Background: The Candida albicans efflux pump Cdr1p causes clinically significant antifungal resistance.
Results: Biochemical mapping of Cdr1p transmembrane domain mutants reveals residues affecting drug transport.
Conclusion: Functional characterization and homology modeling provide insight into the drug binding cavity and substrate promiscuity of Cdr1p.
Significance: A platform is provided for systematic Cdr1p structure/function analysis and the rational design of transport modulators/inhibitors.
Keywords: ABC Transporter, ATPases, Drug Resistance, Membrane Proteins, Multidrug Transporters, Azoles, Fungal Multidrug Resistance, Pleiotropic Drug Resistance, Substrate Specificity, Transmembrane Domains
Abstract
The fungal ATP-binding cassette (ABC) transporter Cdr1 protein (Cdr1p), responsible for clinically significant drug resistance, is composed of two transmembrane domains (TMDs) and two nucleotide binding domains (NBDs). We have probed the nature of the drug binding pocket by performing systematic mutagenesis of the primary sequences of the 12 transmembrane segments (TMSs) found in the TMDs. All mutated proteins were expressed equally well and localized properly at the plasma membrane in the heterologous host Saccharomyces cerevisiae, but some variants differed significantly in efflux activity, substrate specificity, and coupled ATPase activity. Replacement of the majority of the amino acid residues with alanine or glycine yielded neutral mutations, but about 42% of the variants lost resistance to drug efflux substrates completely or selectively. A predicted three-dimensional homology model shows that all the TMSs, apart from TMS4 and TMS10, interact directly with the drug-binding cavity in both the open and closed Cdr1p conformations. However, TMS4 and TMS10 mutations can also induce total or selective drug susceptibility. Functional data and homology modeling assisted identification of critical amino acids within a drug-binding cavity that, upon mutation, abolished resistance to all drugs tested singly or in combinations. The open and closed Cdr1p models enabled the identification of amino acid residues that bordered a drug-binding cavity dominated by hydrophobic residues. The disposition of TMD residues with differential effects on drug binding and transport are consistent with a large polyspecific drug binding pocket in this yeast multidrug transporter.
Introduction
The incidence of fungal infections has increased over the past three decades because of the increased population of immunocompromised patients due to transplantation surgery, cancer chemotherapy, and HIV infection (1). Superficial infections caused by Candida albicans are commonly treated with the azole drugs, whereas life-threatening systemic infections are treated with triazole drugs or the more expensive echinocandins (2). Widespread and prolonged use of antifungals in recent years has led to the rapid emergence of azole- or triazole-resistant strains of Candida, which exhibit multidrug resistance (MDR)4 (3). Various mechanisms that contribute to the development of MDR have been identified. Overexpression of drug efflux pump encoding genes, such as CDR1 or CDR2, which belong to the ABC (ATP-binding cassette) family (4, 5) and MDR1, which belongs to the major facilitator superfamily transporter family (6, 7), represents one strategy that C. albicans strains used to develop drug resistance. Among the ABC transporters, Cdr1p is a major drug transporter in C. albicans (8, 9), and overexpression of Cdr1p is associated with increased drug substrate efflux in azole-resistant clinical isolates. Novel modulators or inhibitors, which can block the drug extrusion mediated by these efflux proteins, represents an attractive approach to reverse MDR (10, 11).
The CDR1 gene encodes a 1501-amino acid integral plasma membrane protein with a molecular mass of ∼170 kDa (4). Cdr1p displays the common architecture of the ABC transporter family, which involves two cytoplasmic nucleotide binding domains (NBDs) and two transmembrane domains (TMDs) (12). Compared with its human ortholog MDR1/P-glycoprotein (ABCB1), the primary sequence of Cdr1p predicts a reverse topology (NBD-TMD)2, with each NBD preceding a TMD. This topology is shared by the PDR class of ABC transporters (13, 14). Cdr1p contains two TMDs of six α-helical transmembrane segments (TMSs) that vectorally bind, then release, drugs and impart substrate specificity to the protein (12, 15). The NBDs bind and hydrolyze the ATP that powers drug efflux. Conformational changes induced by ATP binding and/or hydrolysis are transmitted from the NBDs to the TMDs, which can result in drug translocation, drug efflux, or resetting of the reaction cycle (16).
Elucidating the architecture of the drug-binding sites in ABC transporters is essential for understanding the drug-protein interactions and will assist the design of specific inhibitors (chemosensitizers) of these transporters. Most structure-function studies of ABC transporters have been performed on human P-glycoprotein. Those studies have shown that nearly all the TMSs are directly, or indirectly, involved in drug transport (17, 18). Two distinct substrate-binding sites, H (Hoechst 33342 binding site) and R (Rhodamine 123 binding site), have been pharmacologically defined for P-glycoprotein (19). Competition experiments subsequently suggested that P-glycoprotein could contain at least seven different drug-binding sites (20). The crystal structures of mouse and nematode P-glycoprotein, together with biochemical data, indicate that these proteins contain a large internal binding cavity that can accommodate structurally unrelated compounds of different sizes and shapes (21, 22).
Earlier mutational analysis suggests that Cdr1p probably contains at least three binding sites (23). One site appeared responsible for the efflux of rhodamine 6G (R6G) and azoles, such as ketoconazole (KTC), miconazole (MCZ) and itraconazole (ITC), whereas a separate site(s) interacts with, and transports, fluconazole (FLC) only. A third binding site may exist for the prazosin analog iodoarylazidoprazosin (24). Similarly, several important amino acid residues in Saccharomyces cerevisiae Pdr5p, a close ortholog of Cdr1p, have been identified as critical for drug binding and transport (25, 26). Studies using a range of Pdr5p substrates indicate that this transporter, like Cdr1p, contains at least three drug-binding sites and that some substrates may interact with more than one site (27, 28).
In the present study we have used scanning alanine/glycine mutagenesis to probe the molecular details of putative substrate/drug-binding sites in Cdr1p. Most residue substitutions did not alter phenotype detectably. Substitution in 42% of TMS residues completely, or selectively, eliminated resistance to the drugs tested. Some substitutions enhanced drug susceptibility and reduced both drug efflux and ATPase activity. In other mutants, the ATPase activity appeared uncoupled from drug efflux. Phenotypic profiling of the mutants identified residues from all TMSs that affected drug-binding/efflux in Cdr1p. The identification of numerous amino acid residues critical for drug transport paves the way for understanding of the substrate promiscuity of Cdr1p.
EXPERIMENTAL PROCEDURES
Yeast Strains and Site-directed Mutagenesis
The yeast strains used in this study are listed in supplemental Excel Sheet I. All the yeast strains were grown on yeast extract peptone dextrose (YEPD) plates or in YPD broth at 30 °C. Cdr1p and its mutants were individually overexpressed in AD1–8u− cells (24, 29). Site-directed mutagenesis was performed using the QuikChange Site-directed Mutagenesis Kit as described previously (24). Oligonucleotides used for mutagenesis are listed in supplemental Excel Sheet II. The mutations were introduced into plasmid pPSCDR1-GFP (24, 29) according to the manufacturer's instructions, and the desired nucleotide sequence alterations were confirmed by DNA sequencing of the ORF. Mutated plasmids were maintained in Escherichia coli DH5α. The mutated plasmid pPSCDR1-GFP linearized with XbaI was used to transform AD1–8u− cells by a lithium acetate transformation protocol and selecting for uracil prototrophy (24, 30).
Immunodetection of Cdr1 Protein
Plasma membrane (PM) samples were prepared from S. cerevisiae cells grown in YPD to late exponential phase, as previously described (24, 31). Western blot analysis was performed using an anti-GFP monoclonal antibody (1:5000 dilution as previously described (24).
Confocal Microscopy and Flow Cytometry
Confocal imaging and flow cytometric analysis of GFP-tagged Cdr1p and its mutants were performed with a Bio-Rad confocal microscope (MRC 1024) with a ×100 oil immersion objective and a fluorescence-activated cell sorter (Becton-Dickson Immunocytometry Systems, San Jose, CA), respectively (24).
Drug Susceptibility
The susceptibilities of S. cerevisiae cells expressing native or mutant Cdr1p to different antifungal drugs were measured using MIC80 determination as previously described (32).
Efflux Assays
The functionality of Cdr1p was determined by assaying the energy-dependent efflux of the fluorescent compound R6G. The efflux of R6G was measured using a previously described protocol (24, 33). Competition experiments with ITC and farnesol were determined according to previously described protocols (22, 34). The accumulation of Nile Red by exponential phase yeast cells was determined by modification of a previously described method (35). Nile Red (3.5 mm stock in DMSO) was added to cells in PBS containing glucose to a final concentration of 7 μm. After incubation at 30 °C for 30 min, the samples were excited with a 488-nm laser and PE-Texas Red filters were used to detect Nile Red accumulation in the yeast cells using a Cyan flow cytometer (DakoCytomation, Fort Collins, CO).
ATPase Activity
The ATPase activity of wild-type and mutant Cdr1p in the purified PM was measured as an oligomycin-sensitive release of inorganic phosphate (24, 36).
Time Resolved Fluorescence Spectroscopy (TRFS)
Fluorescence lifetime and anisotropy decay measurements were made in time-correlated single photon counting mode using an FL920 instrument (Edinburgh Instruments, Livingston, UK). The R6G in the sample was excited at 470 nm using a picosecond (ps) pulsed diode laser (pulse width ∼85 ps; instrument response function, ∼110 ps). The fluorescence lifetime decays at an emission wavelength of 555 nm were collected at the magic angle (54°) and at a parallel and perpendicular polarization relative to the vertical polarization of excitation. From the decays measured at parallel and perpendicular polarization, the anisotropy decay incorporating the value of the instrumental G-factor was calculated using Equation 1.
 |
The interaction of R6G with native Cdr1p was measured by determining the time-resolved fluorescence and anisotropy decays of R6G, free in PBS, and then bound to Cdr1p. The data were analyzed using IGOR-Pro software (Wave Metrics, USA). All fluorescence lifetime and anisotropy experiments were performed with the purified PM from cells overexpressing Cdr1p (AD-CDR1) and with PM from host cells that did not express Cdr1p (AD1–8u−).
Bioinformatic Analysis
A full-length multiple sequence alignment was used to identify the TMSs of Cdr1p. The PDR transporters having domain organization similar to Cdr1p, i.e. (NBD-TMS6)2, were extracted from 349 full-length PDR proteins from 55 fungal species (37). Partial or half-size sequences were excluded and sequences suspected to originate from redundant submissions to data bases were eliminated. The sequences of the 85 non-redundant full-length PDR transporters selected were aligned with Cdr1p using the membrane-specific multiple alignment program PRALINETM (38). The boundaries of these TMSs were determined by consensus using topology prediction programs, including SWISS-PROT (39), HMMTOP (40), TMHMM (41), TopPred 2 (42), and TMPred (43). Residue conservation scores were determined for each aligned sequence column using the Jalview 2.4.0.b2. The transmembrane segment conservation score for each TMS, calculated as the mean of scores of the individual residue columns within each domain, is shown in supplemental Table S1.
RESULTS
Drug Susceptibility of Strains with Mutated Cdr1p TMDs
C. albicans CDR1, and mutated versions of CDR1, were heterologously expressed in S. cerevisiae using an expression system that has been shown to achieve reproducible, high-level expression of fungal ABC proteins (44). The entire primary sequence of the two TMDs of Cdr1p, identified by bioinformatic analysis of 85 representative PDR transporters and comprising 12 TMSs (see “Experimental Procedures”), was subjected to alanine scanning mutagenesis with each of the 252 residues replaced with an alanine (Ala). When alanine occurred as the wild-type residue, it was replaced with glycine (Gly). Confirmed mutants were screened for their drug susceptibility profile by liquid minimum growth inhibitory concentration (MIC80) determinations. Supplemental Excel Sheet III shows the susceptibility patterns of the negative (AD1–8u−) and positive (AD-CDR1) control strains and the mutants, which showed increased susceptibility to at least one or more drugs. The drug susceptibility pattern of 146/252 mutants (58%) was the same as wild-type (AD-CDR1) Cdr1p-overexpressing cells. The remaining 106 mutants could be placed into two groups. The first group of 21 totally susceptible mutants lost resistance to all of the drugs tested (supplemental Excel Sheet III), whereas the second group of selectively susceptible mutants showed susceptibility to one or more drugs. The selective susceptibility of variants in the second group and its subgroup suggests that the mutated amino acid residues may contribute to substrate specificity (supplemental Excel Sheet III).
All Cdr1p Mutants Show Proper Surface Localization and Expression
To test the expression and surface localization of the mutant proteins, wild-type and mutant Cdr1p variants were compared using confocal microscopy and Western blot analysis. The confocal images of GFP-tagged Cdr1p confirmed that wild-type and mutant proteins were localized comparably at the cell surface. Immunoblot analysis of the PM fractions with an anti-GFP monoclonal antibody showed that the wild-type and mutant Cdr1p were expressed at similar levels in the PM (data not shown).
We established that the GFP fluorescence did not represent perinuclear ER or “cell surface-like” staining, which can be caused by the cortical ER underlying the PM. Six mutants from each of the two groups of variants were stained with the ER-specific dye ER Tracker (45). Confocal images of the ER Tracker stained cells showed red fluorescence extending throughout the entire yeast cell to close to the PM. Superimposition of the Cdr1p-GFP and ER Tracker fluorescence images was distinguished clearly between the PM-localized Cdr1p protein and the underlying cortical ER (data not shown). Thus, Cdr1p-GFP and ER Tracker co-staining confirmed that all Cdr1p mutants tested were properly surface localized.
Drug Efflux and ATPase Activity of Drug Susceptible Variants
To test whether mutations in the TMSs that increased drug susceptibility also reduced substrate efflux, drug efflux activity was measured using the Cdr1p substrates R6G and Nile Red (35, 45). Because this phenotype could also result from an altered catalytic cycle, basal Cdr1p ATPase activity in purified PMs was also measured (46). It is important to note that the substrate-stimulated ATPase activity of mammalian ABC transporters has yet to be observed in their yeast homologues (46). Our S. cerevisiae overexpression host AD1–8u− contributes a very low background to the basal ATPase activity because it has seven major ABC transporters deleted (47). In addition, contributions from PM, vacuolar, and mitochondrial ATPases were eliminated by measuring the oligomycin-sensitive ATPase of PM preparations in the presence of KNO3 (50 mm) and NaN3 (10 mm) at pH 7.5 (24). Our functional analysis categorized the drug susceptible mutants into five groups (I-V), which show at least 8-fold increased susceptibility to one or more of the drugs tested. Fig. 1 depicts the data pertaining to representative variants from each of the five groups.
FIGURE 1.
Efflux and ATPase activities of representative drug-susceptible mutants. A, the mutants fell into five groups based on their efflux and ATPase activities. Group I mutants showed limited efflux and ATPase activity. Group II mutants showed uncoupled ATPase activity. Group III mutants displayed selective loss of R6G transport. Group IV mutants displayed selective loss of Nile Red (NR) transport. Group V consisted of mutants with differential susceptibilities to xenobiotics but with wild-type efflux and ATPase activities. AD-CDR1 represents the wild-type Cdr1p. The results are averages of at least 3 independent experiments. Error bars represent S.D. Differences between the mean values were analyzed by Student's t test and significant changes are indicated with an asterisk (*). B, Western analysis (anti-GFP antibodies) and confocal images of representative mutants from each group. Western and confocal images show proper expression and membrane localization. Numbers in parentheses indicate the total number of mutants in each group.
Group I included 5 variants with reduced drug resistance that was associated with a substantial reduction or abolition of both drug efflux and ATPase activity. Cdr1p mutants F559A, E564A, G672A, T677A, and C1336A showed significant (51–66%) decreases in R6G efflux compared with cells expressing wild-type Cdr1p. All these mutants were shown by efflux assays to accumulate (57–76%) higher levels of Nile Red (due to lower rates of efflux) compared with cells expressing wild-type Cdr1p. The ATPase activities of these strains were significantly (52–69%) lower than the wild-type Cdr1p-expressing strain (Fig. 1, supplemental Excel Sheet IV).
The second category of mutants gave total or partial uncoupled ATPase activity. For example, the totally susceptible Cdr1p mutants L529A, V532A, A549G, F552A, A553G, V554A, R644A, C1294A, A1347G, F1360A, and G1362A showed 47–78 and 51–78% reductions in R6G and Nile Red efflux, respectively, but retained near normal ATPase activities. The partially susceptible mutants L563A, L565A, A1346G, and T1351A, whereas showing abrogated efflux of R6G and Nile Red retained resistance to some of the drugs, e.g. ITC and FLC, implying partially coupled ATPase activity (Fig. 1, supplemental Excel Sheets III and IV).
Some mutants had normal basal ATPase activities but effluxed either R6G or Nile Red poorly. The Cdr1p mutants L555A, M604A, T661A, K1200A, F1230A, A1286G, A1330G, and T1331A had normal Nile Red efflux but R6G efflux was reduced by 49–75% (Fig. 1, supplemental Excel Sheet IV). In contrast, mutants F551A, N557A, S561A, N609A, L663A, A666G, I675A, P678A, I780A, N1240A, and Q1245A showed a 50–78% decrease in Nile Red transport but R6G efflux was comparable with the control strain (Fig. 1, supplemental Excel Sheet IV). These variants were placed in the third and fourth categories, respectively. The mutants L555A, M604A, F1230A, A1330G, and T1331A of Group III, originally detected as total susceptible, were subsequently assigned to Group III because they were found to effectively transport Nile Red but not R6G.
The largest set of the mutants were selectively susceptible to one or more but not the full set of drugs tested (azoles/triazoles, anisomycin (ANI) and CHY), and retained efflux of both fluorescent substrates and ATPase activity. These variants were placed in a fifth category (Fig. 1, supplemental Excel Sheet IV).
Two subgroups associated with Groups III and V contain mutants that show a more modest increase in susceptibility of at least 4-fold and are shown as subgroups III and V in supplemental Excel Sheet IV. Apart from 7 mutants (G526A, N617A, C632A, C635A, T768A, I1201A, and N1211A), the 35 mutants in these subgroups show resistance to only one of the compounds tested.
Kinetics of Drug Transport Mediated by Representative TMS Variants
A preliminary understanding of the mechanistic effects of the mutations was obtained from a kinetic study of ATPase activity and drug efflux for a representative variant from each of the five groups (Fig. 1). The results are summarized in Table 1. For example, the severely diminished drug transport found in F559A (group I) can be attributed to a decrease in Vmax and substrate turnover (Kcat) (underlined in Table 1). The representative variants of Groups II, III, and IV retained normal ATPase activity. Although none of these mutants showed significant change in Km and Vmax values measured using PM preparations, the Group III M604A mutant had significantly higher Kcat and Kcat/Km values (underlined in Table 1), which could be attributed to a significantly lower amount of Cdr1p in the membrane. As expected, the observed substrate-specific reduction in efflux of either R6G (Group III) or Nile Red (Group IV) was due to reduced affinity (higher Kd) for the respective substrate (underlined in Table 1). The kinetic properties of the group II variant (L529A) also indicated that the reduced affinity for drug binding was responsible for reduced efflux and R6G susceptibility, whereas Nile Red susceptibility could be correlated with reduced efflux (underlined in Table 1). Surprisingly, the kinetic parameters of drug transport were unaffected in the group V mutant (I1237A). These data do not provide a simple explanation of how ATPase activity is uncoupled from drug export in this representative variant.
TABLE 1.
Kinetics of drug transport mediated by representative variants from each of the five mutant groups (I-V) (Fig. 1)
The (−) values means reduced efflux (R6G and Nile Red (NR)) and ATPase activities of the mutants as compared to Cdr1p, while (+) values indicate no significant change. Fifty % and above inhibition is considered as significant. The underline means that the respective parameter is responsible for the susceptible phenotype of the mutant variants. The values are the average ± S.D. of three independent experiments. Km and Vmax were calculated using Graphpad Prism 5 Software.
| Kinetic arameters | Cdr1p (WT) | F559A (Group I) | L529A (Group II) | M604A (Group III) | N609A (Group IV) | I1237A (Group V) |
|---|---|---|---|---|---|---|
| R6G efflux (%) | 100 | 48 ± 7 (−) | 29 ± 7 (−) | 25 ± 6.5 (−) | 72 ± 12 (+) | 94 ± 4.7 (+) |
| NR efflux (%) | 100 | 31 ± 6 (−) | 40 ± 5 (−) | 78 ± 10.6 (+) | 43 ± 7 (−) | 81 ± 6 (+) |
| ATPase (%) | 100 | 48 ± 5 (−) | 87 ± 8 (+) | 76 ± 9 (+) | 82 ± 8 (+) | 87 ± 4 (+) |
| ATP hydrolysis Km (mm) | 0.61 ± 0.14 | 0.64 ± 0.12 | 0.60 ± 0.13 | 0.68 ± 0.19 | 0.69 ± 0.13 | 0.76 ± 0.12 |
| ATP hydrolysis Vmax (nmole/min) | 116 ± 8 | 49 ± 4 | 131 ± 10 | 96 ± 17 | 101 ± 10 | 109 ± 17 |
| Kcat (s−1) | 33.2 ± 2.3 × 103 | 23.5 ± 1.7 × 103 | 30.3 ± 2.4 × 103 | 59.2 ± 10.7 × 103 | 32.3 ± 3.2 × 103 | 32.4 ± 5.1 × 103 |
| Kcat /Km (mm−1 sec−1) | 54.5 ± 3.8 × 106 | 36.7 ± 2.7 × 106 | 50.5 ± 4.0 × 106 | 87.1 ± 15.8 × 106 | 46.8 ± 4.7 × 106 | 42.7 ± 6.7 × 106 |
| R6G binding Kd,R6G (μm) | 5.53 ± 0.56 | 5.7 ± 1.0 | 12.46 ± 1.32 | 11.3 ± 0.75 | 5.48 ± 0.78 | 6.63 ± 0.80 |
| R6G efflux rate (nmol/ml) | 0.62 ± 0.07 | 0.23 ± 0.01 | 0.31 ± 0.01 | 0.37 ± 0.05 | 0.57 ± 0.03 | 0.62 ± 0.04 |
| NR binding Kd,NR (μm) | 0.4 ± 0.04 | 0.6 ± 0.06 | 0.48 ± 0.08 | 0.42 ± 0.12 | 1.73 ± 0.09 | 0.52 ± 0.08 |
| NR efflux rate (nmol/ml) | 98.9 ± 1.9 | 51 ± 47 | 24.9 ± 5.5 | 98.78 ± 0.88 | 46 ± 35 | 99.2 ± 1.4 |
Residues from Different TMSs Affect Drug Transport
The drug susceptibility patterns of the TMS variants identified residues that may contribute to putative drug recognition sites for the azoles (MCZ and KTC) and triazoles (FLC and ITC). A Venn diagram depicting the azole susceptibilities of the variants revealed a set of 26 mutations that affected the efflux of all four azoles (Fig. 2A). Other groups of variants affected the susceptibility to individual azoles or triazoles or particular combinations of these drugs. For example, KTC-specific susceptibility was caused by 18 mutations, MCZ- or ITC-specific susceptibility by 5 mutations, and FLC-specific resistance by 11 mutations. Where pairs of mutations caused azole susceptibility, all mutations affecting MCZ susceptibility gave either KTC (7 mutants) or FLC (2 mutants) cross-susceptibility but not ITC susceptibility. Only 2 mutants conferred KTC plus ITC cross-susceptibility and only 1 mutant conferred ITC plus FLC susceptibility. Although there is overlap between mutations affecting the azole drugs + FLC and mutations affecting azole drugs + ITC, there is no mutation affecting FLC + ITC, except for T768A. Mutations conferring susceptibility to 2 azoles plus a triazole showed a pattern where MCZ, KTC, and FLC were affected by mutations in TMD1, whereas mutations affecting the two azole drugs and ITC, with the exception of Ser-561, were found only in TMD2.
FIGURE 2.
Venn diagram showing association of mutant phenotypes, which indicate overlapping binding sites for different drugs in a common drug binding pocket. A, mutations that have common effects on the efflux of azoles (MCZ and KTC) and/or triazoles (ITC and FLC) or show selective efflux. B, Venn diagram of phenotypes suggesting that R6G and Nile Red (NR) share binding sites with azoles/triazoles.
The Venn diagram in Fig. 2B shows that many of the mutated residues causing susceptibility to all the azoles/triazoles also affect the transport of R6G and Nile Red. The drug susceptibility profiles of the Cdr1p variants not only indicated that many of the affected amino acid residues within the TMDs are polyspecific, but that there is considerable overlap between sites that affect the transport of the azole drugs and the fluorescent dyes such as R6G and Nile Red. In addition, subsets of mutated residues confer susceptibility to particular drugs.
TRFS Reveals Substrate Binding to Native Cdr1p
A paucity of photoaffinity substrates for Cdr1p made it difficult to assess whether defects in drug binding or release cause defective drug efflux. We had limited success labeling Cdr1p with [125I]IAAP (iodoarylazidoprazosin) and [3H]azidopine, which are photoaffinity analogs of the human P-glycoprotein substrate prazosin and its modulator verapamil, respectively (24). This problem was overcome by using TRFS to monitor interactions between Cdr1p and its fluorescent substrate R6G. The use of R6G is particularly advantageous because its efflux involves amino acid residues in common with several azoles (Fig. 2B, supplemental Excel Sheet III) (23, 29). TRFS detects excited-state processes and the dynamics of a molecule as a function of time after excitation. Because the fluorescence lifetime and anisotropy decay of a molecule are sensitive to interaction with the surrounding environment, these properties inform about the interactions of fluorescent compounds with nearby molecules. Thus, fluorescent lifetime and anisotropy decay measurements were used to monitor and characterize the interaction of R6G with Cdr1p.
The binding of R6G to native Cdr1p was defined by comparing the anisotropy decays of free and bound R6G. Free R6G in solution rotates rapidly, resulting in a fast decay of r(t) (“Experimental Procedures,” see Eq. 1) that can be fitted with a single rotational time constant of 345 ps (Table 2). The complex with R6G bound to native Cdr1p rotates more slowly and the r(t) decay shows two rotational time constants: a fast component that correlates with the time constant of free R6G (τfr = 345 ps) and a slow component due to R6G bound to Cdr1p (τbr = 3.1 ns) (Table 2). We found that the anisotropy decay rate of R6G bound to Cdr1p in the PM was slower (with a higher fraction of R6G bound and higher residual anisotropy) than with the PM from AD1–8u− cells, which lack Cdr1p (Table 2). Samples of R6G incubated with PM containing native Cdr1p had less free R6G (afr = 0.65) than R6G samples incubated with the PM of AD1–8u− cells (afr = 0.80) (Table 2). The raw anisotropy decay data for the Cdr1p-containing PM also showed a slower decay rate. Its anisotropy decay curve runs above those of free R6G and R6G with AD1–8u− PM (Fig. 3A), consistent with a slowly rotating R6G-Cdr1p complex. The total fraction, atr(total), of R6G bound to Cdr1p was significantly higher (0.35) than in the AD1–8u− sample (0.20) (Table 2). The background binding observed in the PM of AD1–8u− cells probably reflected nonspecific binding of R6G to other PM proteins.
TABLE 2.
Contributions of free and bound R6G in the different complexes obtained from anisotropy decays analysis
All decays are fitted with the equation r(t) = r0(afr exp(−t/τfr) + abr exp(−t/τbr)) + b by deconvoluting the instrument response function (IRF).
| Sample | Fraction of free R6G (afr) with time-constant (τfr = 345 ps) | Fraction of bound R6G (abr) with time constant (τbr = 3.1 ns) | Residual anisotropy of bound R6G (b) | Total bound contribution (abr + b) |
|---|---|---|---|---|
| R6G in PBS | 1 | 0 | 0 | 0 |
| AD-CDR1 | 0.65 | 0.27 | 0.08 | 0.35 |
| AD1–8u− | 0.80 | 0.15 | 0.05 | 0.20 |
| AD-CDR1 + farnesol | 0.83 | 0.14 | 0.03 | 0.17 |
| AD-CDR1+ ITC | 0.75 | 0.14 | 0.04 | 0.18 |
| V554A | 0.83 | 0.16 | 0.01 | 0.17 |
| M604A | 0.79 | 0.19 | 0.02 | 0.21 |
| I1237A | 0.59 | 0.23 | 0.18 | 0.41 |
| L1358A | 0.60 | 0.31 | 0.09 | 0.40 |
| L529A | 0.80 | 0.20 | 0.01 | 0.21 |
| V532A | 0.83 | 0.17 | 0.001 | 0.17 |
| C1294A | 0.78 | 0.18 | 0.04 | 0.22 |
FIGURE 3.
TRFS reveals substrate binding to native Cdr1p. A, anisotropy decays (with fits) for native Cdr1p. B, fluorescence lifetime decays (with fits) for native Cdr1p (inset, expanded initial time window in linear scale). C, anisotropy decays (with fits) for native Cdr1p in the presence of farnesol (FAR) or ITC. D, fluorescence lifetime decays (with fits) for native Cdr1p in the presence of farnesol (FAR) or ITC (inset, expanded initial time window in linear scale). R6G was used at a final concentration of 3 μm and its efflux was out-competed with a ×100 higher concentration (300 μm) of ITC or farnesol (FAR). Both ITC and farnesol (FAR) compete for R6G binding in cells overexpressing WT Cdr1p (AD-CDR1). E, farnesol (FAR) and ITC competitively inhibit the efflux of R6G by AD-CDR1. For competition assays R6G was used at a final concentration of 10 μm and its efflux was out-competed with a ×5 higher concentration of ITC (50 μm) and a ×10 higher concentration of farnesol (FAR) (100 μm). ITC and farnesol (FAR) were added to the de-energized cells 5 min before the addition of R6G and allowed to equilibrate. The energy-dependent R6G efflux was initiated by adding glucose (2%, indicated by an arrow) and quantified by measuring the absorbance of the supernatant at 527 nm. AD1–8u− was the negative control. AD-CDR1 was the positive control. The results are averages of at least 3 independent experiments. Error bars represent S.D. Differences between the mean values were analyzed by Student's t test and were statistically significant (p ≤ 0.05).
Although anisotropy decay measures the global rotation of macromolecules in solution, fluorescent lifetime decay measures local interactions with molecules in the immediate vicinity of a (fluorescent) solute, i.e. R6G. Small variations in these local interactions directly affect the fluorescence lifetime of the solute. Comparison of the lifetime decays of R6G enabled measurement of the extent of drug-protein interactions in different complexes. The lifetime decay of free R6G in phosphate-buffered saline showed a single exponential decay with a time constant of 3.9 ns (Table 3). In combination with other proteins, the R6G decays gave shorter, multicomponent, lifetimes. These decreased lifetimes reflected electron transfer from nearby amino acids to R6G inside wild-type Cdr1p. The rate of electron transfer depends on electron-donating efficiency from donor (e.g. Cdr1p) to the accepter (i.e. R6G), which is also affected by their relative distance and orientation. The decay of R6G in Cdr1p showed three time constants: one similar to free R6G (3.9 ns) and two faster time constants (0.3 and 1.8 ns) involving electron transfer to R6G from nearby residues in Cdr1p (Table 3). The PM of cells expressing Cdr1p contributed faster time components (greater electron transfer efficiency) than the PM of AD1–8u− cells. This confirmed that R6G interacts strongly with overexpressed native Cdr1p (Fig. 3B) and corroborated the anisotropy decay results.
TABLE 3.
Fluorescence lifetime decay parameters of free and bound R6G in different complexes
All decays are fitted with the equation I(t) = af exp(−t/τf) + (ab1 exp(−t/τb1) + ab2 exp(−t/τb2)) by deconvoluting the instrument response function (IRF).
| Sample | Fraction of free R6G (af) with time-constant (τf = 3.9 ns) | Fraction of bound R6G (ab1) with time-constant (τb1=0.3 ns) | Fraction of bound R6G (ab2) with time-constant (τb2=1.8 ns) | Total contribution of bound R6G time-constants (ab1 + ab2) | Mean lifetime |
|---|---|---|---|---|---|
| R6G in PBS | 1 | 0 | 0 | 0 | 3.91 |
| AD-CDR1 | 0.15 | 0.54 | 0.31 | 0.85 | 1.31 |
| AD1–8u− | 0.36 | 0.35 | 0.29 | 0.64 | 2.03 |
| AD-CDR1 + farnesol | 0.38 | 0.33 | 0.29 | 0.62 | 2.10 |
| AD-CDR1 + ITC | 0.25 | 0.44 | 0.31 | 0.75 | 1.67 |
| V554A | 0.46 | 0.26 | 0.28 | 0.54 | 1.19 |
| M604A | 0.44 | 0.21 | 0.35 | 0.56 | 1.20 |
| I1237A | 0.11 | 0.53 | 0.35 | 0.88 | 1.26 |
| L1358A | 0.17 | 0.50 | 0.33 | 0.83 | 1.40 |
| L529A | 0.28 | 0.43 | 0.29 | 0.72 | 1.74 |
| V532A | 0.40 | 0.28 | 0.32 | 0.60 | 2.20 |
| C1294A | 0.34 | 0.40 | 0.26 | 0.66 | 1.91 |
The specificity of R6G binding to Cdr1p was investigated by measuring the anisotropy and fluorescence lifetime decay of R6G binding in the presence of its quorum-sensing competitor farnesol and of ITC, which share a common binding site(s) with R6G (22, 33). As expected, the fluorescence lifetime and anisotropy measurements confirmed that both farnesol and ITC prevented R6G binding to Cdr1p (Fig. 3, C and D, Tables 2 and 3). This inhibition of R6G binding was supported by efflux assays (Fig. 3E). The efflux of R6G by cells overexpressing Cdr1p is competitively inhibited by both farnesol and ITC (23, 34).
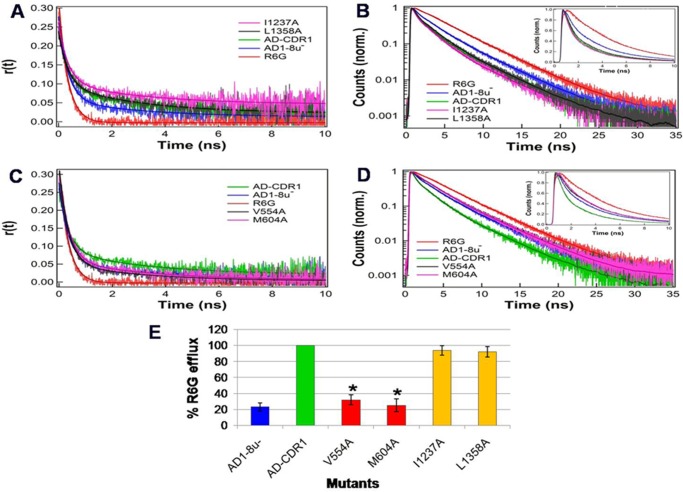
The complete abolition of resistance to R6G in some variants correlated with its reduced efflux (supplemental Excel Sheet IV, Groups I–III). We therefore assessed whether the loss of R6G efflux in these Cdr1p variants was related to poor interaction with the substrate. Two representative pairs of mutants were selected for study. The first pair (V554A and M604A) were sensitive to azole drugs (supplemental Excel Sheet III) and showed reduced R6G efflux (supplemental Excel Sheet IV and Table S1 for M604A). The second pair (I1237A and L1358A) had near normal R6G efflux and was resistant to R6G (supplemental Excel Sheets IV and III, respectively). We hypothesized that binding defects in susceptible mutants may be reflected in their fluorescence lifetime and anisotropy decay measurements. The R6G anisotropy decay rates in both susceptible mutants (V554A and M604A) were faster and the lifetime decays were slower. Because free R6G has a longer fluorescence lifetime, both mutants showed a smaller fraction of R6G bound and a larger fraction of free R6G compared with the native Cdr1p (Tables 2 and 3). This result was also reflected in the raw anisotropy and fluorescence lifetime decay curves of both mutants. Their close correspondence with the results from the PM of AD1–8u− cells implies a defect in R6G binding in these variants (Fig. 4, A and B). In contrast, the anisotropy and fluorescence lifetime decay rates of R6G for the I1237A and L1358A pair were similar to that of native Cdr1p. This suggests that the interactions of R6G with these mutant proteins and the native protein were comparable (Fig. 4, C and D). Both variants also showed a comparable fraction of free R6G as with native Cdr1p (Tables 2 and 3). The R6G binding properties of the two representative pairs of mutants was also supported by R6G efflux data (Fig. 4E).
FIGURE 4.
TRFS reveals reduced substrate binding to drug-susceptible Cdr1p mutants. A, anisotropy decays (with fits) for R6G-susceptible mutants. B, fluorescence lifetime decays (with fits) for susceptible mutants (inset, expanded initial time window in linear scale). C, anisotropy decays (with fits) for R6G-resistant mutants. D, fluorescence lifetime decays (with fits) for R6G resistant-mutants (inset, expanded initial time window in linear scale). E, R6G efflux of susceptible and resistant mutants. The results are averages of at least 3 independent experiments. Error bars represent S.D. Differences between the mean values were analyzed by Student's t test and significant changes are indicated with an asterisk (*).
Structural Modeling of Cdr1p Reveals Features of the Drug-binding Pocket
To aid understanding of the nature and specificity of the drug binding pocket of Cdr1p we obtained structural models of Cdr1p in the ATP-bound (close) and ATP-unbound (open) states using S. cerevisae Pdr5p as input model (49, 50). The 3V program (51) identified a large drug-binding cavity (volume = 13241 Å3, surface area = 4216 Å2) in the open Cdr1p conformation, but a relatively smaller drug-binding cavity (volume = 4891 Å3, surface area = 2234 Å2) in the close Cdr1p conformation at the interface of its two TMDs (Fig. 5, A and B, respectively). In the open conformation, the cavity is exposed to the cytoplasm and shielded from the lipid bilayer and extracellular space by TMSs and the ectodomain. In the close conformation, the cavity is exposed to the extracellular space and shielded from the lipid bilayer and cytoplasm. Evaluation of the surface electrostatic potential of the binding cavity of Cdr1p (surface potential from −10 kT to +10 kT) revealed that the drug-binding cavity contains mainly hydrophobic amino acids and a much smaller number of charged and polar residues (Fig. 5, A and B). In the open conformation, the cytoplasmic ends of the Cdr1p TMSs presents a necklace of acidic and basic and hydrophilic residues around the drug-binding cavity. The walls of the binding cavity surface contributed by the TMSs involves predominantly hydrophobic residues interspersed with patches of hydrophilic residues. The base of the binding cavity contains a mixture of acidic, basic, and hydrophilic residues.
FIGURE 5.
View from the cytoplasm of the open and closed conformations of the TMDs and ectodomain of Cdr1p. A, view from the cytoplasm of the open conformation. Charged/polar and hydrophobic Group I residues are shown in red and orange, respectively, whereas charged/polar and hydrophobic Group II residues are shown in white and gray, respectively. B shows the same view for the closed conformation of the transporter. In both figures, residues that appear to contribute to the drug binding pocket are shown as spheres, whereas residues outside the pocket are shown as sticks. The figures highlight the clustering of residues that most dramatically affect function and their relationship with conformation-dependent structural features suggested by the Cdr1p model. Blue and light blue surfaces are expected to face a lipid environment.
The Cdr1p drug-binding cavity is surrounded by residues from TMS1, TMS3, and TMS6 of TMD1 helices and TMS7, TMS9, TMS11, and TMS12 of TMD2 helices of Cdr1p in both the open and closed conformations. A few residues from TMS2, TMS5, and TMS8 but none from TMS4 (apart from the guanidino group of Arg-644) appear directly involved in the drug-binding cavity of Cdr1p. One face of TMS10 is directly exposed to the drug-binding cavity in the open conformation and is completely excluded in the closed conformation. The model suggest residues Cys-632, Cys-635, Thr-636, and Phe-643 in TMS4 and Tyr-1328, Thr-1329, Ala-1330, and Cys-1336 in TM10 induce indirectly total or selective drug susceptibility.
Fig. 5A shows a view from the cytoplasm of the open conformation of the TMDs and ectodomain of Cdr1p. Fig. 5B shows the same view for the closed conformation of the transporter. The figures highlight the clustering of residues that most dramatically affect function and provide indications of how this might occur (see “Discussion”). The residues critical for drug susceptibility are predominantly clustered on TMS1, TMS2, and TMS11 on one side of the Cdr1p structure and TMS4, TMS5, and TMS8 on other side. TMS3, TMS6, TMS7, TMS9, TMS10, and TMS12 of Cdr1p therefore appear to contribute relatively few residues critical to efflux function. Although some patches of hydrophilic residues are visible, the majority of the residues lining the walls of the proposed drug binding pocket of Cdr1p are hydrophobic and thus are more likely to interact with hydrophobic drugs entering from the lipid bilayer. The residues conferring total sensitivity to the drugs tested are the residues with charged side chains such as Glu-564, Arg-644, and the residues with polar side chains such as Cys-1294 and Thr-1331 (supplemental Excel Sheet IV). The charged residues occur near the cytoplasmic end of the pocket together with Thr-1331, whereas Cys-1294 is deep in the pocket. Glu-564, Arg-644, Cys-1294, and Thr-1331 appear to contribute to the open drug binding pocket, whereas only Arg-644 and Cys-1294 contribute to the closed drug-binding site. Of the 11 hydrophobic residues in the group conferring total sensitivity, 5 residues (Leu-529, Val-532, Val-554, Ala-1347, and Gly-1362) contribute to the open drug binding pocket, whereas the closed binding site loses the contributions from Val-554 and Gly-1362 (supplemental Excel Sheet IV).
Analysis of mutations conferring susceptibilities to structurally related drugs using the model provide insight into how particular structural elements of these drugs may bind. Fig. 6A shows five amino acid mutations in the open conformation of the Cdr1p drug binding pocket that affect the binding of the azole drugs MCZ and KTC (shown as green and yellow, respectively) and one mutation (the S531A) that affects the binding of both drugs (shown as red). The S531A mutation suggests that the azole and dichlorophenyl groups lie deep in the drug binding pocket, whereas the Q1244A mutation (shown as green), which confers susceptibility to MCZ only, suggests the O-linked dichlorophenyl group may project across the drug-binding site toward the center of TMS9. The 4 mutations conferring susceptibility to KTC (shown as yellow) within the binding site suggest that the longer tail of this drug may interact with residues in the middle of TMS3, -6, and -7 and with a residue at the cytoplasmic end of TMS11. In the case of the triazole drugs FLC and ITC (Fig. 6B), only the T768A mutation (shown in red) deep in the binding site on TMS6 confers susceptibility to both FLC and ITC, suggesting that this residue may interact with the triazole group in common between FLC and ITC. The 6 residues in the pocket that confer significant susceptibility to FLC alone (shown as yellow) occur adjacent to Thr-768 (Ala-772 on TMS6) and cluster on TMS2, TMS3, and TMS12 at and below the middle of the binding cavity. These residues may interact with the difluorophenyl group (possibly together with one of the triazole groups) of FLC. Mutations in two residues located deep in the other half of the pocket on TMS5 (Ile-675) and TMS9 (Phe-1233) confer increased susceptibility to ITC alone (shown in green). These results suggest that the long and relatively hydrophobic tail of ITC extends in this direction.
FIGURE 6.

Mutations in the open conformation of the Cdr1p drug binding pocket that affect the binding of the azole and triazole drugs. A, five amino acid mutations in the open conformation of the drug binding pocket that affect the binding of the azole, i.e. MCZ and KTC (shown as green and yellow, respectively), and the single mutation (S531A, shown as red) in the pocket that affects the binding of both drugs. B, mutations in the open conformation of the Cdr1p drug binding pocket that affect the binding of the triazole drugs. Only one mutation (T768A, shown in red) deep in the binding site on TMS6 confers susceptibility to both FLC and ITC. The 6 residues in the pocket that confer significant susceptibility to FLC alone (shown as yellow) occur adjacent to Thr-768 (A772 on TMS6) and cluster on TMS2, TMS3, and TMS12 at and below the middle of the binding cavity. Mutations in two residues located deep in the other half of the pocket on TMS5 (Ile-675) and TMS9 (Phe-1233) confer increased susceptibility to ITC alone (shown in green). Blue and light blue surfaces are expected to face a lipid environment.
DISCUSSION
Despite intensive study of the structure and function of the ABC multidrug transporter Cdr1p, a detailed molecular understanding of its function is lacking. How drugs are sequestered within the TMDs in the open conformation and released from the closed conformation, and how this process is powered by NBD dimerization and ATP hydrolysis is poorly understood. The present study provides insight into these aspects by identifying the 12 TMSs through bioinformatic analysis and subjecting the entire putative primary sequence the TMSs of both TMDs to alanine scanning mutagenesis. The library of mutants was then phenotypically, biochemically, and biophysically mapped. Individual replacements of 58% of the native residues with alanine (or glycine as required) gave phenotypes consistent with the overexpression and correct localization of fully functional wild-type Cdr1p. In contrast, the substitution of 42% of the residues yielded overexpressed and correctly localized Cdr1p variants that were completely, or selectively, drug sensitive. The properties of these mutants provide insight into Cdr1p function.
Enhanced Drug Susceptibility Was Independent of Conservation Status within TMSs
The conservation status of each residue within the 12 TMSs was assessed in relationship to the mutated residues conferring total or selective susceptibility. Residue conservation scores and transmembrane segment conservation scores obtained from multiple sequence alignment (see “Experimental Procedures”) of PDR transporters having domain organization similar to Cdr1p, i.e. (NBD-TMS6)2 (supplemental Fig. S1A), show no direct correlation between the number of sensitivity conferring mutations and the TMS conservation (supplemental Fig. S1B) or conservation of the residues per se (supplemental Fig. 1C). For example, TMS2 is the most conserved TMS and it has the largest number of critical residues whose replacement leads to drug susceptibility. However, TMS1, TMS5, TMS8, and TMS11 also contribute multiple essential residues even though these TMSs are relatively less conserved (supplemental Figs. 1, B and C, and 2). We also observe that physicochemical conservation alone is not necessarily associated with a specific function of substrate binding and efflux. For example, out of the 37 residues in Cdr1p that have the highest conservation scores (from 9 to 11) (supplemental Fig. S3), only 14 give susceptibility phenotypes that affect drug transport (supplemental Fig. S2). Although such conservation could contribute to broad functions such as the hydrophobic residues required for membrane localization (52), the present study suggests neither variation nor conservation within TMSs dominates the substrate promiscuity of multidrug transporters.
Most TMSs Contribute to Drug Transport
Functional data mapped on our Cdr1p model have indicated that, similarly to mammalian ABC transporters, most TMSs of Cdr1p contribute to and affect, multiple, probably overlapping, substrate- and inhibitor-binding sites. Alanine scanning mutagenesis of each residue in the TMDs showed only TMS12 with no residues critical for drug transport (supplemental Fig. S2), even though TMS12 delimits the Cdr1p substrate binding cavity in both the open and closed conformations (Figs. 5 and 6). However, four variants in TMS12 gave modest (4-fold) increases in susceptibility to R6G (F1473A), ANI (I1479A), and FLC (V1482A and A1488G) (supplemental Excel Sheet III). The minor impact of TMS12 on drug specificity and transport is consistent with several earlier findings. First, TMS12 of Cdr1p is replaceable with the TMS12 of its homologue Cdr3p without functional consequences (53). Second, chimeras between Cdr1p and its close homologue Cdr2p indicate that TMS12 does not participate in Cdr1p-specific aspects of substrate recognition (54). Finally, deletion of 79 C-terminal amino acids from Cdr1p, including TMS12, did not affect susceptibility to azole and triazole drugs in crude agar diffusion assays, but did affect susceptibility to anisomycin (55). Although deletion of 23 C-terminal amino acid residues of P-glycoprotein did not affect protein function (56), the TMS12 of P-glycoprotein responds to nucleotide binding and hydrolysis at the NBDs and is directly involved in interdomain communication (57). These properties associated with P-glycoprotein may be a consequence of the different domain topologies of P-glycoprotein and Cdr1p. In P-glycoprotein TMS6, TMS7, and TMS12 are directly involved in interactions with the NBDs, whereas in Cdr1p, TMS1, TMS6, and TMS7 are involved.
Cdr1p Residues Conferring Drug Susceptibility Are Less Likely to be Associated with the Lipid Bilayer
Lipid bilayer-facing surfaces on each TMS of Cdr1p were identified using the empirical scoring function LIPS (LIPid-facing surface), which is based on the lipophilicity and conservation of residues in the helix (http://gila.bioengr.uic.edu//lab/larisa/lips.html) (48). Residues with high LIPS score (red curves on supplemental Fig. 4, A and B, blue and light blue surfaces in Figs. 5 and 6) are expected to face a lipid environment unlike the low scoring non-LIPS residues. Because amino acids facing the substrate-binding site should be non-LIPS residues, their substitution should be more likely to affect the drug sensitivity of cells overexpressing Cdr1p. Although the replacement of some high LIPS scoring residues reduced drug resistance, deleterious mutations were mainly restricted to the non-LIPS residues, consistent with close proximity to the substrate-binding pocket (supplemental Fig. S4, A and B). We note that the orientation of the TMSs of the Cdr1p model is consistent with the LIPS analysis with two possible exceptions (Fig. 5). The LIPS face of TMS1 is rotated 90° from the direction of the lipid bilayer in both open and closed structures, but this may be an artifact of this helix running inside the structure between TMS2 and TMS3. In the open structure TMS4 appears to project the high LIPS face toward the lipid bilayer, but this turns about 45° away in the closed structure. This helix rotation does not occur in TMS10, the analogous helix in TMD2. We also note that TMS6 and TMS12 are substantially protected from the lipid bilayer by other helices. Despite this, the LIPS faces of TMS6 and TMS12 project toward the lipid bilayer between TMS3–4 and TMS9–10. Although imperfectly segregated, the clustering of deleterious and neutral mutations in the TMSs suggests that many non-LIPS residues play significant structural or functional roles in drug recognition and transport, even if not directly contributing to the proposed drug-binding site between the two TMDs.
Several Residues Are Involved in Coupling ATPase Activity to Drug Transport
Biochemical analysis of the TMSs mutants identified 37 variants with reduced drug efflux but near normal ATPase activity (Fig. 1, groups II–IV; supplemental Excel Sheet IV), implying uncoupling of these two processes. This was also supported by the kinetic analysis of representative mutants from each group as none of the mutants showed any significant change in Km and Vmax values, although the M604A mutant may have a significantly higher Kcat. As expected, the observed reduction in efflux was due to either reduced affinity (higher Kd) or the reduced efflux rate for the respective substrate (Table 1). The simplest explanation of these data is that mutated residues relax the stoichiometric cross-talk between the substrate-binding site and the NBD(s) carrying out the ATP hydrolysis required for transport and conformational resetting. For the purposes of this discussion we will consider uncoupled mutants to be those in which both R6G and Nile Red transport are severely affected but ATPase activity is essentially normal. These mutations occur in TMS1 (2 mutations), TMS2 (6 mutations), TMS4 (1 mutation), TMS9 (1 mutation), and TMS11 (5 mutations). The Cdr1p models show that 13 of the 15 uncoupling mutations co-locate in TMS1, TMS2, and TMS11, which together link to, and underlie, NBD1 in the closed conformation of the transporter.
Replacement of Certain Residues Gives Poor Substrate Recognition
TRFS monitoring of fluorescence lifetimes and anisotropy decays enabled characterization of the interaction between R6G and Cdr1p (Fig. 3, A and B). Substrates and inhibitors were found to compete with R6G binding to native Cdr1ps (Fig. 3, C–E). The anisotropy and fluorescence lifetime decays of R6G were unaffected in a representative pair of variants (I1237A and L1358A) that were resistant to R6G (supplemental Excel Sheets III and IV), confirming that these variants have proper substrate-protein interactions (Fig. 4, A and B). In contrast, fluorescence anisotropy and lifetime decays were severely affected in a pair of variants (V554A and M604A; Fig. 4, C and D; Tables 2 and 3) that were susceptible to R6G (supplemental Excel Sheets III and IV), indicating poor substrate binding. The other R6G-sensitive variants R644A and F1360A (supplemental Excel Sheets III and IV) show similar anisotropy decay as in wild-type (AD-CDR1), whereas fluorescence lifetime decays are closer to AD1–8u− (supplemental Fig. S5, A and B, and Tables S2 and S3). Defective drug release or defective coupling of drug efflux with ATPase activity may be the reason for the R6G susceptibility of these mutants. Another mutant E564A conferred drug susceptibility and had reduced R6G efflux and low ATPase activity (supplemental Excel Sheet IV). Because TRFS indicated that R6G still interacts with the mutant protein E564A (supplemental Fig. 5, A and B, and Tables S2 and S3), the poor drug efflux in this case may be due to the low ATPase activity of the mutant. The R6G-related phenotypic defect in these mutants was confirmed by measurements showing reduced R6G efflux (supplemental Fig. 5C).
Our Cdr1p Structural Model Predicts the Critical Residues Involved in the Drug Binding Pocket of Cdr1p
The three-dimensional models of Cdr1p provide a preliminary map of the Cdr1p variants that show broad-spectrum or drug-specific susceptibility profiles for various drugs. The open and closed Cdr1p models enabled the identification of amino acid residues that appear to border the drug-binding cavity in both structures (Figs. 5–7). For example, Leu-529 and Val-532 of TMS1 and Cys1294 of TMS9, which border the depths of the drug-binding cavity (Fig. 7, A and B), shared common drug efflux phenotypes. The mutant cells were susceptible to all the azole and triazole drugs tested (supplemental Excel Sheet III) and were unable to efflux R6G, but had a normal ATPase activity (Fig. 7C, supplemental Excel Sheets III and IV). TRFS revealed that the fluorescence lifetime and anisotropy decays of R6G were severely affected in these variants (Fig. 7, D and E), with the mutated amino acid residues affected drug binding and thus causing drug susceptibility.
FIGURE 7.
Effects of mutations bordering the putative drug binding pocket of Cdr1p. Close up view of open (A) and closed (B) conformation of Cdr1p showing specific residues affecting resistance to drugs that border the drug-binding cavity. The Leu-529 and Val-532 residues of TMS1 and Cys-1294 of TMS9 interact directly with the drug binding pocket of Cdr1p. Cdr1p mutations conferring total and selectively susceptible phenotypes are shown in red and orange colors, respectively. The cavity is shown in gray. C, R6G transport and ATPase activity of the mutant variants. The efflux results are means of at least 3 independent experiments. Error bars represent S.D. Differences between the mean values were analyzed by Student's t test and significant changes are indicated with an asterisk (*). Anisotropy (D) and fluorescence lifetime (E) decays (with fits). Inset, expanded initial time window in linear scale.
Other residues that bordered the putative drug-binding site in both the open and closed conformations and gave modified phenotypes were Ser-531 in TMS1; Ile-565 in TMS2; Asn-609 in TMS3; Val-1205 in TMS7; Gln-1245 in TMS8 and Ala-1346, Ala-1347, Thr-1351, Thr-1355, and Leu-1358 in TMS11. Of even greater interest were the phenotype-affecting residues that bordered the larger putative drug-binding site in the open conformation only. Of these residues, Val-554 and Glu-564 in TMS2, Arg-644 in TMS4, Phe-1230 in TMS8, Ala-1330 and Thr-1331 in TMS10, and Gly-1362 in TMS11 most strongly affected transport function. The remaining residues were Tyr-534 in TMS1, Gly-547 in TMS2, Leu-663 in TMS5, Lys-1200 in TMS7, Asn-1227, Met-1229, Phe-1233, and Ile-1237 in TMS8. These residues gave a range of phenotypes consistent with the modification of different drug binding pockets and will provide the opportunity for further site-directed mutagenesis studies of substrate binding.
Mutations that confer susceptibility to individual azole or triazole drugs together with mutations that confer susceptibility to pairs of triazole drugs indicate that both azole and triazole head groups bind deep in the open drug binding pocket (Fig. 6). Consistent with this observation, the remaining parts of MCZ, KTC, and FLC appear to be directed across the drug-binding site toward residues located at the center of the TMSs. Of particular interest are residues that affect the binding of structurally differing parts of FLC and ITC. These not only bind in different halves of the drug-binding site but the hydrophobic tail of ITC may project toward deep segments of TMS5 and TMS9. These results are consistent with previous experiments that showed that FLC and ITC bind differently to Cdr1p and have different effects on its ATPase and transport activities (17).
It is apparent from the models that, apart from TMS4 and TMS10, all the other TMSs border the drug-binding cavity of both the open and closed Cdr1p structures. The face of TMS10 that includes Thr-1331 is an exception as it borders the drug-binding cavity in the open conformation only. Several residues in TMS4 and TMS10 do not directly interact with the drug-binding cavity, but indirectly induce total (Ala-1330 and Cys-1336 of TMS10) or selective (Phe-625, Tyr-628, and Trp-629 of TMS4) drug susceptibility. Several additional phenotype-modifying residues in the other TMSs are not predicted to be in the drug-binding pocket and may also affect drug transport indirectly. Finally, only five mutations in the TMSs affected ATPase activity. These were F559A and E564A in TMS2, G672A and T677A in TMS5, and C1336A in TMS10 (Fig. 5). Of these only one (E564A) contributed to the putative drug-binding site but appeared more accessible in the open conformation (Fig. 5A, supplemental Excel Sheet III). In addition, the TMS2 helix appears slightly curved in the open conformation and has a double kink bordered by Phe-559 and Glu-564 in the closed conformation (Fig. 5B). Similarly, Gly-672 and Thr-677 border a structural discontinuity in TMS5 of the closed conformation that becomes a continuous helix in the open conformation of Cdr1p. Reciprocal structural transitions in TMS2 and TMS5 may be required for the conformational couplings mediated by ICL1 and ICL2 that enable the full catalytic cycle involving ATP hydrolysis by the NBDs. The phenotypes and biochemical properties of the variants with these residues modified is consistent with this mechanism. A simple explanation for the impact of the Cys-1336 mutation has yet to be found. One possibility is a contribution to dimerization of Cdr1p. It is also noted that several variants that confer total susceptibility to the complete array of drugs tested but have normal ATPase activity are clustered near the structural discontinuity in TM2, e.g. the grouping of Phe-552, Ala-553, and Val-554 is beside Phe-559, whereas Gly-1362 in TM11 is also in close spatial proximity this cluster.
In conclusion, extensive mutagenesis of both TMDs of Cdr1p, combined with phenotypic, biochemical, and biophysical mapping, has identified amino acid residues that contribute structural or functional determinants critical for drug transport. We show for the first time that the majority of residues imparting susceptibility to different drugs of Cdr1p are hydrophobic in nature. The predominance of hydrophobic amino acids in the walls of the drug-binding cavity is consistent with Cdr1p having greater affinity toward hydrophobic rather than hydrophilic drugs. Based on phenotypic and biochemical mapping of the residues of TMSs, we show that Cdr1p harbors overlapping binding sites within a large drug binding pocket. The distribution of essential residues clearly show two distinct regions comprised of TMSs 1/2/11 and 4/5/8 that play important roles in substrate recognition. Despite the promiscuous nature of the transporter, the residues required for the transport of most drugs tested are associated predominantly with TMS2 and TMS11, which border the likely site between the TMDs for entry from the inner leaflet of the lipid bilayer into the drug binding pocket. Elucidation of such contributions to substrate binding and transport is expected to give a platform that will provide a better understanding of the substrate binding pocket of Cdr1p, and enable rational design of its modulators/inhibitors or xenobiotics that are not efflux pump substrates.
Acknowledgments
We thank Suresh Ambudkar, Di Xia, and Robert Rutledge for help during the course of this work. We acknowledge the Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University, India, for the confocal microscopy and TRFS experiments. We thank Ranbaxy Laboratories, Ltd., India, for providing FLC.
This work was supported in part by Department of Biotechnology Grants DBT BT/01/CEIB/10/III/02, DBT BT/PR13641/MED/29/175/2010, and DBT BT/PR14879/BRB10/885/2010 (to R. P.) and Marsden Grant UOO1004 (to B. C. M.) from the Royal Society of New Zealand.

This article contains supplemental Figs. S1–S5, Excel Sheets I–IV, and Tables S1–S3.
- MDR
- multidrug resistance
- TMS
- transmembrane segment
- TMD
- transmembrane domain
- NBD
- nucleotide binding domain
- PDR
- pleiotropic drug resistance
- ABC
- ATP-binding cassette
- MIC
- minimum inhibitory concentration
- LIPS
- lipid-facing surface
- TRFS
- time resolve fluorescence spectroscopy
- ER
- endoplasmic reticulum
- PM
- plasma membrane
- KTC
- ketoconazole
- MCZ
- miconazole
- ITC
- itraconazole
- FLC
- fluconazole.
REFERENCES
- 1. Richardson D. M. (2005) Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56, i5–i11 [DOI] [PubMed] [Google Scholar]
- 2. Perlin D. S. (2011) Current perspectives on echinocandin class drugs. Future Microbiol. 6, 441–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White T. C., Marr K. A., Bowden R. A. (1998) Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11, 382–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad R., De Wergifosse P., Goffeau A., Balzi E. (1995) Molecular cloning and characterisation of a novel gene of C. albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27, 320–329 [DOI] [PubMed] [Google Scholar]
- 5. Sanglard D., Ischer F., Monod M., Bille J. (1997) Cloning of Candida albicans genes conferring resistance to azole antifungal agents. Characterisation of CDR2, a new multidrug ABC transporter gene. Microbiology 143, 405–416 [DOI] [PubMed] [Google Scholar]
- 6. Goldway M., Teff D., Schmidt R., Oppenheim A. B., Koltin Y. (1995) Multidrug resistance in Candida albicans. Disruption of the Benr gene. Antimicrob. Agents Chemother. 39, 422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasrija R., Banerjee D., Prasad R. (2007) Structure and function analysis of CaMdr1p, a MFS antifungal efflux transporter protein of Candida albicans. Identification of amino acid residues critical for drug/H+ transport. Eukaryot. Cell 6, 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins C. F., Linton K. J. (2004) The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 11, 918–926 [DOI] [PubMed] [Google Scholar]
- 9. Holmes A. R., Lin Y. H., Niimi K., Lamping E., Keniya M., Niimi M., Tanabe K., Monk B. C., Cannon R. D. (2008) ABC transporter Cdr1p contributes more than Cdr2p to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 52, 3851–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shukla S., Sauna Z. E., Prasad R., Ambudkar S. V. (2004) Disulfiram is a potent modulator of multidrug transporter Cdr1p of Candida albicans. Biochem. Biophys. Res. Commun. 322, 520–525 [DOI] [PubMed] [Google Scholar]
- 11. Sharma M., Manoharlal R., Shukla S., Puri N., Prasad T., Ambudkar S. V., Prasad R. (2009) Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with antifungals. Antimicrob. Agents Chemother. 53, 3256–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad R., Goffeau A. (2012) Yeast ATP-Binding cassette transporters conferring multidrug resistance. Annu. Rev. Microbiol. 66, 39–63 [DOI] [PubMed] [Google Scholar]
- 13. Cannon R. D., Lamping E., Holmes A. R., Niimi K., Baret P. V., Keniya M. V., Tanabe K., Niimi M., Goffeau A., Monk B. C. (2009) Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22, 291–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ernst R., Kueppers P., Stindt J., Kuchler K., Schmitt L. (2010) Multidrug efflux pumps. Substrate selection in ATP binding cassette multidrug efflux pumps. First come, first served? FEBS J. 277, 540–549 [DOI] [PubMed] [Google Scholar]
- 15. Prasad R., Sharma M., Rawal M. K. (2011) Functionally relevant residues of Cdr1p: a multidrug ABC transporter of human pathogenic Candida albicans. J. Amino Acids 2011, 10.4061/2011/531412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta R. P., Kueppers P., Schmitt L., Ernst R. (2011) The multidrug transporter Pdr5. A molecular diode? Biol. Chem. 392, 53–60 [DOI] [PubMed] [Google Scholar]
- 17. Loo T. W., Clarke D. M. (2005) Do drug substrates enter the common drug-binding pocket of P-glycoprotein through “gates”? Biochem. Biophys. Res. Commun. 329, 419–422 [DOI] [PubMed] [Google Scholar]
- 18. Loo T. W., Bartlett M. C., Clarke D. M. (2007) Suppressor mutations in the transmembrane segments of P-glycoprotein promote maturation of processing mutants disrupt a subset of drug-binding sites. J. Biol. Chem. 282, 32043–32052 [DOI] [PubMed] [Google Scholar]
- 19. Shapiro A. B., Ling V. (1997) Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 250, 130–137 [DOI] [PubMed] [Google Scholar]
- 20. Safa A. R. (2004) Identification and characterization of the binding sites of P-glycoprotein for multidrug resistance-related drugs and modulators. Curr. Med. Chem. Anticancer Agents 4, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aller S. G., Yu J., Ward A., Weng Y., Chittaboina S., Zhuo R., Harrell P. M., Trinh Y. T., Zhang Q., Urbatsch I. L., Chang G. (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin M. S., Oldham M. L., Zhang Q., Chen J. (2012) Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 490, 566–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puri N., Gaur M., Sharma M., Shukla S., Ambudkar S. V., Prasad R. (2009) The amino acid residues of transmembrane helix 5 of multidrug resistance protein CaCdr1p of Candida albicans are involved in substrate specificity and drug transport. Biochim. Biophys. Acta 1788, 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shukla S., Saini P., Smriti, Jha S., Ambudkar S. V., Prasad R. (2003) Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryot. Cell 4, 1361–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tutulan-Cunita A. C., Mikoshi M., Mizunuma M., Hirata D., Miyakawa T. (2005) Mutational analysis of the yeast multidrug resistance ABC transporter Pdr5p with altered drug specificity. Genes to Cells 10, 409–420 [DOI] [PubMed] [Google Scholar]
- 26. Ernst R., Kueppers P., Klein C. M., Schwarzmueller T., Kuchler K., Schmitt L. A. (2008) mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter. Proc. Natl. Acad. Sci. U.S.A. 105, 5069–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golin J., Ambudkar S. V., Gottesman M. M., Habib A. D., Sczepanski J., Ziccardi W., May L. (2003) Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J. Biol. Chem. 278, 5963–5969 [DOI] [PubMed] [Google Scholar]
- 28. Golin J., Ambudkar S. V., May L. (2007) The yeast Pdr5p multidrug transporter. How does it recognize so many substrates? Biochem. Biophys. Res. Commun. 356, 1–5 [DOI] [PubMed] [Google Scholar]
- 29. Saini P., Prasad T., Gaur N. A., Shukla S., Jha S., Komath S. S., Khan L. A., Haq Q. M., Prasad R. (2005) Alanine scanning of transmembrane helix 11 of Cdr1p ABC antifungal efflux pump of Candida albicans. Identification of amino acid residues critical for drug efflux. J. Antimicrob. Chemother. 56, 77–86 [DOI] [PubMed] [Google Scholar]
- 30. Gietz R. D., Woods R. A. (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96 [DOI] [PubMed] [Google Scholar]
- 31. Monk B. C., Kurtz M. B., Marrinan J. A., Perlin D. S. (1991) Cloning and characterization of the plasma membrane H+-ATPase from Candida albicans. J. Bacteriol. 173, 6826–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukhopadhyay K., Kohli A., Prasad R. (2002) Drug susceptibilities of yeast cells are affected by membrane lipid composition. Antimicrob. Agents Chemother. 46, 3695–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maesaki S., Marichal P., Vanden Bossche H., Sanglard D., Kohno S. (1999) Rhodamine 6G efflux for the detection of CDR1 overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 44, 27–31 [DOI] [PubMed] [Google Scholar]
- 34. Sharma M., Prasad R. (2011) The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob. Agents Chemother. 55, 4834–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ivnitski-Steele I., Holmes A. R., Lamping E., Monk B. C., Cannon R. D., Sklar L. A. (2009) Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal. Biochem. 394, 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A.(1992) Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 267, 4854–4858 [PubMed] [Google Scholar]
- 37. Lamping E., Baret P. V., Holmes A. R., Monk B. C., Goffeau A., Cannon R. D. (2010) Fungal PDR transporters. Phylogeny, topology, motifs and function. Fungal Genet. Biol. 47, 127–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pirovano W., Feenstra K. A., Heringa J. (2008) Sequence analysis PRALINETM. A strategy for improved multiple alignment of transmembrane proteins. Bioinformatics 24, 492–497 [DOI] [PubMed] [Google Scholar]
- 39. Boutet E., Lieberherr D., Tognolli M., Schneider M., Bairoch A. (2007) UniProtKB/Swiss-Prot. Methods Mol. Biol. 406, 89–112 [DOI] [PubMed] [Google Scholar]
- 40. Tusnády G. E., Simon I. (1998) Principles governing amino acid composition of integral membrane proteins. Application to topology prediction. J. Mol. Biol. 283, 489–506 [DOI] [PubMed] [Google Scholar]
- 41. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model. Application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 42. von Heijne G. (1992) Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225, 487–494 [DOI] [PubMed] [Google Scholar]
- 43. Hofmann K., Stoffel W. (1993) TMbase. A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler http://opencitations.net/id/expression/DgiWFFAdWhPAlWYk
- 44. Lamping E., Monk B. C., Niimi K., Holmes A. R., Tsao S., Tanabe K., Niimi M., Uehara Y., Cannon R. D. (2007) Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyper-expression in Saccharomyces cerevisiae. Eukaryot. Cell 6, 1150–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandal A., Kumar A., Singh A., Lynn A. M., Kapoor K., Prasad R. (2012) A key structural domain of the Candida albicans Mdr1 protein. Biochem. J. 445, 313–322 [DOI] [PubMed] [Google Scholar]
- 46. Shukla S., Rai V., Banerjee D., Prasad R. (2006) Characterization of Cdr1p, A major multidrug efflux protein of Candida albicans. Purified protein is amenable to intrinsic fluorescence analysis. Biochemistry 45, 2425–2435 [DOI] [PubMed] [Google Scholar]
- 47. Nakamura K., Niimi M., Niimi K., Holmes A. R., Yates J. E., Decottignies A., Monk B. C., Goffeau A., Cannon R. D. (2001) Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45, 3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adamian L., Liang J. (2006) Prediction of transmembrane helix orientation in polytopic membrane proteins. BMC Struct. Biol. 6, 13–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rutledge R. M., Esser L., Ma J., Xia D. (2011) Toward understanding the mechanism of action of the yeast multidrug resistance transporter Pdr5p. A molecular modeling study. J. Struct. Biol. 173, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niimi K., Harding D. R., Holmes A. R., Lamping E., Niimi M., Tyndall J. D., Cannon R. D., Monk B. C. (2012) Specific interactions between the Candida albicans ABC transporter Cdr1p ectodomain and a d-octapeptide derivative inhibitor. Mol. Microbiol. 85, 747–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voss N. R., Gerstein M. (2010) 3V: Cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res. 38, W555–W562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kapoor K., Rehan M., Kaushiki A., Pasrija R., Lynn A. M., Prasad R. (2009) Rational mutational analysis of a multidrug MFS transporter CaMdr1p of Candida albicans by employing a membrane environment based computational approach. PLoS Comput. Biol. 5, e1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saini P., Gaur N. A., Prasad R. (2006) Chimeras of the ABC drug transporter Cdr1p reveal functional indispensability of transmembrane domains and nucleotide-binding domains, but transmembrane segment 12 is replaceable with the corresponding homologous region of the non-drug transporter Cdr3p. Microbiology 152, 1559–1573 [DOI] [PubMed] [Google Scholar]
- 54. Tanabe K., Lamping E., Nagi M., Okawada A., Holmes A. R., Miyazaki Y., Cannon R. D., Monk B. C., Niimi M. (2011) Chimeras of Candida albicans Cdr1p and Cdr2p reveal features of pleiotropic drug resistance transporter structure and function. Mol. Microbiol. 82, 416–433 [DOI] [PubMed] [Google Scholar]
- 55. Krishnamurthy S., Chatterjee U., Gupta V., Prasad R., Das P., Snehlata P., Hasnain S. E., Prasad R. (1998) Deletion of transmembrane domain 12 of CDR1, a multidrug transporter from Candida albicans, leads to altered drug specificity. Expression of a yeast multidrug transporter in Baculovirus expression system. Yeast 14, 535–550 [DOI] [PubMed] [Google Scholar]
- 56. Currier S. J., Ueda K., Willingham M. C., Pastan I., Gottesman M. M. (1989) Deletion and insertion mutants of the multidrug transporter. J. Biol. Chem. 264, 14376–14381 [PubMed] [Google Scholar]
- 57. Crowley E., O'Mara M. L., Kerr I. D., Callaghan R. (2010) Transmembrane helix 12 plays a pivotal role in coupling energy provision and drug binding in ABCB1. FEBS J. 277, 3974–3985 [DOI] [PubMed] [Google Scholar]