Summary
In mice, homozygous deletion of the cardiac sodium channel Scn5a results in defects in cardiac morphology and embryonic death before robust sodium current can be detected. In zebrafish, morpholino knockdown of cardiac sodium channel orthologs scn5Laa and scn5Lab perturbs specification of pre-cardiac mesoderm and inhibits growth of the embryonic heart. It is not known which developmental processes are perturbed by sodium channel knockdown and whether reduced cell number is from impaired migration of cardiac progenitors into the heart, impaired myocyte proliferation, or both. We found that embryos deficient in scn5Lab displayed defects in primary cardiogenesis specific to loss of nkx2.5, but not nkx2.7. We generated kaede reporter fish and demonstrated that embryos treated with anti-scn5Lab morpholino showed normal secondary differentiation of cardiomyocytes at the arterial pole between 30 and 48 hours post-fertilization. However, while proliferating myocytes were readily detected at 48 hpf in wild type embryos, there were no BrdU-positive cardiomyocytes in embryos subjected to anti-scn5Lab treatment. Proliferating myocytes were present in embryos injected with anti-tnnt2 morpholino to phenocopy the silent heart mutation, and absent in embryos injected with anti-tnnt2 and anti-scn5Lab morpholinos, indicating cardiac contraction is not required for the loss of proliferation. These data demonstrate that the role of scn5Lab in later heart growth does not involve contribution of the secondary heart field, but rather proliferation of cardiomyocytes, and appears unrelated to the role of the channel in cardiac electrogenesis.
Keywords: heart, development, sodium channel, myocardium
INTRODUCTION
Voltage-gated sodium channels are key regulators of cardiac electrical activity. In addition, a body of evidence strongly supports the notion that these channels play an indispensable role in cardiac development. In mice, homozygous deletion of the cardiac sodium channel gene Scn5a results in death at 10.5 days of embryonic gestation, before robust sodium current can be detected (Davies et al., 1996; Papadatos et al., 2002). These embryos have small ventricles, lacking trabeculation, but with intact endocardial tissues and vasculature (Papadatos et al., 2002).
The zebrafish genome includes two Scn5a orthologs, with similar sequence homology to Scn5a: scn5Laa (scn12aa) and scn5Lab (scn12ab) (Novak et al., 2006; Chopra et al., 2010). Development of the zebrafish heart begins early in embryogenesis, as fate-mapping reveals regions of blastomeres destined to the cardiac lineage as early as 5 hours post-fertilization (hpf) (Keegan et al., 2004). By 12 hpf, the cardiac-specification gene nkx2.5 identifies bilateral stripes of cardiac progenitors in the anterior lateral plate mesoderm (Chen and Fishman, 1996). Zebrafish in which either scn5Laa or scn5Lab has been knocked down exhibit defects in initial specification of pre-cardiac mesoderm, shown by marked reduction in early expression of nkx2.5 (Chopra et al., 2010). The NK2 homeobox genes nkx2.5 and nkx2.7 (Lee et al., 1996) have similar but not completely redundant functions in zebrafish heart tube elongation and ventricular growth. Other studies have demonstrated morpholino knockdown of nkx2.5 alone has the mild phenotype of a slightly smaller ventricular chamber (Targoff et al., 2008), while knockdown of nkx2.7 results in failure of the developing heart to loop correctly (Tu et al., 2009). Both studies observed dramatic phenotype on double knockdown, including reduced ventricular chamber size, impaired looping and weaker contractions (Targoff et al., 2008; Tu et al., 2009). It is unknown whether nkx2.7 is affected by scn5Lab knockdown.
In a wildtype zebrafish, the bilateral stripes of nkx2.5+ precursors fuse along the midline and will form a beating heart tube at 24 hpf (Stainier, 2001), and by 36 hours post-fertilization, the atrium and ventricle can be easily distinguished by light microscopy (Westerfield, 2000; Stainier, 2001). The heart tube in zebrafish deficient in scn5Lab has significantly fewer cardiomyocytes than in the wildtype zebrafish at 24 hpf (Chopra et al., 2010). As development proceeds, morphant zebrafish possess visibly smaller hearts, fewer ventricular cardiomyocytes, and the heart fails to loop and develop properly (Chopra et al., 2010). The embryos develop pericardial edema and die by 5 days post-fertilization (dpf) (Chopra et al., 2010).
The initial cardiomyocyte population of the heart tube at 24 hpf differentiates from mesodermal precursors in the lateral plate mesoderm (Serbedzija et al., 1998; Stainier, 2001). Recent work has identified a second heart field in zebrafish (Hami et al., 2011), containing an initially extra-cardiac population of atrial and ventricular myocyte progenitors. The venous and arterial poles of the heart are largely comprised of cardiomyocytes from this late-differentiating (24-36 hpf) population of precursors, which expresses the early heart-field marker nkx2.5 (Zhou et al., 2011) as well as other cardiac-specific genes (Lazic and Scott, 2011). Among other pathways, Fgf signaling within this region has been demonstrated as critical to the differentiation and development of outflow tract myocardium (de Pater et al., 2009). By 48 hpf, differentiation of myocardium is largely complete, and further increases in cardiomyocyte number are a result largely of cellular proliferation (Chernyavskaya et al., 2012).
Our group has previously shown that scn5Lab is required for early specification of pre-cardiac mesoderm, and that this effect appears unrelated to the channel's role in electrogenesis. During the early stages of cardiac development, expression of scn5Lab has been detected by in situ hybridization (Novak et al., 2006; Chopra et al., 2010), but has been demonstrated to not contribute to action potential generation (Chopra et al., 2010). Here, we test the competing hypotheses that impaired cardiac growth and decreased cardiomyocyte number in scn5Lab-deficient embryos are due to impaired secondary differentiation, or arrested proliferation and growth of previously-differentiated cardiomyocytes.
RESULTS
scn5Lab morphant hearts acquire reduced numbers of cardiomyocytes
Zebrafish deficient in scn5Lab develop with visibly smaller, un-looped hearts, slow heart rates, and pericardial edema. To determine if reduced heart size resulted from fewer cells, we counted cardiomyocytes at each stage of early heart development. Beginning with initial peristaltic contractions of the wildtype heart tube at 24 hpf, uninjected and morpholino-injected embryos were formaldehyde-fixed at 12 hour-increments and immunofluorescence was performed to identify dsRed+ cardiomyocytes. Compared to uninjected wildtype embryos, zebrafish deficient in scn5Lab (morphants) had significantly reduced numbers of cardiomyocytes at 24 hpf (Fig. 1a,e,i; 129.2±2.4 vs. 83.6±6.1, n=6,7). Following initial differentiation and establishment of the heart tube, wildtype zebrafish embryos continued to add cardiomyocytes throughout the second day (24-48 hpf) of development (Fig. 1a-c,i). During this same period, however, morphant hearts also continued to grow (Fig. 1e-g,i), with a similar increase in cardiomyocyte number as the uninjected embryos. After 48 hpf, uninjected and morphant hearts again diverged in cardiomyocyte number, as wildtype hearts continued to acquire dsRed+ cells (Fig. 1d,i), while morphant hearts failed to add a similar number of cardiomyocytes (Fig. 1h,i). Additionally, wildtype hearts underwent normal cardiac looping (Fig. 1d) while adding myocytes, whereas morphant hearts remained un-looped or partially looped (Fig. 1g,h).
Fig. 1.
Throughout development, morpholino-injected embryos have fewer cmlc2+-cardiomyocytes than uninjected embryos. At 24 hpf (a,e,i), the number of cells in the initial heart tube is dramatically decreased in morphant embryos (129.2±2.4 vs 83.6±6.1, n=6,7, p<.001). As development progresses, both morphant and wildtype embryos continue to add cardiomyocytes. At 36 hpf (b,f,i), uninjected embryos continue to have a significantly greater number of cardiomyocytes (182±4.2 vs 143.5±3.0, n=6, p<.001). Between 48 hpf(c,g,I: 198.5±5.2 vs. 162.5±2.5, n=6, p<.001) and 60 hpf (d,h,i: 219.8±3.8 vs. 170.5±4.2, n=7,6, p<.001) wildtype embryos continue to add cardiomyocytes to the hearts, while morphant embryos have slowed in growth. A = atrium, V = ventricle, W = widtype, M = morphant.
scn5Lab is required for specific expression of nkx2.5 but not nkx2.7 in primary cardiac differentiation
We have previously demonstrated a requirement for voltage-gated sodium channels in expression of nkx2.5 in the anterior lateral plate mesoderm (Chopra et al., 2010). However the observed cardiac phenotype in scn5Lab-morphant embryos is more suggestive of the double nkx2.5/nkx2.7 knockdown. To investigate the specificity of the role for scn5Lab in expression of selected cardiac transcription factors and determine if other cardiac specification pathways were affected by loss of scn5Lab, we performed in situ hybridization for nkx2.5 and nkx2.7 in 12 hpf embryos. At this stage, nkx2.5 expression in the anterior lateral plate mesoderm is nearly absent in scn5Lab-morphant embryos (Chopra et al., 2010) compared to wildtype (Fig. 2a-b). However, embryos from the same clutch showed normal nkx2.7 expression in both wildtype and morpholino-injected embryos (Fig. 2c-h). This finding suggests the role for scn5Lab in expression of early cardiac transcription factors is specific for nkx2.5, or its upstream mediators, rather than playing a more general role in which many cardiac transcription factors would be affected by its absence. Additionally, differentiated cardiomyocytes in the heart tube at 24 hpf displayed normal expression of nkx2.5 (Fig. 2c-d), suggesting the role for scn5Lab in expression of nkx2.5 is limited to the specification of pre-cardiac mesoderm. Given the severe phenotype suggestive not only of loss of nkx2.5, but of additional gene expression defects, we attempted to rescue the morphant phenotype with microinjection of nkx2.5 mRNA. Embryos injected with mRNA alone developed with slightly enlarged hearts (Fig. 3a-c), as expected from previous reports (Chen and Fishman, 1996). However, embryos injected with scn5Lab active morpholino and nkx2.5 mRNA (Fig. 3f) displayed no rescue of the morphant phenotype (Fig. 3d,e), suggesting other cardiac specification genes are affected by scn5Lab knockdown. Thus, while nkx2.5 is an important readout of impaired cardiac specification in scn5Lab morphants, other unidentified pathways contribute to the phenotype as well.
Fig. 2.
Embryos injected with active morpholino show impaired cardiac specification. In situ hybridization for the cardiac-specification transcription factor nkx2.5 (a-b) shows markedly reduced expression in the anterior lateral plate mesoderm. Expression of the related homeobox gene nkx2.7 (e-j) was unaffected in embryos from the same injection clutch. Cardiomyocytes at 26 hpf (c,d) have restored expression of nkx2.5, within the smaller heart tube. MO = morphants, WT = uninjected
Fig 3.
nkx2.5 mRNA is insufficient to rescue loss of scn5Lab: Wildtype embryos (a), or embryos injected with 150pg nkx2.5 mRNA (b) or mRNA with scn5Lab-MIS morpholino (c) show no gross morphological phenotype. scn5Lab-MO injected embryos (d,e) are not rescued by co-injection of nkx2.5 (f).
Secondary cardiomyocyte differentiation is normal in scn5Lab morphant embryos
Loss of gastrula-stage expression of nkx2.5 helps to explain a decrease in differentiated cardiomyocytes in the zebrafish heart tube at 24 hpf. To determine if scn5Lab is required for late differentiation of myocardium, we generated the Tg(cmlc2:kaede) line as previously reported (de Pater et al., 2009); this line and others used in these studies are described in further detail in Methods below. Cmlc2+ myocytes (positive for the cardiac marker cmlc2) expressing the green fluorescent Kaede at 30 hpf were permanently photo-converted to red fluorescence, marking the initial phase of cardiac differentiation. As embryos continued to develop, both primary and secondary cardiomyocytes expressed green (new) Kaede, marking primary cardiomyocytes as both red and green fluorescent, while secondary myocytes expressed green Kaede only. Using this approach, we found that the extent of secondary differentiation at the arterial pole between 30 and 48 hpf was similar in uninjected wildtype (Fig. 4a) and scn5Lab morphant (Fig. 4b) embryos. Photo-conversion at 30 hpf followed by imaging at 48 hpf displayed in clustering of FITC+, CY3- myocytes at the ventricular outflow tract in wildtype, as expected based on previous studies of secondary differentiation (de Pater et al., 2009; Lazic and Scott, 2011; Zhou et al., 2011), and this was unchanged in morphants.
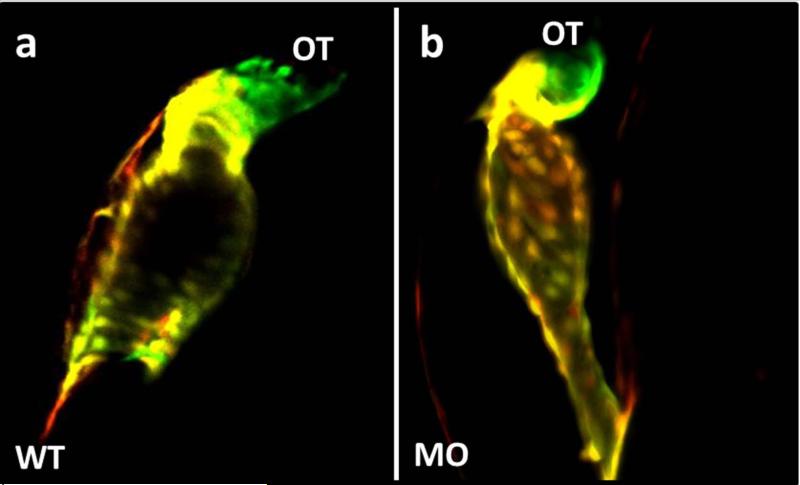
Fig. 4.
(a) Wildtype (WT) and (b) morpholino-injected embryos (MO) were exposed to UV irradiation at 30 hpf and allowed to develop until 48 hpf. Stacked confocal imaging shows photoconverted primary (yellow) cardiomyocytes, and new (green) cardiomyocytes. Between 30 and 48 hpf, secondary differentiation of cardiomyocytes occurred exclusively at the arterial pole outflow tract (OT) of the ventricle in wildtype and morphant embryos. Both groups appeared to efficiently add cells between 30 and 48 hpf.
Morphant embryos add normal number of cells to the arterial pole between 30 and 48 hpf
To test the hypothesis that differentiation of outflow tract myocardium proceeds normally in embryos deficient in scn5Lab, we quantitated secondary differentiation in wildtype and morphant ventricles. Stable transgenic zebrafish tg(UAS:Kaede) were crossed with a cardiac-specific driver line tg(cmlc2:Gal4) to generate robust expression of Kaede protein. Embryos were photo-converted as above at 30 hpf and then allowed to grow to 48 hpf and fixed. Embryos were stained with the nuclear marker TO-PRO-3-Iodide to quantitatively assay secondary cardiac differentiation at the arterial pole (Fig. 5a-e). There was no difference in the number of arterial pole myocytes differentiating between 30 and 48 hpf in wildtype versus morphant embryos (34.8±1.3 vs. 33.1±1.6, n≥8, p>0.2, Fig. 5f). The overall average number of new myocytes (34.1±1.0, n=18) observed at this stage was consistent with previously published counts (Lazic and Scott, 2011). Thus, despite impaired early specification and differentiation of pre-cardiac mesoderm, scn5Lab-morphant embryos exhibited a normal secondary phase of cardiac differentiation. This result indicates perturbation of the second heart field is not responsible for sub-physiological number of ventricular myocytes in the embryos.
Fig. 5.
Formaldehyde-fixed tg(cmlc2:gal4, UAS:kaede) embryos maintain green (a) and red (b) fluorescence after processing. Topro3-I marks nuclei (c) without affecting identification of secondary cardiomyocytes. Secondary cardiomyocytes (d) form the arterial pole of the ventricle, clustering at the outflow tract and appearing green with blue nuclei (arrow), while older, primary cardiomyocytes appear as yellow with white nuclei (arrowhead). At high-powered magnification, primary (arrow) and secondary cardiomyocytes can be identified and counted. Wildtype (34.8±1.3, n=10) and morpholino-injected (33.1±1.6, n=8) embryos (e) add equal numbers of cells to the arterial pole between 30 and 48 (p>0.2).
Ventricular cardiomyocyte proliferation is impaired in morphant embryos at 48 hpf
After 48 hpf, cardiac differentiation in the zebrafish is largely complete (Reifers et al., 2000; de Pater et al., 2009), and ventricle size and cell number increases predominantly through growth and proliferation of myocardium (Mably et al., 2006; Qu et al., 2008; Chernyavskaya et al., 2012). One potential contributor to the continued cardiomyocyte deficit in morphant embryos after initial heart tube formation is the loss of ability of differentiated cardiomyocytes to divide. To examine proliferation in the embryonic heart, we examined embryos exposed to BrdU for a five-hour window at 48-53 hpf, a time point reflecting completed secondary differentiation of myocardium. Embryos were formaldehyde-fixed, and immunofluorescence was performed, to evaluate mismatch and active morpholino-injected embryos for altered cellular proliferation. While both wildtype (5.8±0.6, n=14) and mismatch (4.0±0.9, n=11) (Fig. 6a-f, Fig. 7) hearts showed double positive (BrdU+, dsRed+) cardiomyocytes, there were no proliferating cardiomyocytes (n= 12, Kruskal-Wallis test p<10-5) from 48-53 hpf in morphant embryos (Fig. 6g-i, Fig. 7). The results indicate that proliferation of post-differentiation myocardium requires the voltage-gated sodium channel scn5Lab.
Fig. 6.
BrdU-injected tg(cmlc2:dsRed) embryos are positive for proliferating cardiomyocytes in wildtype(a-c), 5 base mismatch morpholino-injected (d-f), and morphant (g-i) embryos. Embryos injected with BrdU at 48 hpf, and incubated for 5 hours, show colocalization (arrows: c,f) of the BrdU (green) and dsRed (red)signals. Embryos injected with active morpholino do not show any colocalization of the two signals (i). Despite having no proliferation in the myocardium, morphant embryos do appear to have normal proliferation in other tissues (arrowheads).
Fig 7.
Quantification of BrdU-positive cardiomyocytes suggests embryos deficient in Scn5Lab have a proliferation defect. Embryos were imaged and sections hyperstacked in ImageJ. Cells positive for both BrdU and dsRed were counted. Embryos injected with active morpholino had no evidence of myocardial proliferation (0 double-positive cells, n=12) compared to uninjected (5.8±0.6, n=14) and mismatch-injected (4±0.9, n=11) hearts. *= pairwise significance p<0.001
Impaired cardiac contractility is not responsible for impaired myocardial proliferation in scn5La morphants
To evaluate the role of cardiac contraction in growth and proliferation of cardiomyocytes, we phenocopied the silent heart mutant zebrafish line using previously described morpholino against cardiac troponin (tnnt2-MO) (Arnaout et al., 2007). Despite complete loss of cardiac contraction, the hearts appeared to develop normally through 60 hpf development. Using morpholinos in the Tg(cmlc2:dsRed-nuc) line, we injected BrdU into the pericardium of the tnnt2-MO, tnnt2-MO + scn5Lab-mismatch (MIS), and tnnt2-MO + scn5Lab-morpholino (MO) embryos, and counted proliferating cardiomyocytes (Fig. 8). Proliferation appeared slightly reduced in tnnt2-MO compared to wildtype embryos (2.1±0.5 vs 1.1±0.3, n= 24,22, p=0.08). Co-injection of scn5Lab-MIS with tnnt2-MO appeared to have no effect on proliferation vs. tnnt2-MO alone (p=0.69); however, the effect of co-injection of active scn5Lab morpholino was identical to that observed in the non-silent heart fish: there was nearly complete absence of proliferation (1.2±0.3 vs. 0.1±0.1, n=12,10, p=0.007).
Fig 8.
Impaired cardiac contraction is not responsible for loss of proliferation in scn5Lab-MO injected embryos. WT, tnnt2-MO, tnnt2-MO+scn5Lab-MIS embryos all have detectable proliferation at 48 hpf, while scn5Lab-MO embryos had none (Kruskal Wallis test p=0.004). Mann-Whitney U test identified proliferation as significantly decreased in tnnt2-MO/scn5Lab-MO co-injected embryos vs. tnnt2-MO/scn5Lab-MIS (1.1±0.3 vs 0.1±0.1, n=12,10; p=0.007) and wildtype (2.2±0.5, n=10,24; p=0.001) hearts. These four sets of experiments were performed at the same time.
DISCUSSION
scn5Lab is required for zebrafish cardiogenesis at distinct phases of differentiation and proliferation
The voltage-gated sodium channel scn5Lab is required for normal development of the heart in embryonic zebrafish. Development of the embryonic zebrafish heart, as with much of vertebrate organogenesis, undergoes distinct phases of specification, differentiation and proliferation to form and grow the functional organ (Shu et al., 2002; Tao and Peng, 2009). Prior studies provided evidence that scn5Lab is required for expression of nkx2.5 in the specification of pre-cardiac mesoderm. Here we provide additional evidence that the morphant defect is specific to nkx2.5 and not nkx2.7. The phenotype resulting from loss of scn5Lab during cardiac development is similar to that seen in the loss of both nkx2.5 and nkx2.7 (Tu et al., 2009). Altered anterior lateral plate mesoderm gene expression resulting from loss of scn5La may be a direct effect of scn5La on activating transcription factors of nkx2.5, or through a yet-undefined network regulating cardiac specification, of which nkx2.5 is a small readout. Failure of nkx2.5 mRNA to rescue the morphant phenotype, with normal expression of nkx2.7, suggests that scn5Lab is required for the function of other critical cardiac specification pathways earlier during development. In addition to a role for sodium channels in specification of pre-cardiac mesoderm, data here implicate scn5Lab in a later role in cardiac development, being required for proliferation of differentiated cardiomyocytes. We also demonstrate that late-differentiation of cardiomyocytes from an extra-cardiac second heart field-like population is unaffected by morpholino knockdown on scn5Lab.
Scn5Lab is required for development of normal numbers of primary but not secondary cardiac cardiomyocytes in the zebrafish
Cardiomyocytes expressing markers of terminal differentiation form the heart tube at 24 hpf, completing the primary phase of cardiac differentiation. As we have previously shown and demonstrate here, embryos deficient in scn5Lab possess a significant deficit in cardiac cell number at this stage. A second phase of differentiation, induced by a separate set of controlling transcription factors, increases cardiac cell number by adding cells from the second heart field to the atrial and ventricular (outflow tract) poles. We initially hypothesized that impaired differentiation from the second heart field could be responsible for impaired ventricular growth, both in myocyte number and function (de Pater et al., 2009; Hami et al., 2011). This hypothesis was supported by prior evidence that scn5Lab is required for physiologic differentiation of the cardiac tube (Chopra et al., 2010). Interestingly, despite cardiac defects in primary differentiation, and later defects in cardiac growth, adequate secondary cardiomyocytes were added to the heart by 48 hpf by morphant embryos. In addition, the second heart field-derived myocytes were added to the distal third of the ventricle, appearing to form a normal functional outflow tract, as previously reported for normal cardiogenesis (de Pater et al., 2009; Lazic and Scott, 2011). While scn5Lab is required for specification of cardiomyocytes forming the initial heart tube at 24 hpf, different factors likely govern the differentiation of later (second heart field) cardiomyocytes, a mechanism that is unaffected by morpholino knockdown of the channel.
Scn5Lab is required for proliferation of cardiomyocytes and maintenance of physiological ventricular cell number
By 36 hpf, both early and late differentiation are largely complete, and morphant and control embryos have added cardiomyocytes at the arterial pole of the ventricle. Evidence suggests the remaining cardiac growth occurs primarily through hypertrophy (Mably et al., 2006; Lin et al., 2012) and phases of increased and decreased proliferation (Qu et al., 2008; Chernyavskaya et al., 2012) contributing to looping and completing the form of the larval heart. At this stage, from 48 hpf onward, an additional deficit in cardiac cell number is revealed in morphant embryos, which are unable to continue cardiac growth. The data we present here support the hypothesis that loss of myocardial proliferation is an important contributing mechanism to an increasing cardiomyocyte deficit in morphant embryos. While adult myocardium is not considered a proliferative tissue at baseline, proliferation is an important facet of the development of the embryonic heart (Qu et al., 2008; Chernyavskaya et al., 2012), and regeneration of adult zebrafish cardiac tissue (Jopling et al., 2010). Importantly, proliferation and early growth of the heart does not appear dependent on myocardial contraction, as silent heart (tnnt2) mutants are able to grow normally in the absence of a heart beat (Sehnert et al., 2002), obtaining adequate perfusion of tissues through diffusion of oxygen from its aquatic environment.
A mutant zebrafish line with a silent ventricle, tell tale heart (Rottbauer et al., 2006) was reported to have identical cardiomyocyte numbers to wildtype fish through 72 hpf (Rottbauer et al., 2001). Data presented here suggest that while proliferation may be decreased in silent hearts, BrdU-positive cardiomyocytes can easily be detected at 48 hpf in tnnt2-MO injected embryos. By contrast, embryos injected with scn5Lab-MO showed a nearly total absence of detectable myocardial proliferation. This experiment provides further evidence that impaired growth of the zebrafish heart due to sodium channel knockdown is not simply a secondary phenotype from impaired cardiac contraction, but rather a defect specific to sodium channel knockdown. In addition, the observed morphant phenotype, including impaired heart tube differentiation and myocardial proliferation, occurs before observed phenotypes in the silent heart phenocopy line, suggesting the requirement for scn5Lab in heart development precedes the requirement for cardiac function in maturation of the myocardium. Action potentials in the developing heart of both zebrafish and mice are supported by depolarizing current through calcium channels, with voltage-gated sodium channel activity contributing only later in development (Davies et al., 1996; Rottbauer et al., 2001; Chopra et al., 2010). Whether or not the morphant phenotype arises from a loss of sodium ion permeation through scn5Lab, it is unlikely to be due to the role of NaV1.5 in promoting action potentials responsible for cardiac contractility.
scn5Lab may influence cardiac growth independently of sodium ion permeation
Thus, the data we present above suggest the role for scn5Lab in cardiac development of zebrafish may be independent of its role in sodium permeation and action potential generation. Ion flux-dependent and independent roles ion channels in zebrafish have already been described (Kessler et al., 2012). Calcium ion signaling through the L -type calcium channel is critical for cardiac development in zebrafish independent of cardiac function (Rottbauer et al., 2001). Mutations in potassium channels on the other hand, present with an electrophysiology phenotype with no morphologic abnormalities (Kessler et al., 2012). Here, we have demonstrated a requirement for the voltage-gated sodium channel scn5Lab in both specification of pre-cardiac mesoderm, and for proliferation and growth of the developing heart. Both developmental processes occur well before sodium ion influx has been observed to be required for action potential generation and propagation, at 4-5 dpf (Chopra et al., 2010) . While the mechanism of the requirement for scn5Lab in proliferation of embryonic myocardium is unknown, evidence cited above indicates that this function is likely independent of the channel's contribution to the cardiac action potential, as even asystolic hearts display normal early development (Sehnert et al., 2002; Rottbauer et al., 2006). An alternative role promoting cellular proliferation is not without precedent for voltage-gated sodium channels. Ion channels are increasingly becoming implicated in tumor initiation and progression (Fiske et al., 2006). mRNA for NaV1.5 was recently shown to be significantly increased in some human ovarian cancers, with protein expression possibly correlated to grade and metastasis status of the tumor (Gao et al., 2010). Additionally, protein shown to bind to NaV1.5 in some cell lines, the sigma-1 receptor, has been shown to promote cancer proliferation and invasiveness (Balasuriya et al., 2012). Voltage-gated sodium channels are an integral component of the mature cardiomyocyte and as such could serve non-electrogenic roles such as acting as a critical checkpoint for myocardial division.
Conserved domains of the voltage-gated sodium channels may provide insight to its role in cellular proliferation
Voltage-gated sodium channels interact with multiple protein partners, and the c-terminus, highly conserved across orthologs including scn5Lab, is especially rich with predicted protein-binding motifs. The channels are well-characterized in their associations with β–subunits, molecules which not only modulate gating properties of sodium permeation, but serve cell-adhesion functions (Isom, 2001). A possible mechanism for the involvement in development is that through protein binding-partners, scn5Lab interacts with integral structural components of cardiomyocytes. For example, disrupted association of the SCN5A complex with the sarcomere protein syntrophin has been implicated in a variant of long QT syndrome (Ueda et al., 2008). Another possible mechanism involves participation, either directly or indirectly, of scn5Lab in signaling pathways critical to cardiac development. This is not unprecedented, as the C-terminus of the L-type voltage-gated calcium channel has been shown to function directly as a transcription factor in function and differentiation for neuronal cells (Gomez-Ospina et al., 2006). The zebrafish cardiac sodium channel scn5Lab possesses putative interaction domains for FHF1b, Nedd4-like ubiquitin ligases, and calmodulin, as well as an EF hand Ca2+-binding motif (Abriel, 2010). Electrochemical signaling has also been demonstrated to activate transcription factors directly. For example, CREB (cAMP response element-binding protein) has been shown in neurons to be directly activated by calcium ion waves, rather than through calmodulin translocation to the nucleus (Hardingham et al., 2001). This mechanism seems unlikely, as defects in zebrafish and in mice appear to arise earlier than channel expression or function can be detected. Further, specific blockers of sodium permeation have no effect on lateral plate mesodermal expression of nkx2.5 and early cardiac growth (Chopra et al., 2010).
Limitations
Zebrafish possess two isoforms of cardiac sodium channels, scn5Laa and scn5Lab. While these studies focus on the role for scn5Lab in cardiac development, there is evidence that scn5Laa is also important for cardiac development, in particular for expression of nkx2.5 in the lateral plate mesoderm (Chopra et al., 2010). Just what role each channel plays, and why knockdown of either channel results in a similar early phenotype is a question that remains to be answered. It is possible that perturbed expression of one channel affects the expression of the other channel, making it difficult to elucidate the exact mechanisms through which these genes promote cardiac development. To date, expression of the full-length channel (nearly 2,000 amino acids) in vivo has not been accomplished, so rescue of the morphant phenotype has yet to be accomplished using co-injection of mRNA. While mounting evidence suggests the role for scn5Lab (and scn5Laa) is independent of the channels’ function in action potential propagation, mRNA rescue using a non-permeant mutated channel, could definitively answer the question.
Conclusion
We demonstrate that the cardiac sodium channel scn5Lab modulates the proliferation of the ventricle following the completion of differentiation, and this effect occurs before the channel assumes a prominent role in cardiac electrogenesis. Interestingly, despite a demonstrated requirement for scn5Lab in the expression of nkx2.5 in primary specification pre-cardiac mesoderm, differentiation from the second heart field proceeds as normal in morphant embryos.
METHODS
Morpholinos
Previously validated (Chopra et al., 2010) active morpholino (AGTTTGTGCTGACCGGTGGTCTGGG, Gene Tools LLC) targeting the exon 25 splice site of scn5Lab was microinjected (1.8 ng) into the yolk of fertilized ova. Control experiments were conducted by injecting a 5-base mismatch (AcTTTcTGCTGAgCGGTGcTCTGcG), at the same injection volume (140 μm droplet) and concentration (150 μM) as active morpholino. Mismatch-morpholino injection had no detectable effect on cardiac development. Injections of antisense oligonucleotides can produce non-specific developmental effects, which can be largely mitigated using co-injection of anti-p53 morpholino (Robu et al., 2007). Accordingly, to limit toxicity, both active and mismatch morpholinos were co-injected with standard p53 morpholino (4.5 ng, Gene Tools LLC). As previously described, injection of the p53 morpholino had no detectable effect on morphant cardiac phenotype (Chopra et al., 2010). Translation of zebrafish cardiac troponin (tnnt2) was inhibited using previously validated morpholino (CATGTTTGCTCTGATCTGACACGCA), at a dose of 4ng (Sehnert et al., 2002).
Zebrafish Lines Used
For counting and proliferation studies, we used zebrafish with a transgene for the red-fluorescent protein dsRed localized to the nucleus and driven by the cardiac myosin light chain promoter (cmlc2:dsRed2-nuc) (Mably et al., 2003). For qualitative secondary differentiation studies, we generated a line of zebrafish expressing Kaede under control of the cardiac-specific cmlc2 promoter. The Kaede gene from Trachyphyllia geoffroyi is a photo-convertible fluorescent protein (), which upon exposure to ultraviolet (420nm) irradiation permanently converts from green (native) to red fluorescence (Ando et al., 2002; Sato et al., 2006). The previously identified (Huang et al., 2003) promoter region for cardiac myosin light chain (cmlc2/myl7) was first isolated using standard PCR protocols. The region was then subcloned into the 5E-MCS element of the Tol2Kit (Kwan et al., 2007), and combined with a middle element containing the sequence for the photo-convertible Kaede to generate an insertion vector. Single-cell embryos were injected with the vector, selected for transient Kaede expression. Adult zebrafish were then test-crossed to verify germline insertion, and bred to homozygosity, and multiple lines were crossed to maximize fluorescence. In addition, we utilized the Gal4-UAS transactivator system to increase the cardiac expression of Kaede so that a more accurate quantitative evaluation of secondary differentiation could be performed. The cardiac myosin light chain (cmlc2) promoter (Huang et al., 2003) was cloned upstream of Gal4 transactivator sequence in a Tol2 based vector (kind gift from M.Nonet, Wash U St. Louis). This construct was then utilized to create a stable transgenic line that had cardiac restricted expression of the Gal4 protein. This line (cmlc2:Gal4) was then crossed with the previously described tg(UAS:Kaede)s1999t line (Scott et al., 2007). Resulting double transgenic embryos had robust cardiac restricted expression of Kaede, which maintained red (CY3+) and green (FITC+) fluorescence after formaldehyde fixation and processing.
Cardiomyocyte counting
To count cardiomyocytes, zebrafish with nuclear cardiomyocyte fluorescence cmlc2:dsRed2-nuc were grown to specific time-points during development, and fixed for 1 hour in 4% formaldehyde/PBST, followed by dechorionation and 10 minute fixation in acetone. Fixed embryos were then permeabilized with Triton X (0.5% in PBS) and washed in blocking buffer (2mg/ml BSA, 2% normal goat serum, 1% DMSO). Sequential primary (anti-dsRed antibody, 1:300, Rockland) and secondary (goat-anti-Rabbit Alexa Fluor 568, 1:200, Invitrogen) antibody washes were followed by partial dissection and embedding in mounting medium on bridge slides. Hearts were imaged with an Olympus FluoView 1000 confocal microscope, and nuclei of differentiated cardiomyocytes were counted using ImageJ software (Abramoff et al., 2004) (http://rsbweb.nih.gov/ij/).
In situ hybridization
In situ hybridizations were performed using a standard protocol (Westerfield, 2000) with digoxigenin-labeled probes. Embryos at appropriate stages were fixed in 4% PBST/formaldehyde, and incubated with previously-published antisense probes nkx2.5 (Chopra et al., 2010), or cloned using published gene sequences (nkx2.7 , NM_131419). Probes were detected with mouse-anti-DIG antibody conjugated to alkaline phosphatase (Roche, 1:5000), and developed in BM Purple AP substrate (Roche). Except where otherwise noted, embryos were examined throughout development, and fixed at identical time points. ‘Hours post-fertilization’ refers to the developmental stage of the wildtype embryo. For embryos fixed during somitogenesis, individual somites were counted for each group to ensure identical developmental stages.
Assessment of secondary differentiation of ventricular cardiomyocytes
Qualitative evaluation of secondary differentiation of cardiomyocytes was performed on embryos with FITC+ hearts from the tg(cmlc2:Kaede) zebrafish described above. As previously described by others (de Pater et al., 2009), wildtype and morpholino-injected embryos were exposed to UV laser irradiation at 30 hpf, to allow cardiomyocytes of the heart tube (primary cardiac differentiation) to produce sufficient Kaede protein. Embryos were visualized as UV laser irradiation (330-385 nm) photo-converted cardiomyocytes from green (FITC+ fluorescence) to red (CY3-positive fluorescence), and focused along the z axis until complete throughout the entire heart (approximately 30 seconds per embryo). Embryos then were allowed to develop to 48 hpf, anesthetized and mounted in 1% methyl cellulose. Hearts were imaged in live anesthetized embryos on an Olympus FluoView 1000 confocal microscope, using FITC (488nm, green) and CY3 (568nm, red) lasers to detect primary (red, green) and secondary (green only) cardiomyocytes.
Quantification of secondary differentiation
The reporter line described above tg(UAS:Kaede)s1999t (Scott et al., 2007) was crossed to the tg(cmlc2:Gal4) driver line to increase expression of Kaede protein in cmlc2-expressing cardiomyocytes. Wildtype and morpholino-injected embryos were exposed to UV irradiation at 30 hpf, then grown to 48 hpf and fixed in 4% paraformaldehyde. Embryos were then permeabilized using 0.5% Triton-X in PBS, and washed in blocking buffer containing 1μM TO-PRO-3-Iodide (Invitrogen). Samples were partially dissected to expose the heart, and placed in mounting medium on bridge slides and imaged on an Olympus FluoView 1000 confocal microscope. Nuclei of secondary-differentiated cardiomyocytes were identified as FITC+ (expressing Kaede) and CY3- (not expressing Kaede before 30 hpf) and were counted using the ImageJ software (Abramoff et al., 2004).
Quantification of Cardiomyocyte Proliferation
BrdU injection to 48 hpf embryos was performed as previously described (Ribeiro et al., 2007; Qu et al., 2008). Briefly, tg(cmlc2:mCherry) embryos were injected with 2nL (1mg/mL) BrdU into the pericardiac region, and incubated for five hours. Embryos were then fixed in 4% paraformaldehyde and immunofluorescence was performed with mouse-anti-BrdU (Developmental Studies Hybridoma, 1:300) and rabbit-anti-dsRed (Rockland, 1:300). Embryos were then stripped of their heads, mounted on bridge slides, and imaged on an Olympus FluoView 1000 confocal microscope. Images were analyzed with ImageJ software package (Abramoff et al., 2004), using the cell counting feature to identify and mark proliferating cardiomyocytes.
Drug delivery to zebrafish circulation
Modulators of voltage-gated sodium channel function were delivered directly to the circulation of 48 hpf zebrafish. Embryos were lightly anesthetized with tricaine methanesulfonate to inhibit movement without affecting heart rate, and placed in 1.5% methyl cellulose in zebrafish egg water (Westerfield, 2000). Borosilicate needles were used to deliver 2 nL (25ng/mL in H2O) anemone toxin (ATX II, Sigma) directly into the left common cardinal vein (Isogai et al., 2001) to avoid volume-related disruption of cardiac function. Phenol red dye was used to evaluate for efficient delivery of the toxin. Embryos were then immediately transferred to egg water without anesthesia and evaluated for altered cardiac rhythm. The L-type calcium channel blocker nifedipine (2nL, 15mM) and H2O (2nL) were used as positive and negative controls, respectively.
nkx2.5 mRNA Injections
A zebrafish cDNA library was used to isolate and clone nkx2.5 using primers aligned to the published sequence (NCBI: NM_131421.1) into the pcGlobin2 vector (Ro et al., 2004). mRNA was transcribed in vitro using the T7 mMessage mMachine kit (Ambion). Transcript was purified and verified on 1% agarose gel, and 75 or 150 pg was microinjected into single cell embryos, as previously described (Lee et al., 1996).
Statistical Methods Used
Data are presented as mean ± standard error. Pair-wise comparisons of normally-distributed data were performed using Student's t-test. For grouped comparisons of non-parametric data, the Kruskal-Wallis test was used, followed by Mann-Whitney U test for pair-wise comparisons between individual groups. Statistically significant differences (p<0.05) are noted with asterisks.
Animal Care
All organisms were cared for in accordance with guidelines set by the Vanderbilt University Institutional Animal Care and Use Committee (IACUC) and the Office of Animal Welfare Assurance (OAWA).
ACKNOWLEDGEMENTS
We would like to acknowledge Charles Hong for critical reading of the manuscript, as well as the laboratory of Josh Gamse for providing the Tg(UAS:Kaede) zebrafish lines. This work was supported in part by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical Scientist Training Program. Experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126).
REFERENCES
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A. 2007;104:11316–11321. doi: 10.1073/pnas.0702724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasuriya D, Stewart AP, Crottes D, Borgese F, Soriani O, Edwardson JM. The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. J Biol Chem. 2012;287:37021–37029. doi: 10.1074/jbc.M112.382077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JN, Fishman MC. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122:3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- Chernyavskaya Y, Ebert AM, Milligan E, Garrity DM. Voltage-gated calcium channel CACNB2 (beta2.1) protein is required in the heart for control of cell proliferation and heart tube integrity. Dev Dyn. 2012;241:648–662. doi: 10.1002/dvdy.23746. [DOI] [PubMed] [Google Scholar]
- Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, Yang T, Zhong TP, Roden DM. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res. 2010;106:1342–1350. doi: 10.1161/CIRCRESAHA.109.213132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78:15–25. doi: 10.1161/01.res.78.1.15. [DOI] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development. 2009;136:1633–1641. doi: 10.1242/dev.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25:493–500. doi: 10.1007/s10555-006-9017-z. [DOI] [PubMed] [Google Scholar]
- Gao R, Shen Y, Cai J, Lei M, Wang Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol Rep. 2010;23:1293–1299. doi: 10.3892/or_00000763. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hami D, Grimes AC, Tsai H-J, Kirby ML. Zebrafish cardiac development requires a conserved secondary heart field. Development. 2011;138:2389–2398. doi: 10.1242/dev.061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Isom LL. Sodium channel beta subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan BR, Meyer D, Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004;131:3081–3091. doi: 10.1242/dev.01185. [DOI] [PubMed] [Google Scholar]
- Kessler M, Just S, Rottbauer W. Ion flux dependent and independent functions of ion channels in the vertebrate heart: lessons learned from zebrafish. Stem Cells Int. 2012;2012:462161. doi: 10.1155/2012/462161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lazic S, Scott IC. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev Biol. 2011;354:123–133. doi: 10.1016/j.ydbio.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Lee KH, Xu Q, Breitbart RE. A new tinman-related gene, nkx2.7, anticipates the expression of nkx2.5 and nkx2.3 in zebrafish heart and pharyngeal endoderm. Dev Biol. 1996;180:722–731. doi: 10.1006/dbio.1996.0341. [DOI] [PubMed] [Google Scholar]
- Lin YF, Swinburne I, Yelon D. Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Dev Biol. 2012;362:242–253. doi: 10.1016/j.ydbio.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably JD, Chuang LP, Serluca FC, Mohideen MA, Chen JN, Fishman MC. santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–3146. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13:2138–2147. doi: 10.1016/j.cub.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Novak AE, Taylor AD, Pineda RH, Lasda EL, Wright MA, Ribera AB. Embryonic and larval expression of zebrafish voltage-gated sodium channel α-subunit genes. Developmental Dynamics. 2006;235:1962–1973. doi: 10.1002/dvdy.20811. [DOI] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Jia H, Garrity DM, Tompkins K, Batts L, Appel B, Zhong TP, Baldwin HS. Ndrg4 is required for normal myocyte proliferation during early cardiac development in zebrafish. Dev Biol. 2008;317:486–496. doi: 10.1016/j.ydbio.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers F, Walsh EC, Leger S, Stainier DY, Brand M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar). Development. 2000;127:225–235. doi: 10.1242/dev.127.2.225. [DOI] [PubMed] [Google Scholar]
- Ribeiro I, Kawakami Y, Buscher D, Raya A, Rodriguez-Leon J, Morita M, Rodriguez Esteban C, Izpisua Belmonte JC. Tbx2 and Tbx3 regulate the dynamics of cell proliferation during heart remodeling. PLoS One. 2007;2:e398. doi: 10.1371/journal.pone.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H, Soun K, Kim EJ, Rhee M. Novel vector systems optimized for injecting in vitro-synthesized mRNA into zebrafish embryos. Mol Cells. 2004;17:373–376. [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottbauer W, Baker K, Wo ZG, Mohideen MA, Cantiello HF, Fishman MC. Growth and function of the embryonic heart depend upon the cardiac-specific L-type calcium channel alpha1 subunit. Dev Cell. 2001;1:265–275. doi: 10.1016/s1534-5807(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Wessels G, Dahme T, Just S, Trano N, Hassel D, Burns CG, Katus HA, Fishman MC. Cardiac myosin light chain-2: a novel essential component of thick-myofilament assembly and contractility of the heart. Circ Res. 2006;99:323–331. doi: 10.1161/01.RES.0000234807.16034.fe. [DOI] [PubMed] [Google Scholar]
- Sato T, Takahoko M, Okamoto H. HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis. 2006;44:136–142. doi: 10.1002/gene.20196. [DOI] [PubMed] [Google Scholar]
- Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Meth. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet. 2002;31:106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Chen JN, Fishman MC. Regulation in the heart field of zebrafish. Development. 1998;125:1095–1101. doi: 10.1242/dev.125.6.1095. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Stainier DYR. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Tao T, Peng J. Liver development in zebrafish (Danio rerio). J Genet Genomics. 2009;36:325–334. doi: 10.1016/S1673-8527(08)60121-6. [DOI] [PubMed] [Google Scholar]
- Targoff KL, Schell T, Yelon D. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev Biol. 2008;322:314–321. doi: 10.1016/j.ydbio.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C-T, Yang T-C, Tsai H-J. Nkx2.7 and Nkx2.5 Function Redundantly and Are Required for Cardiac Morphogenesis of Zebrafish Embryos. PLoS One. 2009;4:e4249. doi: 10.1371/journal.pone.0004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOSSCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of Zebrafish (Danio rerio) Univ. of Oregon Press; Eugene: 2000. The zebrafish book. [Google Scholar]
- Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE, Heideman W, Burns CE, Burns CG. Latent TGF-beta binding protein 3 identifies a second heart field in zebrafish. Nature. 2011;474:645–648. doi: 10.1038/nature10094. [DOI] [PMC free article] [PubMed] [Google Scholar]