Abstract
Sarcopenia, defined as muscle weakness and fiber atrophy, of respiratory muscles such as the diaphragm (DIAm) has not been well characterized. The DIAm is the main inspiratory muscle and knowledge of DIAm sarcopenia is important for establishing the effects of aging on respiratory function. We hypothesized that aging is associated with a loss of DIAm force and reduced fiber cross-sectional area (CSA), and that these changes vary across fiber types. DIAm sarcopenia was assessed in young (5 month; n=11) and old (23 month; n=12) wild-type mice reflecting ~100 and 75% survival, respectively. In addition, DIAm sarcopenia was evaluated in BubR1H/H mice (n=4) that display accelerated aging (~60% survival at 5 months) as a result of expression of a hypomorphic allele (H) of the mitotic checkpoint protein BubR1. Maximum specific force (normalized for CSA) of the DIAm was 34% less in old mice and 57% lower in BubR1H/H mice compared to young mice. Mean CSA of type IIx and/or IIb DIAm fibers was 27% smaller in old wild-type mice and 47% smaller in BubR1H/H mice compared to young mice. Mean CSA of type I or IIa fibers was not different between groups. Collectively these results demonstrate sarcopenia of the DIAm in aging wild-type mice and in BubR1H/H mice displaying accelerated aging. Sarcopenia may limit the ability of the DIAm to accomplish expulsive, non-ventilatory behaviors essential for airway clearance. As a result, these changes in the DIAm may contribute to respiratory complications with aging.
Keywords: contractile properties, cross-sectional area, fiber type, myosin heavy chain
1. Introduction
The physiological process of aging significantly impacts an individual's quality of life, as consequence of the gradual functional, structural, and biochemical changes due to aging. The age-related decline in skeletal muscle function is defined as sarcopenia, which is characterized by muscle weakness and fiber atrophy (Cesari 2012; Fielding 2011). Persons with sarcopenia have a greater incidence of functional disability including loss of independence and higher risks of falling (Landers 2001) and sarcopenia is a substantial predictor of mortality in aging individuals (Fielding 2011).
Respiratory complications, such as pneumonia and respiratory infections, are common in aging individuals (Fein 1994; Houston 1997) and are a common cause of death in this population (Heron 2011). Decreased function of the diaphragm muscle (DIAm), the main inspiratory muscle, may contribute significantly to the increased susceptibility to respiratory complications during aging. In aging adults, respiratory muscle force is decreased by 13-25% as measured by the maximal sniff and Mueller maneuvers (Polkey 1997; Tolep 1995). Thus, DIAm sarcopenia may compound age-related lung and chest wall changes reducing an individual's ability to perform the large forces generated during expulsive, non-ventilatory behaviors important for airway clearance.
While sarcopenia of limb muscles is well characterized and defined (Brooks 1988), the presence of DIAm sarcopenia remains controversial. Previous research in rodent models of aging has been both limited and conflicting in regards to age-related changes in the DIAm. For example, cross-sectional area (CSA) of rat DIAm fibers reportedly does not change (Kavazis 2012) or increases ~28% (in type IIa fibers) (Kawai 2012) with age. In aging mice, DIAm force shows either no difference (Lynch 1997) or a trend for a reduction (Dupont-Versteegden 1992; Faulkner 2008). None of these studies, however, comprehensively examined the DIAm in aging animals to confirm sarcopenia.
Rodent models are useful to examine genetic and molecular determinants of aging and characterizing aging effects on specific muscle groups, and in particular respiratory muscles such as the DIAm is important. The goal of this project was to systematically determine the effects of aging on the DIAm, specifically those properties defining sarcopenia (i.e., specific force and CSA). Additionally, we sought to determine if sarcopenia is fiber-type specific, i.e., more pronounced in type IIx and/or IIb DIAm fibers (fiber type classification based on myosin heavy chain – MyHC isoform expression). We hypothesized that the DIAm is susceptible to sarcopenia, resulting in both a reduction in force and fiber atrophy, with greater effects at type IIx and/or IIb fibers.
Additionally, we examined a recently developed mouse model of accelerated aging that exhibits physiologically relevant reductions in the mitotic checkpoint protein kinase BubR1 (budding uninhibited by benzimidazole-related 1). This novel mouse model expresses a hypomorphic allele (H) of BubR1 (BubR1H/H) and displays many aging-related phenotypes such as failure to grow, lipodystrophy, alopecia, decreased dermal thickness, cataracts, and atherosclerotic changes in addition to a shortened lifespan (Baker 2004). Decreasing BubR1 is thought to play a relevant role in aging since protein levels of BubR1 decrease naturally in healthy tissues with age, including skeletal muscles (Baker 2004; Baker 2008).
2. Methods
2.1. Animals
All wild-type (strain background C57BL/6 × 129) and BubR1H/H mice were bred at colonies maintained at the Mayo Clinic. Mice were group housed by genotype and maintained on a 12 hr light-dark schedule under specific pathogen-free conditions with ad libitum access to food and water. Wild-type mice were examined at ~5 and 23 months of age (mo), representing 100% and ~75% survival (young and old), respectively (Flurkey 2009; Turturro 1999). BubR1H/H mice were used at 5 mo representing ~60% survival (Baker 2004; Baker 2008). Considerations for animal use in gerontological research were followed (Miller 2000). Subsets of mice were used for analysis of in vitro force and histo-morphological analysis of the DIAm muscle. At the terminal experiment, mice were anesthetized with an intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and euthanized by exsanguination. Harvested midcostal DIAm segments were stored at −80 °C until further analysis. All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with National Institute of Health guidelines.
2.2. Diaphragm muscle in vitro contractility
Isometric force was measured using DIAm strips from a subset of mice, as previously described (Ameredes 2000; Gosselin 1996; Lewis 1986; Miyata 1995; Sieck 2012). Briefly, ~3 mm midcostal DIAm segments were carefully dissected, with the rib origin of the muscle stabilized by minutien pins and the central tendon insertion attached to a force transducer (model 6350, Cambridge Technology, Cambridge, MA). Muscle segments were incubated in Reese-Simpson buffer (pH 7.4) with 95% O2 and 5% CO2 and maintained at 26 °C. Muscles were then set to optimal length, at which all contractile measurements were conducted. Maximum twitch force was elicited by stimulating the muscle with a 0.5-ms pulse (Grass S88 stimulator delivered through a SIU5D stimulus isolation unit; Grass Telefactor) via plate electrodes. Isometric tetanic force was elicited by stimulating muscles for 1000 ms and 120 Hz. The relationship between force and stimulation frequency of DIAm segments was determined with a protocol of 1-s trains of 0.5-ms stimuli at 10, 25, 50, 75, 100, 125, and 150 Hz. The stimulator and servomotor system were controlled by computer using a data acquisition interface board and custom-developed LabView software (National Instruments). Specific force was analyzed as maximal isometric force normalized to physiological CSA defined as DIAm mass / (optimal length × muscle density).
2.3. Contractile protein analyses
Midcostal DIAm sections were homogenized on ice in 10 mM phosphate buffer (pH 7.0). Homogenates were assayed to determine noncollagenous protein content using the Lowry method (Bio-Rad, Hercules CA). Homogenate samples were further electrophoresed on SDS-polyacrylamide gels to determine total contractile protein content (myosin and actin), as previously reported (Geiger 2003; Greising 2011).
2.4. Diaphragm muscle histo-morphological analysis
Strips of DIAm were dissected and saved for histological analysis of MyHC isoform classification, fiber type-specific CSA, and fraction of total muscle CSA comprised by interstitial space. Samples were serially cut into 10μm thick cross-sections for analysis. Gross histologic examination of DIAm cross-sections was conducted on hematoxylin and eosin-stained sections. Muscle sections were labeled using primary antibodies for MyHC isoforms: anti-MyHCSlow (Vector Labs VP-M667) and anti-MyHC2A (SC-71 obtained from Developmental Studies Hybridoma Bank, Iowa City IA) and laminin (Sigma L9393) to visualize the sarcolemma. Sections were treated with appropriate fluorescently-conjugated secondary antibodies. Individual muscle fibers were classified as type I, type IIa and type IIx and/or IIb, based on expression of MyHCSlow, MyHC2A, and the absence of staining, respectively. A single image of each DIAm section was obtained via confocal microscopy using a Nikon Eclipse C1 laser scanning confocal microscope system (Nikon Instruments Inc., Melville NY) equipped with Argon (488 nm) and solid state (405 and 561 nm) lasers capable of simultaneous multi-label fluorescence imaging. Confocal images were saved separately for each fluorescence channel using Nikon C1 software as 8-bit grayscale-TIFF files. All images were pseudo-colored and merged in NIS-Elements software (Nikon Instruments) for analysis. As previously described (Sieck 2012), ~ 400 type-identified fibers per DIAm section were randomly sampled to determine fiber type counts and measure fiber CSA using the morphometric tool in NIS-Elements software. The total number of DIAm fibers was then used to determine the proportion of fibers of each type.
Separate muscle sections were labeled with wheat germ agglutinin to determine the fraction of total muscle CSA comprised by interstitial space, with modification of a previous methodology (Gosselin 1993). Briefly, DIAm sections were incubated with 1 μg/ml of wheat germ agglutinin conjugated to Alexa Fluor 488 (Molecular Probes W11261). Single confocal images were obtained for each DIAm section (as above) and were analyzed using the threshold, binarize and morphometric analysis functions in MetaMorph (Universal Imaging Corp.). Briefly, wheat germ agglutinin fluorescence was thresholded manually such that all DIAm fibers were clearly outlined. Images were binarized and both the total muscle CSA and area comprised by interstitial space were determined. On average, four non-continuous sections of DIAm were analyzed per animal and the fraction of total muscle CSA comprised by interstitial space was determined as the ratio of the sum of areas comprised by interstitial space and the total muscle CSA for each animal.
For the purpose of image presentation, individual images were exported into Adobe Photoshop (Adobe Systems Inc., San Jose CA) as TIFF files and cropped to highlight representative areas. Fluorescence images were pseudocolored by changing the color gamut (RGB) while preserving original contrast and brightness.
2.5. Statistical analysis
All data was analyzed using JMP (JMP version 8.0; SAS Institute Inc., Cary NC). Data for body mass, DIAm contractility, and DIAm fiber CSA and fraction of total muscle CSA comprised by interstitial space were analyzed using one-way ANOVA comparing across groups (young, old, and BubR1H/H mice). Force-frequency relationships were compared using a one-way ANOVA with Bonferroni correction. The proportion of DIAm fiber types and the relative fiber type contribution to total DIAm CSA were analyzed by two-way ANOVA (group × fiber type). When appropriate, post hoc analyses were conducted using Tukey-Kramer's honestly-significant difference test. Chi-squared was used to analyze the distribution of DIAm fiber CSAs. Unless otherwise specified, all data reported as mean ± standard error (SE). Significance was accepted at the α < 0.05 level.
3. Results
3.1. Mouse characteristics
Naturally aged wild-type mice were examined for markers of DIAm sarcopenia at 5 mo and 23 mo (young and old mice, respectively) representing 100% and ~75% survival. In addition accelerated-aging BubR1H/H mice at 5 mo of age (~60% survival) were examined (Table 1). At the time of analysis, old mice had 21% greater body mass than young mice, whereas BubR1H/H mice were 31% smaller (Table 1).
Table 1.
Group characteristics of natural and accelerated aging mouse models
| Young | Old | BubR1H/H | One-way ANOVA | |
|---|---|---|---|---|
| (n=11) | (n=12) | (n=4) | P-value | |
| Age (mo) | 5.0±0.2 | 23.3±0.4 | 5.1±0.4 | |
| Body Mass (g) | 31.2±1.6 | 37.8±1.4* | 21.6±1.7*† | <0.001 |
Mean±SE.
Significantly different than young mice
significantly different than old mice.
3.2. Diaphragm muscle in vitro contractility
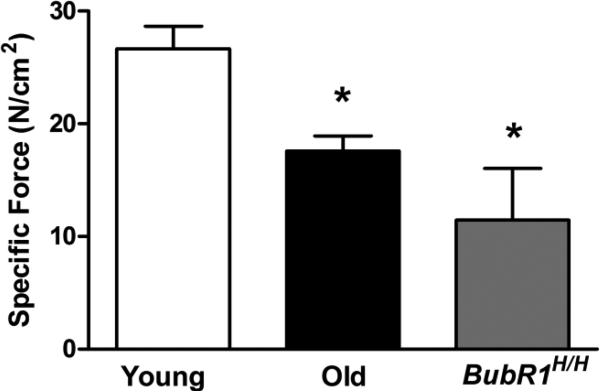
The first component of sarcopenia analyzed was DIAm force loss. Sarcopenia was evident in the DIAm of aged mice, both in the old and BubR1H/H mice. Specific force (Po) of the DIAm for young mice was 26.7±2.0 N/cm2 (Fig. 1), which was greater than both aged groups. Specifically, old mice had 34% less DIAm force generating capacity and BubR1H/H mice had 57% less force than young mice (P<0.001; Fig. 1). Compared to young mice, the specific maximum twitch force (Pt) generated by the DIAm was less in both the old and BubR1H/H mice (8.8±1.4, 5.8±0.6, and 4.7±1.4 N/cm2 for young, old, and BubR1H/H mice, respectively; P=0.049). There was a difference in the ratio of specific Pt to Po for BubR1H/H mice in comparison to both young and old mice (0.32±0.03, 0.33±0.02, and 0.50±0.12 for young, old, and BubR1H/H mice, respectively; P=0.039).
Figure 1.
Contractility of the DIAm from natural and accelerated aging mice (mean±SE). Specific forces are corrected for physiological cross-sectional area. Both naturally-aged mice at 75% survival (old; n=10) and the BubR1H/H accelerated-aging model (n=4) at ~60% survival displayed less force generating capacity than young mice (n=7). Data analyzed by one-way ANOVA. *Significantly different than young mice.
Analysis of the force-frequency relationship in DIAm segments was performed for young and old wild-type mice. There were no effects of aging present. The frequency at which maximal DIAm force was elicited was ~115 Hz and 50% force was elicited at 16 Hz for both the old and young mice (P≥0.892 for comparisons across groups). Accordingly, the normalized force-frequency relationship was not significantly different between young and old mice (P≥0.341 across the range of stimulation frequencies). Likewise there was no difference in the slope of the force-frequency relationship between age groups (P=0.700).
3.3 Contractile protein expression
Protein content was measured in midcostal DIAm segments obtained from young, old and BubR1H/H mice. There was a slight decrease in total protein content in the old mice compared to young mice, however there was no difference in the fraction of contractile proteins myosin and actin (expressed per total protein) across groups (Table 2).
Table 2.
Effects of natural aging and accelerated aging on the DIAm
| Young | Old | BubR1H/H | One-way ANOVA | |
|---|---|---|---|---|
| (n=8) | (n=7) | (n=5) | P-value | |
| Total protein per muscle mass (mg/mg) | 0.141±0.010 | 0.097±0.009* | 0.115±0.016 | 0.030 |
| Contractile protein per total protein (mg/mg) | 0.605±0.087 | 0.551±0.118 | 0.562±0.036 | 0.991 |
Mean±SE.
Significantly different than young mice.
3.4. Diaphragm muscle histo-morphological analysis
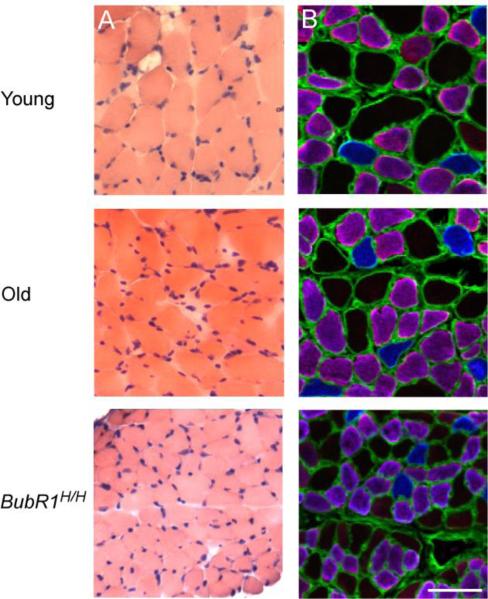
The second component of sarcopenia analyzed was the presence of muscle atrophy. Evaluation of hematoxylin and eosin-stained DIAm cross-sections showed no signs of degenerating fibers, fibrosis, or centrally located nuclei (Fig. 2). Additionally, in young mice 22.9±1.0 % of the total muscle CSA was comprised by interstitial space, while both old (26.0±2.1 %) and BubR1H/H (25.5±2.5 %) mice displayed a minimal but insignificant increase (P=0.361).
Figure 2.
Representative serial cross-sections of DIAm fibers from natural and accelerated aging mice. A) Hematoxylin and eosin stained cross-sections, indicating a normal and healthy appearance for all age groups. B) Fiber types were classified according to myosin heavy chain (MyHC) expression based on immunoreactivity to isoforms MyHCslow (blue) and MyHC2A (purple), the absence of staging (black) was classified as MyHC IIx and/or IIb. Anit-laminin (green) was used to define the fiber sarcolemma. Scale bar represents 50 μm.
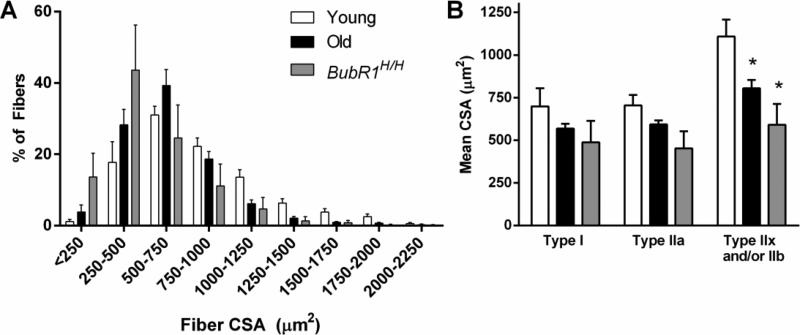
In general, young mice displayed the greatest DIAm fiber size, followed by old and then BubR1H/H mice. Overall, the mean CSA of the DIAm fibers was less in both old (648±28μm2) and BubR1H/H (523±113 μm2) mice in comparison to young (847±67 μm2) mice (P=0.022). Accordingly, there was a significant leftward shift in distribution of DIAm fiber CSAs (Fig. 3; P<0.001). This was consistent with fiber type-specific changes in fiber CSA. A modest reduction in type IIx and/or IIb fiber CSA (Fig. 3) of old (804±49 μm2) and BubR1H/H (590±123 μm2) was evident when compared to young (1108±99 μm2) mice (P=0.009). For both types I and IIa of DIAm fibers there were no significant differences in CSA (Fig. 3; P≥0.060).
Figure 3.
Fiber cross-sectional area (CSA) of DIAm muscles from natural and accelerated aging mice (mean±SE). A) The distribution of fiber CSAs of the DIAm with significantly shifted toward the left in both old (n= 5) and BubR1H/H (n= 4) mice compared to young (n= 9) mice, distribution analyzed by chi-squared analysis. B) Fiber CSA of type identified DIAm fibers; there was a significant decrease in the CSA of type IIx and/or IIb fibers of old and BubR1H/H mice compared to young mice. Data analyzed by one-way ANOVA. *Significantly different than young mice. n=4-9 per group.
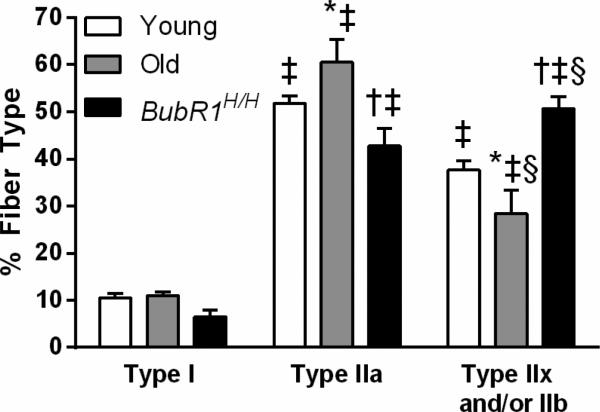
The proportion of fiber types in the DIAm was affected by age (Fig. 4; interaction of group × fiber type, P<0.001). There was a significant difference in the overall distribution of fiber types with ~10, 52, and 38% for type I, type IIa, and type IIx and/or IIb fibers, respectively (mean across age groups). For all groups, type I fibers were less abundant than type IIa and type IIx and/or IIb fibers. Old and BubR1H/H mice had a modest alteration in the proportion of type IIa and type IIx and/or IIb fibers such that old mice had more type IIa fibers than type IIx and/or IIb, while BubR1H/H mice had the inverse proportion of type II fibers. Specifically, in old mice type IIa fibers accounted for 60% and type IIx and/or IIb fibers accounted for 28% of the total in contrast to BubR1H/H mice that had 43 and 51%, respectively. There were also differences across groups in the proportion of fibers for the same fiber type. Both the young and BubR1H/H mice had significantly fewer type IIa DIAm fibers compared to old mice. Conversely, both the young and BubR1H/H mice had significantly more type IIx and/or IIb DIAm fibers than old mice. Collectively, these results indicated an age-related change in the proportion of DIAm fiber types, although there was no difference when comparing type I and all type II (i.e., IIa, IIx and/or IIb) fibers.
Figure 4.
The proportion of DIAm fibers by fiber type and age. Data were analyzed by two-way ANOVA (group × fiber type). Interaction post hoc results are as follows: ‡significantly different than type I fibers of the same group; §significantly different than type IIa fibers of the same group;*significantly different than young mice within the same fiber type; †significantly different than old mice within the same fiber type. n=4-9 per group.
The relative contribution of each fiber type to total DIAm CSA showed significant differences across age and fiber type groups in agreement with the modest changes in type-specific fiber CSA and fiber type proportions (interaction of group × fiber type, P<0.001). For all groups, there was significantly less contribution of type I fibers compared to both type IIa and type IIx and/or IIb fibers, with ~8, 45, and 47% of total DIAm CSA accounted for by type I, IIa, and IIx and/or IIb fibers, respectively (mean across age groups). In comparison to the old mice, both young and BubR1H/H mice displayed significantly decreased contribution of type IIa fibers and increased contribution of type IIx and/or IIb fibers to total DIAm CSA. Specifically in young mice type IIa fibers contribute to 43±2% and type IIx and/or IIb fibers 49±2%, while in old mice they respectively account for 55±5% and 35±5% of total DIAm CSA.
4. Discussion
The presence or absence of respiratory muscle sarcopenia (i.e., loss of DIAm specific force and fiber CSA) is likely a central factor in contributing to respiratory failure among the aged. The results of this study present novel systematic data on age-related changes in the DIAm that indicate sarcopenia. How the respiratory system, specifically the DIAm, responds to age-related changes has previously been unclear, especially in mouse models. Both old wild-type and BubR1H/H (accelerated aging) mice displayed reduced DIAm specific force and smaller fiber CSA compared to young mice, confirming both components of sarcopenia. There was also an age-related change in the proportion of fiber types and the relative contribution of each fiber type to total DIAm CSA. As hypothesized, sarcopenia was more pronounced in type IIx and/or IIb DIAm fibers, such that in both old and BubR1H/H mice there was an alteration of the relative contribution of type IIa and type IIx and/or IIb fibers. Taken together these findings indicate that sarcopenia is present in the mouse DIAm, and the maximal force generating capacity of the DIAm is restricted. These results suggest that DIAm sarcopenia may impact age-related respiratory function by limiting the range of forces generated by the DIAm and hampering the ability to perform non-ventilatory behaviors necessary for airway clearance.
Sarcopenia is characterized by two main components: muscle weakness and atrophy (Cesari 2012; Fielding 2011). Maximal specific force was reduced 34-50% in old and BubR1H/H mice compared to young mice. Estimating the maximal force of the DIAm from the product of specific Po and mean fiber CSA, it is evident that a considerable decrement in force for both old (~50% deficit) and BubR1H/H (~74% deficit) mice from young mice would be present. Previous reports of age-related changes in DIAm force have been conflicting (Dupont-Versteegden 1992; Faulkner 2008; Kavazis 2012; Kawai 2012; Lynch 1997). Most of the previous research on age-related loss of DIAm force was conducted in aging rats, where collectively there was approximately a 12-24% decrease in DIAm force (for age groups corresponding to 65-25% survival) compared to young rats (Criswell 1997; Criswell 2003; Gosselin 1994; Imagita 2009; Powers 1996; Smith 2002). Although it is possible that some of the difference relates to the selection of age groups with somewhat different survival rates, there is also likely a difference in the age-related changes in DIAm force between rats and mice. However, very few previous studies have examined age-related changes in the DIAm force of mice. Dupont-Versteegden and McCarter (1992) examined mice at various ages between 2 weeks and ~20 months of age, reporting no significant difference in DIAm force. However, they reported a large variation in specific forces (16-30 N/cm2) through the various time points measured. This variation in specific force is much greater than we observed in the present study. A report from Lynch and colleagues (1997) found no difference in DIAm specific force of mice at ~5 and 24 months of age. Although not reported, survival rates are likely comparable to those in the current study. Thus, the results of the current study are in contrast to these previous studies conducted in mice. This conflict highlights the importance of including longitudinal assessments with multiple time-points when studying the combined effects of an intervention or disease and aging. Similar considerations apply when using animal models of accelerated aging such as the BubR1 model.
The second component of sarcopenia, muscle fiber atrophy, was also examined in the present study. Although there was a significant overall effect on the size of DIAm fibers as a result of natural and accelerated aging, remarkably the effect was fiber type specific. In particular, there was an age-related decrease in the size of the type IIx and/or IIb DIAm fibers for both the old and BubR1H/H mice. Old wild-type mice had a modest increase in both the proportion and relative contribution of type IIa fibers to total DIAm CSA, with a concomitant decrease for type IIx and/or IIb fibers. The decrease in proportion of type IIx and/or IIb fibers may be due to an actual loss of these DIAm fibers although further research is needed to determine if fiber loss occurs with DIAm sarcopenia. In BubR1H/H mice there was a decrease in the relative contribution of type IIa fibers to total DIAm CSA and an increase for type IIx and/or IIb fibers. It is possible that fibers co-express MyHC isoforms, and immunohistochemical techniques used herein cannot exclude co-expression of MyHC2X or MyHC2B with MyHCSlow or MyHC2A.Of note, we did not find evidence of MyHCSlow and MyHC2A co-expression in any of the samples analyzed. However, an increased incidence of MyHC isoform co-expression in DIAm fibers has been observed in other conditions, such as denervation and hypothyroidism (Geiger 2002; Geiger 2001; Mantilla 2009). Much like age-related DIAm force loss, previous studies have provided conflicting results related to age-related atrophy of DIAm fibers. Kavazis and colleagues (2012) found no difference in DIAm fiber CSA for rats at 100% and ~35% survival. However, a previous report from the same group and substantiated by others (Kawai 2012) indicated an age-related increase in DIAm fiber CSA, specifically of type IIa fibers, in rats with comparable survival rates (Kavazis 2007). No previous reports have examined DIAm fiber CSA in a mouse model of aging.
In the present study, there was a small, but significant, age-related decrease in total protein content of the DIAm but no change in contractile protein (i.e., myosin and actin) expressed as a fraction of total protein between young and aged mice. This is the first paper to examine DIAm protein content in old mice. However protein content has been previously examined in aged rats with conflicting results. Most previous research has reported no age-related change in DIAm protein content (Criswell 2003; Smith 2002), although these findings are inconsistent (Criswell 1997). Indeed, Criswell and colleagues (1997) normalized DIAm force for protein content in light of age-related loss of DIAm force. This normalization eliminated the difference in specific force (i.e., force per DIAm CSA) indicating a decrease in DIAm protein content with increasing age, but this data was not shown. Although there was a small change determined in total DIAm protein content in the current study, it is possible that the combination of reduced in DIAm force and CSA of MyHC type IIx and/or IIb fibers may reflect reductions in MyHC content per half sarcomere in these fibers that are undetectable at the whole muscle protein level. It is worthwhile noting that histo-morphological analyses of the DIAm reveal minimal age-related change in the fraction of total DIAm CSA comprised by interstitial space, suggesting that any protein loss reflects changes at the myofiber level. Single, type-identified DIAm fibers (in rats) have been shown to produce varying amounts of specific force depending on MyHC expression such that type I and IIa fibers exert less specific force than type IIx and IIb fibers (Geiger 2000). Previous research in rodent models of DIAm inactivity (e.g., via spinal hemisection, unilateral phrenic nerve denervation, and chronic systemic corticosteroid administration) have shown changes in CSA of type IIx and/or IIb fibers in parallel with changes in DIAm specific force, albeit to varying degrees (Lewis 1996; Mantilla 2013; Miyata 1995). To further determine the specific mechanisms of DIAm sarcopenia it will be necessary to examine single fiber mechanics for specific force, MyHC content per half sarcomere, and MyHC co-expression in single type-identified DIAm fibers.
Taken together the results of the present study indicate that sarcopenia is present in the DIAm, as represented by reductions in DIAm force and fiber size. Sarcopenia may have substantial effects on the ability of the DIAm to accomplish a broad range of ventilatory and non-ventilatory behaviors. It is possible that an increase in the percentage of fibers recruited for resting breathing (i.e., eupnea) and a decrease in the ability of the DIAm to suitably perform near maximal forces generated during non-ventilatory behaviors (e.g., sneezing or coughing) may occur. Additionally, if there is decreased functional reserve capacity of the DIAm due to sarcopenia, it is possible that external stresses placed on the DIAm by other aging-associated pulmonary pathologies may not be tolerated well. In combination, this may limit the ability of the DIAm to maintain airway clearance, especially in light of any age-related changes in respiratory system mechanics. Future studies are needed to examine the extent of disability for ventilatory and non-ventilatory behaviors imposed by sarcopenia of respiratory muscles such as the DIAm, throughout the lifespan.
Highlights.
Aging is associated with force loss in the diaphragm muscle
Type IIx and/or IIb diaphragm muscle fibers display decreased size with age
Accelerated aging in BubR1H/H mice exhibits diaphragm sarcopenia
Acknowledgments
We thank Dr. Jan van Deursen and Dr. Darren Baker for kindly providing BubR1H/H mice used in this study. We would also like to acknowledge Yun-Hua Fang for technical assistance in the completion of this project. This research was supported by grants from National Institute of Health R01-AG044615 (CBM & GCS) and T32-HL105355 (SMG), and the Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameredes BT, Zhan W-Z, Vanderboom R, Prakash YS, Sieck GC. Power fatigue of the rat diaphragm muscle. J Appl Physiol. 2000;89:2215–2219. doi: 10.1152/jappl.2000.89.6.2215. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Perez-Terzic C, Jin F, Pitel K, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, Van Kan GA, Anker SD, Rutkove S, Vrijbloed JW, Isaac M, Rolland Y, M'Rini C, Aubertin-Leheudre M, Cedarbaum JM, Zamboni M, Sieber CC, Laurent D, Evans WJ, Roubenoff R, Morley JE, Vellas B. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell DS, Powers SK, Herb RA, Dodd SL. Mechanism of specific force deficit in the senescent rat diaphragm. Respir Physiol Neurobiol. 1997;107:149–155. doi: 10.1016/s0034-5687(96)02509-1. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest. 2003;124:2302–2308. doi: 10.1378/chest.124.6.2302. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, McCarter RJ. Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve. 1992;15:1105–1110. doi: 10.1002/mus.880151008. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Ng R, Davis CS, Li S, Chamberlain JS. Diaphragm muscle strip preparation for evaluation of gene therapies in mdx mice. Clinical and Experimental Pharmacology and Physiology. 2008;35:725–729. doi: 10.1111/j.1440-1681.2007.04865.x. [DOI] [PubMed] [Google Scholar]
- Fein AM, Niederman MS. Severe pneumonia in the elderly. Clin Geriatr Med. 1994;10:121–143. [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, editor. The Jackson Laboratory Handbook on Genetically Standardized Mice. Bar Harbor, ME: 2009. [Google Scholar]
- Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol. 2003;95:611–619. doi: 10.1152/japplphysiol.00862.2002. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2002;92:1506–1514. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Effect of unilateral denervation on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2001;90:1196–1204. doi: 10.1152/jappl.2001.90.4.1196. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med. 1994;150:174–178. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Martinez DA, Vailas AC, Sieck GC. Interstitial space and collagen alterations of the developing rat diaphragm. J Appl Physiol. 1993;74:2450–2455. doi: 10.1152/jappl.1993.74.5.2450. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Zhan WZ, Sieck GC. Hypothyroid-mediated changes in adult rat diaphragm muscle contractile properties and MHC isoform expression. J Appl Physiol. 1996;80:1934–1939. doi: 10.1152/jappl.1996.80.6.1934. [DOI] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110:109–115. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011;59:1–95. [PubMed] [Google Scholar]
- Houston MS, Silverstein MD, Suman VJ. Risk factors for 30-day mortality in elderly patients with lower respiratory tract infection. Community-based study. Arch Intern Med. 1997;157:2190–2195. [PubMed] [Google Scholar]
- Imagita H, Yamano S, Tobimatsu Y, Miyata H. Age-related changes in contraction and relaxation of rat diaphragm. Biomed Res. 2009;30:337–342. doi: 10.2220/biomedres.30.337. [DOI] [PubMed] [Google Scholar]
- Kavazis AN, Deruisseau KC, Gordon DM. The senescent rat diaphragm does not exhibit age-related changes in caspase activities, DNA fragmentation, or myonuclear domain. Eur J Appl Physiol. 2012;112:3983–3990. doi: 10.1007/s00421-012-2380-2. [DOI] [PubMed] [Google Scholar]
- Kavazis AN, DeRuisseau KC, McClung JM, Whidden MA, Falk DJ, Smuder AJ, Sugiura T, Powers SK. Diaphragmatic proteasome function is maintained in the ageing Fisher 344 rat. Exp Physiol. 2007;92:895–901. doi: 10.1113/expphysiol.2007.038307. [DOI] [PubMed] [Google Scholar]
- Kawai M, Saitsu K, Yamashita H, Miyata H. Age-related changes in satellite cell proliferation by compensatory activation in rat diaphragm muscles. Biomed Res. 2012;33:167–173. doi: 10.2220/biomedres.33.167. [DOI] [PubMed] [Google Scholar]
- Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56:B443–448. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- Lewis MI, LoRusso TJ, Zhan W-Z, Sieck GC. Interactive effects of denervation and malnutrition on diaphragm structure and function. J Appl Physiol. 1996;81:2165–2172. doi: 10.1152/jappl.1996.81.5.2165. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC, Fournier M, Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol. 1986;60:596–603. doi: 10.1152/jappl.1986.60.2.596. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol. 1997;272:C2063–2068. doi: 10.1152/ajpcell.1997.272.6.C2063. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, Hamnegard CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997;155:1560–1564. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Herb RA, Demirel H, Dodd S. Age-related increases in diaphragmatic maximal shortening velocity. J Appl Physiol. 1996;80:445–451. doi: 10.1152/jappl.1996.80.2.445. [DOI] [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WN, Dirks A, Sugiura T, Muller S, Scarpace P, Powers SK. Alteration of contractile force and mass in the senescent diaphragm with beta(2)-agonist treatment. J Appl Physiol. 2002;92:941–948. doi: 10.1152/japplphysiol.00576.2001. [DOI] [PubMed] [Google Scholar]
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med. 1995;152:677–682. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]