Abstract
AIM: To examine surgical and medical outcomes for patients with cholangiocarcinoma using a population-based cancer registry.
METHODS: Using the California Cancer Registry’s Cancer Surveillance Program, patients with intrahepatic cholangiocarcinoma treated in Los Angeles County from 1988 to 2006 were identified and evaluated for clinical and pathologic factors and therapies received (surgery, radiation, and chemotherapy). The surgical cohort was further categorized into three treatment groups: patients who received adjuvant chemotherapy, adjuvant chemoradiation, or underwent surgery alone (no chemotherapy or radiation administered). Survival was assessed by Kaplan-Meier method; and Cox proportional hazard modeling was used in multivariate analysis.
RESULTS: Of 825 patients, 60.2% received no treatment. Of the remaining 328 patients, 18.5% chemotherapy only, 7.4% chemoradiation, and 13.8% underwent surgery. More male patients underwent surgical resection (P = 0.004). Surgical patients were younger than the patients receiving chemotherapy or chemoradiation (P < 0.001). Of the surgical cohort (n = 114), 60.5% underwent surgery alone while 39.5% underwent surgery plus adjuvant therapy (chemotherapy, n = 20; chemoradiation, n = 21) (P < 0.001). Median survival for all patients in the study was 6.6 mo. Median survival was highest for patients who underwent surgery (23 mo), whereas both chemotherapy (9 mo) and chemoradiation (8 mo) alone were each less effective (P < 0.001). By multivariate analysis, extent of disease, receipt of surgery, and administration of chemotherapy (with/without surgery) were independent predictors of overall survival.
CONCLUSION: This study demonstrates that surgery is a critical treatment modality. Multimodality treatment has yet to be standardized, but play a role in optimal therapy for cholangiocarcinoma.
Keywords: Cholangiocarcinoma, Chemotherapy, Surgery, Survival, Therapies
Core tip: Cholangiocarcinoma is an aggressive biliary tract cancer with few treatment options. Surgical resection has been the only available curative-intent treatment in early disease. This study demonstrates that multimodality therapy may provide the best improvement in survival.
INTRODUCTION
Cholangiocarcinoma (CCA) is an aggressive malignancy of the biliary tract that has two major anatomic subtypes: intrahepatic and ductal/perihilar[1]. It is the 2nd most common primary hepatic malignancy worldwide and afflicts nearly 2000-3000 people in the United States annually[2,3]. Partly due to improved diagnostic measures as well as a change in diagnosis coding, the incidence of CCA in the United States has increased by nearly 165% over the past several decades[4-7]. In fact, CCA was often coded as hepatocellular carcinoma until the late 1980s; therefore, the true incidence of cholangiocarcinoma prior to that time remains unknown[8]. Despite improvements in diagnostic techniques, many patients are asymptomatic until the disease has become advanced. Symptomatic patients commonly present with jaundice, but non-specific symptoms (e.g., weight loss, loss of appetite, and abdominal pain) are also frequently reported[9-12].
Surgical resection has long been considered the mainstay of curative treatment for CCA. Unfortunately, most patients with CCA present with advanced unresectable disease. Even when patients are eligible for surgical intervention, overall survival is poor, with 5-year survival rates of 20%-43%[13]. For patients who are eligible for systemic therapy for unresectable disease or adjuvant therapy, the current recommended therapy of gemcitabine and cisplatin provides a very minimal increase in median overall survival (OS) to 11.7 mo[14,15]. The lack of effective therapies has led to the frequent administration of systemic agents in non-standardized regimens[16].
Lack of better standardized therapeutic regimens and prior inconsistencies in coding of CCA have made it difficult to assess multimodality regimens for this disease. The absence of data from prospective randomized trials specifically limited to CCA precludes appropriate clinical practice guidelines[17]. In this investigation, we sought to examine common CCA therapies (i.e., surgical resection, chemotherapy, and radiation therapy) to assess treatment outcomes and to determine which therapeutic regimen(s) have offered improvements in overall survival in a large population based cohort.
MATERIALS AND METHODS
Cancer surveillance program for Los Angeles County
After obtaining City of Hope Institutional Review Board approval, we used the California Cancer Registry’s Cancer Surveillance Program (CSP) to identify patients diagnosed with cholangiocarcinoma. CSP for Los Angeles County is a population-based cancer registry that includes data on nearly all cancer diagnoses. The collected data is focused on cancer diagnosis and treatment with the stated CSP aim of improving cancer care in the State of California. This focus on comprehensive data collection for cancer therapy (i.e., chemotherapy, radiotherapy, and curative-intent surgery) distinguishes CSP from other regional and national databases.
CSP reporting for CCA location, staging, and differentiation is based on the International Classification of Diseases for Oncology. After restricting our analysis to liver (C22.0) and intrahepatic bile duct (C22.1) topologies, we selected only cases with histology codes 8160 (bile duct adenocarcinoma) and 8162 (Klatskins tumor). CSP extent of disease was reported as localized, regional [extension only; nodes only; extension and nodes; not otherwise specified (NOS)], distant or unknown. Tumor grade was categorized by CSP as well, moderate, poor, undifferentiated, or unknown. Chemotherapy was classified as negative (none; recommended, not given; or refused) or positive (single agent, multiple agent, or NOS). Radiation data was also classified as negative (none) or positive (beam, implants, isotopes, combination, or NOS). All patients who underwent curative-intent surgical therapy (defined by CSP codes 10, 13, 16, 20-80) were included in the surgery group. Patients who did not have surgery or who underwent non-curative surgery (defined by CSP codes 11, 12, 15, 17 and 90) were included in the non-surgical group.
Statistical analysis
Inclusion criteria consisted of patients diagnosed with CCA from 1988 to 2006 who survived 30 d or longer post-diagnosis that received some form of treatment for their disease. Patients were only included in whom complete survival data was available. Treatment groups were defined as chemotherapy alone, chemoradiation, and surgery. The surgical cohort was then further categorized into three treatment groups: patients who received adjuvant chemotherapy, adjuvant chemoradiation, or underwent surgery alone (no chemotherapy or radiation administered). All quantitative data were expressed as the median and range, unless otherwise indicated. Clinical and pathologic characteristics were compared across the different treatment arms by chi-square analyses.
Cox-proportional hazards modeling was used to evaluate the role of chemoradiation and other variables on overall survival as represented by hazard ratios (HR) with 95% confidence intervals (CI). Variables included in the univariate and multivariate analyses were age, sex, race/ethnicity, socioeconomic status, tumor location, tumor size, T-stage, N-stage, M-stage, tumor grade, American Joint Commission on Cancer 7th edition stage, lymph node number, chemotherapy administration, and radiation administration. Overall survival for the treatment arms was calculated by the Kaplan-Meier method and differences in survival were compared by the log-rank test. Two-sided P-values < 0.05 were considered to be statistically significant. All statistical analyses were completed using SAS software (SAS institute Inc. Cary, NC, United States).
RESULTS
Patient characteristics
There were 825 patients identified with CCA. Of this group, 60.2% (n = 497) did not receive treatment, 18.5% (n = 153) received chemotherapy only, 7.4% (n = 61) received chemoradiation, and 13.8% (n = 114) underwent curative-intent surgery. Of the patients who underwent surgery, 8.4% (n = 69) received surgery only, and 5.5% (n = 45) received surgery plus additional adjuvant treatment (Table 1). Of these, 20 (17.5%) surgical patients received adjuvant chemotherapy, 21 (18.4%) underwent adjuvant chemoradiation, and 4 (3.5%) underwent adjuvant radiation. Given the small sample size of the adjuvant radiation group, this group was excluded from our analysis.
Table 1.
Definition of study groups n (%)
| Total study population | n = 825 |
| No treatment | 497 (60.2) |
| Treatment groups | |
| Chemotherapy | 153 (18.5) |
| Chemoradiation | 61 (7.4) |
| Surgery | 114 (13.8) |
| Surgical subgroups | |
| Surgery alone | 69 (60.5) |
| Adjuvant radiation | 4 (3.5) |
| Adjuvant chemotherapy | 20 (17.5) |
| Adjuvant chemoradiation | 21 (18.4) |
The 825 patients included in this study were categorized by type of treatment received. Treatment groups were divided into chemotherapy, chemoradiation, and surgical groups. The surgical group was then subdivided into surgery alone, surgery with adjuvant chemotherapy, and surgery with adjuvant chemoradiation.
Comparison of treatment arms
Of the three treatment groups (chemotherapy, chemoradiation, and surgical groups), the characteristics that were different between the surgical patients and the non-surgical patients were age, gender, tumor location, and stage (Table 2). Patients in the surgery cohort were more often male, white, and young. Not surprisingly, there were expected differences in extent of disease and type of disease between the groups, with more early stage intrahepatic CCA disease in the surgical cohort. Additionally, over one-half of the chemotherapy group (n = 68; 53.1%) had metastatic disease at presentation, compared to 12.7% (n = 14) of surgical patients.
Table 2.
Characteristics of patient and treatment groups n (%)
| Chemo only (n = 153 ) | Chemoradiation (n = 61) | Surgery (n = 114 ) | P value | |
| Age group (yr) | < 0.0001 | |||
| 18-49 | 29 (19.0) | 10 (16.4) | 29 (25.4) | |
| 50-64 | 62 (40.5) | 20 (32.8) | 44 (38.6) | |
| 65-79 | 55 (35.9) | 31 (50.8) | 39 (34.2) | |
| ≥ 80 | 7 (4.6) | 0 (0.0) | 2 (1.8) | |
| Sex | 0.000 | |||
| Men | 57 (37.3) | 36 (59.0) | 69 (60.5) | |
| Women | 96 (62.7) | 25 (41.0) | 45 (39.5) | |
| Race | 0.002 | |||
| Non-hispanic white | 74 (48.4) | 26 (42.6) | 53 (46.5) | |
| Black | 9 (5.9) | 7 (11.5) | 4 (3.5) | |
| Hispanic white | 37 (24.2) | 8 (13.1) | 29 (25.4) | |
| Other | 33 (21.6) | 20 (32.8) | 28 (24.6) | |
| Socioeconomic status | 0.046 | |||
| Highest | 38 (24.8) | 18 (29.5) | 33 (28.9) | |
| Middle | 96 (62.7) | 35 (57.4) | 65 (57.0) | |
| Lowest | 19 (12.4) | 8 (13.1) | 16 (14.0) | |
| Tumor | 0.000 | |||
| Liver | 21 (13.7) | 3 (4.9) | 22 (19.3) | |
| Location | ||||
| Bile duct | 132 (86.3) | 58 (95.1) | 92 (80.7) | |
| Grade | 0.685 | |||
| Well differentiated | 12 (19.4) | 4 (17.4) | 19 (21.1) | |
| Moderately differentiated | 20 (32.3) | 5 (21.7) | 33 (36.7) | |
| Poorly differentiated | 30 (48.4) | 14 (60.9) | 38 (42.2) | |
| Tumor size (cm) | 0.060 | |||
| ≤ 5 | 10 (27.8) | 7 (58.3) | 34 (50.0) | |
| > 5 | 26 (72.2) | 5 (41.7) | 34 (50.0) | |
| Summary | 0.000 | |||
| Local | 17 (15.3) | 9 (21.4) | 35 (36.1) | |
| Stage | ||||
| Regional | 29 (26.1) | 16 (38.1) | 36 (37.1) | |
| T stage | 0.001 | |||
| T1 | 23 (24.5) | 8 (19.5) | 37 (37.4) | |
| T2 | 2 (2.1) | 3 (7.3) | 14 (14.1) | |
| T3 | 19 (20.2) | 10 (24.4) | 18 (18.2) | |
| T4 | 50 (53.2) | 20 (48.8) | 30 (30.3) | |
| N stage | 0.211 | |||
| N0 | 53 (72.6) | 22 (68.8) | 71 (79.8) | |
| N1 | 20 (27.4) | 10 (31.3) | 18 (20.2) | |
| M stage | < 0.0001 | |||
| M0 | 60 (46.9) | 32 (68.1) | 96 (87.3) | |
| M1 | 68 (53.1) | 15 (31.9) | 14 (12.7) | |
| AJCC7 stage | < 0.0001 | |||
| I | 13 (14.4) | 2 (6.3) | 34 (36.6) | |
| II | 1 (1.1) | 2 (6.3) | 9 (9.7) | |
| III | 18 (11.8) | 7 (11.5) | 23 (20.2) | |
| IV | 58 (64.4) | 21 (65.6) | 27 (29.1) | |
| Chemotherapy | 153 (100.0) | 61 (100.0) | 41 (36.0) | < 0.0001 |
| Radiation | 0 (0.0) | 61 (100.0) | 25 (21.9) | < 0.0001 |
In general, characteristics that were different between the surgical patients and the non-surgical patients were age, gender, tumor location, and stage. Patients in the surgery cohort were more often male, white, and young. chemo: Chemotherapy; AJCC: American Joint Committee on Cancer.
Comparison of surgical groups
The characteristics found to be significantly different between surgical patients who received surgery alone and those who underwent surgery and additional adjuvant therapy were age, gender, tumor location, and stage (Table 3). Patients in the adjuvant chemoradiation group were male and much younger in age compared to the other groups. Whereas 80.9% of the adjuvant chemoradiation group were < 65 years old, more patients (39.1%) were older than 65 in the surgery alone subset.
Table 3.
Characteristics of surgical groups n (%)
| Surgery alone (n = 69) | Adjuvant chemotherapy (n = 20) | Adjuvant chemoradiation (n = 21) | P value | |
| Age group (yr) | < 0.0001 | |||
| 18-49 | 13 (18.8) | 6 (30.0) | 10 (47.6) | |
| 50-64 | 29 (42.0) | 6 (30.0) | 7 (33.3) | |
| 65-79 | 25 (36.2) | 8 (40.0) | 4 (19.0) | |
| ≥ 80 | 2 (2.9) | 0 (0.0) | 0 (0.0) | |
| Sex | 0.018 | |||
| Men | 41 (59.4) | 13 (65.0) | 14 (66.7) | |
| Women | 28 (40.6) | 7 (35.0) | 7 (33.3) | |
| Race | 0.497 | |||
| Non-hispanic white | 33 (47.8) | 11 (55.0) | 9 (42.9) | |
| Black | 2 (2.9) | 0 (0.0) | 2 (9.5) | |
| Hispanic white | 20 (29) | 3 (15.0) | 5 (23.8) | |
| Other | 14 (20.3) | 6 (30.0) | 5 (23.8) | |
| Socioeconomic status | 0.603 | |||
| Highest | 18 (26.1) | 7 (35.0) | 7 (33.3) | |
| Middle | 41 (59.4) | 9 (45.0) | 12 (57.1) | |
| Lowest | 10 (14.5) | 4 (20.0) | 2 (9.5) | |
| Tumor | 0.000 | |||
| Liver | 17 (24.6) | 3 (15.0) | 2 (9.5) | |
| Location | ||||
| Bile duct | 52 (75.4) | 17 (85.0) | 19 (90.5) | |
| Grade | 0.684 | |||
| Well differentiated | 13 (23.6) | 3 (20.0) | 3 (16.7) | |
| Moderately differentiated | 21 (38.2) | 4 (26.7) | 6 (33.3) | |
| Poorly differentiated | 21 (38.1) | 8 (53.3) | 9 (50.0) | |
| Tumor size (cm) | 0.825 | |||
| ≤ 5 | 24 (54.5) | 3 (25.0) | 6 (60.0) | |
| > 5 | 20 (45.5) | 9 (75.0) | 4 (40.0) | |
| Summary | 0.016 | |||
| Local | 23 (38.3) | 3 (20.0) | 7 (38.9) | |
| Stage | ||||
| Regional | 24 (40.0) | 5 (33.3) | 6 (33.3) | |
| Distant | 13 (21.7) | 7 (46.7) | 5 (27.8) | |
| T stage | 0.071 | |||
| T1 | 24 (40.0) | 5 (29.4) | 7 (36.8) | |
| T2 | 9 (15.0) | 3 (17.6) | 2 (10.5) | |
| T3 | 12 (20.0) | 3 (17.6) | 3 (15.8) | |
| T4 | 15 (25.0) | 6 (35.3) | 7 (36.8) | |
| N stage | 0.396 | |||
| N0 | 44 (83.0) | 10 (62.5) | 14 (82.4) | |
| N1 | 9 (17.0) | 6 (37.5) | 3 (17.6) | |
| AJCC7 stage | < 0.0001 | |||
| I | 22 (40.0) | 5 (29.4) | 6 (33.3) | |
| II | 7 (12.7) | 1 (5.9) | 1 (5.6) | |
| III | 13 (23.6) | 3 (17.7) | 5 (27.8) | |
| IV | 13 (23.7) | 8 (47.0) | 6 (33.4) |
Characteristics found to be significantly different between surgical patients were age, gender, tumor location, and stage. Patients in the adjuvant chemoradiation group were male and much younger in age compared to the other groups. AJCC: American Joint Committee on Cancer.
Survival according to treatment group
Median survival (MS) for all patients in the overall cohort was 6 mo, with a 5-year survival of 5.7% (Figure 1A). When comparing outcomes by treatment groups, MS was highest for surgical patients, with an MS of 23 mo (Figure 1B) compared to 9 and 8 mo for chemotherapy and chemoradiation groups, respectively (P < 0.0001). When evaluating the surgical subgroups, surgery alone patients had the highest survival (MS 28 mo) (Figure 1C), with MS of 18 and 22 mo for the adjuvant chemotherapy and adjuvant chemoradiation groups, respectively (P = 0.3085). Of note, patients who did not receive treatment had an MS of 3.0 mo.
Figure 1.
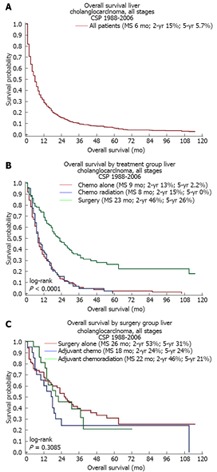
Kaplan-Meier survival of treatment groups. A: Median survival (MS) for all patients in overall cohort was 6 mo; B: MS was highest for surgical patients, with an MS of 23 mo, compared to 9 mo and 8 mo for chemotherapy (chemo) and chemoradiation groups, respectively; C: Surgery alone patients had the highest survival (MS 28 mo), with MS of 18 and 22 mo for the adjuvant chemotherapy and adjuvant chemoradiation groups, respectively. CSP: Cancer Surveillance Program.
Univariate and multivariate analysis of patient cohort
A Cox regression analysis was performed to identify predictors of survival for CCA (Table 4). On univariate analysis, older age, black race, lower socioeconomic status, undifferentiated tumors, and T4 tumors were significantly associated with poorer survival. Upon multivariate analysis, older age (i.e., above 80 years of age), advanced disease stage, poorly differentiated or undifferentiated tumors, and lymph node involvement were independent predictors of poorer survival in all treatment groups. Our analysis demonstrated a significant improvement in survival associated with surgical resection (HR = 0.44, 95%CI: 0.34-0.59, P < 0.0001) and receipt of chemotherapy (HR = 0.70, 95%CI: 0.59-0.83 P = 0.001). In contrast, receipt of radiation therapy was not associated with improved survival.
Table 4.
Univariate and stepwise analysis for overall survival n (%)
| Univariate hazard ratio (95%CI) | Stepwise hazard ratio (95%CI) | ||
| Age1 (yr) | |||
| 18-49 | 107 (13.0) | 1.00 (reference) | 1.00 (reference) |
| 50-64 | 230 (27.9) | 1.43 (1.11-1.85) | 1.64 (1.27-2.13) |
| 65-79 | 327 (39.6) | 1.40 (1.10-1.78) | 1.47 (1.15-1.89) |
| ≥ 80 | 161 (19.5) | 2.19 (1.68-2.86) | 1.85 (1.40-2.46) |
| Sex1 | |||
| Men | 390 (47.3) | 1.00 (reference) | |
| Women | 435 (52.7) | 1.02 (0.88-1.18) | |
| Race | |||
| Non-hispanic white | 373 (45.2) | 1.00 (reference) | |
| Ethnicity1 | |||
| Black | 64 (7.8) | 1.50 (1.14-1.97) | |
| Hispanic white | 215 (26.1) | 1.04 (0.87-1.24) | |
| Other | 170 (20.6) | 1.00 (0.82-1.21) | |
| Socioeconomic status1 | |||
| Highest | 184 (22.3) | 1.00 (reference) | 1.00 (reference) |
| Middle | 497 (60.2) | 1.25 (1.04-1.49) | 1.18 (0.98-1.41) |
| Lowest | 144 (17.5) | 1.61 (1.28-2.03) | 1.52 (1.20-1.92) |
| Grade | |||
| Well differentiated | 52 (6.3) | 1.00 (reference) | |
| Moderately differentiated | 99 (12.0) | 1.05 (0.72-1.53) | |
| Poorly differentiated | 135 (16.4) | 1.61 (1.13-2.29) | |
| Undifferentiated | 8 (1.0) | 2.96 (1.38-6.32) | |
| Unknown | 531 (64.4) | 1.71 (1.24-2.35) | |
| Tumor size (cm) | |||
| ≤ 5 | 95 (11.5) | 1.00 (reference ) | |
| > 5 | 94 (11.4) | 1.03 (0.76-1.39) | |
| T Stage | |||
| T1 | 150 (18.2) | 1.00 (reference) | |
| T2 | 28 (3.4) | 0.91 (0.57-1.47) | |
| T3a | 64 (7.8) | 1.10 (0.80-1.51) | |
| T3b | 13 (1.6) | 1.37 (0.72-2.63) | |
| T4 | 198 (24) | 2.23 (1.76-2.83) | |
| TX | 372 (45.1) | 1.85 (1.50-2.29) | |
| N stage | |||
| N0 | 278 (33.7) | 1.00 (reference ) | |
| N1 | 77 (9.3) | 1.62 (1.24-2.12) | |
| NX | 470 (57) | 1.76 (1.50-2.07) | |
| AJCC7 group1 | |||
| I | 93 (11.3) | 1.00 (reference) | 1.00 (reference) |
| II | 15 (1.8) | 0.80 (0.41-1.56) | 1.07 (0.54-2.09) |
| III | 79 (9.6) | 1.37 (0.98-1.91) | 1.31 (0.93-1.84) |
| IV | 204 (24.7) | 2.81 (2.11-3.72) | 2.56 (1.90-3.44) |
| Unknown | 434 (52.6) | 2.23 (1.72-2.89) | 1.52 (1.16-2.00) |
| Treatment groups | |||
| Chemo alone | 153 (18.5) | 1.00 (reference) | |
| Chemoradiation | 61 (7.4) | 0.98 (0.72-1.34) | |
| Surgery | 114 (13.8) | 0.44 (0.34-0.59) | |
| None | 497 (60.2) | 1.50 (1.24-1.81) | |
| Surgery groups | |||
| Surgery Alone | 69 (8.4) | 1.00 (reference) | |
| Adjuvant chemo | 20 (2.4) | 1.44 (0.82-2.55) | |
| Adjuvant chemoradiation | 21 (2.5) | 1.02 (0.56-1.85) | |
| Adjuvant radiation | 4 (0.5) | 1.53 (0.55-4.26) | |
| No surgery | 711 (86.2) | 3.19 (2.35-4.33) | |
| Surgery1 | |||
| No | 711 (86.2) | 1.00 (reference ) | 1.00 (reference ) |
| Yes | 114 (13.7) | 0.34 (0.27-0.43) | 0.42 (0.32-0.54) |
| Chemo1 | |||
| No | 570 (69.1) | 1.00 (reference ) | 1.00 (reference ) |
| Yes | 255 (30.9) | 0.73 (0.62-0.85) | 0.70 (0.59-0.83) |
| Radiation1 | |||
| No | 707 (85.7) | 1.00 (reference ) | |
| Yes | 118 (14.3) | 0.74 (0.60-0.90) |
Included in step-wise model. On univariate analysis, older age, black race, lower socioeconomic status, undifferentiated tumors, and T4 tumors were significantly associated with poorer survival. Upon stepwise analysis, older age (> 80 year), advanced disease stage, poorly differentiated or undifferentiated tumors, and lymph node involvement were independent predictors of poorer survival in all treatment groups. There was a significant improvement in survival associated with surgical resection and receipt of chemotherapy. chemo: Chemotherapy; AJCC: American Joint Committee on Cancer.
DISCUSSION
Surgery has been the traditional mainstay of curative-intent therapy for patients with early stage, resectable CCA. Such practice has been largely established on the backbone of single institution reports touting the benefits of surgical resection[10,11,18]. Our current population-based investigation supports this growing body of evidence, also highlighting the superior outcomes when patients with CCA are eligible for and undergo surgical resection. For those surgical patients, favorable prognostic factors include absence of tumor at the resection margins, elevated serum CA19-9 levels, solitary lesion, absence of lymph node involvement, presence of well-differentiated adenocarcinoma, and absence of vascular invasion[19-21]. Nevertheless, the relatively low survival even with appropriate surgical intervention underscores the pressing need for multidisciplinary approach to the management of patients with CCA.
Treatment guidelines suggest that systemic chemotherapy should be the treatment of choice for patients with good performance status who have extensive disease not amenable to surgical resection. In the setting of unresectable or metastatic CCA, the aims of treatment should include prolongation of life but also maintenance of quality of life. Prior studies have reported rates of OS ranging between 5.6 and 14 mo[22-28]. Patients that did not receive treatment in our study had an MS of 3 mo, whereas patients who received chemotherapy or chemoradiation had MS of 8 and 9 mo respectively. The identification of chemotherapy in our study as an independent variable for survival is consistent with prior reports.
There is an inherent limitation with the CSP database, in that the exact chemotherapy regimen and its timing and period of administration are not available for analysis. A phase III trial recently demonstrated the benefit of combined gemcitabine and cisplatin over gemcitabine alone with improved OS and progression-free survival[15]. Although the study included all hepatobiliary cancers, including gallbladder and ampullary cancers[15], a benefit to combination chemotherapy was seen in the CCA subgroup. The National Comprehensive Cancer Network (NCCN) now recommends the combination therapy of gemcitabine and cisplatin as the standard treatment for unresectable CCA based on the results of the randomized phase III trial ABC-02 and retrospective and pooled data[15,29-31].
The NCCN also provides guidelines, albeit non-specific, on the use of adjuvant therapies in the management of patients with resectable CCA disease. Depending on the completeness of surgical resection, adjuvant therapies in clinical trial settings or case-by-case individualized therapies are recommended[31]. The lack of definitive recommendations for adjuvant treatment in patients with curatively resected cholangiocarcinoma is based on the absence of level 1 evidence for such conditions. In a recent systematic review and meta-analysis of adjuvant chemotherapy, radiation, or chemoradiation in 6712 patients with resected biliary tract cancer, a significant benefit in OS was observed in favor of any adjuvant treatment in CCA patients with either lymph node positivity or with positive resection margins[32]. In our study, we did not see any positive impact to chemotherapy or chemoradiation in patients with resected cholangiocarcinoma. The reasons for the lack of adjuvant treatment benefits may be related to a true lack of efficacy in adjuvant therapies for patients with CCA. This patient population is typically underpowered to identify a benefit; or patient selection bias may exist wherein patients with favorable T1-T2N0 tumors are selected for surgery only groups.
Radiation therapy must also be considered in the multimodal management of patients with CCA. Although prior small cohort studies have shown disappointing results for radiation alone, chemoradiation therapies in select patients should continue to be investigated in future trials. Nevertheless, the addition of radiation to chemotherapy regimens did not improve survival in our study. Nonsurgical patients had similar MS after receiving either chemotherapy alone (9 mo) or chemoradiation (8 mo). This is consistent with a prospective study by Pitt et al[33], who reported that radiation did not provide a survival advantage in patients with perihilar CCA.
In other published retrospective and population-based studies, adjuvant treatment with chemoradiation using fluoropyrimidine-based chemotherapy showed possible improvement in local control and potential improvement in short-term survival in patients with CCA without long-term benefit[34-36]. For intrahepatic CCA, retrospective and population reviews have suggested that adjuvant chemoradiation may provide a slight survival benefit in R1 resections[31]. However, based on the paucity of data, current guidelines can only recommend that chemoradiation be used in unresectable patients without metastatic disease[31].
Novel approaches not examined in our study should also be considered in patients with CCA. Advancements in radiation treatments have led to novel approaches to deliver radiation to patients with CCA including radioembolization with Yttrium-90 microspheres[37,38]. Single-institution data appear promising for patients with liver predominant disease[37]. However, the definitive role of radioembolization should be evaluated in randomized studies prior to its inclusion as a standard measure. We could not fully evaluate the role of radioembolization in our study since this treatment was not integrated to any extent in patients with CCA prior to 2006.
Our study is one of the few that shed light on how multimodal therapy impacts patients with intrahepatic CCA also highlighting the inherent complexity in treating CCA. Our results verify that surgical resection remains the most important variable associated with improved survival for patients with resectable intrahepatic CCA, but our results also indicate that further work is necessary to identify more effective systemic/targeted and regional therapies.
COMMENTS
Background
Cholangiocarcinoma is an aggressive cancer of the bile ducts and liver. Unfortunately, most patients are asymptomatic and only present when symptoms suggestive of advance disease, such as jaundice, presents. Surgery remains the best curative-intent treatment, but is only possible in patients with early stage disease. There are few studies evaluating multimodality treatment: surgery, chemotherapy, and radiation.
Research frontiers
Multimodality therapies are now being investigated for the treatment of cholangiocarcinoma, such as radioembolization of the liver; yet these therapies are not standard of care.
Innovations and breakthroughs
The mainstay of treatment is chemotherapy with gemcitabine and cisplatin. Some studies are have evaluated other chemotherapy, including fluoropyramidine-based regimens, with similar results. In single-center trials, radioembolization with Yttrium-90 has been shown to be a safe.
Applications
This study indicates that surgery provides the best survival benefits for patients with cholangiocarcinoma and should continue to be a critical component in the multimodality treatment.
Terminology
Cholangiocarcinoma: an adenocarcinoma of the bile duct system. This may include the bile duct outside of the liver (perihilar) or the smaller bile ducts within the liver (intrahepatic). Adjuvant chemotherapy: chemotherapy given after surgical resection.
Peer review
The authors investigated surgical and medical outcomes for patients with cholangiocarcinoma using a population-based cancer registry. They demonstrated that surgical resection is a critically important modality of treatment for this disease.
Footnotes
P- Reviewers Ker CG, Wakai T S- Editor Gou SX L- Editor A E- Editor Lu YJ
References
- 1.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003084-pdf.pdf.
- 3.Vogt P, Raab R, Ringe B, Pichlmayr R. Resection of synchronous liver metastases from colorectal cancer. World J Surg. 1991;15:62–67. doi: 10.1007/BF01658964. [DOI] [PubMed] [Google Scholar]
- 4.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594–603. doi: 10.1016/j.jamcollsurg.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 6.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 7.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 8.Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, Nuzzo G, Belghiti J, Pruvot FR, Regimbeau JM. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 9.Zografos GN, Farfaras A, Zagouri F, Chrysikos D, Karaliotas K. Cholangiocarcinoma: principles and current trends. Hepatobiliary Pancreat Dis Int. 2011;10:10–20. doi: 10.1016/s1499-3872(11)60001-5. [DOI] [PubMed] [Google Scholar]
- 10.Saiura A, Yamamoto J, Kokudo N, Koga R, Seki M, Hiki N, Yamada K, Natori T, Yamaguchi T. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201:203–208. doi: 10.1016/j.amjsurg.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Lanthaler M, Biebl M, Strasser S, Weissenbacher A, Falkeis C, Margreiter R, Nehoda H. Surgical treatment of intrahepatic cholangiocarcinoma--a single center experience. Am Surg. 2010;76:411–417. [PubMed] [Google Scholar]
- 12.Friman S. Cholangiocarcinoma--current treatment options. Scand J Surg. 2011;100:30–34. doi: 10.1177/145749691110000106. [DOI] [PubMed] [Google Scholar]
- 13.Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, Zöpf T, Trarbach T, Malagó M, Baba HA, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, Baka S, Maraveyas A, Corrie P, Falk S, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer. 2009;101:621–627. doi: 10.1038/sj.bjc.6605211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 16.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford) 2005;7:278–282. doi: 10.1080/13651820500373028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, Serra C, Brandi G, Pinna AD. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252:107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- 19.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 20.Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Yamazaki O, Kinoshita H. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:969–974. doi: 10.1046/j.0007-1323.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 21.Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374–2383. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn R, Hribaschek A, Eichelmann K, Rudolph S, Fahlke J, Ridwelski K. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs. 2002;20:351–356. doi: 10.1023/a:1016209901417. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Park YH, Lee JN, Bang SM, Cho EK, Shin DB, Lee JH. Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer. 2006;106:361–365. doi: 10.1002/cncr.21621. [DOI] [PubMed] [Google Scholar]
- 24.Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 25.André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 26.Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, Heo JS, Park YS, Kang WK, Park K. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–1346. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 27.Murad AM, Guimarães RC, Aragão BC, Rodrigues VH, Scalabrini-Neto AO, Padua CA, Moore FC. Phase II trial of the use of gemcitabine and 5-fluorouracil in the treatment of advanced pancreatic and biliary tract cancer. Am J Clin Oncol. 2003;26:151–154. doi: 10.1097/00000421-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–1312. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 29.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 31.Benson AB. Hepatobiliary Cancers. NCCN Guidelines Version 2. 2012. Available from: http://www.NCCN.org. [Google Scholar]
- 32.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 33.Pitt H, Nakeeb A, Abrams R, Coleman J, Piantadosi S, Yeo C, Lillemore K. Cameron, J. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:778–798. doi: 10.1097/00000658-199506000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, Hurwitz HI, Bendell JC, Morse MA, Clough RW, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2009;73:148–153. doi: 10.1016/j.ijrobp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes MA, Frassica DA, Yeo CJ, Riall TS, Lillemoe KD, Cameron JL, Donehower RC, Laheru DA, Hruban RH, Abrams RA. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys. 2007;68:178–182. doi: 10.1016/j.ijrobp.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 36.Fuller CD, Wang SJ, Choi M, Czito BG, Cornell J, Welzel TM, McGlynn KA, Luh JY, Thomas CR. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer. 2009;115:5175–5183. doi: 10.1002/cncr.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, Rauch B, Trumm CT, Jakobs TF, Helmberger TK, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–116. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 38.Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]


