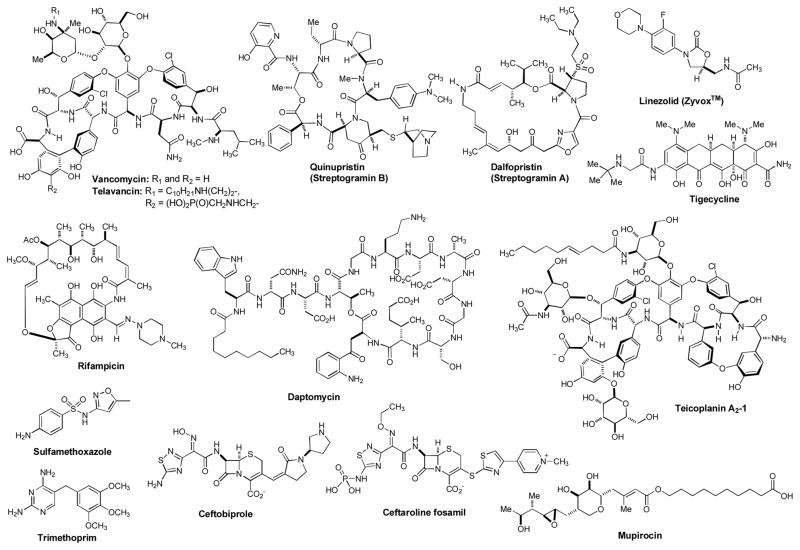
The increasing drug resistance among Gram-positive bacteria is a significant problem because they are responsible for one third of nosocomial infections. Drug resistance in Gram-positive organisms (i.e. staphylococci, pneumococci, vancomycin resistance in enterococci, and mycobacteria) have achieved prominence in last 15 years [1,2,3,4,5,6,7,8]. Methicillin-resistant S. aureus (MRSA) is one of most frequently reported nosocomial pathogens in developed countries. MRSA infections are responsible for more deaths (20,000 deaths per year) in the U.S. each year than AIDS [9]. S. aureus commonly colonizes the external portion of nose (anterior nares), throat, respiratory tract, urinary tract, and soft skin tissues. Most often, S. aureus infections are associated with medical insertion of foreign metals, plastic or vascular devices such as those used for hemodialysis, venous catheterization, or artificial prostheses. S. aureus also causes wound infections and has the potential to induce osteomyelitis, endocarditis and bacteremia, leading to infections in any of the major organs of the body. Some CA-MRSA (community-associated methicillin resistant S. aureus) strains can affect vital organs and lead to widespread infection (sepsis), toxic shock syndrome, and necrotizing pneumonia. These life-threatening medical conditions are believed to be due to toxins carried by CA-MRSA strains, such as Panton-Valentine leukocidin (PVL) and phenol-soluble modulins (PSM) [10,11,12]. In addition, treatment of many chronic S. aureus biofilm related infections including endocarditis, osteomyelitis and indwelling medical device infections are very difficult. About 75 percent of CA-MRSA infections are localized to skin and soft tissue and CA-MRSA can usually be treated effectively. However, CA-MRSA strains display enhanced virulence, spreading more rapidly and causing illness much more severe than traditional HA-MRSA (healthcare-associated methicillin resistant S. aureus) infections, which can affect vital organs and lead to widespread infections [10]. Both CA-MRSA and HA-MRSA are resistant to traditional anti-staphylococcal β-lactam antibiotics [11]. Antibacterial agents available to treat MRSA infections are summarized in Figure 1. Linezolid was the first oxazolidinone antibacterial agent to be licensed for treatment of Gram-positive bacterial infections in 2000 [13,14,15]. The indication for linezolid approved by the U.S. FDA is the treatment of vancomycin-resistant Enterococcus faecium infections, nosocomial pneumonia (healthcare-associated) and CA-pneumonia caused by S. aureus or S. pneumonia, complicated skin and skin structure infections (cSSSI), and uncomplicated skin and soft tissue infections (uSSSI) caused by S. pyogenes or S. aureus [16,17,18,19]. Daptomycin is a cyclic lipopeptide antibiotic used in the treatment of certain CA-MRSA and HA-MRSA infections. Daptomycin is approved in the U.S. for skin, skin structure, and soft tissue infections caused by Gram-positive bacteria, S. aureus bacteremia and right-sided S. aureus endocarditis. Daptomycin represents another addition to the Gram-positive armamentarium, with a novel mechanism of action (disrupting bacterial cell membrane function) and an acceptable safety profile. However, defining its place in therapy is made difficult by the lack of clinical data in conditions such as nosocomial pneumonia, bacteremia, osteomyelitis, and endocarditis. Until further data are available, daptomycin is restricted to the treatment of serious infections where standard therapy is not possible due to organism resistance and/or patient intolerance [20,21]. It is worth noting that daptomycin cannot be used for community-associated pneumonia as it is inactivated by pulmonary surfactant [22]. Vancomycin remains the choice for the treatment of systemic infection caused by MRSA due to the fact that 1) vancomycin shows relatively safe profile and 2) limited anti-MRSA regimens are available (the lack of other approved alternatives). However, the oral absorption of vancomycin is very low, and thus, vancomycin must be administered intravenously to control systemic infections [23,24,25,26]. Thus, treatment of MRSA infection with vancomycin can often be complicated, due to its inconvenient route of administration. Several newly discovered strains of MRSA show antibiotic resistance even to vancomycin and teicoplanin (a glycopeptide antibiotic). These new evolutions of the MRSA bacterium have been called vancomycin intermediate-resistant Staphylococcus aureus (VISA). For the moment, there are limited and rather expensive therapeutic options for the infections by S. aureus in the critically ill (e.g. chemotherapy with linezolid) [27,28]. Antibiotic regimens such as linezolid, quinupristin/dalfopristin, and daptomycin are used to treat more severe infections which do not respond to glycopeptides such as vancomycin. Tigecycline (a glycylcycline antibiotic) was developed in response to the growing prevalence of antibiotic resistance in Staphylococcus aureus and Acinetobacter baumannii. However, tigecycline is not recommended in the treatment of severe infections caused by glycopeptide-resistant strains due to an association with increased mortality rates [29]. Ceftobiprole (a fifth-generation cephalosporin) has been approved for use in limited countries. Ceftobiprole shows activity against systemic MRSA infections when given intravenously [30]. Ceftaroline fosamil (teflaro), a prodrug of ceftaroline, is a new broad-spectrum cephem that was approved in the US in 2010 for community-associated bacterial pneumonia and acute bacterial skin and skin structure infections including MRSA [31,32]. The high affinity of ceftaroline for penicillin-binding proteins is responsible for the potent activity observed against clinically relevant pathogens. Teflaro is so far approved as a monotherapeutic agent that should be administered by IV over approximately 1 hour (t1/2 2.13–2.89 h) for adult patients [33]. Instances of linezolid resistant in MRSA have been reported when the patient was placed on oral linezolid therapy [34]. Several new generation drugs against MRSA have been developed based on the existing drugs. For example, telavancin (a lipoglycopeptide) was approved by the U.S. FDA for complicated skin and skin structure infections [35]. The continuing development of currently available antibiotic arsenals to be effective against drug resistant bacteria remains important, especially for 1) redefining infectious disease therapy (i.e. combination products with superior efficacy over either product alone), 2) shortening length of therapy, 3) reducing side effects profile, and 4) reducing drug resistance profile of currently available antibacterial products. β-Lactams, macrolides, aminoglycosides, fluoroquinolones, and diaminopyrimidines are important pharmacophores for the development of newer generation antibactericidals to combat drug-resistant bacteria [26]. However, the ultimate goal of drug development for MRSA infections is to discover novel antibacterial agents which interfere with unexploited bacterial molecular target(s). This review article provides an update on antistaphylococcal drugs in clinical trials and the investigational molecules effective that exhibited significant bacterial growth inhibitory activities against Gram-positive bacteria including MRSA. The structural information of new antibacterial agents and their mode of actions summarized here are very useful for designing drug leads to develop into new anti-MRSA drugs.
Figure 1.
Antibacterial agents used to treat MRSA infections.
2. Antibacterial agents and non-antibiotic therapeutics in clinical trials for MRSA and other related bacterial infections
Antibacterial agents currently under clinical development for Gram-positive bacterial infections are summarized in several review articles [23,36]. This section summarizes antibacterial agents and non-antibacterial drug therapies in clinical studies that were developed for MRSA infections or have the potential to treat infections caused by MRSA (Table 1). The up-to-date information of clinical trial studies for MRSA and related infections were obtained from the ClinicalTrials.gov web site, PubMed database, Annual Update in Intensive Care and Emergency Medicine, Annual Reports in Medicinal Chemistry, and companies’ web sites.
Table 1.
Antibacterial agents and non-antibiotic therapeutics in clinical trials for MRSA and other related bacterial infections.
| Name | Mode of Action | Conditions | Route(s) | Status | Investigator(s)/Ownership |
|---|---|---|---|---|---|
| Oritavancin | Inhibition of peptidoglycan biosynthesis (a glycopeptide) | Wound infections, Abscess, Systemic inflammation, cellulitis | IV | Phase III | The Medicine Co. (Parsippany, NJ) |
| Dalbavancin | Inhibition of peptidoglycan biosynthesis (a lipoglycopeptide) | Abscess, wound infection, surgical site infection, cellulitis | IV | Phase III | Pfizer |
| TD-1792 (Theravance) | Inhibition of peptidoglycan biosynthesis | Staphylococcal skin infection | IV | Phase II | Theravance (San Francisco, CA) |
| Mupirocin and Chlorhexidine | Inhibition of bacterial protein synthesis by inhibiting isoleucyl tRNA Synthase | MRSA skin infection | Intranasal, body wash | Phase III | Los Angeles Biomedical Research institute, (Los Angeles, CA) |
| Iclaprim | Inhibition of Dihydrofolate reductase | Complicated skin and skin structure infections | IV | Phase III (development terminated) | Acino Holding (Basel, Switzerland) |
| XF-73 | Depolarization of cytoplasmic membrane | Staphylococcal skin infection | Intranasal | Phase II | Destiny Pharma (Brighton, UK) |
| TP-434 | Inhibition of bacterial protein synthesis by targeting ribosome | Complicated intra-abdominal infections | IV | Phase II | Tetraphase (Watertown, MA) |
| BC-3781 | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | Skin infections and bacterial pneumonia caused by MRSA | Oral, IV | Phase I | Nabriva Therapeutics (Vienna, Austria) |
| BC-7013 | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | Skin and skin structure infections and CA-pneumonia | Topical | Phase I | Nabriva Therapeutics (Vienna, Austria) |
| BC-3205 | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | Skin and skin structure infections and CA-pneumonia | Oral | Phase I | Nabriva Therapeutics (Vienna, Austria) |
| RX-1741 | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | CA-pneumonia, Staphylococcal skin infections, Streptococcal infections, Abscess | Oral | Phase II | Rib-X Pharmaceuticals (New Haven, CT) |
| Tedizolid | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | Skin and subcutaneous tissue infections | Oral, IV | Phase III | Trius Therapeutics (San Diego, CA)/Bayer HealthCare |
| Tomopenem | Inhibition of bacterial cell wall synthesis by binding to Penicillin Binding protein | Nosocomial infections due to Gram-negative and –positive bacteria, including MRSA | IV | Phase II | Daiichi-Sankyo (Tokyo, Japan) |
| Cethromycin (Restanza™) | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | CA-bacterial pneumonia (The US FDA designated cethromycin an orphan drug for the prophylactic treatment of patients exposed to inhalation anthrax and in May 2007) | Oral | Phase III | Abbott/Advanced Life Sciences, Inc. (Chicago, IL) |
| EDP-420 | Inhibition of bacterial protein synthesis by targeting 50S ribosomal subunit | CA-pneumonia | Oral | Phase II | Enanta (Watertown, MA) |
| NXL 103 (XRP 2868) | Inhibition of bacterial protein synthesis by targeting ribosomal subunits (synergistic effect) | Acute bacterial skin and skin structure infections, CA- pneumonia | Oral | Phase II | AstraZeneca |
| Delafloxacin | Inhibition of bacterial Gyrase | Acute bacterial skin and skin structure infections, Complicated skin and skin structure infections | Oral, IV | Phase II | Rib-X (New Haven, CT) |
| LBM-415 | Inhibition of bacterial protein synthesis by inhibiting peptide deformylase | CA-respiratory tract infections | Oral | Phase I (development terminated) | Novartis |
| GSK1322322 | Inhibition of bacterial protein synthesis by inhibiting peptide deformylase | Bacterial skin and skin structure infections and hospitalized CA- pneumonia | Oral | Phase II | GlaxoSmithKline |
| Locilex™ (MSI-78 topical cream) | Formation of an amphipathic α-helical peptide on membranes that induces pore or changes membrane permeability | Diabetic foot infections caused by drug-sensitive and –resistant bacteria including MRSA, VRE, extended-spectrum β-lactamase producing bacteria | Topical | Phase III | Dipexium Pharmaceuticals (White Plains, NY) |
| OligoG CF-5/20 | Immunomodulating activity | Lung infections in cystic fibrosis | Inhalation | Phase I | Algipharma (Sandvika, Norway) |
| F598 | Inhibition of virulence by targeting the PNAG carbohydrate of the bacterial capsule | S. aureus and other clinically relevant bacteria | IV | Phase I | Sanofi (Paris, France) |
2.1. Antibacterial agents in clinical trial studies for MRSA or related infections
Lipoglycopeptides are a class of antibacterial agents that possess a hydrophobic side-chain linked to glycopeptide (e.g. vancomycin). The U.S. FDA approved telavancin (Figure 1), a semi-synthetic derivative of vancomycin, in 2009 for complicated skin and skin structure infections (vide supra). Vancomycin and its derivatives inhibit the peptidoglycan (PG) biosynthesis of most of Gram-positive bacteria by forming strong hydrogen bond interactions with the D-Ala-D-Ala moiety of PG biosynthetic precursors (e.g. lipid II). Vancomycin has been applied for the treatment of life-threatening infections caused by Gram-positive bacteria. Several semi-synthetic glycopeptide antibacterial agents, oritavancin (The Medicines Company, Parsippany, NJ) and dalbavancin (Pfizer) underwent new phase III clinical studies for the treatment of patients with acute bacterial skin and skin structure infections [37,38,39].
TD-1792 (Theravance) is a new multivalent glycopeptide-cephalosporin antibiotic with potent activity against Gram-positive bacteria being developed by Theravance, Inc, (San Francisco, CA). The in vitro activity of TD-1792 was tested against 527 S. aureus isolates, including multidrug-resistant isolates. TD-1792 was evaluated in a phase II clinical study for the treatment of complicated skin and skin structure infections caused by Gram-positive bacteria, including resistant strains such as MRSA. Phase III clinical study was recently completed and clinical efficacy and safety were evaluated within an expanded patient population [40,41,42,43].
Mupirocin and Chlorhexidine: Mupirocin (bactroban, Figure 1) has been used as a topical treatment for bacterial skin infections. The mechanism of mupirocin differs from other clinical antibiotics. Mupirocin interferes with the isoleucyl t-RNA synthase in Gram-positive bacteria, resulting in inhibition of bacterial protein synthesis [44]. Mupirocin is rapidly metabolized when administered intravenously. Shortly after the introduction of 2% mupirocin ointment in 2002 for the topical treatment of impetigo caused by S. aureus and S. pyogenes, mupirocin-resistant S. aureus strains emerged. Los Angeles Biomedical Research Institute (Los Angeles, CA) is conducting a multi-center clinical trial to compare the efficacy and safety of body and environmental decolonization regimens in the prevention of CA-MRSA infection via chlorhexidine (once a day for 14 days) and mupirocin (twice a day for 7 days) [45,46,47]. Chlorhexidine is an antiseptic (prevent or inhibit growth of bacteria) and has been a component of mouthwash and skin cleansers.
Iclaprim is a diaminopyrimidine dihydrofolate reductase (DHFR) inhibitor being developed for the treatment of complicated skin and soft tissue infections caused by drug-resistant Gram-positive bacteria. Iclaprim is structurally related to trimethoprim (TMP, Figure 1) that was developed based on a rational drug design by Arpida (Basel, Switzerland). In Phase III clinical trial, intravenously-administered iclaprim was found to be effective in people with skin and soft tissue infections caused by MRSA [48,49,50,51]. Acino Holding Ltd. (Basel, Switzerland) acquired all assets of iclaprim including intellectual property rights from Arpida in 2009. However, the company terminated its development for MRSA infections [52].
XF-73 is a phase II clinical drug candidate being developed by Destiny Pharma Ltd (Brighton, UK). XF-73 is a dicationic molecule that is synthesized from 5,15-bis(4-hydroxyphenyl)porphyrin. XF-73 is active against a broad range of Gram-positive bacteria including MRSA (e.g. MIC 1μg/mL against S. aureus SH1000). Interestingly, XF-73 kills S. aureus much faster than the other antistaphylococcal agents. Although more studies are required to understand rapid bactericidal activity, XF-73 seems to interfere with the staphylococcal cytoplasmic membrane and cause depolarization. Unlike daptomycin (Figure 1), this process does not require calcium ions (a Ca-independent mechanism) [53,54]. The clinical development of XF-73 is targeted at intranasal administration to reduce the risks caused by MRSA and other Gram-positive bacteria [55].
TP-434 is a novel fluorocycline antibiotic being developed by Tetraphase Pharmaceuticals, Inc. (Watertown, MA). TP-434 was tested against panels of recent clinical aerobic and anaerobic isolates, and TP-434 demonstrated broad-spectrum activities against Gram-negative and -positive bacteria including MRSA and VRE[56,57]. TP-434 is currently being evaluated intravenously in a phase II clinical study for the treatment of CA-associated complicated intra-abdominal infections [58].
BC-3781 is a semi-synthetic pleuromutilin derivative that shows significant bactericidal activities against Gram-positive bacteria. Pleuromutilins were isolated from the fungus Clitopilus passeckerianus and other fungi in 1950’s. Tiamulin was the first pleuromutilin compound to be approved for a veterinary use in 1979, followed by valnemulin (econor) in 1999. They have been used to treat infections of swine (e.g. dysentery, ileitis, pulmonary infections, colitis and pneumonia). On the other hand, retapamulin is the first pleuromutilin approved for use in humans in 2007. However, retapamulin is limited to topical application for infections caused by Gram-positive bacteria including MRSA. Pleuromutilins selectively inhibit bacterial protein synthesis by interacting at a site on the 50S subunit of the bacterial ribosome. Two semi-synthetic pleuromutilins, BC-3205 and BC-7013 were developed by Nabriva Therapeutics (Vienna, Austria). BC-3205 is potent antibacterial activity with favorable pharmaceutical properties, making this compound suitable for oral and intravenous delivery [59,60]. The clinical studies have demonstrated its efficacy against skin and skin structure infections (SSSI) and community-associated pneumonia [61].
RX-1741 is an oxazolidinone antibiotic being developed by Rib-X Pharmaceuticals, Inc. (New Haven, CT) that exhibits activity against MRSA and other Gram-positive organisms. RX-1741 has demonstrated greater spectrum and potency of activity than linezolid (Zyvox®) [62,62,63,64]. In a phase II clinical study, the safety and efficacy of RX-1741 have been evaluated in the treatment of adult patients with mild to moderate severity of community-associated pneumonia [65].
Tedizolid (torezolid, TR-701) is an oxazolidinone drug being developed by Trius Therapeutics (San Diego, CA) for complicated skin and skin-structure infections caused by MRSA [66,67]. Tedizolid phosphate had completed a phase III clinical trial (a six-day regimen of tedizolid 200 mg once-daily against a ten-day regimen of linezolid 600 mg twice-daily) [68]. Trius Therapeutics has partnered with Bayer HealthCare for the commercialization and development of Tedizolid phosphate outside of the U.S., Canada and the European Union.
Tomopenem (CS-023, RO4908463) is a carbapenem with improved activity against diverse hospital pathogens, including Pseudomonas aeruginosa and MRSA. Tomopenem has a half-life about twice longer than the half-lives of other carbapenems such as imipenem and meropenem. The antibacterial activity of tomopenem against the clinical isolates of MRSA and P. aeruginosa is attributed to its high affinity for PBP 2a [69]. Tomopenem is an injectable carbapenem antibiotic that was originally developed by Daiichi-Sankyo (Tokyo, Japan). Although further study is needed to provide more detailed information regarding the clinical efficacy of tomopenem against various strains, it is thought to be a promising compound for nosocomial infections [70,71,72]. Tomopenem is currently in phase II development. At present, meropenem and imipenem/cilastatin have been widely used for treatment of several nosocomial infections, but are excluded for MRSA infections. As of today over 10 new carbapenems were developed into clinical drugs; only two [entapenem (inavanz) and doripenem] were approved in 2001 and 2007, respectively, in the U.S. Although carbapenems show ultra-broad spectrum of activities, a very few are effective against MRSA in vivo. Tomopenem and ME1036 (CP5609, currently in preclinical development in the U.S.) are highly active against MRSA strains.
Cethromycin (Restanza™, ABT-773) is a once-daily ketolide antibiotic developed by Abbott and Taisho Pharmaceutical (Tokyo, Japan) for the treatment of community-associated pneumonia (CAP) and other infections caused by Gram-positive pathogens. In vitro, cethromycin displayed more potent antibacterial effects than its precursor, telithromycin. Cethromycin exhibits potent inhibition of both Gram-positive and -negative respiratory pathogens by binding to the 23S rRNA of the 50S ribosomal subunit. Cethromycin was acquired by Advanced Life Sciences Inc. (Chicago, IL) for further development. In 2007, the company successfully completed the Phase III clinical trials. Cethromycin has been tested in approximately 5,600 human subjects in clinical trials by 2008. In the same year, the company submitted a new drug application for the use of cethromycin in community-acquired bacterial pneumonia (CABP). In the following year, the FDA indicated that cethromycin cannot be approved to treat CABP and requires additional clinical data to demonstrate efficacy with defined statistical methodology. In 2010, the company reached an agreement with the FDA on the design of Phase III study of cethromycin to treat CABP. The U.S. Department of Defense has supported Advanced Life Sciences Inc to evaluate cethromycin’s efficacy in combating Category A and B bioterror agents. If cethromycin is proved to be safe with regard to hepatotoxicity, it would be a promising alternative to current standard therapy for community-associated respiratory infections, especially pneumonia [73,74,75].
EDP-420 is a bridged bicyclic ketolide that was developed by Enanta Pharamceuticals (Watertown, MA). EDP-420 is currently in clinical development for the treatment of respiratory tract infections. Extensive comparative in vivo efficacy studies were performed between EDP-420 and its parental molecule, telithromycin, against H. influenzae, S. pneumoniae, S. aureus, and S. pyogenes. EDP-420 effectively kills many strains of resistant S. pneumonia. EDP-420 possesses an excellent PK profile across multiple species, with a half-life 2–3 times longer as compared to those of clarithromycin and telithromycin. The long half-life and high systemic exposure of EDP-420 support once-daily dosage [76,77,78]. Recently, the company reported a relatively efficient large-scale synthesis of EDP-420 [79].
NXL 103 (formerly XRP 2868) is a mixture of two semi-synthetic streptogramins. This combination of semi-synthetic streptogramin antibiotics were developed by Novexel (Romainville, France). The streptogramins are naturally occurring bacteriostatic antibiotics derived from Streptomyces pristinaspiralis. Flopristin blocks an early step in protein synthesis by forming a bond with a ribosome to prevent elongation of the peptide chain. Linopristin blocks a later step by preventing the extension of peptide chains and causing incomplete chains to be released. NXL 103 is orally bioavailable streptogramines composed of a 30:70 ratio of linopristin (RPR 202868) and flopristin (RPR 132552), and has potent activity against certain Gram-positive bacteria including MRSA as well as the important respiratory pathogens including β-lactam-, macrolide-, and quinolone-resistant strains. Forward-looking results have been reported from a phase II trial for treatment of acute bacterial skin and skin structure infections [80,81,82,83]. AstraZeneca acquired Novexel in 2010 and will perform further studies of NXL 103.
Delafloxacin (RX-3341) is a fluoroquinolone antibacterial agent that was developed by Rib-X Pharmaceuticals, Inc. (New Haven, CT). Delafloxacin shows a broad spectrum of activity and is intended for use as an effective and convenient first-line therapy primarily in hospitals prior to the availability of a specific diagnosis. This molecule has the potential to offer broad-spectrum coverage as a monotherapy for serious Gram-negative and Gram-positive bacterial infections including MRSA, with both intravenous and oral formulations [84]. Phase II clinical trials have been completed. Delafloxacin met its primary and secondary efficacy endpoints based on the draft guidance from the FDA. A Phase III trial for acute bacterial skin and skin structure infections will begin shortly [85].
GSK1322322 is a novel peptide deformylase (PDF) inhibitor in the early phase of development for treatment of complicated bacterial skin and skin structure infection and hospitalized community-associated pneumonia. Peptide deformylase, a highly conserved metalloproteinase, has been observed to be critical to the maturation of proteins during translation in prokaryotic cells. Failure to remove the N-formyl group prevents the action of methionine aminopeptidase, which is essential for cell growth, but PDF is not found in mammalian protein biosynthesis. Therefore, PDF represents a selective and promising target for the development of new antibacterial agents. Although the compounds having a metal-chelating functionality inhibit the cell free enzyme, only molecules containing hydroxamic acid or N-formyl hydroxylamine exhibit reasonable antibacterial activity. Several review articles summarize known and new PDF inhibitors [86,87,88,89,90]. GSK1322322 demonstrates in vitro activity against MDR respiratory and skin infection caused by Gram-positive bacteria, including MDR Streptococcus pneumoniae and MRSA. GSK completed studies of the safety, tolerability and efficacy of GSK1322322 in subjects with acute bacterial skin and skin structure infection in a Phase II clinical trial in 2011 [91]. Previously, potent peptide deformylase inhibitors, BB-83698 and LBM415 (NVP PDF-713) were developed by British Biotech Pharmaceuticals (Oxford, UK) in collaboration with Genesoft Pharmaceuticals (South San Francisco, CA) and Novartis, respectively. Both molecules were evaluated in phase I clinical studies, but are no longer being developed for the treatment of community-associated respiratory tract infections and various uncomplicated and complicated Gram-positive infections [92, 93].
Antimicrobial peptides (host defense peptides) are small molecular weight polypeptides with broad spectrum of antimicrobial activity against bacteria, viruses, and fungi. Antimicrobial peptides are generally between 12 and 50 amino acids. They are usually positively charged and have both a hydrophobic and hydrophilic side that enables the molecule to be soluble in aqueous environments yet also enter lipid-rich membranes. The peptide kills bacterial cells through diverse mechanisms, including disrupting membranes, interfering with metabolism, and targeting cytoplasmic components. Of the potential antimicrobial peptides, a few have developed into clinical trial drugs to date [94,95]. MSI-78 (pexiganan) was the first antimicrobial peptide that was developed into a phase III clinical trial drug by Genaera Corporation [formerly Magainin Pharmaceutical Inc. (Plymouth Meeting, PA)] in collaboration with MacroChem Corporation (Lexington, MA). MSI-78 is a synthetic 22-amino-acid analogue of magainin II, a cationic peptide isolated from the skin of the African clawed frog Xenopus laevis. MSI-78 acetate cream (Locilex™) was designed to treat patients with diabetic foot infections and demonstrated its efficacy in lysing MDR bacteria including MRSA, vancomycin-resistant enterococcus (VRE) and other resistant pathogens [96]. In clinical trials, the safety profile of MSI-78 was proved. Importantly, no antimicrobial resistance against MSI-78 has been reported to date. Unfortunately, the U.S. FDA rejected topical application of Locilex™ for the treatment of diabetic foot ulcers in 1999. Dipexium Pharmaceuticals (White Plains, NY) acquired the rights to Locilex™ in 2010 and further developed formulation of MSI-78. The company filed updated CMC Amendment with FDA in 2012 which includes several new data of the enhanced formulation of MSI-78 [97].
2.2. Non-antibiotic therapeutics in clinical trials for MRSA and related bacterial infections
OligoG CF-5/20 is a potential antibacterial agent against lung infections in cystic fibrosis (CF) patients. OligoG CF-5/20 is an alginate oligomer, consisting of 1,4-linked β-D-mannuronic acid and α-L-guluronic acid residues. Alginate oligomers have been widely used as thickening and gelling agents in food-industry and medical products. Small fragments of these polysaccharides have recently shown to have immunomodulating activity. S. aureus, Haemophilus influenzae, and Pseudomonas aeruginosa are the most common organisms causing lung infections in CF patients. AlgiPharma (Sandvika, Norway) demonstrated that OligoG CF-5/20 could disrupt P. aeruginosa biofilms. In addition, the company demonstrated better biofilm susceptibilities of series of antibacterial agents in the presence of OligoG CF-5/20. Although more studies are required for evaluating effectiveness of OligoG CF-5/20 against other bacteria that cause lung infections in CF, inhaled OligoG CF-5/20 has shown to reduce sputum from CF patients in a phase I clinical study [98,99]. This non-toxic oligosaccharide may be a very useful asset to develop combination therapy with other antimicrobial agents including anti-MRSA drugs (an expert’s opinion).
F-598 is a human IgG mono-clonal antibody that was developed by Alopexx Phamaceuticals (Cambridge, MA). F-598 targets against MRSA and other serious infectious diseases. Monoclonal antibodies are not expected to cause bacterial resistance to the therapy. The target of F-598 is a carbohydrate on the bacterial capsule (PNA). Sanofi (Paris, France) elected to exercise its option to acquire an exclusive and worldwide license of Alopexx’s F-598 antibody. To date, several disappointing results of IgG mono-clonal antibody therapies have been reported. However, vaccine/antibody therapies are ultimate assets to combat the pathogens (e.g. MRSA) that are resistant to current antibacterial agents [100,101,102,103].
Phage therapy is the application of “bacterium-specific” viruses with the goal of reducing or eliminating pathogenic bacteria. It is therefore necessary in many cases to take a swab from the patient and culture the bacteria prior to treatment. Occasionally, isolation of therapeutic phages can require a few months to complete, but clinics generally keep supplies of phage cocktails for the most common bacterial strains in a geographical area. Phages in practice are applied orally, topically on infected wounds or spread onto surfaces, or used during surgical procedures. The direct human use of phages is likely to be very safe; suggestively, in August 2006, the U.S. Food and Drug Administration approved spraying meat with phages (targeting against Listeria monocytogenes). Historically, the first human phage therapy took place in the 1920s. By the 1940s, the human phage therapy was in steep decline despite early promise. On the other hand, the phage therapy traditions and practice have been continued in the former Soviet Republic of Georgia, and Phage Therapy Center in Tbilisi Georgia is accepting patients. Kutter et al. summarized the phage therapy in clinical practice in their article [104]. Several articles regarding phage therapy indicate that more clinical and microbiological research is needed to meet current standards. Nonetheless, in 2007 a Phase I and II clinical trials were completed at the Royal National Throat, Nose and Ear Hospital, London for Pseudomonas aeruginosa infections (otitis). Phase I clinical trials have been completed in the Southwest Regional Wound Care Center, Lubbock, Texas for an approved cocktail of phages against bacteria, including P. aeruginosa, S. aureus and E. coli. The phage cocktails for the clinical trials were developed and supplied by Intralytix (MD, USA) [105,106].
3. Drug leads for MRSA and other Gram-positive bacterial infections
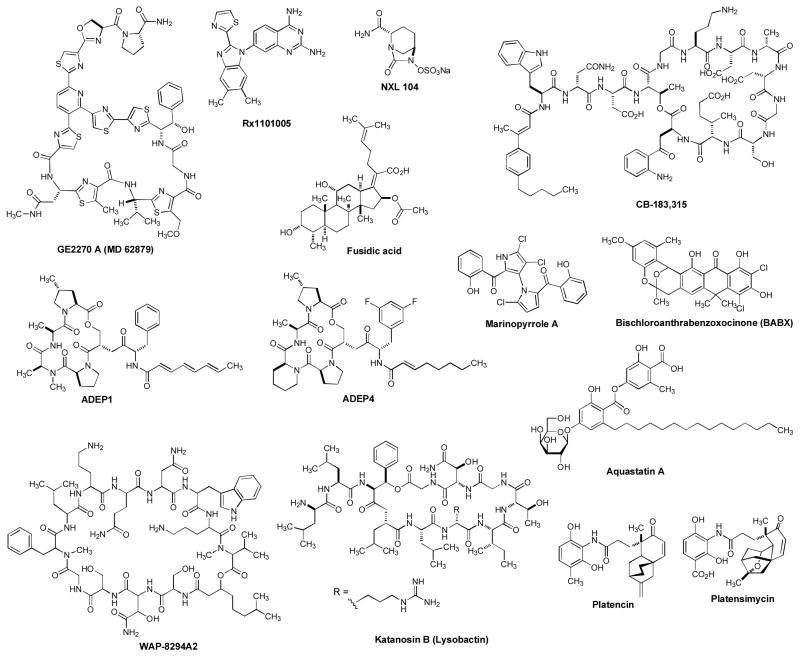
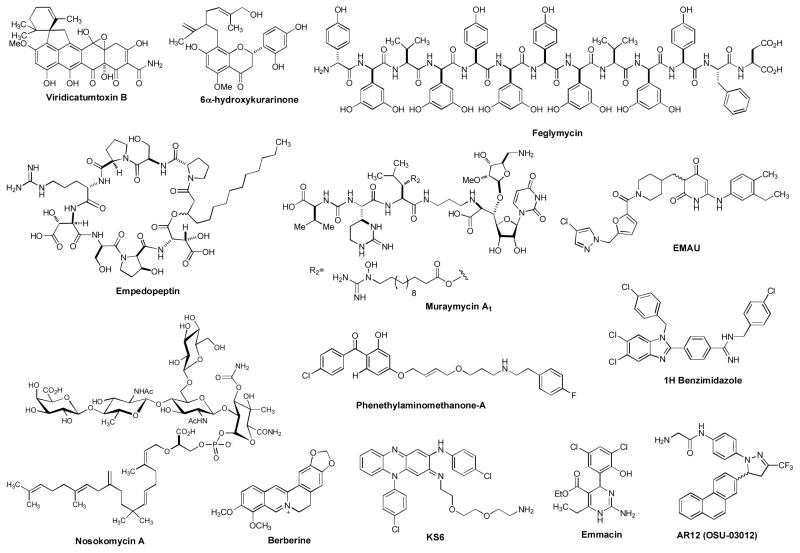
Natural products have historically been a rich source for discovery of new antibacterial agents [107,108]. Some antibiotics were discovered several decades ago, but further development was discontinued due to the change of business strategies in pharmaceutical industries or to the finding of their inappropriate physicochemical characteristics as drug candidates (e.g. undesired toxicities in vivo, poor PK/PD profile, or low-therapeutic index). Over the past decade, significant advances have been made in synthetic organic chemistry, pharmaceutical formulations, and drug delivery systems for drug discovery. Drug discovery programs via such modern drug discovery platforms have resulted in reinvestigation of previously discovered molecules to be the FDA approved drugs or clinical trial drugs [109,110]. This chapter summarizes investigational drugs for Gram-positive pathogens, natural product-based molecules, and new small molecule inhibitors that have the potential to be new anti-MRSA agents (Figure 3).
Figure 3.
Drug leads for MRSA and other related bacterial infections
GE2270 A (MD 62879) is an antibiotic, isolated from the fermentation broth of a strain of Planobispora rosea. Structural characteristics showed similarities between GE2270 A and thiazolyl peptide natural products such as micrococcin P1. Micrococcin P1 is known to have powerful antibiotic, anticancer, and antimalarial activities. Moreover, micrococcin P1 is active against microorganisms resistant to vancomycin. The antibacterial activity of micrococcin P1 is attributed to inhibition of elongation factor G (EF-G) and initiation factor 2 (IF2) that prevent the binding of EF-G to the ribosome. A group at Novartis reinvestigated GE2270 A and its derivatives for Gram-positive bacterial infections. The water solubility of two analogs of GE2270 A was significantly improved and these molecules showed very strong anti-staphylococcal activities (0.06–0.25 μg/mL against a MRSA strain). In vivo studies have been demonstrated that two GE2270 A analogs are potent antibacterial agents with efficacious doses at concentrations lower than those of daptomycin. The low frequency of resistance and lack of cross-resistance with other antibacterial agents in clinical use highlight the potential of this class of natural products to develop into new drug to combat drug resistant Gram-positive pathogens including MRSA [108,111].
Rx101005 is a new dihydrofolate reductase (DHFR) that was developed by Trius Therapeutics (San Diego, CA) that shows strong antimicrobial activities against S. aureus including trimethoprim (TMP) resistant strain (F99Y mutant). This compound has a reasonable pharmacokinetic profile and exhibited in vivo dose-dependent efficacy in a S. aureus infected mouse model. Folate biosynthesis is one of the four established drug targets in antibacterial drug development and its inhibitor is expected to have synergistic effect with a sulfa drug such as sulfamethoxazole (SMX) [112,113]. Indeed, Rx101005 showed synergistic effects against S. aureus with SMX at lower concentrations than the known combination drugs of TMP/SMX [114].
NXL104 is an injectable broad-spectrum β-lactamase inhibitor that was originally developed by Novexel (Romainville, France). This company was acquired by AstraZeneca in 2010. In combination with the cephalosporin antibiotic, ceftazidime, NXL104 has demonstrated activity against a broad-range of Gram-negative bacterial pathogens. The phase II clinical trials with the NXL104/ceftazidime combination were conducted in adult patients with complicated urinary tract infections in the U.S. Preclinical data indicated that NXL104 inhibits both class A (inhibited by approved β-lactamase such as tazobactam, clavulanate, and sulbactam) and class C β-lactamases. NXL104 can also inhibit carbapenemases. Ceftaroline is a new-generation cephalosporin (vide supra) and active against MRSA and pneumococci, but is labile to common β-lactamases, including AmpC and extended-spectrum types. NXL104 has been applied with ceftaroline to counteract β-lactamases. Thus, NXL104 and other type of β-lactamase inhibitors have great potential to improve efficacy of β-lactam antibacterial drugs [115,116,117,118]. Alternatively, boron-based β-lactamase inhibitors (e.g. benzo[b]-thiophene-2-boronic acid derivatives) have been designed and tested their efficacies against Gram-positive and -negative bacteria [119].
CB-183,315 is a daptomycin analog that was developed by Cubist Pharmaceuticals (Lexington, MA). CB-183,315 has been studied for the treatment of a severe and life-threatening diarrhea caused by Clostridium difficile and C. difficile-associated diarrhea. The phase II clinical trial for CB-183,315 was completed in 2011. Not surprisingly, CB-183,315 showed excellent activity against MRSA and other Gram-positive pathogens. Since approval of daptomycin for the treatment of complicated skin and soft tissue infections caused by susceptible strains of S. aureus, including MRSA, and other Gram-positive bacteria in 2003, only a few strains of S. aureus isolates had decreased susceptibility to daptomycin [120,121]. However, the increased prevalence of drug resistant strains due to the extensive use of daptomycin as a monotherapy is an expected natural phenomenon. Whole genome analyses of daptomycin nonsusceptible strains revealed that they had mutations in phospholipid biosynthesis genes (mprF and pgsA) that resulted in formation of a thicker cell wall and increase in membrane lysyl-phosphatidylglycerol [122,123]. Discovery of new daptomycin analogs for nonsusceptible strains will be continued.
Fusidic acid is an antibiotic that has been used to treat a number of bacterial skin infections since 1960s. The molecule has a steroid-like structure but does not possess any steroidal activity. Fusidic acid has been applied as cream and ointment for skin infections caused by drug sensitive and resistant S. aureus. Fusidic acid has been widely used in Europe, Canada, Australia and some Asian countries for decades [124]. Although this drug has not been approved for use in the U.S., Cempra Pharmaceuticals (Morrisville, NC) has designed a dosing regimen (the intravenous formulation) for the use of sodium fusidate (TAKSTA™) as monotherapy. Fusidic acid prevents the turnover of elongation factor G (EF-G) from the ribosome that ultimately inhibits protein biosynthesis. Significantly, the prevalence of resistance to fusidic acid in S. aureus remained low for decades after its clinical introduction [125].
Acyldepsipeptides (ADEPs) have strong antibacterial activity against Gram-positive bacteria in vitro (MIC 0.01–0.05 μg/mL) and in several rodent models of bacterial infections. In addition, ADEPs showed potent antibacterial activity against multidrug-resistant bacteria. A mechanism of action of ADEP does not fall into the known targets of other antibacterial drugs approved by FDA, but involves inhibition of cell division. Brötz-Oesterhelt et al. determined that ADEPs inhibited the function of the caseinolytic proteases (Clp proteases) [126]. ADEPs were realized to target the proteolytic core of Clp, ClpP. Clp is a critical bacterial intracellular protease that regulates protein turnover in many bacterial species. Clp proteases are comprised of ClpP and ClpX, which are regulatory ATPases [127,128]. A mixture of eight ADEPs were first isolated from Streptomyces hawaiiensis NRRL 15010 in 1985. The congeners of ADEPs, enopeptin A and B, were isolated from a culture broth of Streptomyces spp. in 1991. The core structure of ADEPs is a cyclic depsipeptide composed of only 5 amino acids, and the congeners of ADEPs can be diversified by hydrophobic side chain via chemical synthesis. Total chemical synthesis of ADEPs has been reported by several research groups and the SAR study of ADEPs was performed by Hinzen et al. in 2006 [129]. Chemically more stable ADEP4 exhibited equal or superior enzyme and bacterial growth inhibitory activities. Although extensive in vivo toxicity tests need to be performed for ADEPs, biological activities and pharmacological properties of ADEPs attract a number of scientists for the development of a novel antibacterial agent for untreatable infections caused by Gram-positive pathogens including MRSA.
Marinopyrrole A is a recently reported alkaloid endowed with promising antibiotic activities against MRSA. Marinopyrrole A showed less toxicity to mammalian cell lines even at concentrations of > 20 × MIC. A variety of marinopyrrole analogs have been isolated from marine-derived Streptomyces. However, high protein binding characteristics of these molecules have been reported. It is likely that marinopyrrole A could be used as a topical agent or in local therapy of device-related Gram-positive bacterial infections [130,131,132].
Aquastatin A is a natural product isolated from Sporothrix spp. FN611. Aquastatin A showed moderate enzyme inhibitory activities against S. aureus FabI (IC50 3.2μM) and a FabK isoform, enoyl-ACP reductase. Aquastatin A also exhibited growth inhibitory activity against MRSA [133]. Fatty acid biosynthesis in bacteria is crucial for the production of a number of lipid-containing components, including the cell membrane. The bacterial fatty acid system (FAS II) employs discrete monofunctional enzymes which operate in conjunction with acyl carrier protein (ACP)-associated substrates, whereas mammalian fatty acid synthase (FAS I) is a multidomain, multifunctional homodimeric protein (a single multifunctional enzyme-ACP complex). FAS I carries out all of the enzymatic steps needed for de novo synthesis of long chain fatty acids. Thus, the differences in prokaryote and eukaryote fatty acid biosynthesis provide an attractive opportunity for the development of new antibacterial agents. Cerulenin (FabF/B inhibitor), thiolactomycin and triclosan are known FAS II inhibitors that have been utilized as investigational tools [134]. To date, several structurally interesting natural products have been identified as FAS II enzyme inhibitors. Bischloroanthrabenzoxocinone (BABX) showed in vitro enzyme inhibitory activity with IC50 values of 11.4 and 35.3 μg/ml in the S. aureus and E. coli FAS II assays. BABX also showed potent antibacterial activities against S. aureus and permeable E. coli strains with MICs ranging from 0.2 to 0.4 μg/ml [135,136]. Homodimeric naphthopyranone natural products (e.g. cephalochromin), vinaxanthone, epigallocatechin gallate (EGCG), and flavonoids were identified as natural Fab I inhibitors [137]. One of the most attractive FAS II inhibitors from natural resources is platencin or its analog platensimycin that were isolated Streptomyces platensis in 2007. They are potent and non-toxic natural products active against Gram-positive pathogens, including drug-resistant strains and Mycobacterium tuberculosis. They are very strong inhibitors of fatty acid biosynthesis of type II. Platensimycin inhibits the elongation-condensing enzyme FabF, whereas platencin inhibits both FabF and FabH. For these antibiotics to become successful drugs, their pharmacokinetics must be improved [138]. They have too high clearance rate in the body, yielding a low degree of systemic exposure [139]. Nonetheless, the natural products that showed FAS II inhibitory activities will be useful pharmacophore to develop novel antibacterial agents for MRSA infections.
WAP-8294A2 is a complex depsipeptide antibiotic isolated from a fermentation broth of Lysobacter spp. WAP-8294A2 was active against Gram-positive bacteria including MRSA (MIC 0.78 μg/mL), and did not show acute toxicity in mice when the molecule was administered via oral (200 mg/kg), intravenous (50 mg/kg), and intraperitoneal (100 mg/kg) route. In addition, WAP-8294A2 was active against MRSA in vivo using a mouse infection model. WAP-8294A2 seems to target phospholipids in bacterial cell membrane, resulting in bacterial cell membrane damage. These forward-looking in vitro and in vivo data for WAP-8294A2 warrant further investigation [140].
Berberine is an isoquinoline alkaloid found in plants such as Berberis spp. Berberine is a natural dye (a color index of 75160) that shows a strong yellow fluorescence under a UV light. As a traditional medicine or dietary supplement, berberine has shown a wide range of biological activities against fungal, parasites, and bacterial, and viral infections. Berberine showed antimicrobial activity against MRSA strains with the MIC values of 32 to 128 μg/mL. Although the bacterial growth inhibitory activity is moderate, berberine was found to exhibit synergistic effect with oxacillin. Lack of apparent toxicity of berberin could be a very unique additive for a certain antibacterial chemotherapy for MRSA infections [141].
Katanosin B (Lysobactin) is a depsipeptide antibiotic that has displayed strong antibacterial activity against MRSA and the other drug resistant Gram-positive bacteria such as vancomycin-resistant enterococci with (MICs) ranging from 0.39 to 0.78 μg/mL. Katanosin B and its congeners (e.g. plusbacin A3) have been isolated from a strain related to the genus Cytophaga and a strain of Pseudomonas. Significantly, they showed therapeutic effects in mice infected with S. aureus when administered subcutaneously. Katanosin B inhibits peptidoglycan formation; however, this activity was not antagonized in the presence of N-acyl-L-Lys-D-Ala-D-Ala, the binding domain of vancomycin. Thus, the mode of action of katanosin B is different from vancomycin, and the natural products in this family are promising agents to develop a new class of antibacterial agent against drug resistant strains [142,143,144].
Viridicatumtoxin B was isolated as an anti-MRSA agent from Penicillium spp. FR11 in 2008. Viridicatumtoxin B is structurally related to a tetracycline analog, viridicatumtoxin, isolated from P. viridicatum. Viridicatumtoxin B inhibited the growth of drug-sensitive and -resistant S. aureus with the MIC value of 0.5 μg/mL. Toxicological and pharmacological studies of viridicatumtoxin B are necessary to develop this molecule as an anti-MRSA agent [145].
Kurarinons are bioactive ingredients in Sophora flavescens which have been utilized as an antibacterial herb in Chinese traditional medicine. For example, 6a-hydroxykurarinone is a prenylated flavone that shows anti-MRSA activity (MICs 2–8 μg/mL) with lower cytotoxicity than other flavanones [146]. These findings encourage reinvestigation of anti-staphylococcal flavones.
Muraymycins inhibit lipid I formation in peptidoglycan biosynthesis and have demonstrated activity against Gram-positive bacteria and MRSA (MIC, 2 μg/mL). Animal efficacy studies indicated that muraymycin A1 was active in a S. aureus lethal-infection model (50% effective dose, 1.1 mg/kg) [147,148]. To date, a large number of uridine-containing natural products that show significant antibacterial activities have been isolated from the culture of Streptomyces spp. Ribosamino-uridine-containing compounds (liposidomycins, caprazamycins, muraymycins, and FR-900493) are not toxic (or low toxic) and are specific inhibitors of MraY, which catalyzes the transformation of Park’s nucleotide to lipid I [149]. Several simplified analogs of the complex natural products have been demonstrated their powerful inhibitory activity against MraY, along with encouraging antibacterial activities [150,151,152,153]. Since peptidoglycan (PG) is an essential bacterial cell-wall polymer, the machinery for PG biosynthesis provides a unique and selective target for antibiotic action. However, only a few enzymes in PG biosynthesis such as the penicillin binding proteins (PBPs) are extensively studied. Thus, the machinery for PG synthesis is still considered to be a source of unexploited drug targets. Although no drug has been developed based on the other early stages of PG biosynthesis enzymes (MurB, C, D, E, and F, MraY, and MurG) to date, these enzymes have been considered as targets of interest for the discovery of novel antibacterials. MurA is pharmacologically validated; fosfomycin is a clinically useful antibiotic which interferes with MurA. Over 60 publications and patents, which described the development of inhibitors against earlier PG biosynthetic enzymes including MurA-F, MraY, and MurG, are identified in 2000–2012.
Empedopeptin (BMY-28117) is a natural lipodepsipeptide antibiotic with potent antibacterial activity against multidrug-resistant Gram-positive bacteria including MRSA and penicillin-resistant Streptococcus pneumoniae in vitro and in animal models of bacterial infection. Empedopeptin selectively interferes with late stages of cell wall biosynthesis by forming the complex with lipid II (a 1:2 molar stoichiometry) in the presence of calcium ions. Details of toxic effects of empedopeptin have not been reported other than lethal dose value [(ED50 560 mg/kg mouse (IV)]. Empedopeptin is not absorbed orally, thus, may be developed as an injection drug or as a topical medicine [154,155].
Feglymycin is a peptide antibiotic discovered from Streptomyces spp. DSM 11171 and displayed activities against Gram-positive bacteria including MRSA (MIC 2 μg/mL against MRSA). Importantly, feglymycin exhibited no cytotoxicity against a mammalian cell line at high concentrations. A complete lack of cross-resistance of feglymycin strongly suggests a novel mode of action. Feglymycin killed MRSA by targeting MurA and MurC enzymes at low μM concentrations [156,157,158]. Linear peptides have generally problems with stability against metabolic enzymes in host and pharmacokinetics. However, feglymycin consists of only 7 different amino acids and all building blocks except for D-3,5-dihydroxyphenylglycine are commercially available. Thus, feglymycin is amenable to medicinal chemistry to improve PK/PD properties and metabolic stability.
Nosokomycins were discovered from a culture broth of Streptomyces spp K04-0144 that inhibited the growth of MRSA with MIC values of 0.125 μg/mL. Nosokomycin A was found to be effective in an in vivo system using a mouse model. Nosokomycins are structurally related to moenomycins, a known inhibitor of the transglycosylase of penicillin-binding protein [159].
Emmacin is an anti-MRSA molecule that was identified from a 223-membered library molecule. Emmacin did not display apparent in vitro cytotoxicity at high concentrations and inhibits S. aureus proliferation by acting as a prokaryote-selective dihydrofolate reductase (DHFR) inhibitor. This molecule is a new structural subclass of bacterial DHFR inhibitor, which may potentially be exploited in the development of new antibacterial agents for Gram-positive pathogens [160,161,162].
1H-Benzimidazolecarboxamidines were found to exhibit strong anti-MRSA activity (MIC, 0.78 μg/mL). A series of 1H-benzimidazolecarboxamidines also showed activities against the other drug resistant S. aureus (e.g. vancomycin-resistant enterococci). The mode of action of these molecules has not been thoroughly studied. However, some amidinobenzimidazoles have been identified as inhibitors of bacterial KinA/Spo0F two-component system that includes a histidine protein kinase and a response regulator. Some histidine protein kinases are essential for Gram-positive bacterial growth. 1H-Benzimidazolecarboxamidines have potential for development as a new class of potent anti-MRSA drug [163,164].
Phenethylaminomethanone-A exhibited growth of drug resistant Gram-positive bacteria including S. aureus macrolide-resistant strain, vancomycin-resistant E. faecalis, and MRSA at low concentrations (MICs 2–4 μg/mL). The molecule kills Gram-positive bacteria by targeting MenA (a menaquinone biosynthesis enzyme). The lipid-soluble electron carriers (lipoquinones) occupy a central and essential role in electron transport, and thus, ATP synthesis. The lipoquinones involved in the respiratory chains of bacteria consist of menaquinones and ubiquinones. Majority of Gram-positive bacteria utilize only menaquinone in their electron transport systems, and menaquinone biosynthesis is essential for survival of non-fermenting Gram-positive bacteria. On the other hand, in the electron transport systems Gram-negative organisms utilize ubiquinone (CoQ) under aerobic conditions and menaquinone under anaerobic conditions. Moreover, the electron transport chain in humans does not utilize menaquinone. Therefore, inhibitors of menaquinone biosynthesis or specific inhibitors of enzymes associated with electron transport systems have great potential to develop novel and selective drugs against MDR Gram-positive pathogens. Phenethylaminomethanone-A will be a very useful pharmacophore to develop pharmacologically acceptable new MenA inhibitors [165,166,167,168,169].
KS6 is a water soluble clofazimine (CFZ) analog that exhibits antibacterial activity against Mycobacterium tuberculosis and Gram-positive bacteria. Clofazimine is a riminophenazine dye and has been used in combination with rifampicin and diamino-diphenyl sulfone (dapsone) for the treatment of leprosy [158]. The mechanism of action of CFZ is rather unique. Clofazimine inhibits type II NADH oxidoreductase (NDH-2) that ultimately results in inhibition of ATP synthesis. NDH-2 does not exist in human mitochondria, and thus KS6 is a selective bacterial electron transport system inhibitor [170].
EMAU are series of N-3-alkylated 6-anilinouracil analogs that have been identified as selective inhibitors of bacterial DNA polymerase IIIC (Pol IIIC). Pol IIIC is one of the two essential replication-specific DNA polymerases for the replication of chromosomal DNA in Gram-positive bacteria. One of EMAU showed the MIC value of 4 μg/ml against a series of Gram-positive bacteria including S. aureus. An analog of EMAU also showed promising in vivo antibacterial efficacy in a staphylococcal sepsis model in mice [171].
AR-12 (formerly OSU-03012) is an inhibitor of PDK-1, phosphatidyl inositol-3-kinase (PI3K)/Akt pathway. This molecule was also found to inhibit growth of series of bacteria including MRSA. AR-12 is an orally available anti-cancer phase I clinical drug. Structural simplicity and a new mode of action of AR-12 make it an attractive chemical entity for the development of new MRSA drug [172].
4. Conclusion
According to the CDC, more than 90,000 Americans become infected each year with life-threatening infections caused by drug-resistant MRSA. Several clinical options that currently exist to treat MRSA are summarized in Introduction [173]. MRSA treatment guideline was prepared by an Expert Panel of the Infectious Diseases Society of America (IDSA) [174]. A substantial rate of clinical failures has been reported for the treatment of MRSA infections with vancomycin due to the complex pharmacokinetic profile of vancomycin. However, with limited choices for the treatment of serious MRSA infections, clinicians have traditionally relied on vancomycin. At this moment, therapy using linezolid alone is quite expensive; the cost for a course of treatment ranges from several thousands of dollars. The MRSA therapeutic market still offers significant opportunities for new therapies with notable improvements over current treatment options. As summarized in Section 2, the number of pipeline drugs presently undergoing clinical trials is not particularly encouraging. Some critics regarding the current pipeline drugs for bacterial infections have been reported [22]. New antimicrobials should provide clear benefit and advances in treatment of infections compared to currently available therapies. Most MRSA drugs that are currently in the late-stage phase clinical trials may not dramatically advance in their ability to treat the infections caused by MRSA. To date, a large number of natural products and small molecules that effectively inhibit growth of MRSA and other Gram-positive bacteria have been reported. The drug leads summarized in Section 3 require significant efforts to obtain critical pharmacological data or to improve their structure to be exploitable drug leads. We have to emphasize that it is not possible to summarize all anti-MRSA agents reported in literatures and/or poster presentations. In this review, we aim to summarize 1) promising drug targets for MRSA infections with specific inhibitor molecules (validated molecular targets), and 2) anti-MRSA agents that have not extensively been studied. The list of molecules in Section 3 includes a new DHF inhibitor, β-lactamase inhibitors, FAS II inhibitors, early-stage peptidoglycan biosynthesis inhibitors, an electron transport inhibitor, a menaquinone biosynthesis inhibitor, PolIIIC inhibitors, a bacterial two-component system inhibitor, molecules that interfere with bacterial cell membrane, and antibiotics possessing strong antistaphylococcal activity and/or unique antibacterial characteristics. In order to develop “truly novel anti-MRSA agents” (novel molecules that kill MRSA by targeting new molecular targets), increasing investments by private or public sponsors are apparently required. It is very important to support exploratory/developmental researches aiming to discover novel and progressable drug leads against unexploited molecular targets found in MRSA [22]. The exploration to discover novel treatment options for MRSA infections should widely be performed in both academic and industrial research laboratories.
5. Expert Opinion
Despite the availability of anti-MRSA drugs, therapeutic options to treat MRSA infections are limited. A few investigational drugs are novel and many are modifications of known antibacterial agents. However, a combination therapy with front-line and/or next-generation front-line drugs is very important to combat MRSA strains that cannot be treated with monotherapy. For example, sulfa drugs are still the drugs of choice used in combination with the DHFR inhibitor for the treatment of MRSA and Pneumocystis carinii infections in HIV [175,176,177,178,179,180]. Based on the demonstrated clinical efficacy and excellent safety profile of FDA-approved drugs, industries have continued to drive towards drug discovery programs aimed at discovering the next-generation drugs with improved pharmacological profile that are effective against drug-resistant S. aureus strains [30,181]. MRSA infections often require systemic drug therapy. Aminoglycosides have played an important role as combination drugs for cell-wall biosynthesis inhibitor, DNA gyrase inhibitor, or folate biosynthesis inhibitor molecules [182,183]. As such, aminoglycosides have been important additional drugs for β-lactam antibiotics and vancomycin in the management of MRSA infections. Accordingly, we believe that reinvestigations of aminoglycosides (e.g. arbekacin) having potent activity against Gram-positive bacteria will result in finding a new combination chemotherapy regimen for MRSA infections. Needless to say, oral chemotherapy offers several advantages over systemic chemotherapy, but not all antibacterial agents. Development of orally bioavailable anti-MRSA drugs will continue by improving PK/PD profiles of FDA-approved drugs and by discovering new small (or synthetically amenable) molecules that interfere with unexploited essential bacterial drug targets.
To date, many researchers have reported antistaphylococcal vaccines and immunoglobulins that are effective in vitro. However, both active and passive immunization strategies have thus far failed to show efficacy in humans. Several other factors that cause the failure of S. aureus vaccine therapy are discussed in an article reported by Bagnoli et al. [184]. Because the vaccine strategy is believed to be an ultimate protection from MRSA infections, efforts to develop effective S. aureus vaccine effective in humans should continue. As stated in Section 2, phage therapy has been attempted for the treatment of a variety of bacterial infections, and to date, a large number of phase clinical studies have been reported. Despite clinical use precedence in Georgia and some other countries, efficacy of phage therapy for infections is still a matter of controversy [104]. Much research has to be done to establish effective phage therapy for MRSA infections in clinical use. Phage therapy is highly specific to bacterial spp. and is safe in humans. These favorable characteristic of bacteriophages should encourage developing more attractive therapeutic agents for MRSA infections.
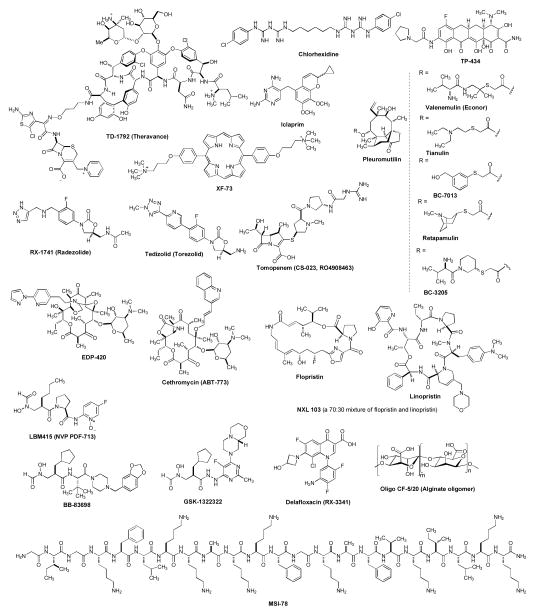
Figure 2.
Clinical trial drugs for MRSA and other related bacterial infections.
Article highlights.
Drugs for the treatment of MRSA infections
Clinical trial drugs for MRSA and other related bacterial infections
Drug leads for MRSA and other Gram-positive bacterial infections
Comprehensive reviews of antistaphylococcal agents
This box summarizes key points contained in the article.
Acknowledgments
The authors thank the reviewers’ useful suggestions and critiques to improve this manuscript. Some of data described in the article were generated from the studies supported by the National Institutes of Health (NIAID grant AI084411) and University of Tennessee.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Grenet K, Guillemot D, Jarlier V, et al. Antibacterial resistance, Wayampis Amerindians, French Guyana. Emerg Infect Dis. 2004;10:1150–4. doi: 10.3201/eid1006.031015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Cosgrove SE, Fowler VG. Management of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:S386–93. doi: 10.1086/533595. Overview of the management of MRSA. [DOI] [PubMed] [Google Scholar]
- 3.Cunha BA. Vancomycin revisited: a reappraisal of clinical use. Critical Care Clinics. 2008;24:393–420. doi: 10.1016/j.ccc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 4•.Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti-Infect Ther. 2007;5:961–81. doi: 10.1586/14787210.5.6.961. Review of MRSA infections. [DOI] [PubMed] [Google Scholar]
- 5.Tacconelli E, Cataldo MA. Antimicrobial therapy of Staphylococcus aureus bloodstream infection. Exp Opin Pharmacother. 2007;8:2505–18. doi: 10.1517/14656566.8.15.2505. [DOI] [PubMed] [Google Scholar]
- 6.Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42:389–91. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Healthcare Epidemiol. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 8.Kluytmans-Vabdenbergh MFQ, Kluytmans JAJW. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clini Micro Infect. 2006;12:9–15. doi: 10.1111/j.1469-0691.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 9.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Diseas. 2007;13:1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eady EA, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin-resistant Staphylococcus aureus-an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003;16:103–24. doi: 10.1097/00001432-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 12.Lina G, Piémont Y, Godail-Gamot F. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 13.Diederen BMW, Kluytmans JAJW. The emergence of infections with community-associated methicillin resistant Staphylococcus aureus. J Infect. 2006;52:157–68. doi: 10.1016/j.jinf.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14•.Barbachyn MR, Ford CW. Oxazolidinone structure-activity relationships leading to linezolid. Angew Chem Int Eng. 2003;42:2010–23. doi: 10.1002/anie.200200528. Overview of the oxazolidinone antibacterial agent. [DOI] [PubMed] [Google Scholar]
- 15••.Wilcox MH. Update on linezolid: the first oxazolidinone antibiotic. Exp Opin Pharmacother. 2005;6:2315–26. doi: 10.1517/14656566.6.13.2315. The oxazolidinone antibacterial agent. [DOI] [PubMed] [Google Scholar]
- 16.Schentag JJ, Hyatt JM, Carr JR, et al. Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium, and the importance of antibiotic management and infection control. Clin Infect Dis. 1998;26:1204–14. doi: 10.1086/520287. [DOI] [PubMed] [Google Scholar]
- 17•.Donadio S, Soiso M. Biosynthesis of glycopeptides: Prospects for improved antibacterials. Curr Top Med Chem. 2008;8:654–66. doi: 10.2174/156802608784221541. Interesting review of glycopeptide antibiotics. [DOI] [PubMed] [Google Scholar]
- 18.Pace JL, Yang G. Glycopeptides: Update on an old successful antibiotic class. Biochem Pharmacol. 2006;71:968–80. doi: 10.1016/j.bcp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Gales AC, Sader HS, Andrade SS, et al. Emergence of linezolid-resistant Staphylococcus aureus during treatment of pulmonary infection in patient with cystemic fibrosis. International J Antimicro Agents. 2007;27:300–2. doi: 10.1016/j.ijantimicag.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20••.Woodworth JR, Nyhart EH, Brier GL, et al. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 1992;36:318–25. doi: 10.1128/aac.36.2.318. Overview of the daptomycin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henken S, Bohling J, Martens-Lobenhoffer M, et al. Efficacy profiles of daptomycin for treatment of invasive and noninvasive pulmonary infections with Streptococcus pneumonia. Antimicrob Agents Chemother. 2010;54:707–17. doi: 10.1128/AAC.00943-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthony KB, Fishman NO, Linkin DR, et al. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46:567–70. doi: 10.1086/526775. [DOI] [PubMed] [Google Scholar]
- 23.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 24.Rybak MJ. The Pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42:S35–9. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 25.Robles-Piedras AL, González-López EH. Therapeutic drug monitoring of vancomycin. Proc West Pharmacol Soc. 2009;52:21–3. [PubMed] [Google Scholar]
- 26.Micek ST. Alternatives to vancomycin for the treatment of Methicillin-Resistant Staphylococcus aureus Infections. Clin Infect Dis. 2007;45:S184–90. doi: 10.1086/519471. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Ioanas M, De Roux A, Lode H. New antibiotics for the treatment of severe staphylococcal infection in the critically ill patient. Curr Opin Crit Care. 2005;11:481–6. doi: 10.1097/01.ccx.0000176690.18433.22. [DOI] [PubMed] [Google Scholar]
- 28.Rayner C, Munchhof WJ. Antibiotics currently used in the treatment of infections caused Staphylococcus aureus. Inter Med J. 2005;35:S3–16. doi: 10.1111/j.1444-0903.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- 29.Safety information of tygeciline, see US FDA web site at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm224626.htm
- 30.Jacqueline C, Navans D, Batard E, et al. In vitro and in vivo synergistic activities of linezolid combined with subinhibitory concentrations of imipenem against methicillin-resistant Staphylococcus aureus. Antimicro Agents Chemothe. 2005;49:45–51. doi: 10.1128/AAC.49.1.45-51.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollef MH. New antimicrobial agents for methicillin-resistant Staphylococcus aureus. Crit Care Resusc. 2009;11:282–6. [PubMed] [Google Scholar]
- 32.Kanafani ZA, Corey GR. Ceftaroline: a cephalosporin with expanded Gram-positive activity. Future Microbiol. 2009;4:25–33. doi: 10.2217/17460913.4.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Steed ME, Rybak MJ. Ceftaroline: A new cephalosporin with activity against resistant Gram-positive pathogens. Pharmacotherapy. 2010;30:375–89. doi: 10.1592/phco.30.4.375. [DOI] [PubMed] [Google Scholar]
- 34.García MS, Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303:2260–4. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 35.Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis. 2009;49:1908–14. doi: 10.1086/648438. [DOI] [PubMed] [Google Scholar]
- 36.Donadio S, Maffioli S, Monciardini P, et al. Antibiotic discovery in the twenty-first century: current trends and future perspectives. J Antibiot. 2010;63:423–30. doi: 10.1038/ja.2010.62. [DOI] [PubMed] [Google Scholar]
- 37.Belley A, McKay GA, Arhin FF, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother. 2010;54:5369–71. doi: 10.1128/AAC.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clini Infect Diseas. 2012;54:S214–19. doi: 10.1093/cid/cir920. [DOI] [PubMed] [Google Scholar]
- 39.Chen AY, Zervos MJ, Vazquez JA. Dalbavancin: a novel antimicrobial. Int J Clin Pract. 2007;61:853–63. doi: 10.1111/j.1742-1241.2007.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blais J, Lewis SR, Krause KM, et al. Antistaphylococcal activity of TD-1792, a multivalent glycopeptide-cephalosporin antibiotic. Antimicrob Agents Chemother. 2012;56:1584–7. doi: 10.1128/AAC.05532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leuthner KD, Vidaillac C, Cheung CM, et al. In vitro activity of the new multivalent glycopeptide-cephalosporin antibiotic TD-1792 against vancomycin-nonsusceptible Staphylococcus isolates. Antimicrob Agents Chemother. 2010;54:3799–803. doi: 10.1128/AAC.00452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde SS, Okusanya OO, Skinner R, et al. Pharmacodynamics of TD-1792, a novel glycopeptide-cephalosporin heterodimer antibiotic used against Gram-positive bacteria, in a neutropenic murine thigh model. Antimicrob Agents Chemother. 2012;56:1578–83. doi: 10.1128/AAC.05382-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Announcement of clinical efficacy of TD-1792, see http://www.evaluatepharma.com/Universal/View.aspx?type=Story&id=132180
- 44.Hughes J, Mellows G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Echerichia coli by pseudomonic acid. Biochem J. 1978;176:305–18. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grare M, Dibama HM, Lafosse S, et al. Cationic compounds with activity against multidrug-resistant bacteria: interest of a new compound compared with two older antiseptics, hexamidine and chlorhexidine. Clin Microbiol Infect. 2010;16:432–438. doi: 10.1111/j.1469-0691.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 46.Coates T, Bax R, Coates A. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J Antimicrob Chemother. 2009;64:9–15. doi: 10.1093/jac/dkp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanakunakorn C, Axelson C, Bota B, et al. Mupirocin ointment with and without chlorhexidine baths in the eradication of Staphylococcus aureus nasal carriage in nursing home residents. American J Infect Control. 1995;23:306–9. doi: 10.1016/0196-6553(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 48.Schneider P, Hawser S, Islam K. Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria. Bioorg Med Chem Lett. 2003;13:4217–21. doi: 10.1016/j.bmcl.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Morgan A, Cofer C, Stevens D. Iclaprim: a novel dihydrofolate reductase inhibitor for skin and soft tissue infections. Future Microbiol. 2009;4:131–44. doi: 10.2217/17460913.4.2.131. [DOI] [PubMed] [Google Scholar]
- 50.Peppard W, Schuenke C. Iclaprim, a diaminopyrimidine dihydrofolate reductase inhibitor for the potential treatment of antibiotic-resistant Staphylococcal infections. Current Opin Investig Drugs. 2008;9:210–25. [PubMed] [Google Scholar]
- 51.Kohlhoff S, Sharma R. Iclaprim. Expert Opin Investig Drugs. 2007;16:1441–8. doi: 10.1517/13543784.16.9.1441. [DOI] [PubMed] [Google Scholar]
- 52.see http://clinicaltrials.gov/show/NCT00543608
- 53.Ooi N, Miller K, Hobbs J, et al. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother. 2009;64:735–40. doi: 10.1093/jac/dkp299. [DOI] [PubMed] [Google Scholar]
- 54.Farrell DJ, Robbins M, Rhys-Williams W, et al. Investigation of the potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus isolates during a 55-passage study. Antmicro Agents Chemother. 2011;55:1177–81. doi: 10.1128/AAC.01285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar K, Chopra S. New drugs for methicillin-resistant Staphylococcus aureus: an update. J Antimicrob Chemother. 2013;68:1–6. doi: 10.1093/jac/dkt045. [DOI] [PubMed] [Google Scholar]
- 56.Grossman T, Starosta A, Fyfe C, et al. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother. 2012;56:2559–64. doi: 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christ D, Sutcliffe J. TP-434 is Metabolically stable and has low potential for drug-drug interactions. Poster 181 F1-2162, 50th Annual ICAAC 2010. http://www.tphase.com/files/F1-2162.pdf.
- 58.Horn PT, Sutcliffe JA, Walpole S, et al. Pharmacokinetics, safety and tolerability of a novel fluorocycline, TP-434, following multiple dose oral administration with and without food. IDSA Annual Meeting Boston, Poster Abstract Session; October, 2011. [Google Scholar]
- 59.Sader H, Paukner S, Schoenfeld Z, et al. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs) J Antimicrob Chemother. 2012;67:1170–5. doi: 10.1093/jac/dks001. [DOI] [PubMed] [Google Scholar]
- 60.Ross J, Sader H, Schoenfeld Z, et al. Disk diffusion and MIC quality control ranges for BC-3205 and BC-3781, two novel pleuromutilin antibiotics. J Clin Microbiol. 2012;50:3361–64. doi: 10.1128/JCM.01294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Declaration of clinical research on BC-3781, see http://www.biotech-intelligence.com/html/html/pool_7/88ba7b2fec852709f7c5df2e8fb3511e.html
- 62.Skripkin E, McConnell T, DeVito J, et al. RX-01, a New family of oxazolidinones that overcome ribosome-based linezolid resistance. Antimicrob Agents Chemother. 2008;52:3550–57. doi: 10.1128/AAC.01193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence L, Danese P, DeVito J, et al. In vitro activities of the RX-01 oxazolidinones against hospital and community pathogens. Antimicrob Agents Chemother. 2008;52:1653–62. doi: 10.1128/AAC.01383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J, Bhattacharjee A, Chen S, et al. Design at the atomic level: Design of biaryloxazolidinones as potent orally active antibiotics. Bioorg Med Chem Lett. 2008;18:6175–78. doi: 10.1016/j.bmcl.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 65.see http://www.clinicaltrials.gov/ct2/results?term=RX-1741+&Search=Search
- 66.Louie A, Liu W, Kulawy R, et al. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob Agents Chemother. 2011;55:3453–60. doi: 10.1128/AAC.01565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown SD, Traczewski MM. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob Agents Chemother. 2010;54:2063–9. doi: 10.1128/AAC.01569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prokocimer P, Bien P, Surber J, et al. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob Agents Chemother. 2011;55:583–92. doi: 10.1128/AAC.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koga T, Masuda N, Kakuta M, et al. Potent in vitro activity of tomopenem (CS-023) against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:2849–54. doi: 10.1128/AAC.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanka K, Mikamo H, Nakao K, et al. In vitro activity of tomopenem (CS-023/RO4908463) against anaerobic bacteria. Antimicrob Agents Chemother. 2009;53:319–22. doi: 10.1128/AAC.00595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomozawa T, Sugihara C, Kakuta M, et al. In vitro postantibiotic effects of tomopenem (CS-023) against Staphylococcus aureus and Pseudomonas aeruginosa. J Med Microbiol. 2010;59:438–41. doi: 10.1099/jmm.0.017905-0. [DOI] [PubMed] [Google Scholar]
- 72.Sugihara K, Sugihara C, Matsushita Y, et al. In vivo pharmacodynamic activity of tomopenem (formerly CS-023) against Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. Antimicrob Agents Chemother. 2010;54:5298–302. doi: 10.1128/AAC.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rios AM, Mejias R, Chavez-Bueno S, et al. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob Agents Chemother. 2004;48:2897–904. doi: 10.1128/AAC.48.8.2897-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia I, Pascual A, Ballesta S, et al. Accumulation and activity of cethromycin (ABT-773) within human polymorphonuclear leucocytes. J Antimicrob Chemother. 2003;52:24–8. doi: 10.1093/jac/dkg290. [DOI] [PubMed] [Google Scholar]
- 75.Kim MK, Zhou W, Tessier PR, et al. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine Pneumococcal pneumonia model. Antimicrob Agents Chemother. 2002;46:3185–92. doi: 10.1128/AAC.46.10.3185-3192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bermudez LE, Motamedi N, Chee C, et al. EDP-420, a bicyclolide (bridged bicyclic macrolide), is active against Mycobacterium avium. Antimicrob Agents Chemother. 2007;51:1666–70. doi: 10.1128/AAC.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo X, Wu R, Wang M, et al. Comparative efficacy of EDP-420, azithromycin, telithromycin, clindamycin, trimethoprim/sulfamethoxazole, linezolid and levofloxacin against murine skin abscess induced by methicillin-resistant Staphylococcus aureus USA300 PVL+ mecA strain. 18th European Congress of Clinical Microbiology and Infectious Diseases; 2008. [Google Scholar]
- 78.Paknikar SS, Narayana S. Newer antibacterials in therapy and clinical trial. North Am J Med Sci. 2012;4:537–47. doi: 10.4103/1947-2714.103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu G, Tang D, Gai Y, et al. An efficient large-scale synthesis of EDP-420, a First-in-class bridged bicyclic macrolide (BBM) antibiotic drug candidate. Org Proc Res Develop. 2010;4:504–10. [Google Scholar]
- 80.Pankuch GA, Kelly LM, Lin G, et al. Activities of a new oral streptogramin, XRP 2868, compared to those of other agents against Streptococcus pneumoniae and haemophilus species. Antimicrob Agents Chemother. 2003;47:3270–4. doi: 10.1128/AAC.47.10.3270-3274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andes D, Craig WA. Pharmacodynamics of a new streptogramin, XRP 2868, in murine thigh and lung infection models. Antimicrob Agents Chemother. 2006;50:243–9. doi: 10.1128/AAC.50.1.243-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mabe S, Champney WS. A comparison of a new oral streptogramin XRP 2868 with quinupristin-dalfopristin against antibiotic-resistant strains of haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae. Curr Microbiol. 2005;51:363–6. doi: 10.1007/s00284-005-0027-9. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein E, Citron D, Merriam V, et al. Comparative in vitro Activities of XRP 2868, pristinamycin, quinupristin-dalfopristin, vancomycin, daptomycin, linezolid, clarithromycin, telithromycin, clindamycin, and ampicillin against anaerobic Gram-positive species, actinomycetes, and lactobacilli. Antimicrob Agents Chemother. 2005;49:408–13. doi: 10.1128/AAC.49.1.408-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemaire S, Tulkens P, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-Gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:649–58. doi: 10.1128/AAC.01201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Announcement of phase study results for delafloxacin, see http://www.rib-x.com/pipeline/delafloxacin.php
- 86.Jones RN, Sader HS, Fritsche TR. Antimicrobial activity of LBM415 (NVP PDF-713) tested against pathogenic Neisseria spp (Neisseria gonorrhoeae and Neisseria meningitidis) Diagn Micr Infec Dis. 2005;51:139–41. doi: 10.1016/j.diagmicrobio.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 87.Rolan P, Sun H, MacLeod C, et al. Pharmacokinetics and Unexpected Safety Issues of LBM415, a Novel Oral Peptide Deformylase Inhibitor. Clin Pharmacol Ther. 2011;90:256–62. doi: 10.1038/clpt.2011.101. [DOI] [PubMed] [Google Scholar]
- 88.Bell JM, Turnidge JD, Inoue M, et al. Activity of a peptide deformylase inhibitor LBM415 (NVP PDF-713) tested against recent clinical isolates from Japan. J Antimicrob Chemother. 2005;55:276–8. doi: 10.1093/jac/dkh547. [DOI] [PubMed] [Google Scholar]
- 89.Osborne CS, Neckermann G, Fischer E, et al. In vivo characterization of the peptide deformylase inhibitor LBM415 in murine infection models. Antimicrob Agents Chemother. 2009;53:3777–81. doi: 10.1128/AAC.00026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ednie LM, Pankuch G, Appelbaum PC. antipneumococcal activity of LBM415, a new peptide diformylase inhibitor, compared with those of other agents. Antimicrob Agents Chemother. 2004;48:4027–32. doi: 10.1128/AAC.48.10.4027-4032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ross J, Scangarella-Oman N, Miller N, et al. Determination of disk diffusion and MIC quality control ranges for GSK1322322, a novel peptide deformylase inhibitor. J Clin Microbiol. 2011;49:3928–30. doi: 10.1128/JCM.01213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verma SK, Jat RK, Nagar N, et al. A novel antibacterial target: Peptide deformylase. Pharmacophore. 2011;2:114–23. [Google Scholar]
- 93.Bogdanovich T, Smith KA, Clark C, et al. Activity of LBM415 compared to those of 11 other agents against Haemophilus Species. Antimicrob Agents Chemother. 2006;50:2323–29. doi: 10.1128/AAC.00106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Gordon JY, Romanowski EG. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005;30:505–15. doi: 10.1080/02713680590968637. A review of antimicrobial peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andres E, Dimarcq JL. Cationic antimicrobial peptides: update of clinical development. J Intern Med. 2004;255:519–20. doi: 10.1046/j.1365-2796.2003.01278.x. [DOI] [PubMed] [Google Scholar]
- 96.Hallock KJ, Lee D-K, Ramamoorthy A. MSI-78, an Analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J. 2003;84:3052–60. doi: 10.1016/S0006-3495(03)70031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.www.dipexiumpharmaceuticals.com/aboutLocilex.html
- 98.Rehm BHA. Microbial production of alginate: Biosynthesis and applications in Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press; 2009. [Google Scholar]
- 99.See http://algipharma.no/images/Marketing/News/Sept/ICAAC_2012_poster_F-2062-AlgiPharmas_Oligo-G_bacterial_motility_study_at_CardiffU.pdf
- 100.Ebrt T, Smith S, Pancari G, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies. 2010;19:113–28. doi: 10.3233/HAB-2010-0235. [DOI] [PubMed] [Google Scholar]
- 101.Henck JO, Byrrn SR. Designing a molecular delivery system within a preclinical timeframe. Drug Discovery Today. 2007;12:189–199. doi: 10.1016/j.drudis.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Kelly-Quintos C, Cavacini LA, Posner MR, et al. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74:2742–50. doi: 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly-Quintos C, Kropec A, Briggs S. The role of epitope specificity in the human opsonic antibody response to the Staphylococcal surface olysaccharide poly N-acetyl glucosamine. J Infect Dis. 2005;192:2012–9. doi: 10.1086/497604. [DOI] [PubMed] [Google Scholar]
- 104.Kutter E, De Vos D, Gvasalia G, et al. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 105.Matsuzaki S, Rashel M, Uchiyama J, et al. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother. 2005;11:211–9. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 106.Mi dzybrodzki R, Fortuna W, Weber-D browska B, et al. Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment. Postepy Hig Med Dosw. 2007;61:461–465. [PubMed] [Google Scholar]
- 107.Winter JM, Behnken S, Hertweck C. Genomics-inspired discovery of natural products. Curr Opini Chem Biol. 2011;15:22–31. doi: 10.1016/j.cbpa.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 108•.Goldstein BP, Berti M, Ripamonti F, et al. In vitro antimicrobial activity of a new antibiotic, MDL 62,879 (GE2270 A) Antimicrob Agents Chemother. 1993;37:741–5. doi: 10.1128/aac.37.4.741. Summarizes new antibacterial natural products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raja A, LaBonte J, Lebbos J, et al. Daptomycin. Nature Rev Drug Disc. 2003;2:943–4. doi: 10.1038/nrd1258. [DOI] [PubMed] [Google Scholar]
- 110.Crowley PJ, Martini LG. Formulation design: new drugs from old. Drug Disc Today: Ther Strat. 2004;1:537–42. [Google Scholar]
- 111.LaMarche MJ, Leeds JA, Dzink-Fox J, et al. 4-Aminothiazolyl analogues of GE2270 A: antibacterial lead finding. J Med Chem. 2011;54:2517–21. doi: 10.1021/jm101602q. [DOI] [PubMed] [Google Scholar]
- 112.Ohlsen K. Novel antibiotics for the treatment of Staphylococcus aureus. Expert Rev Clin Pharmacol. 2009;2:661–72. doi: 10.1586/ecp.09.26. [DOI] [PubMed] [Google Scholar]
- 113•.Roccaforte JS, Bittner MJ, Stumpf CA, et al. Attempts to eradicate methicillin-resistant Staphylococcus aureus colonization with the use of trimethoprim-sulfamethoxazole, rifampin, and bacitracin. Am J Infect Contr. 1988;16:141–6. doi: 10.1016/0196-6553(88)90024-7. Describes the combination chemotherapy. [DOI] [PubMed] [Google Scholar]
- 114.See http://www.triusrx.com/pdfs/Advanced-Microbiology-and-In-Vivo-Efficacy-of-Rx101005-a-Novel-2-4-Diaminoquinazoline-DHFR-Inhibitor.pdf
- 115.Mushtaq S, Warner M, Williams G, et al. Activity of chequerboard combinations of ceftaroline and NXL104 versus β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2010;65:1428–32. doi: 10.1093/jac/dkq161. [DOI] [PubMed] [Google Scholar]
- 116.Xu H, Hazra S, Blanchard JS. NXL104 Irreversibly Inhibits the β-Lactamase from Mycobacterium tuberculosis. Biochemistry. 2012;51:4551–7. doi: 10.1021/bi300508r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Louie A, Castanheira M, Liu W, et al. Pharmacodynamics of β-Lactamase inhibition by NXL104 in combination with ceftaroline: Examining organisms with multiple types of β-lactamases. Antimicrob Agents Chemother. 2012;56:258–70. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiskirchen DE, Crandon JL, Furtado GH, et al. In vivo efficacy of a human-simulated regimen of ceftaroline combined with NXL104 against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55(3):220–5. doi: 10.1128/AAC.00024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119•.Nafsika GH. β-Lactamase inhibitors: evolving compounds for evolving resistance targets. Exp Opini Investigational Drugs. 2004;13:1307–18. doi: 10.1517/13543784.13.10.1307. Describes the β-lactamase inhibitors. [DOI] [PubMed] [Google Scholar]
- 120.Snydman DR, Jacobus NV, McDermott LA. Activity of a novel cyclic lipopeptide, CB-183,315, against resistant Clostridium difficile and other Gram-positive aerobic and anaerobic intestinal pathogens. Antimicrob Agents Chemother. 2012;56:3448–52. doi: 10.1128/AAC.06257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carmela MTM, Lawrence MI, Kare HT, et al. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob Agents Chemother. 2012;56:5023–30. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mangili A, Bica I, Snydman DR, et al. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40:1058–60. doi: 10.1086/428616. [DOI] [PubMed] [Google Scholar]
- 123.Peleg AY, Miyakis S, Ward DV, et al. Whole genome characterization of the mechanisms of daptomycin resistance in clinical and laboratory derived isolates of Staphylococcus aureus. PLoS One. 2012;7:e28316. doi: 10.1371/journal.pone.0028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Falagas ME, Grammatikos AP, Michalopoulos A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev Anti Infect Ther. 2008;6:593–600. doi: 10.1586/14787210.6.5.593. [DOI] [PubMed] [Google Scholar]
- 125.McLaws FB, Larsen AR, Skov RL, et al. Distribution of fusidic acid resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:1173–6. doi: 10.1128/AAC.00817-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brots-Oesterhelt H, Beyer D, Kroll HP, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med. 2005;11:1082–7. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 127.Li DHS, Chung YS, Gloyd M, et al. Acyldepsipeptide antibiotics induce the formation of a structured axial channel in ClpP: A model for the ClpX/ClpA-bound state of ClpP. Chem Biol. 2010;17:959–69. doi: 10.1016/j.chembiol.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128•.Lee BG, Park EY, Lee KE, et al. Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat Struct Mol Biol. 2010;17:471–9. doi: 10.1038/nsmb.1787. Describes the mode of action of acyldepsipeptide. [DOI] [PubMed] [Google Scholar]
- 129.Hinzen B, Raddatz S, Paulsen H, et al. Medicinal chemistry optimization of acyldepsipeptides of the enopeptin class antibiotics. Chem Med Chem. 2006;1:689–93. doi: 10.1002/cmdc.200600055. [DOI] [PubMed] [Google Scholar]
- 130.Haste NM, Hughes CC, Tran DN, et al. Pharmacological properties of the marine natural product marinopyrrole A against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:3305–12. doi: 10.1128/AAC.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doi K, Li R, Sung SS, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287:10224–35. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nicolaou KC, Simmons NL, Chen JS, et al. Total synthesis and biological evaluation of marinopyrrole A and analogs. Tetrahedron Lett. 2011;52:2041–3. doi: 10.1016/j.tetlet.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwon YJ, Fang Y, Xu GH, et al. Aquastatin A, a new Inhibitor of enoyl-acyl carrier protein reductase from Sporothrix sp. FN611 Biol Pharm Bull. 2009;32:2061–4. doi: 10.1248/bpb.32.2061. [DOI] [PubMed] [Google Scholar]
- 134.Yong K, Jayasuriya H, Ondeyka J, et al. Discovery of FabH/FabF inhibitors from natural products. Antimicrob Agents Chemother. 2006;50:519–26. doi: 10.1128/AAC.50.2.519-526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kithsiri HB, Hiranthi J, Ziqiang G, et al. Anthrabenzoxocinones from Streptomyces sp. as liver X receptor ligands and antibacterial agents. J Nat Prod. 2005;68:1437–40. doi: 10.1021/np050176k. [DOI] [PubMed] [Google Scholar]
- 136.Srinivas K, Andrew G, Katherine Y. Determination of selectivity and efficacy of fatty acid synthesis inhibitors. J Biol Chem. 2005;280:1669–77. doi: 10.1074/jbc.M406848200. [DOI] [PubMed] [Google Scholar]
- 137.Zheng CJ, Sohn MJ, Kim WG. Vinaxanthone, a new FabI inhibitor from Penicillium sp. J Antimicrob Chemother. 2009;63:949–53. doi: 10.1093/jac/dkp058. [DOI] [PubMed] [Google Scholar]
- 138•.Wang J, Kodali S, Lee SH, et al. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci. 2007;104:7612–16. doi: 10.1073/pnas.0700746104. Mode of action of platencin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Evan M, Arnold DL. Platensimycin and platencin: promising antibiotics for future application in human medicine. J Antibiot. 2011;64:705–10. doi: 10.1038/ja.2011.80. [DOI] [PubMed] [Google Scholar]
- 140.Kato A, Nakaya S, Kokubo N, et al. A new anti-MRSA antibiotic complex, WAP-8294A I. Taxonomy, isolation and biological activities. J Antibiot. 1998;51:929–35. doi: 10.7164/antibiotics.51.929. [DOI] [PubMed] [Google Scholar]
- 141.Hyeon-Hee Y, Kang-Ju K, Jeong-Dan C, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–61. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 142.Bonner DP, O’Sullivan J, Tanaka KS, et al. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J Antibiot. 1988;41:1745–51. doi: 10.7164/antibiotics.41.1745. [DOI] [PubMed] [Google Scholar]
- 143.Franz N, Sonja A, Jordi B-B, et al. Structure and total synthesis of lysobactin (katanosin B) Angew Chem Int Ed. 2007;46:2039–42. doi: 10.1002/anie.200604232. [DOI] [PubMed] [Google Scholar]
- 144.Hall EA, Kuru E, VanNieuwenhze MS. Solid-phase synthesis of lysobactin (katanosin B): Insights into structure and function. Org Lett. 2012;14:2730–3. doi: 10.1021/ol300926d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng CJ, Yu HE, Kim EH, et al. Viridicatumtoxin B, a new anti-MRSA agent from Penicillium sp. FR11 J Antibiot. 2008;61:633–7. doi: 10.1038/ja.2008.84. [DOI] [PubMed] [Google Scholar]
- 146.Ma X-C, Sun C, Huang S-S, et al. Preparative isolation and purification of four prenylflavanones from microbial biotransformation of kurarinone by high-speed counter-current chromatography. Separ Puri Technol. 2010;76:140–5. [Google Scholar]
- 147.McDonald LA, Barbieri LR, Carter GT, et al. Structures of muraymycins, novel peptidoglycan biosynthesis inhibitors. J Am Chem Soc. 2002;124:10260–1. doi: 10.1021/ja017748h. [DOI] [PubMed] [Google Scholar]
- 148.Lin YI, Li Z, Francisco GD, et al. Muraymycins, novel peptidoglycan biosynthesis inhibitors: semisynthesis and SAR of their derivatives. Bioorg Med Chem Lett. 2002;12:1341–4. doi: 10.1016/s0960-894x(02)00469-9. [DOI] [PubMed] [Google Scholar]
- 149.Bouhss A, Crouvoisier M, Blanot D, et al. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J Biol Chem. 2004;279:29974–80. doi: 10.1074/jbc.M314165200. [DOI] [PubMed] [Google Scholar]
- 150.Silver LL. Does the cell wall of bacteria remain a viable source of targets for novel antibiotics? Biochem Pharmacol. 2006;71:996–1005. doi: 10.1016/j.bcp.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 151.Dini C. MraY inhibitors as novel antibacterial agents. Curr Topics Med Chem. 2005;5:1221–36. doi: 10.2174/156802605774463042. [DOI] [PubMed] [Google Scholar]
- 152.Aleiwi BA, Schneider CM, Kurosu M. Synthesis of ureidomuraymycidine derivatives for structure-activity relationship studies of muraymycins. J Org Chem. 2012;77:3859–67. doi: 10.1021/jo300205b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Winn M, Goss JM, Kimura K, et al. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure–function studies and nucleoside biosynthesis. Nat prod Rep. 2010;27:279–304. doi: 10.1039/b816215h. [DOI] [PubMed] [Google Scholar]
- 154.Konishi M, Sugawara K, Hanada M, et al. Empedopeptin (BMY-28117), a new depsipeptide antibiotic. I. Production, isolation and properties. J Antibiot. 1984;37:949–57. doi: 10.7164/antibiotics.37.949. [DOI] [PubMed] [Google Scholar]
- 155.Müller A, Münch D, Schmidt Y, et al. Lipodepsipeptide empedopeptin inhibits cell wall biosynthesis through Ca2+-dependent complex formation with peptidoglycan precursors. J Biol Chem. 2012;287:20270–80. doi: 10.1074/jbc.M112.369561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vértesy L, Aretz W, Knauf M, et al. Feglymycin, a novel inhibitor of the replication of the human immunodeficiency virus. Fermentation, isolation and structure elucidation. J Antibiot. 1999;52:374–82. doi: 10.7164/antibiotics.52.374. [DOI] [PubMed] [Google Scholar]
- 157.Dettner F, Hänchen A, Schols D, et al. Total synthesis of the antiviral peptide antibiotic feglymycin. Angew Chem Int Ed. 2009;48:1856–61. doi: 10.1002/anie.200804130. [DOI] [PubMed] [Google Scholar]
- 158.Rausch S, Hänchen A, Denisiuk A, et al. Feglymycin is an inhibitor of the enzymes MurA and MurC of the peptidoglycan biosynthesis pathway. Chem BioChem. 2011;12:1171–3. doi: 10.1002/cbic.201100120. [DOI] [PubMed] [Google Scholar]
- 159.Uchida R, Iwatsuki M, Kim YP, et al. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. I: Fermentation, isolation and biological properties. J Antibiot. 2010;63:151–5. doi: 10.1038/ja.2010.9. [DOI] [PubMed] [Google Scholar]
- 160.Wyatt EE, Galloway WRJD, Thomas GL, et al. Identification of an anti-MRSA dihydrofolate reductase inhibitor from a diversity-oriented synthesis. Chem Commun. 2008;44:496–24. doi: 10.1039/b812901k. [DOI] [PubMed] [Google Scholar]
- 161.DaCunha EFF, Ramalho TC, Maia ER, et al. The search for new DHFR inhibitors: a review of patents January 2001–February 2005. Exp Opin Ther Patents. 2005;15:967–86. [Google Scholar]
- 162.Hawser S, Lociuro S, Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochem Pharmacol. 2006;71:941–8. doi: 10.1016/j.bcp.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 163.Karatas H, Alp M, Yildiz S, et al. Synthesis and potent in vitro activity of novel 1H-benzimidazoles as anti-MRSA agents. Chem Biol Drug Design. 2012;80:237–44. doi: 10.1111/j.1747-0285.2012.01393.x. [DOI] [PubMed] [Google Scholar]
- 164••.Kurosu M, Begari E. Bacterial protein kinase inhibitors. Drug Develop Res. 2010;71:168–87. A comprehensive review of bacterial protein kinase inhibitor molecules. [Google Scholar]
- 165••.Kurosu M, Begari E. Vitamin K2 in electron transport system: Are enzymes involved in vitamin K2 biosynthesis promising drug targets? Molecules. 2010;15:1531–53. doi: 10.3390/molecules15031531. A comprehensive review of electron transport inhibitor molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kurosu M, Narayanasamy P, Biswas K, et al. Discovery of 1,4-dihydroxy-2-naphthoate prenyltransferase inhibitors: New drug leads for multidrug-resistant Gram-positive pathogens. J Med Chem. 2007;50:3973–4. doi: 10.1021/jm070638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Debnath J, Siricilla S, Wan B, et al. Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis. J Med Chem. 2012;55:3739–55. doi: 10.1021/jm201608g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dhiman RK, Mahapatra S, Slayden RA, et al. Menaquinone synthesis is critical for maintaining Mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol Microbiol. 2009;72:85–97. doi: 10.1111/j.1365-2958.2009.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kashyap A, Sehgal VN, Sahu A, et al. Anti-leprosy drugs inhibit the complement-mediated solubilization of pre-formed immune complexes in vitro. Internat J Immunopharmacol. 1992;14:269–73. doi: 10.1016/0192-0561(92)90039-n. [DOI] [PubMed] [Google Scholar]
- 170.Yano T, Kassovska-Bratinova S, The JS, et al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase A. Pathway for the generation of bacterial levels of reactive oxygen species. J Biol Chem. 2011;286:10276–87. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kuhl A, Svenstrup N, Ladel C. Biological characterization of novel inhibitors of the Gram-positive DNA polymerase IIIC enzyme. Antimicro Agents Chemother. 2005;49:987–95. doi: 10.1128/AAC.49.3.987-995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee TX, Packer MD, Huang J, et al. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. Eur J Cancer. 2009;45:1709–20. doi: 10.1016/j.ejca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chiua H-C, Leeb S-L, Kapuriya N. Development of novel antibacterial agents against methicillin-resistant Staphylococcus aureus. Bioorg Med Chem. 2012;15:4653–60. doi: 10.1016/j.bmc.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in us intensive care units, 1997–2007. J Am Med Associ. 2009;301:727–36. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 175.Bush LM. Best alternative to vancomycin for serious methicillin-resistant Staphylococcus aureus infections: Let’s just say it. Clin Infect Diseas. 2011;53:965–6. doi: 10.1093/cid/cir528. [DOI] [PubMed] [Google Scholar]
- 176.Pexer JC, Tancrede C, Tancrede D. In vitro study of the bactericidal activity of a sulfamethoxazole-trimethoprim combination of 59 hospital strains. Therapie. 1970;25:13–28. [PubMed] [Google Scholar]
- 177.Schiraldi O, Sforza E, Piaia F. Effect of a new sulfa-trimethoprim combination (trimethoprim -sulfamethopyrazine) in typhoid fever. A double-blind study on 72 adult patients. Chemother. 1985;31:68–75. doi: 10.1159/000238316. [DOI] [PubMed] [Google Scholar]
- 178.Bohni E. Comparative bacteriological investigations with the combination trimethoprim/sulfamethoxazole in vitro and in vivo. Chemother. 1969;14:1–21. [PubMed] [Google Scholar]
- 179.Miyamoto E. Oral trimethoprim-sufamethoxazole for methicillin-resistant Staphylococcus aureus infections: the evidence behind the use. Drug Ther Topics. 2004;33:69–74. [Google Scholar]
- 180.Graham S, Coote PJ. Potent, synergistic inhibition of Staphylococcus aureus upon exposure to a combination of the endopeptidase lysostaphin and the cationic peptide ranalexin. J Antimicrob Chemother. 2007;59:759–62. doi: 10.1093/jac/dkl539. [DOI] [PubMed] [Google Scholar]
- 181.Soeda H. Pharmacokinetics and therapeutic drug monitoring of anti-MRSA drugs. Kagaku Ryoho no Ryoiki. 2011;28:1702–8. [Google Scholar]
- 182.Chaudhary M, Shrivastava SM, Saurabh S, et al. In vitro antimicrobial activity of cefepime, amikacin and their fixed dose combination against some pathogenic bacteria. J Ecophysiol Occupati Health. 2008;8:161–5. [Google Scholar]
- 183.Neu HC. Antibiotics in the second half of the 1980s. Areas of future development and the effect of new agents on aminoglycoside use. Am J Med. 1986;80:195–203. doi: 10.1016/0002-9343(86)90501-2. [DOI] [PubMed] [Google Scholar]
- 184.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16. doi: 10.3389/fcimb.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]