Abstract
Regulatory T-cells (Tregs) play a pivotal role in the maintenance of immune tolerance and hold great promise as cell therapy for a variety of immune-mediated diseases. However, the cellular mechanisms that regulate Treg maintenance and homeostasis have yet to be fully explored. While Tregs express Granzyme-B (GrB) to suppress effector T-cells via direct-killing, the mechanisms by which they protect themselves from GrB-mediated self-inflicted damage are unknown. We show, for the first time, that both iTregs and nTregs increase their intracellular expression of GrB and its endogenous inhibitor, Serine Protease Inhibitor-6 (Spi6) upon activation. Sub-cellular fractionation and measurement of GrB activity in the cytoplasm of Tregs show that activated Spi6−/− Tregs had significantly higher cytoplasmic GrB activity. We observed an increase in GrB-mediated apoptosis in Spi6−/− nTregs and impaired suppression of alloreactive T-cells in vitro. Spi6−/− Tregs were rescued from apoptosis by the addition of a GrB inhibitor (Z-AAD-CMK) in vitro. Furthermore, adoptive transfer experiments showed that Spi6−/− nTregs were less effective than WT nTregs in suppressing Graft-versus-host-disease (GVHD) due to their impaired survival, as shown in our in vivo bioluminescence imaging. Finally, Spi6-deficient recipients rejected MHC class II-mismatch heart allografts at a much faster rate and showed a higher rate of apoptosis among Tregs, as compared to WT recipients. Our data demonstrate, for the first time, a novel role for Spi6 in Treg homeostasis by protecting activated Tregs from GrB-mediated injury. These data could have significant clinical implications for Treg-based therapy in immune-mediated diseases.
Keywords: Regulatory T-cells, T cell homeostasis, Tolerance
Introduction
Regulatory T-cells (Tregs) play a critical role in mediating immune tolerance, not only to self-antigens, but also to alloantigens, allergens and tumor antigens(1, 2). Treg-therapy represents a promising tolerogenic approach for a wide variety of immune-mediated conditions, including autoimmune disease, Graft-Versus-Host-Disease (GVHD) and transplantation tolerance(3-5). Although our knowledge of the wide spectrum of their suppressive properties is constantly expanding(6, 7), the homeostatic mechanisms involved in maintaining the population and function of Tregs have been largely unexplored(8). Given the increasing number of clinical trials testing the safety and efficacy of Treg-based immunotherapy, the need to establish the mechanisms that determine Treg survival is paramount(9).
One of the mechanisms for Treg-mediated suppression is the direct cytolysis of target effector cells(10): Tregs possess Granzyme B (GrB), enabling them to induce apoptosis in effector T-cells(11, 12); indeed, GrB-mediated suppression by Tregs plays an important role in maintaining transplant and tumor tolerance(11, 13). Endogenous serine protease inhibitors (serpins) of GrB have been shown to protect target cells from GrB secreted by effector T-cells(14, 15). However, the existence of specific regulatory mechanisms that protect Tregs against self-inflicted GrB-mediated damage, and, in particular, the role of endogenous GrB inhibitors therein, has yet to be determined(16). Knowledge of the molecular mechanisms controlling Treg survival have the potential to greatly advance our understanding of Treg biology, thereby facilitating the design of more effective Treg therapy for multiple immune diseases. In this study, we focus on the mechanistic role of the GrB-inhibitor Serine Protease Inhibitor 6 (Spi6) in controlling the homeostasis and tolerance-inducing properties of Tregs in murine models of GVHD and heart transplantation.
Material and Methods
Mice
C57BL/6(thy1.1+) and C57BL/6(thy1.2+), BALB/c(H-2d) and B6.C-H2bm12/KhEg(bm12) mice were obtained from the Jackson Laboratory. Spi6−/− C57BL/6 mice were maintained in our animal facility(15). All animals were used at 6–10 weeks of age (20–25 g) and were housed in accordance with institutional and NIH guidelines. The Harvard Medical School Animal Management Committee approved all animal experiments.
Flow Cytometric Analysis
Anti-mouse Abs against CD4, CD8, CD25, FoxP3, H2Kb, thy1.2, CD44, CD62L, Ki67, Annexin and GrB were purchased from BD Biosciences, San Jose, CA. Intracellular anti-Spi6 was purchased from Hycult-Biotech. Cells recovered from spleens and peripheral lymphoid tissues were analyzed by flow cytometry with a FACS Canto-II flow-cytometer (BD-Biosciences) and analyzed using FlowJo software version 9.3.2 (Treestar, Ashland, OR).
Treg generation assay
Using a 96-well flat-bottom plate, 100 μl of anti-CD3-Ab diluted in PBS (1 μg/ml, BD Biosciences) was added to each well and incubated at 37°C for 4 hours. Soluble anti-CD28-Ab (1 μg/ml, BD Biosciences), soluble recombinant human TGF-β (1 ng/ml, R&D Systems) and recombinant murine IL-2 (2 ng/ml) were then added to each well in the presence of 2.5×104 CD4+ T-cells (MACS-separation, Miltenyi Biotec) from WT or Spi6−/− C57BL/6 mice. A GrB inhibitor, Z-AAD-CMK, was obtained from Calbiochem (Cambridge, MA) and used at a 1μM concentration (15).
Treg isolation and suppression assay
CD4+CD25+ Tregs were purified from WT and Spi6−/− C57BL/6 splenocytes using the Regulatory T-cell Isolation Kit-II (Miltenyi-Biotec), according to the manufacturer’s instructions. Tregs were then co-cultured in varying ratios with freshly isolated CD4+CD25− T-cells from C57BL/6 mice (Teff, 1×105; Teff/Treg ratios 1/1, 1/2 and 1/4) in the presence of T cell-depleted splenocytes (1×105) and anti-CD3 (1 μg/ml) for 96 h. 3H-TdR was added 16 h before the cells were harvested for analysis.
Sub-cellular Fractionation of Tregs and measurement of GrB-activity
CD4+CD25+ Tregs were stimulated in vitro with anti-CD3/CD28 for 24 hours. Cells were then lysed by sonication in hypotonic buffer (50 mM PIPES, 50 mM KCL, 5 mM EGTA, 2 mM MgCl2, 5 mM DTT [pH 7.6]), and centrifuged at 3,000 × g for 20 min to remove nuclei, followed by a further 30 min at 15,000 × g to produce cytosol (supernatant) and granule (pellet) fractions. The granule pellet was re-suspended in 1% Triton X-100, 10 mM Tris.HCl and 150 mM NaCl (pH 7.6) for 30 min on ice. Colorimetric assays for GrB were performed in reaction-buffer with Ac-IEPD-pNA at 0.2 mM, as previously described(15, 17).
Confocal Microscopy
For subcellular localization of CD107 and GrB, the cells were fixed and permeabilized with a kit from BD Biosciences and were then stained with FITC-labeled anti-GrB and PE-labeled anti-CD107 (eBiosciences) for 20 min, then washed and mounted on slides. The cells were then visualized with a Zeiss LSM510 META laser scanning confocal microscope with a 633 water immersion objective (NA 1.2). Separate filter sets for FITC (488 nm excitation, a 505–550 nm band pass emission) and PE (543 nm excitation, 560 nm long pass emission) were used for image acquisition.
GVHD model
BALB/c hosts underwent total body irradiation (9 Gy) at 85 rad/min and received 2 × 106 bone marrow cells and either 0.5 × 106 or 2 × 106 CD25-depleted T-cells derived from WT or SPI6−/−C57BL/6 mice via tail vein injection. Splenocytes were harvested from recipient mice on day 4 and day 10 as indicated for flow-cytometry analysis. To study the survival of nTregs, irradiated BALB/c hosts received 2 × 106 bone marrow cells and 0.5 × 106 CD25-depleted T-cells derived from Thy1.1 WT mice via tail vein injection. Mice also received 0.5 × 106 nTregs from either Thy1.2 WT or Spi6−/−C57BL/6 mice. Splenocytes were harvested from recipient mice on day 4 as indicated for flow cytometry analysis.
Bioluminescence-molecular imaging to track the survival of Tregs
Tregs were isolated from naïve mice as discussed above and cultured with Dynabeads (11452D, Gibco) at 37°C overnight; the lentivirus-vector was then added directly to the cells (at a multiplicity of infection of 5 transducing-units-per-cell) in the presence of Polybrene (8 μg/ml). The cells were centrifuged (at 300× g) for 2 hours at 32 °C and then rested for another 6 hours prior to their injection into irradiated-BALB/c mice, along with WT CD3+CD25− T-cells and WT bone-marrow cells. To ensure >90% transduction efficiency of CD4+ T-cells, a test sample was collected 24 hours later to permit examination of the cells for GFP-expression using fluorescence-microscropy.
Gluc secretion by live Tregs in the BALB/c mice was analyzed at days 2 and 8 via a 5 μL blood sample taken simply through a minor tail-cut. The addition of coelenterazine to the blood sample containing Gluc induced a light-producing chemical reaction measured by a luminometer. For the in-vivo bioluminescence imaging, BALB/c mice were injected i.v. with coelenterazine (4 mg/kg body weight) 10 days after receiving the Gluc-transduced Tregs and imaged using a cooled-charge-coupled devise (CCD) camera.
Murine Cardiac transplantation
Vascularized intra-abdominal heterotopic transplantation of cardiac allografts was performed using microsurgical techniques(18). Graft survival was assessed by daily palpation; rejection was defined as complete cessation of cardiac contractility as determined by direct visualization, and was confirmed by histological examination.
Mixed Lymphocyte Reaction (MLR) and Luminex assay
Recipient splenocytes plus irradiated (30Gy) allogeneic BM12 splenocytes (0.5×106 each) were cultured for 48 hours. Cell-free supernatants of individual wells were removed after 48 h of incubation and analyzed by a multiplexed cytokine bead–based immunoassay using a preconfigured 21-plex mouse cytokine detection kit (Millipore). All samples were tested in triplicate wells.
Isolation of Lymphocytes from hearts
Cardiac allografts were removed, perfused with PBS, minced finely with a razor blade and digested at 37 °C with 1 mg/ml collagenase in 1 ml complete medium for 1 hour. Cells in the supernatant were washed twice, centrifuged at 620 × g using Percoll solutions at 33% (cell suspension) and 66 %. Lymphocytes were aspirated at the interface.
Histological and Immunohistochemical Assessment
Five-micron-thick, formalin-fixed, paraffin-embedded sections were stained with standard Hematoxylin/Eosin stain (H&E). Acute cellular rejection was semi-quantitatively graded (0R-3R) according to the revised International Society of Heart and Lung Transplantation (ISHLT) guidelines(19). The extent of cellular infiltration and myocyte loss was also assessed.
Statistics
Kaplan-Meier survival graphs were constructed, and a log-rank comparison of the groups was used to calculate p-values. The unpaired t-test was used for comparison of experimental groups. Differences were considered to be significant for p≤0.05. Prism-software was used for data analysis and drawing graphs (GraphPad-Software, Inc., San-Diego, CA). Data represent mean ± SEM.
Results
Tregs express GrB and Spi6 upon activation in vitro
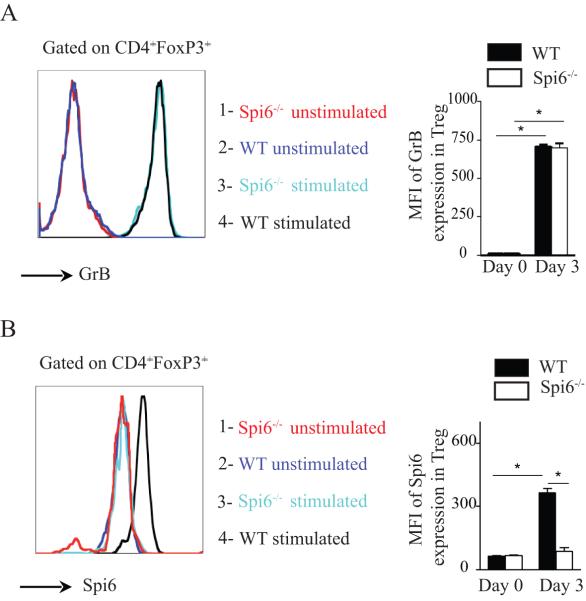
We first examined the dynamics of GrB and Spi6 expression in Tregs, both at rest and following activation by anti-CD3/CD28 stimulation in the presence of IL-2 and TGF-β in our Treg generation assay. Unstimulated CD4+FoxP3+ Tregs from naïve WT or Spi6−/− mice did not express GrB or Spi6 (figures 1A and 1B, respectively). Following activation of CD4+ T-cells for 72 hours, however, both WT and Spi6−/− Tregs showed a significant increase in their GrB expression (figure 1A); as compared to Spi6−/− Tregs, WT Tregs increased their expression of Spi6 following stimulation (figure 1B). Our data show, for the first time, that, in addition to increasing their expression of GrB, activated Tregs also increase their intracellular expression of Spi6, compared to resting Tregs.
Figure 1. GrB and Spi6 expression in naive and activated T regulatory cells in vitro.
CD4+ T cells isolated from WT and Spi6−/− C57BL/6 mice were stimulated in vitro in a Treg generation assay. (A) Representative histograms of GrB expression in CD4+ regulatory T cells (gated on CD4+FoxP3+) from unstimulated Spi6−/− (red color) and WT (Dark blue) mice at baseline (day 0); and day 3 post-stimulation (Spi6−/−: Light blue and WT: black). Both WT and Spi6−/− Tregs comparably increased their expression of GrB at day 3 as compared to day 0. Bar graph shows the MFI of GrB expression. (*p<0.05; n=3-5 mice; data representative of 3 separate experiments). (B) Representative histograms of Spi6 expression in CD4+ regulatory T cells (gated on CD4+FoxP3+) from WT and mice at day 0 (Spi6−/− : red color and WT : Dark blue) and day 3 (Spi6−/− : Light blue and WT : black). As compared to Spi6−/− Tregs, WT Tregs increased their expression of Spi6 at day 3. Bar graph shows the MFI of Spi6 expression at day 0 and day 3. (*p<0.05; n=3-5 mice; data representative of 3 separate experiments).
Impaired survival and function of Spi6−/− nTregs in vitro
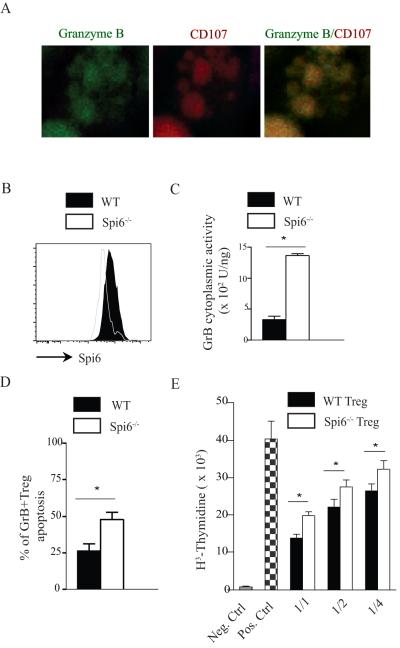
nTregs (CD4+CD25+) isolated from naïve WT mice and stimulated in vitro with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 72 hours showed significant production of GrB, as shown by confocal imaging in figure 2A. While figure 1B shows that nTregs from naïve WT mice showed no Spi6 expression, nTregs stimulated with anti-CD3/CD28 for 72 hours increased their expression of Spi6 as compared to stimulated Spi6−/− nTregs (figure 2B). As Spi6 is shown to bind and inactivate intra-cytoplasmic GrB, we isolated the cytoplasmic fractions of both WT and Spi6−/− activated nTregs by subcellular-fractionation, as previously described(15), to permit measurement of GrB activity therein. Indeed, levels of GrB activity in the cytoplasm of activated Spi6−/− Tregs were shown to be 3-fold greater than those in WT Tregs (figure 2C), indicating intra-cytoplasmic leakage of GrB from their own cytotoxic granules. To determine whether this intra-cytoplasmic GrB leakage resulted in impaired survival of GrB+Tregs in vitro in the presence of Spi6 deficiency, we examined the rate of apoptosis of Spi6−/− and WT Tregs by determining their phosphotidyl-serine surface expression, as measured by Annexin-V staining(20). Indeed, Spi6−/− Tregs underwent more apoptosis than WT Tregs, as evidenced by the increased percentage of Tregs expressing Annexin-V at 72 hours (figure 2D).
Figure 2. Spi6−/− Tregs suppress T cell proliferation less efficiently than WT Tregs.
CD4+CD25+ Tregs from WT were activated with anti-CD3 and anti-CD28 antibodies for 24 hr, permeabilized, and stained with anti-GrB FITC and anti-CD107 PE (granular marker). The cells were then visualized by laser-scanning confocal microscopy. (B) Representative histograms of Spi6 expression in CD4+CD25+ Tregs from WT and Spi6−/− mice at day 3 post stimulation with anti-CD3 and anti-CD28 antibodies. WT nTregs increased expression of Spi6 after stimulation in vitro. (C) Subcellular fractionation studies of stimulated WT and Spi6−/− Tregs showed higher intracytoplasmic GrB activity in Spi6−/− Tregs compared to WT Tregs. (D) Graph shows the percentage of Annexin expression (marker of apoptosis) by stimulated GrB+ WT and Spi6−/− Tregs at day 3 post-stimulation. (E) Graph shows WT and Spi6−/− Treg suppression of T cell proliferation at varying Treg/Teff ratios (Teff, 1×105; Treg/Teff ratios 1/1, 1/2, and 1/4). (*p<0.05; n=3-5 mice; data representative of 2 separate experiments).
To compare their suppressive abilities, WT and Spi6−/− CD4+CD25+ nTregs isolated from naïve mice were then added to WT CD4+CD25− T-cells in varying ratios (1/1, 1/2, and 1/4), in the presence of T-cell-depleted BALB/c splenocytes and anti-CD3. As shown in figure 2E, Spi6−/− Tregs suppressed T-cells less efficiently than WT Tregs at all ratios tested.
Defect in Treg generation in the absence of Spi6
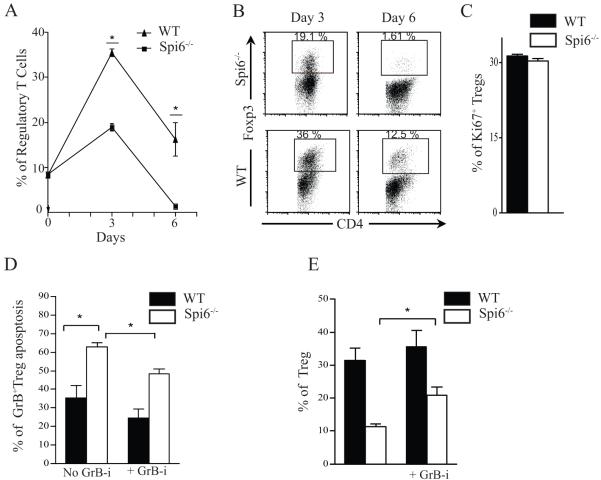
As there is great interest in using ex vivo generated Tregs as cell therapy, we sought to study the role of Spi6 in Treg generation in vitro. Consequently, isolated CD4+ T-cells from WT and Spi6−/− mice were stimulated in a Treg generation assay: Figures 3A and 3B show a significant reduction in the percentage of Spi6−/− Tregs compared to WT Tregs at day 3 (18.95 ± 0.87 vs. 35.50 ± 0.86%, respectively; p<0.01) and day 6 (1.46 ± 0.13 vs. 16.25 ± 3.75%, respectively; p<0.01). The absolute number of Spi6−/− Tregs was also significantly lower than WT Tregs at day 3 (20.43 × 103 ± 1.00 vs. 28.35 × 103 ± 2.3, respectively, p=0.02) and day 6 (2.37 × 103 ± 0.28 vs. 12.23 × 103 ± 3.58, respectively, p=0.01). To examine defects in Treg proliferation, we then measured the expression of Ki67 in WT and Spi6−/− Tregs in our Tregs generation assay by flow cytometry (21). As shown in figure 3C, no difference in the percentage of Ki67+ Tregs was observed between WT and Spi6−/− Tregs. Nonetheless, activated Tregs lacking Spi6 had a higher rate of apoptosis (% of Tregs expressing Annexin-V) compared to WT Tregs at day 3 (62.98 ± 2.28 vs. 35.23 ± 6.76%, respectively; p=0.005). Given that Spi6 is an inhibitor of GrB, we next determined whether the impaired survival of Spi6−/− Tregs was due to increased GrB-mediated apoptosis. Treatment of Spi6−/− T cells with the GrB inhibitor, Z-AAD-CMK, reduced apoptosis of treated Spi6−/− Tregs compared to untreated ones, as evidenced by annexin V staining (48.33 % ± 2.72 vs. 62.98 % ± 2.28, respectively; p=0.004; figure 3C). Furthermore, treatment with GrB inhibitor increased the percentage of generated Tregs as well (31.40 ± 3.75 vs. 11.35 ± 0.81, respectively; p<0.001; figure 3D).
Figure 3. Defect in Treg generation in the absence of Spi6.
(A) Graph shows that the percentage of Spi6−/− CD4+ Tregs was significantly less compared to WT Tregs at day 3 and day 6 in a Treg generation assay (*p<0.05; n=3-5 mice; data represents one of three separate experiments). (B) Representative examples of FACS staining of CD4 and FoxP3 from the Treg generation assay using WT and Spi6−/− CD4+ T cells at different time points. (C) The ratio of proliferating Tregs in the Treg generation assay as measured by percentage of Ki67+ Tregs by flow cytometry at day 2. (D) Graph shows the percentage of Annexin expression (marker of apoptosis) by GrB+Tregs from WT and Spi6−/− mice in the Treg generation assay at day 3 post-stimulation with or without GrB inhibitor (Z-AAD-CMK) at 1 μM. (D) Graph shows the percentage of Tregs from WT and Spi6−/− mice at day 3 post-stimulation with (left) or without (right) GrB inhibitor (Z-AAD-CMK) at 1 μM. (*p<0.05; n=3-5 mice; data representative of 2 separate experiments).
Our data indicate that activated Tregs exhibit GrB-activity and that the presence of Spi6 is critical for their protection against GrB and, consequently, their survival.
Spi6 is critical to the survival of activated Tregs in vivo
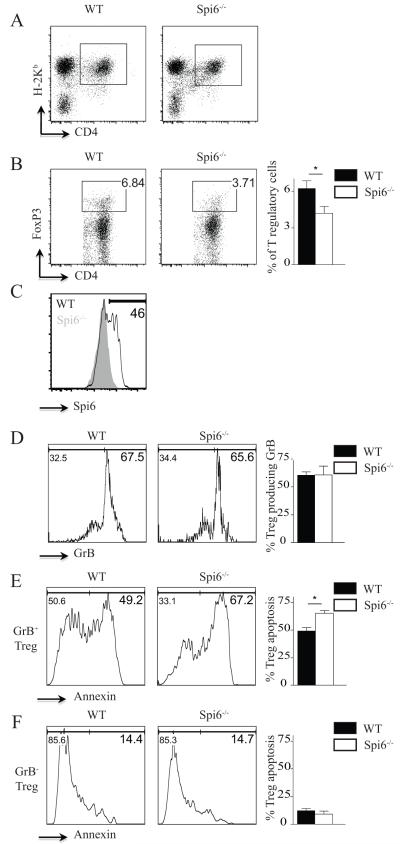
To examine the role of Spi6 in the survival of induced Tregs (iTregs) in an allogeneic setting in vivo, lethally irradiated BALB/c mice were reconstituted with 2 × 106 bone-marrow cells and 2 × 106 CD3+CD25− T-cells derived from WT (H-2Kb) or Spi6−/− (H-2Kb) C57BL/6 mice. Splenocytes were harvested from recipient mice on day 4, and donor-derived Tregs (H-2Kb+CD4+Foxp3+) were analyzed by flow cytometry (figures 4A and 4B). As shown in figure 4B, the percentage of WT Tregs was double that of Spi6−/− Tregs. The absolute numbers of WT Tregs were also more than twice those of Spi6−/− Tregs (130.90 × 103 ± 37.88 vs. 48.67 × 103 ± 10.73, respectively; p=0.05). As compared to Spi6−/− Tregs, WT Tregs increased their expression of Spi6 at day 4, showing that more than 40 % of alloactivated Tregs (46.83 ± 6.30 %) do express Spi6 in vivo (figure 4C). We then examined the expression of GrB and Annexin-V to measure the apoptosis of allo-activated Tregs. As shown in figure 4D, while more than 60% of both WT and Spi6−/− Tregs expressed GrB, GrB+Spi6−/− Tregs expressed a higher level of Annexin-V compared to GrB+WT Tregs (65.13 ± 2.62 vs. 49.05 ± 3.26%, respectively; p<0.01; figure 4E). No difference in Annexin-V expression was observed between GrB−Spi6−/− Tregs and GrB-WT Tregs (9.08 ± 2.80 vs. 12.28 ± 1.96%, respectively; p=0.2; figure 4F), indicating that, in the absence of GrB, Treg apoptosis was both minimal and unaffected by the presence or absence of Spi6. However, GrB−Spi6−/− Tregs expressed significantly lower levels of Annexin-V than GrB+Spi6−/− Tregs (9.08 ± 2.80 vs. 65.13 ± 2.62%, respectively; p<0.01), suggesting that the effect of Spi6 deficiency on Treg survival is directly related to the presence of GrB. Collectively, these data suggest a critical role for Spi6 in protecting GrB-producing Tregs from GrB-mediated apoptosis in vivo.
Figure 4. Spi6 as a key molecule in maintaining the survival of alloactivated Tregs in vivo.
Lethally irradiated BALB/c mice were reconstituted with 2 × 106 bone marrow cells and 2 × 106 T cells derived from WT (H-2Kb+) or Spi6−/− (H-2Kb+) mice. Splenocytes were harvested from recipient mice on day 4 for flow cytometry analysis of GrB and Annexin expression on donor-derived Tregs (H-2Kb+CD4+FoxP3+). (A) Representative examples of FACS staining of recipient splenocytes gated on H-2Kb+ and CD4+ are shown. (B) Representative examples of FACS staining of recipient splenocytes gated on H-2Kb+, CD4+ and FoxP3+ are shown. The percentage of WT Tregs was significantly higher than Spi6−/− Tregs. Bar graph represents the percentage of WT and Spi6−/− Tregs (H-2kb+) in recipient splenocytes. (*p<0.05; n=6-8 mice/group). (C) Representative examples of flow plots gated on H-2Kb+, CD4+, FoxP3+ and Spi6+ of recipient splenocytes. As compared to Spi6−/− Tregs, more than 40 % of WT Tregs expressed Spi6. (D) Representative examples of flow plots, gated on H-2Kb+, CD4+, FoxP3+ and GrB+ of recipient splenocytes. No difference was observed in the percentage of Tregs expressing GrB+ between Spi6−/− and WT groups. Bar graph represents the percentage of GrB+WT and GrB+Spi6−/− Tregs of recipient splenocytes (*p<0.05; n=6-8 mice/group). (E) Representative examples of flow plots, gated on H-2Kb+, CD4+, FoxP3+, GrB+ and Annexin of recipient splenocytes. Spi6−/− Tregs expressing GrB were more apoptotic compared to WT Tregs expressing GrB. Bar graph represents the percentage of apoptosis of GrB+ WT and Spi6−/− Tregs of recipient splenocytes (*p<0.05; n=6-8 mice/group). (F) Representative examples of flow plots, gated on H-2Kb+, CD4+, FoxP3+, GrB− and Annexin of recipient splenocytes. GrB−Spi6−/− and GrB−WT groups showed no difference in their rate of apoptosis. (n=6-8 mice/group).
Transfer of WT Tregs fails to rescue GrB-producing Spi6−/− Tregs from apoptosis in vivo
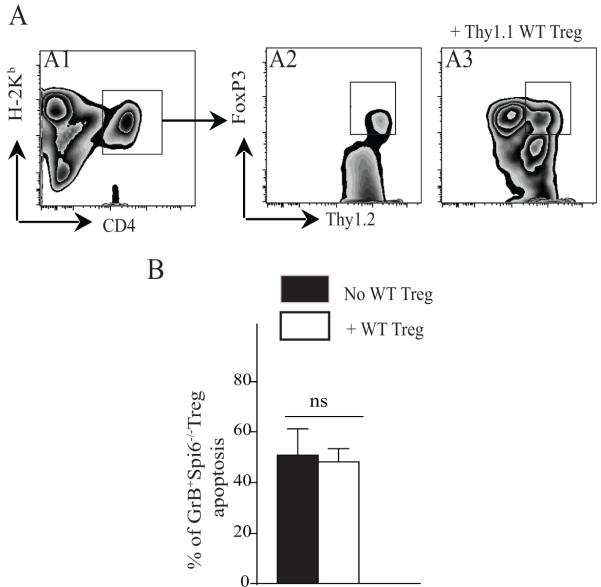
We then sought to determine whether the increased apoptosis observed in GrB+Spi6−/− iTregs was subject to any extrinsic influence by the severity of GVHD, rather than the result of an inherent defect. Irradiated BALB/c mice were reconstituted with 0.5 × 106 CD3+CD25− T-cells from WT (H-2Kb-thy1.2) or Spi6−/− (H-2Kb-thy1.2) C57BL/6 mice, either alone or with an additional 0.5 × 106 WT Tregs. These WT Tregs, added to moderate the severity of GVHD, were isolated from C57BL/6 (H-2Kb) mice that expressed the surface marker thy1.1; any iTregs generated were therefore clearly distinguished by their surface expression of thy 1.2, ensuring analysis of apoptosis was limited therein. The mice were sacrificed on day 10 post adoptive transfer, whereupon flow cytometry analysis of splenocytes revealed that the absolute number of induced Tregs (CD4+FoxP3+thy1.2+) was significantly lower in mice that received Spi6−/− T-cells rather than WT T-cells (5.00 × 103 ± 1.52 vs. 38.46 ± 0.29, respectively; p=0.04). We then analyzed the extent of apoptosis among induced Tregs in mice recipient of thy1.2+ WT (H-2Kb) or Spi6−/− (H-2Kb) C57BL/6 T-cells; further comparison was made to those mice recipient of an additional transfer of thy1.1+ WT Tregs to determine the influence thereof. The flow cytometry gating strategy for the splenocytes isolated from the different groups is shown in figure 5A. As shown in figure 5B, limitation of the severity of GVHD, achieved by the co-transfer of thy1.1+WT Tregs, did not rescue induced GrB+Spi6−/− thy1.2+ iTregs from their increased susceptibility to cell death, as measured by the percentage of Tregs expressing Annexin-V (50.67 ± 10.49 vs. 48.23 ± 5.18%, respectively; p=0.4). This data suggests that the increased apoptosis observed in GrB+Spi6−/− iTregs is the result of an inherent defect.
Figure 5. Adoptive Transfer of WT Tregs does not rescue GrB producing Spi6−/− Tregs from cell death.
(A) Representative example of flow cytometry gating on splenocytes derived from irradiated BALB/c mice reconstituted with T cells derived from WT (H-2Kb+thy1.2+) or Spi6−/− (H-2Kb+thy1.2+) mice and co-injected with WT (H-2Kb+thy1.1+) Tregs at 1/1 ratio. Control mice only received T cells without Tregs. Mice were sacrificed at day 10 post-adoptive transfer. Gating on Thy1.2 expression permits differentiation between induced Tregs (thy1.2+) and transferred WT Tregs (thy1.1+). A1 is a representative example of FACS staining of recipient splenocytes gated on H-2Kb+ and CD4+ (donor CD4+ T cells). A2 and A3 are representative examples of FACS staining of recipient splenocytes gated on H-2Kb+, CD4+, thy1.2+ and FoxP3+ (induced Tregs from donor CD4+T cells). A2 represents a mouse recipient of thy1.2+ T cells only. A3 represents a recipient mouse that received thy1.2+T cells in addition to WT thy1.1+Tregs. (B) Graph represents the percentage of apoptosis as measured by Annexin expression in induced Tregs from Spi6−/− T-cells, with or without co-transferred WT Tregs. Transfer of WT Tregs did not rescue induced GrB+ Tregs from accelerated cell death. (n=4-6 mice/group)
Spi6−/− nTregs were less efficient than WT nTregs in suppressing GVHD
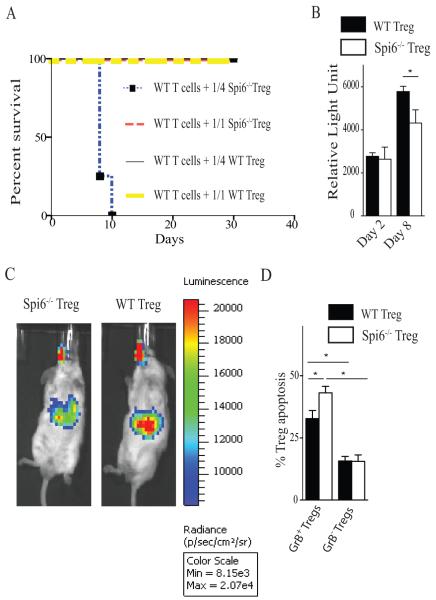
To compare the functionality of Spi6−/− Tregs to WT Tregs in vivo, two groups of lethally irradiated BALB/c mice were reconstituted with 2 × 106 CD3+CD25− T-cells derived from WT C57BL/6 mice. Mice were co-injected with Spi6−/− nTregs or WT nTregs at a Treg/Teff ratio of 1/1 (2 × 106 Tregs) or 1/4 (0.5 × 106 Tregs). As shown in figure 6A, mice that received WT Tregs at ratios of either 1/1 and 1/4 survived long-term. Mice that received Spi6−/− Tregs at a 1/1 ratio also survived long-term; however, none of the mice recipient of Spi6−/− Tregs at a ratio of 1/4 survived beyond day 10 post-adoptive transfer due to severe GVHD. As the remaining groups all survived long-term, we examined their relative weight loss as indicator of GVHD severity. Mice that received Spi6−/− Tregs at a ratio of 1/1 weighed significantly less relative to their initial weight compared to the mice recipient of WT Tregs at a ratio of either 1/1 (68.00 ± 4.0 vs. 80.50 ± 0.50%; p<0.05) or 1/4 (68.00 ± 4.0 vs. 84.00 ± 3.0%; p<0.05), indicating GVHD of greater clinical severity. Therefore, Spi6−/− Tregs were impaired in their ability to suppress GVHD.
Figure 6. Spi6−/− Tregs were less potent in suppressing GVHD as compared to WT Tregs and experienced more apoptosis.
Thy1.2 WT or Spi6−/− Tregs were adoptively transferred into irradiated BALB/c mice reconstituted with 2 × 106 bone marrow cells and 2 × 106 T cells derived from Thy1.1 WT mice at Treg:Teff ratios of 1/1 and 1/4. (A) Graph shows percent survival of the different groups. All the mice from the different groups survived long-term except the group that received Spi6−/− Tregs at a ratio of 1/4, in which all died earlier. (B) Gluc activity measured in the blood at day 2 and 8 of the lethally irradiated BALB/c mice that received WT T cells in addition to either Gluc transduced WT or Spi6−/− Tregs. (C) Bioluminescent imaging of those mice showed a clear signal in the splenic area that is more intense in mice that received Spi6−/− Tregs compared to WT Tregs. (*p<0.05; n=3-4 mice/group). (D) Bar graph represents the percentage of apoptosis as measured by flow cytometry of the adoptively transferred Thy1.2 WT and Spi6−/− nTregs in splenocytes of recipient mice. Recipient splenocytes were gated on H-2Kb+, Thy1.2+, CD4+, FoxP3+ and GrB+ to identify the subpopulation of GrB positive (GrB+) and negative (GrB−) nTregs from Spi6−/− and WT. (*p<0.05; n=4 mice/group).
We also utilized our live imaging technique to compare the survival of Spi6−/− and WT Tregs by transducing them with a lentivirus-vector encoding Gluc, a naturally secreted luciferase from the marine copepod Gaussia princeps(22); as previously described, the concentration of Gluc in blood increases linearly with respect to cell number in vivo(22, 23). Irradiated BALB/c mice reconstituted with 2 × 106 CD3+CD25− T-cells derived from C57BL/6 mice were co-injected with transduced Gluc+ Spi6−/− or WT-Tregs; blood samples were collected from mice at day 2 and 8, and analyzed for Gluc activity using its substrate coelenterazine and a luminometer. As shown in figure 6B, while Gluc activity was similar in the blood of mice that received Gluc+ Spi6−/− or WT Tregs at day 2, it was significantly lower in mice that received Gluc+ Spi6−/− Tregs rather than WT Tregs at day 8 (figure 6B). Furthermore, whole body bioluminescent imaging showed much higher signal intensity around the spleen in recipients of WT Tregs compared to Spi6−/− Tregs at day 10 (figure 6C). These data show the key role of Spi6 in maintaining the survival of Tregs in vivo.
To study the role of Spi6 in nTreg survival, irradiated BALB/c hosts received 0.5 × 106 nTregs from either Thy1.2 WT or Spi6−/−C57BL/6 mice. Both groups received Thy1.1 WT CD25-depleted T cells (0.5 × 106) and bone marrow cells (2 × 106). Mice were sacrificed at day 4 and splenocytes were recovered to examine the rate of apoptosis in the subpopulations of GrB+ and GrB− nTregs within the WT or Spi6−/− nTregs. While both WT and Spi6−/− nTregs showed similar levels of GrB expression (52.38 ± 1.14 vs. 47.78 ± 2.95 respectively, p=0.2), GrB+Spi6−/− nTregs expressed significantly higher levels of Annexin-V as compared to GrB+WT Tregs (figure 6D). However, no difference in Annexin-V expression was observed between GrB−Spi6−/− Tregs and GrB−WT Tregs (figure 6D), indicating that in the absence of GrB, nTreg apoptosis was unaffected by the presence or absence of Spi6. Furthermore, WT and Spi6−/− GrB+ Tregs expressed significantly higher levels of Annexin-V compared to both WT and Spi6−/− GrB−Tregs as well (figure 6D). These data suggest that the effect of Spi6 deficiency on Treg survival is mediated by GrB. Furthermore, no difference in Ki67 expression was seen between WT and Spi6−/− Tregs (33.95 % ± 1.434 vs. 33.53 ± 0.8873 respectively, p=0.4)
Spi6-deficiency accelerates rejection of MHC class II-mismatched heart transplants
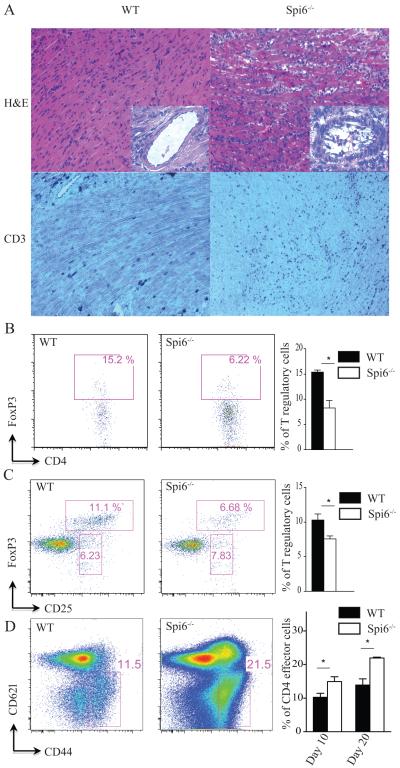
To examine the role of Spi6 in GrB+Tregs in transplantation tolerance, we used the single, class II MHC-mismatch heart transplant model (B6.H-2bm12 heart→C57BL/6 recipient), in which Tregs play a central role in protecting allografts from acute rejection(24-26). Spi6−/− recipients of B6.H-2bm12 hearts rejected their graft significantly earlier than WT recipients (MST (mean survival time) 20 vs. 70 days, respectively; p=0.02; n=4-6 per group). Cardiac allograft histology showed higher grades of myocyte loss, CD3 infiltration and vasculitis in the Spi6−/− recipients compared to WT mice at day 10 post-transplant (figure 7A). The ISHLT (International Society of Heart and Lung Transplantation) score was 2.75 ± 0.25 and 1.33 ± 0.33 for Spi6−/− and WT recipients, respectively (p=0.01; n=3-4 mice per group).
Figure 7. Lack of Spi6 accelerates rejection in a Treg-dependent MHC class-II mismatch heart transplant model.
(A) Representative examples of cardiac allograft histology at day 10 post-transplantation show higher grades of acute cellular rejection (upper panels, H&E stain, original magnification × 20), and more CD3 infiltration (lower panels, original magnification × 20) in the Spi6−/− recipients compared to WT mice (n=4 mice/group). Vasculitis was also more frequently encountered in Spi6−/− compared to WT mice (insets). (B) Representative examples of FACS staining from lymphocytes isolated from BM12 heart allografts from Spi6−/− and C57BL/6 recipients. Cells are gated on CD4 and FoxP3. Lymphocytes from heart allografts of Spi6−/− recipients had lower percentage of Tregs compared to WT. Bar graph represents the percentage of Tregs (*p<0.05; n=3-4 mice/group). (C) Representative examples of flow plots from draining LNs of Spi6−/− and WT mice recipients of BM12 hearts, gated on CD4+, CD25+ and FoxP3+. LNs from Spi6−/− recipients had lower percentage of Tregs (CD4+CD25+FoxP3+) compared to WT. Bar graph represents the percentage of Tregs in draining LN of Spi6−/− and WT mice. (* p<0.05; n=3-4 mice/group). (D) Representative examples of flow plots from spleens of Spi6−/− and WT mice recipients of BM12 hearts at day 20 post transplant, gated on CD4+, CD44high and CD62llow. Splenocytes from Spi6−/− recipients had higher percentage of effector T cells (CD4+CD44highCD62llow) compared to WT. Bar graph represents the percentage of effector T cells in splenocytes of Spi6−/− and WT mice at days 10 and 20 post transplant. (* p<0.05; n=3-4 mice/group).
Lymphocytes isolated from B6.H-2bm12 heart allografts of Spi6−/− recipients showed lower percentages of Tregs compared to WT recipients at day 10 post-transplant (figure 7B). Furthermore, Spi6−/− Tregs showed a higher rate of apoptosis compared to WT Tregs, as measured by Annexin-V expression (36.65 ± 3.35 vs. 10.25 ± 9.75%; p=0.06). FACS analysis of draining LNs also showed a significantly lower percentage of Tregs in Spi6−/− recipients compared to WT recipients (figure 7C). Furthermore, we examined the percentage of effector T cells (CD4+CD44highCD62llow) and allo-reactive IFNγ production at days 10 and 20 post-transplantation. As shown in figure 7D, there was a higher percentage of effector T cells noted in the spleen of Spi6−/− recipients as compared to WT at day 10 and day 20 post transplant. In addition, splenocytes from WT and Spi6−/− recipients at day 10 and 20 post transplant were cultured in a MLR with irradiated BM12 splenocytes and IFNγ cytokine was measured 48 hours later by a Luminex assay. While at day 10 post transplant, IFNγ produced by Spi6−/− alloreactive splenocytes was slightly higher than WT (9.86 pg/ml ± 2.14 vs. 4.98 pg/ml ± 2.17 respectively, p=0.09), it was significantly higher at day 20 post transplant (96.33 pg/ml ± 19.26 vs. 11.68 pg/ml ± 2.28 respectively, p< 0.001). Collectively, our data show that Spi6 plays a major role in the survival of Tregs and in the promotion of tolerance.
Discussion
The suppressive properties of Tregs play an important role in maintaining tolerance and are key to the therapeutic potential of Treg therapy, which is considered to hold great promise in the treatment of a wide variety of immune-mediated diseases, including transplantation(9). While the mechanisms of suppression have been the focus of intense research, one of the key unmet needs is a better understanding of the cellular mechanisms that determine Treg survival, which would permit the design of more effective Treg therapy. The ability of Tregs to secrete GrB is known to be a potent tool in their suppressive arsenal, but the mechanisms by which GrB-producing Tregs protect themselves from GrB-mediated damage remain to be fully explored. Spi6, an endogenous inhibitor of GrB, has been reported to play a key role in protecting target cells from the apoptotic effects of GrB by covalently binding and inactivating GrB(15, 27); its role in Treg homeostasis, has, heretofore, been unknown.
Here, we examined the specific role of Spi6 in Treg survival and function, and, consequently, its influence on transplantation tolerance. Our data indicate that activated iTregs and nTregs increase their intracellular expression of Spi6, in addition to their expression of GrB, compared to resting Tregs in vitro and in vivo. In the absence of Spi6, activated Spi6−/− nTregs showed higher intra-cytoplasmic GrB activity, indicating a critical role of Spi6 in reducing Treg intracytoplasmic GrB activity. This increased GrB activity resulted in an increase in apoptosis and a decrease in the suppressive function of Spi6−/− nTregs as compared to WT Tregs. These data reveal, for the first time, a critical role for Spi6 in protecting GrB-producing Tregs from GrB-mediated apoptosis and providing a supportive cellular platform to exert their immunoregulatory function.
While there is great interest in using ex vivo-generated Tregs as cell therapy in various diseases, we sought to study the role of Spi6 in Treg generation in vitro. Spi6−/− CD4+ T-cells generated less Tregs and demonstrated a higher percentage of apoptosis as compared to WT Tregs. Our data indicate that activated Tregs exhibit GrB-activity and that the presence of Spi6 was critical for their protection against GrB, and, consequently, their survival.
To determine the role of Spi6 in the survival and function of Tregs in vivo, we used an established model of GVHD(28). Spi6−/− T-cells transferred into irradiated, fully MHC-mismatched hosts generated less alloactivated iTregs compared to WT T-cells. Although >60% of both WT and Spi6−/− Tregs expressed GrB post-transfer, GrB+Spi6−/− Tregs displayed higher rates of apoptosis compared to GrB+WT Tregs; no difference in apoptosis was observed between GrB−Spi6−/− Tregs and GrB-WT Tregs, demonstrating that, in the absence of GrB secretion, Treg apoptosis was unaffected by the presence or absence of Spi6. However, GrB−Spi6−/− Tregs displayed significantly lower rates of apoptosis than GrB+Spi6−/− Tregs, indicating that the effect of Spi6 deficiency on Treg survival was directly related to the presence of GrB. Importantly, Spi6−/− Tregs were also less efficient than WT Tregs in suppressing GVHD, as indicated by relative weight loss. Our in vivo imaging studies showed a decreasing number of Spi6−/− Tregs over time, suggesting a progessive decline in Treg survival in the absence of Spi6 in vivo. Furthermore, although the co-transfer of WT Tregs in addition to Spi6−/− Tregs suppressed GVHD, it failed to rescue GrB-producing Spi6−/− Tregs from undergoing apoptosis in vivo. Collectively, these data highlight the functional importance of the Spi6-GrB pathway in determining the survival and function of Tregs in vivo, and also suggest that the defect in GrB+Spi6−/− Treg survival is an intrinsic phenomenon.
Finally, to examine the role of Spi6 in transplant tolerance, we used a single, class II MHC-mismatch heart allograft model, in which Tregs play a central role in protecting allografts from acute rejection(24-26). Spi6−/− recipients of heart allografts demonstrated accelerated acute rejection, and, furthermore, aggravated chronic vascular injury/rejection, a major obstacle to the long-term survival of organ transplant patients. In keeping with both our in vitro data and in vivo GVHD data, GrB-expressing Tregs in the heart and draining LNs displayed higher rates of apoptosis in the absence of Spi6. It is important to note that the Proteinase inhibitor 9 (PI9) human homologue of Spi6 was suggested to be an inhibitor of caspase 1 that regulates key steps in inflammation (29, 30). Therefore there is a possibility that some of the effects observed in our in vivo mouse models could be related to a defect in IL-1beta production.
However, the consistency of our data across multiple alloimmune models highlights the major role that Spi6 plays in the survival and function of Tregs. Given their central role in maintaining tolerance and the therapeutic potential of Treg therapy, we believe the enhanced understanding of Treg biology provided by our data will have a significant impact on the design of more effective Treg therapy. Indeed, much progress has been made in the development of various therapeutic molecules to influence the SERPINB9:GrB system; we believe that these advancements have the potential to greatly improve Treg-based therapy, and, consequently, the treatment of multiple immune-mediated diseases.
Acknowledgements
None
This work was supported in part by the National Institute Of Allergy and Infectious Diseases of the National Institutes of Health under Award Number RO1AI091930 (RA). The content is solely the responsibilities of the authors and does not necessarily represent the official views of National Institute of Health.
References
- 1.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, Wang Z, Thomson AW. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184:624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 7.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 9.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Lovo E, Zhang M, Wang L, Ashton-Rickardt PG. Serine protease inhibitor 6 is required to protect dendritic cells from the kiss of death. J Immunol. 2012;188:1057–1063. doi: 10.4049/jimmunol.1102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 17.Liu N, Raja SM, Zazzeroni F, Metkar SS, Shah R, Zhang M, Wang Y, Bromme D, Russin WA, Lee JC, Peter ME, Froelich CJ, Franzoso G, Ashton-Rickardt PG. NF-kappaB protects from the lysosomal pathway of cell death. EMBO J. 2003;22:5313–5322. doi: 10.1093/emboj/cdg510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corry AM. 1973 meeting in kansas city. Bull Med Libr Assoc. 1973;61:39–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Ansari AW, Temblay JN, Alyahya SH, Ashton-Rickardt PG. Serine protease inhibitor 6 protects iNKT cells from self-inflicted damage. J Immunol. 2010;185:877–883. doi: 10.4049/jimmunol.1000651. [DOI] [PubMed] [Google Scholar]
- 21.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, Wu Z, Sandner S, Gorbachev AV, Fukamachi K, Heeger PS, Sayegh MH, Turka LA, Fairchild RL. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 25.Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci U S A. 2007;104:11382–11387. doi: 10.1073/pnas.0704853104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Riella LV, Boenisch O, Popoola J, Robles S, Watanabe T, Vanguri V, Yuan X, Guleria I, Turka LA, Sayegh MH, Chandraker A. Paradoxical functions of B7: CD28 costimulation in a MHC class II-mismatched cardiac transplant model. Am J Transplant. 2009;9:2837–2844. doi: 10.1111/j.1600-6143.2009.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton-Rickardt PG. Serine protease inhibitors and cytotoxic T lymphocytes. Immunol Rev. 2010;235:147–158. doi: 10.1111/j.0105-2896.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 28.Cai SF, Cao X, Hassan A, Fehniger TA, Ley TJ. Granzyme B is not required for regulatory T cell-mediated suppression of graft-versus-host disease. Blood. 2010;115:1669–1677. doi: 10.1182/blood-2009-07-233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. The Journal of experimental medicine. 2000;191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annand RR, Dahlen JR, Sprecher CA, De Dreu P, Foster DC, Mankovich JA, Talanian RV, Kisiel W, Giegel DA. Caspase-1 (interleukin-1beta-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. The Biochemical journal. 1999;342(Pt 3):655–665. [PMC free article] [PubMed] [Google Scholar]