Abstract
Cerebral microdialysis is used to study anti-cancer drug penetration in the central nervous system (CNS) and brain tumors in animal models. Genetically engineered mouse models (GEMMs) have been recently used to study many aspects of CNS tumors as they represent a more relevant model than orthotopic brain tumor xenograft models. However, it is challenging to implant microdialysis cannula in these animals because T2-weighted MRI imaging does not show the reference point (bregma) traditionally used to obtain stereotactic coordinates. Thus, an alternative reference point that can be visualized on MRI images is needed. In this study a novel reference point, identified as the intersection between the olfactory bulb/frontal lobe border and the midline between cerebral hemispheres on T2-weighted MRI images, was used to calculate anterior-posterior (AP) and medial-lateral (ML) coordinates of brain tumors in a GEMM. This point overlies a visible crossover between the rostral rhinal vein and the midline suture on the mouse skull, allowing for the conversion of the MRI coordinates into surgical stereotactic coordinates. Post-mortem MRI and histological examination confirmed accurate probe placement. This procedure will facilitate the accurate and precise implantation of microdialysis probes for the study of anti-cancer drug penetration in brain tumors of GEMMs.
Keywords: microdialysis, MRI, spontaneous tumors, glioma
Introduction
Cerebral microdialysis sampling is used to monitor CNS anti-cancer drug disposition in mouse models1,2. This approach provides advantages over other sampling methods (e.g., whole tissue homogenization) including sampling from discrete anatomic compartments such as the brain extracellular fluid (ECF) or ventricular cerebrospinal fluid (vCSF)3, sampling from normal brain or brain tumor tissue, and acquisition of unbound or pharmacologically active drug moieties4. Furthermore, microdialysis enables serial sampling from individual animals, thereby reducing the number of animals required for pharmacokinetic investigations5.
Microdialysis has been frequently employed in studying anti-cancer drug penetration within brain tumors, particularly high-grade gliomas 6–9. These studies are most often performed using murine orthotopic xenograft models in which human glioma cells are stereotactically injected into a defined brain location and the microdialysis guide cannula is simultaneously implanted. Tumor tissue then develops and surrounds the cannula6–8. Although orthotopic tumor xenograft models are easy to use, relatively inexpensive, and reproducible, in many cases the genetics and histology of human tumors are not adequately recapitulated10. In addition, the tumor microenvironment and host immune responses are likely altered in immunodeficient mice, which diminish the ability of xenograft models to truly recapitulate the features observed in human tumors11. In contrast, genetically engineered murine models (GEMMs) of brain tumors bestow many of the genetic and histological features of malignant human brain tumors11. In general, these mouse models develop tumors spontaneously upon alteration of signaling pathways critical for the development of the specific tumor of interest11,12. Thus, molecular and other complex processes including specific contributions of the tissue microenvironment, such as tumor angiogenesis, can appropriately mimic human disease in these spontaneous tumor models.
In GEMMs, heterogeneous tumor growth patterns and development of tumors in different brain regions pose several technical challenges to using these models for CNS pharmacokinetic investigations12. For example, a major challenge to using cerebral microdialysis to study anti-cancer drug penetration in tumors of GEMMs is the difficulty of acquiring stereotactic coordinates to accurately place a microdialysis cannula in the tumor. Traditionally, the coordinates of the intersection of the coronal and sagittal sutures (bregma point) are used as a reference point for the placement of the microdialysis cannula3,13. However, this approach is not practical in GEMMs because the bregma does not appear on images derived by MRI, which is the imaging method used to identify the size and location of the spontaneously arising tumors in the brain. The objective of the current study was to use in vivo MRI imaging to identify a reference point for implanting microdialysis cannula in spontaneously arising tumors in a GEMM for high-grade glioma. The accuracy of cannula placement was verified by post-mortem MRI and histological examination.
Materials and Methods
Animals
Transgenic mice used in this study were previously described14. Briefly, conditional deletion of Pten, Tp53, and Rb1 in Gfap-expressing cells was induced by I.P injection of tamoxifen (Sigma, St. Louis, MO) to GFAP-CreER™; Ptenflox/flox;Tp53 flox/flox;Rb1flox/flox post-natal day 30 (P30) mice at a dosage of 9 mg/40 gm weight daily for 3 consecutive days. Mice were maintained on a 12 h light/dark cycle with free access to food and water. Mice developed tumors within 4 to 6 months. Other mouse strains (C57BL6 and CD1 athymic nude mice) were also used for validation of reference point studies. All animal studies were carried out in compliance with the Animal Care and Use Committee at St. Jude Children's Research Hospital.
Magnetic Resonance Imaging (MRI)
MRI exams were performed using a 7-Tesla Bruker Clinscan animal MRI scanner and a 4-channel phased-array surface coil (Bruker BioSpin MRI GmbH, Germany). Animals were anesthetized using isoflurane during data acquisition. Turbo Spin Echo (TSE) protocols (TR 2000–2500 ms; TE 40 ms) were used to produce T2 weighted transverse images with slice thickness of 0.5 mm. Turbo Gradient Spin Echo protocols (TR 2000 ms; TE 40 ms; Flip angle 180 degree) were used to produce fast T2-weighted images in the coronal plane with slice thicknesses of 0.7 mm. For post-mortem MRI, the mouse heads were submerged in Fluorinert FC-770 liquid (3M, Belgium) and imaged by a TSE protocol with a rat head 4-channel phased-array surface coil.
Magnetic Resonance Angiography (MRA)
MRA data were produced from a Bruker FL2D Time-of-Flight (TOF) protocol (TR 7.6 ms; TE 3.77 ms; Flip angle 90 degree). The raw data were acquired in the coronal plane followed by Maximum-Intensity Projection (MIP) reconstruction on a Siemens workstation using Syngo MR B15 software (Siemens, Erlangen, Germany). Image processing procedures were similar for both angiography and post-mortem MRI studies.
Stereotactic Surgery for Microdialysis Cannula Insertion
The general surgical procedure has been previously described15. Briefly, a mouse was anesthetized with 50 mg/kg ketamine (Hospira, Inc., Lake Forest, IL) and 10mg/kg xylazine (VEDCO, St. Joseph, MO). The head of the mouse was fixed in position on a KOPF stereotactic apparatus (Tujunga, CA). A small incision on the scalp was made and the rostral rhinal vein was identified. Using MRI images, tumor coordinates were calculated using View-Dimensions tool in Syngo MR B15 software. A microdialysis guide cannula was implanted in the center of a tumor. The mouse was allowed to recover for 3–5 days. On the day of the microdialysis study, a microdialysis probe (MBR-1–5, Bioanalytical Systems) was inserted through the cannula. The probe was perfused with artificial cerebrospinal fluid (aCSF15) at 0.5 μL/min, and the probe membrane was allowed to equilibrate for 1 hour before starting the microdialysis experiment. Microdialysis experiments were performed as previously described2.
Histology
At the end of the microdialysis experiment, the animal was euthanized and the brain was removed and fixed in 10% neutral buffered formalin (NBF) for at least 24 hr. Cannula and probe were removed and the brain was sliced into sagittal sections around the location of the implanted cannula, dehydrated, cleared, and embedded in a paraffin block. Sections were cut on a microtome at 4 μm thickness and every tenth section was collected and stained with haematoxylin and eosin. All sections from a given block were examined microscopically to verify the accurate placement of the microdialysis probe via the track in the tumor.
Results and Discussion
Identification of the rostral rhinal vein as a correlative reference point
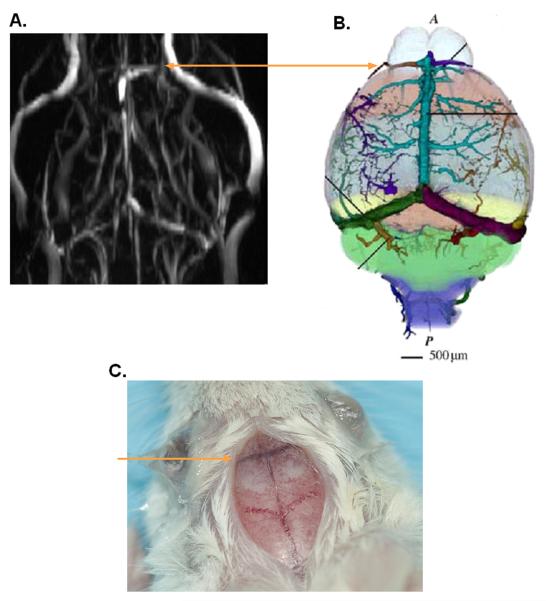
Images of blood vessels of the mouse brain acquired using angiography revealed the presence of a large vein overlying the border between the olfactory bulb and the frontal lobe (Fig. 1A and 1B). Using the mouse cerebral vasculature atlas16, this vein was identified as the rostral rhinal vein, which is readily apparent to the unaided eye and traverses both the dorsal and ventral surfaces following the olfactory bulb/frontal lobe border (Fig. 1C). The removal of skulls (n≥6 per strain used) confirmed that this vein overlies the border between the olfactory bulb and the frontal lobe. Thus, we designated our reference point as the intersection of the midline suture and the rostral rhinal vein on the mouse brain surface. This point correlated with the intersection between the midline and the olfactory bulb/frontal lobe border visualized on MRI T2-weighted images of the brain. The rostral rhinal vein was present in all mouse strains evaluated including C57BL6 and CD1 athymic nude. Anatomical dissection of these strains confirmed that this vein overlies the olfactory bulb/frontal lobe border and thus this method may be applied to target tumors in other mouse strains (data not shown).
Fig. 1. Presence of the rostral rhinal vein on the surface of a mouse brain.
A, Angiogram of a non-tumor bearing GFAP-CreER™;Ptenflox/flox;Tp53flox/flox;Rb1flox/flox mouse showing a large vein across the olfactory bulb/frontal lobe border. B, Diagrammatic illustration of veins on mouse brain surface illustrating the rostral rhinal vein on the border between the olfactory bulb and the mouse brain frontal lobe. Reproduced with permission from16. C, Brain surface of a GFAPCreER™;Ptenflox/flox;Tp53flox/flox;Rb1flox/flox mouse. Lines indicate location of the rostral rhinal vein.
Determination of Tumor coordinates and confirmation of probe placement
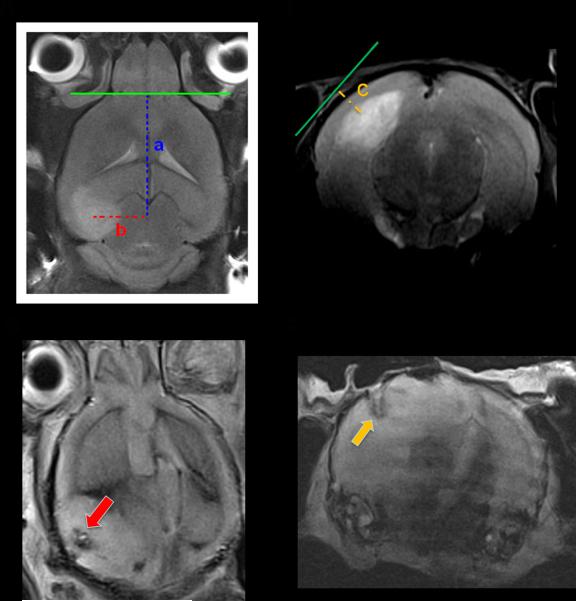
Tamoxifen injection induced Cre activity and spontaneous glioma formation in GFAPCreER™;Ptenflox/flox;Tp53flox/flox;Rb1flox/flox mice14. Induced mice were monitored biweekly for tumor development by MRI using T2-weighted imaging beginning at P120. Tumors developed in several locations within the brain including the forebrain, midbrain, and the cerebellum. When a tumor was large enough for microdialysis cannula/probe implantation (around 2.5–4 mm in diameter), placement coordinates were determined using the View-Dimensions tool in Syngo MR B15 software. The X and Y axes were determined using the transverse plane (Fig. 2A) while the depth of implantation (Z-axis) was determined using the coronal plane (Fig. 2B). Postmortem MRI (Fig. 2C and 2D) and histochemical examination (Fig. 2E) confirmed targeted probe placement in all mice studied (n=6). Mice used in this study developed tumors that arose within several regions of the brain including the forebrain (33%), midbrain (50%), and the cerebellum (17 %). Probe recovery values for the conducted experiments were 21% ± 4.5% (n=6).
Fig. 2. Derivation of MRI-guided stereotactic coordinates for cannula implantation in a spontaneously arising brain tumors.
A, Transverse section on a T2-weighted MRI demonstrating a tumor in the left hippocampus. Tumor margins are enclosed by a dashed line. The intersection between the olfactory bulb/frontal lobe border and the midbrain was used as a reference point to determine the coordinates on the Y-axis (a) and the X-axis (b). B, Coronal section on a T2-weighted MRI for calculation of the depth coordinates, i.e. Z-axis (c). In the provided example, values for a, b, and c were 7.6, 3, and 1.3 mm, respectively. C–D, Postmortem MRI showing track of the microdialysis probe in the tumor in the X and Y axes (red arrow), and on the Z-axis (yellow arrow). E, Hematoxylin and eosin staining confirming probe location in the targeted area (yellow dots limit tumor margins, blue dashes limit cannula track, and red dashes limit probe track; lines were drawn with Adobe Photoshop V11.0).
Conclusion
It is a challenge to accurately place microdialysis cannulae in GEMMs that spontaneously develop brain tumors because the traditional reference point commonly used to derive stereotactic co-ordinates for cannula implantation, the bregma, is not visible by MRI. By using a combination of angiography and T2-weighted imaging by MRI, we identified the rostral rhinal vein as a reference point to derive coordinates for accurate placement of microdialysis cannula in tumors developing in several regions of the mouse brain.
Acknowledgments
We thank Monique Payton and Melissa Johnson from the Animal Imaging Center for their assistance in imaging the mice using MRI and for their helpful discussion.
This work was supported in part by grants CA23099, CA21765 and CA096832 from the U.S. Public Health Service and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Boschi G, Scherrmann J. Microdialysis in mice for drug delivery research. AdvDrug DelivRev. 2000;45(2–3):271–281. doi: 10.1016/s0169-409x(00)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-Binding Cassette and Solute Carrier Transporters in Erlotinib CNS Penetration and Intracellular Accumulation. Clin Cancer Res. 17(1):89–99. doi: 10.1158/1078-0432.CCR-10-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen J, Carcaboso AM, Hubbard KE, Tagen M, Wynn HG, Panetta JC, Waters CM, Elmeliegy MA, Stewart CF. Compartment-specific roles of ATP-binding cassette transporters define differential topotecan distribution in brain parenchyma and cerebrospinal fluid. Cancer Res. 2009;69(14):5885–5892. doi: 10.1158/0008-5472.CAN-09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange EC, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Jr., Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BW, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24(5):1014–1025. doi: 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 5.Pan YF, Feng J, Cheng QY, Li FZ. Intracerebral microdialysis technique and its application on brain pharmacokinetic-pharmacodynamic study. Arch Pharm Res. 2007;30(12):1635–1645. doi: 10.1007/BF02977335. [DOI] [PubMed] [Google Scholar]
- 6.Carcaboso AM, Elmeliegy MA, Shen J, Juel SJ, Zhang ZM, Calabrese C, Tracey L, Waters CM, Stewart CF. Tyrosine kinase inhibitor gefitinib enhances topotecan penetration of gliomas. Cancer Res. 70(11):4499–4508. doi: 10.1158/0008-5472.CAN-09-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J, Li S, Reed K, Guo P, Gallo JM. Pharmacodynamic-mediated effects of the angiogenesis inhibitor SU5416 on the tumor disposition of temozolomide in subcutaneous and intracerebral glioma xenograft models. J Pharmacol Exp Ther. 2003;305(3):833–839. doi: 10.1124/jpet.102.048587. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Pulfer S, Li S, Chu J, Reed K, Gallo JM. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res. 2001;61(14):5491–5498. [PubMed] [Google Scholar]
- 9.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91(1):51–58. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech. 2008;1(2–3):78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66(7):3355–3358. doi: 10.1158/0008-5472.CAN-05-3827. discussion 3358–3359. [DOI] [PubMed] [Google Scholar]
- 12.de Vries NA, Beijnen JH, van Tellingen O. High-grade glioma mouse models and their applicability for preclinical testing. Cancer Treat Rev. 2009;35(8):714–723. doi: 10.1016/j.ctrv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Bert L, Favale D, Jego G, Greve P, Guilloux JP, Guiard BP, Gardier AM, Suaud-Chagny MF, Lestage P. Rapid and precise method to locate microdialysis probe implantation in the rodent brain. J Neurosci Methods. 2004;140(1–2):53–57. doi: 10.1016/j.jneumeth.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, Broniscer A, Ellison DW, Baker SJ. Cooperativity within and among Pten, p53, and Rb Pathways Induces High-Grade Astrocytoma in Adult Brain. Cancer Cell. 19(3):305–316. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leggas M, Zhuang Y, Welden J, Self Z, Waters CM, Stewart CF. Microbore HPLC method with online microdialysis for measurement of topotecan lactone and carboxylate in murine CSF. J Pharm Sci. 2004;93(9):2284–2295. doi: 10.1002/jps.20134. [DOI] [PubMed] [Google Scholar]
- 16.Dorr A, Sled JG, Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007;35(4):1409–1423. doi: 10.1016/j.neuroimage.2006.12.040. [DOI] [PubMed] [Google Scholar]