Background: Extranuclear ER plays an important role in cancer cell growth regulation through activation of kinase cascades.
Results: MEMO modulates extranuclear functions of ER as well as breast cancer cell growth.
Conclusion: MEMO is a new component of extranuclear ER signalosome and is essential for the regulation of ER-positive breast cancer cell growth.
Significance: MEMO might be an interesting target for ER-positive breast cancer therapy.
Keywords: Breast Cancer, Cell Proliferation, Estrogen Receptor, Growth Factors, Signal Transduction, ERBB2, IGF1R, MEMO
Abstract
In addition to nuclear estrogen receptor (ER) acting as a transcription factor, extranuclear ER also plays an important role in cancer cell growth regulation through activation of kinase cascades. However, the molecular mechanisms by which extranuclear ER exerts its function are still poorly understood. Here, we report that mediator of ERBB2-driven cell motility (MEMO) regulates extranuclear functions of ER. MEMO physically and functionally interacted with ER. Through its interaction with the growth factor receptors IGF1R and ERBB2, MEMO mediated extranuclear functions of ER, including activation of mitogen-activated protein kinase (MAPK) and protein kinase B/AKT, two important growth regulatory protein kinases, and integration of function with nuclear ER. Activation of MAPK and AKT was responsible for MEMO modulation of ER phosphorylation and estrogen-responsive gene expression. Moreover, MEMO increased anchorage-dependent and -independent growth of ER-positive breast cancer cells in vitro and was required for estrogen-induced breast tumor growth in nude mice. Together, our studies identified MEMO as a new component of extranuclear ER signalosome and suggest an essential role for MEMO in the regulation of ER-positive breast cancer cell growth.
Introduction
Estrogen plays a critical role in the development and progression of estrogen-related cancers such as breast cancer (1, 2). Estrogen exerts its biological responses through estrogen receptor (ER)4 α and β to regulate networks of gene transcription. ER belongs to a superfamily of ligand-activated transcription factors that share structural similarity characterized by several functional domains. The N-terminal estrogen-independent and C-terminal estrogen-dependent activation function domains (AF1 and AF2, respectively) contribute to the transcriptional activity of the two receptors. The DNA binding domain of ER is centrally located. The ligand binding domain, overlapping AF2, shows 58% homology between ERα and ERβ. The DNA binding domain is identical between the two receptors except for three amino acids. However, the AF1 domain of ERβ has only 28% homology with that of ERα.
Traditionally, ER is thought to be ligand-activated nuclear transcription factors that bind to estrogen-response elements (ERE) of target genes. Upon binding to DNA, ER regulates target gene transcription through recruitment of coactivators and corepressors. Although the majority of ER is in the nucleus mediating both ligand-dependent and ligand-independent gene transcription, evidence from an increasing number of studies clearly shows that a small population of ER is localized at or near the plasma membrane in the presence or absence of estrogen and mediates rapid extranuclear functions of ER (3–7). Extranuclear ER signaling pathway involves insulin-like growth factor 1 receptor (IGF1R) and epidermal growth factor receptor (EGFR), resulting in the activation of many signaling molecules, such as mitogen-activated protein kinase (MAPK), protein kinase B/AKT, and intracellular second messengers (8). MAPK and AKT can phosphorylate ERα, thereby increasing ERα transcriptional activity (9–14). In cultured cancer cells, membrane-initiated ERα signaling mediates the proliferative effects of estrogen. However, the molecular mechanisms by which extranuclear ER exerts its function are still poorly understood.
The mediator of ERBB2-driven cell motility (MEMO) is a 297-amino acid protein and is not homologous to any known signaling protein (15, 16). The biological function of MEMO is largely unknown. MEMO interacts with ERBB2 and is required for breast cancer cell migration (15, 17). ERBB2 is a member of the epidermal growth factor (EGF, also known as ErbB) family of receptor tyrosine kinases, which also includes EGFR, ErbB3, and ErbB4 (18). MEMO controls ERBB2-regulated microtubule dynamics (19). In this study, we have identified MEMO as a novel ER-interacting protein. Through its interaction with IGF1R and ERBB2, MEMO regulates extranuclear functions of ERα, such as activation of MAPK and AKT pathways and integration of function with nuclear ERα that functions as a transcription factor. Importantly, MEMO is required for estrogen-mediated breast tumor growth.
EXPERIMENTAL PROCEDURES
Plasmids and siRNAs
The estrogen-responsive reporter ERE-Luc and eukaryotic expression vectors for FLAG-tagged ERα and ERβ have been described previously (20). The extranuclear ERα mutant ERα (H2+NES) was constructed as reported previously (21). Other mammalian expression vectors encoding FLAG or Myc fusion proteins tagged at the N terminus were constructed by inserting PCR-amplified fragments into pcDNA3 (Invitrogen) or pIRESpuro2 (Clontech). Enhanced green fluorescent protein-tagged MEMO construct was generated by inserting MEMO cDNA into pEGFP-C1 (Clontech). Plasmids encoding GST fusion proteins were made by cloning PCR-amplified sequences into pGEX-KG (Amersham Biosciences). The cDNA target sequences of siRNAs for MEMO were GATGAACACAGTATTGAAA (MEMO siRNA1) and GCAATTACTTGAAGAAATA (MEMO siRNA2) and were inserted into pSilencer2.1-U6neo (Ambion) or pSIH-H1-puro (System Biosciences). Expression vectors for siRNA-resistant MEMO containing a silent mutation in the 3′-nucleotide of a codon in the middle of the siRNA-binding site were generated by recombinant PCR. Recombinant lentivirus vectors for ERBB2 siRNA and IGF1R siRNA were used for knockdown of endogenous ERBB2 and IGF1R, respectively, according to the manufacturer's instructions (Santa Cruz Biotechnology).
Yeast Two-hybrid Assay
The bait plasmid pGBKT7-ERα-AF1(1–167) was used to screen a human mammary gland cDNA library fused to the GAL4 activation domain in pACT2 according to the manufacturer's instructions (Clontech). The bait plasmid and the mammary cDNA library were sequentially transformed into AH109 yeast cells as described previously (22).
GST Pulldown Assay
The GST alone and GST fusion proteins were expressed in bacteria and purified according to the manufacturer's instructions (Amersham Biosciences). HEK293T extracts expressing ERα or ERβ were incubated with 10 μg of purified GST derivatives bound to glutathione-Sepharose beads, and the adsorbed proteins were examined as described previously (23).
Coimmunoprecipitation
Cell extracts were prepared, immunoprecipitated, and analyzed as described previously (23). Immunoprecipitation was performed with anti-FLAG M2 affinity gel (Sigma), anti-c-Myc affinity gel (Sigma), anti-ERα (Millipore), anti-MEMO (Abcam), anti-ERBB2 (Santa Cruz Biotechnology), or anti-IGF1R (Santa Cruz Biotechnology).
Cell Culture, Transfection, and Luciferase Reporter Assay
HEK293T embryonic kidney cells and MCF7, ZR75-1, T47D, and SKBR3 breast cancer cells were purchased from American Type Culture Collection (ATCC) and were routinely grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone). For hormone treatment experiments, cells were grown in medium containing phenol red-free DMEM supplemented with 10% charcoal-stripped FBS. Lipofectamine 2000 reagent was used for transfections according to the manufacturer's protocol (Invitrogen). Lentiviruses were produced by cotransfection of HEK293T cells with recombinant lentivirus vectors and pPACK Packaging Plasmid Mix (System Biosciences) using Megatran reagent (Origene). Lentiviruses were added to the medium of target cells with 8 μg/ml Polybrene (Sigma). Stable cell lines were selected in 500 μg/ml G418 or 1 μg/ml puromycin. Pooled clones or individual clones were screened by standard immunoblot protocols and produced similar results. Luciferase reporter assays were performed as described previously (24).
Real Time RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen). Real time PCR was performed as described previously with the primers listed in supplemental Table 1A (25).
Chromatin Immunoprecipitation Assay (ChIP)
ChIP assays were performed as described previously (22) using anti-ERα (Millipore). The primers used for ChIP are listed in supplemental Table S1B.
Anchorage-dependent and -independent Growth Assays
Anchorage-dependent cell growth was determined by a crystal violet assay as described previously (22). For anchorage-independent growth (22), 1 × 104 cells were plated on 6-cm plates containing a bottom layer of 0.6% low melting temperature agar in DMEM and a top layer of 0.3% agar in DMEM. Colonies were scored after 3 weeks of growth.
Animal Experiments
Animal studies were approved by the Institutional Animal Care Committee of Beijing Institute of Biotechnology. Two days after implantation of estrogen pellets (E2, 0.36 mg/pellet, 60-day release) (Innovative Research of America), 1 × 107 tumor cells were injected into the abdominal mammary fat pad of 6-week-old female nude mice. Tumor growth was monitored by caliper measurements. Excised tumors were weighed, and portions were frozen in liquid nitrogen or fixed in 4% paraformaldehyde for further study.
Statistical Analysis
Differences between variables were assessed by two-tailed Student's t test or one-way analysis of variance. Statistical calculations were performed using SPSS13.0. A p value <0.05 was considered statistically significant.
RESULTS
MEMO Interacts with ER in Vitro and in Vivo
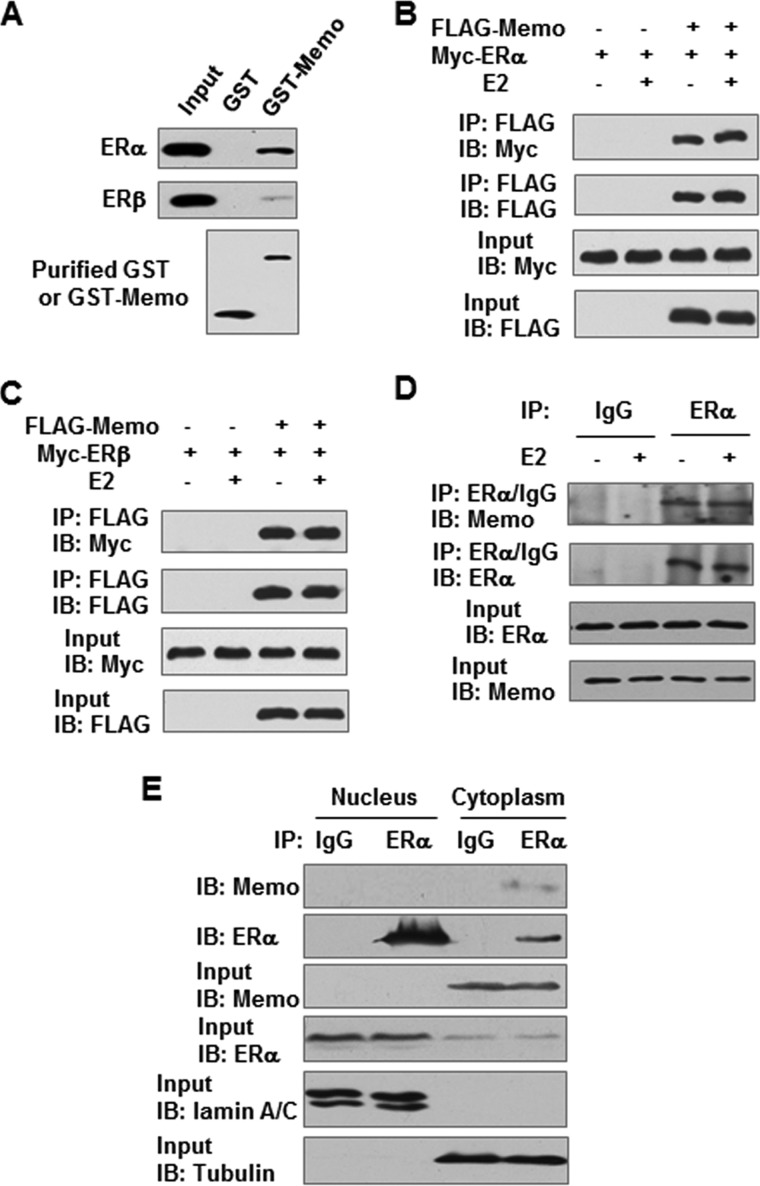
Yeast two-hybrid screening of a human mammary cDNA library, with the AF1 domain of ERα as bait, identified human MEMO as an ERα-interacting protein (supplemental Fig. 1). Transformation of yeast cells with MEMO together with the controls did not activate the His, Ade, and lacZ reporter genes, indicating the specific interaction of MEMO with ERα in yeast cells. GST pulldown and coimmunoprecipitation assays further demonstrated that MEMO interacted with ERα and ERβ in vitro and in 293T cells in the absence or presence of 17β-estradiol (E2) (Fig. 1, A–C). Importantly, endogenous MEMO associated with ERα in MCF7 breast cancer cells (Fig. 1D). Moreover, subcellular fractionation experiments showed that in MCF7, ZR75-1, and T47D breast cancer cells MEMO resided in the cytoplasm but not in the nucleus (supplemental Fig. 2). Endogenous cytoplasmic MEMO also interacted with endogenous cytoplasmic ERα in MCF7 cells (Fig. 1E).
FIGURE 1.
MEMO interacts with ER in vitro and in vivo. A, GST pulldown analysis of purified GST or GST-MEMO fusion protein incubated with lysates of HEK293T cells expressing FLAG-tagged ERα or ERβ. Bound proteins were subjected to Western blot with anti-FLAG antibody. B and C, HEK293T cells transiently transfected with FLAG-tagged MEMO and Myc-tagged ERα (B) or ERβ (C) in the presence of 10 nm E2 were immunoprecipitated (IP) with anti-FLAG followed by immunoblotting (IB) with the indicated antibodies. D, coimmunoprecipitation analysis of the endogenous interaction of MEMO with ERα in the presence of 10 nm E2. Cell lysates from MCF7 cells were immunoprecipitated with antibodies specific for ERα, followed by immunoblotting with the indicated antibodies. IgG, normal serum. E, MCF7 cells were fractionated, and proteins were immunoprecipitated from cytoplasmic and nuclear fractions, with the indicated antibodies or preimmune control serum (IgG). Precipitates were analyzed by immunoblot using the indicated antibodies. Lamin A/C and α-tubulin were used as the nuclear and cytoplasmic marker, respectively.
MEMO Activates MAPK and AKT and Subsequent ERα Phosphorylation
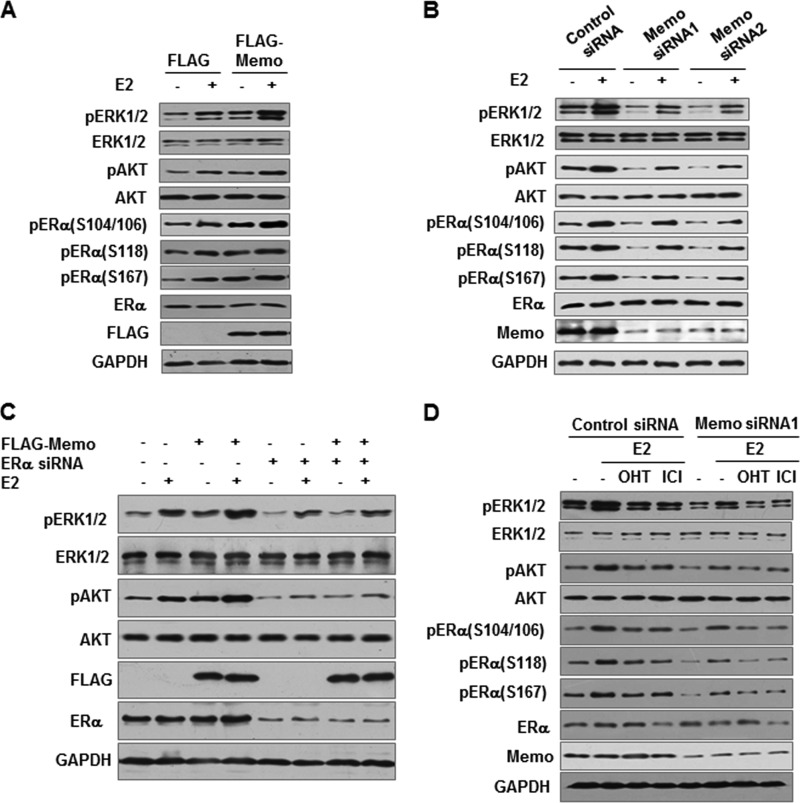
Because MEMO associates with extranuclear ERα, we tested whether MEMO regulates rapid extranuclear functions of ER, such as activation of MAPK/extracellular signal-regulated kinases 1 and 2 (ERK1/2) and AKT (6). As expected, E2 rapidly increased phosphorylation of ERK1/2 and AKT in MCF7 cells, which was seen from 5 to 30 min after E2 treatment (Fig. 2A and data not shown). Importantly, overexpression of MEMO enhanced phosphorylation of ERK1/2 and AKT in both the absence and the presence of E2. In contrast, stable knockdown of MEMO with MEMO siRNAs reduced ERK1/2 and AKT phosphorylation (Fig. 2B).
FIGURE 2.
MEMO activates ERK1/2 and AKT and subsequent ERα phosphorylation. A and B, immunoblot analysis of MCF7 cells stably transfected with FLAG-tagged MEMO (A) and MEMO siRNA1 or MEMO siRNA2 (B) and treated with 10 nm E2 for 30 min. GAPDH was used as a loading control. p, phosphorylation. C, immunoblot analysis of FLAG-MEMO-expressing MCF7 cells transfected with ERα siRNA. D, immunoblot analysis of MCF7 cells stably transfected with MEMO siRNA1 and treated with 10 nm E2, 10 nm E2 plus 100 nm 4-OHT, or 10 nm E2 plus 10 nm ICI182,780 for 30 min.
Although ERβ phosphorylation is largely unknown, ERα has been shown to be phosphorylated at serines (Ser) 104, 106, and 118 by MAPK (9–12). ERα Ser-167 can be phosphorylated by AKT (13, 14). Because MEMO can activate MAPK and AKT, we determined whether MEMO increases phosphorylation of ERα. Indeed, overexpression of MEMO in MCF7 cells increased phosphorylation of ERα at serines 104, 106, 118, and 167 (Fig. 2A), whereas stable knockdown of MEMO decreased ERα phosphorylation at these sites (Fig. 2B). Similar results were obtained in ZR75-1 cells (supplemental Fig. 3, A and B). Moreover, ERα is required for MEMO activation of MAPK and AKT because knockdown of endogenous ERα in MCF7 cells abolished the ability of MEMO to regulate MAPK and AKT (Fig. 2C), suggesting the importance of the interaction between MEMO and ERα. Similar results were observed in ERα-negative HEK293T cells. More importantly, MEMO could activate MAPK and AKT through extranuclear ERα because the previously reported extranuclear ERα mutant ERα(H2+NES) was sufficient for MEMO modulation of MAPK and AKT (21) (supplemental Fig. 3C).
To determine whether estrogen antagonists affect MEMO-induced MAPK and AKT activation as well as ERα phosphorylation, the mixed agonist/antagonist 4-OHT and the pure antagonist ICI182,780 were used. Tamoxifen and ICI182,780 treatment in control siRNA-transfected MCF7 cells had similar effects to those in MEMO siRNA-transfected MCF7 cells, i.e. tamoxifen and ICI182,780 antagonized estrogen-mediated effects in both control siRNA- and MEMO siRNA-transfected MCF7 cells (Fig. 2D).
MEMO Enhances ERα Transcriptional Activity through Increased ERα Phosphorylation and ERα Recruitment to Estrogen-responsive Promoters
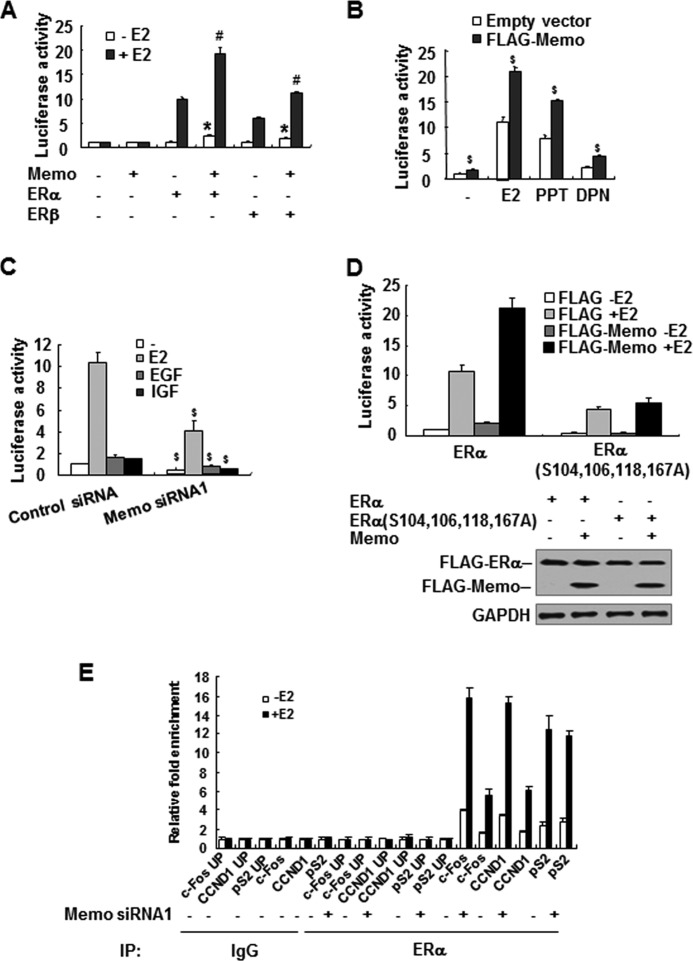
It has been shown that extranuclear ER can indirectly regulate ER transcriptional activity through ER phosphorylation (26). As MEMO can regulate ER phosphorylation, we determined whether MEMO affects ER transcriptional activity. MEMO overexpression in ERα- and ERβ-negative SKBR3 breast cancer cells increased transcription of a luciferase reporter construct containing the estrogen-responsive element (ERE) in an ERα- or ERβ-dependent manner in the presence or absence of E2 (Fig. 3A). Moreover, MEMO increased ER transcriptional activity in ERα- and ERβ-positive MCF7 cells regardless of E2, the ERα-specific agonist propylpyrazole triol, and the ERβ-specific agonist diarylpropionitrile (Fig. 3B and supplemental Fig. 4C), suggesting that MEMO regulates ER transcriptional activity in a ligand-independent manner. Similar results were observed in ERα-positive ZR75-1 and T47D cells (supplemental Fig. 4, B and C). Consistent with the results of MEMO overexpression, knockdown of endogenous MEMO in MCF7 cells decreased ERE-Luc reporter activity independently of ligands, including EGF and IGF, which have been reported to stimulate ER transcriptional activity through ER phosphorylation (Fig. 3C) (10). Importantly, MEMO increased wild-type ERα-mediated ERE-LUC reporter transcription much greater than the ERE-LUC reporter transcription mediated by mutant ERα in which serines 104, 106, 118, and 167 were mutated to alanine (Fig. 3D). These data suggest that phosphorylation of ERα at these sites is critical for the enhancement of ERα transcriptional activity by MEMO.
FIGURE 3.
MEMO increases ER transcriptional activity as well as ER recruitment to estrogen-responsive promoters. A and B, luciferase reporter assays of ERα and ERβ transcriptional activity in SKBR3 (A) or MCF7 (B) cells transfected with ERE-LUC and FLAG-tagged MEMO with or without ERα or ERβ and treated with 10 nm E2, 1 nm propylpyrazole triol, or 1 nm diarylpropionitrile for 24 h. The results shown are means ± S.D. of three independent experiments. *, p < 0.01 versus ERα or ERβ in the absence of E2. #, p < 0.01 versus ERα or ERβ in the presence of E2. $, p < 0.01 versus corresponding to empty vector. C, luciferase reporter assays of ER transcriptional activity in MEMO knockdown MCF7 cells (Fig. 2B) transfected with ERE-LUC and treated with 10 nm E2, 100 ng/ml EGF, or 100 ng/ml IGF1. The results shown are means ± S.D. of three independent experiments. $, p < 0.01 versus corresponding control siRNA. D, luciferase reporter assays of ERα transcriptional activity in SKBR3 cells transfected with ERE-LUC and FLAG-tagged MEMO with wild-type ERα or ERα(S104A,S106A,S118A,S167A). The results shown are means ± S.D. of three independent experiments. Bottom, immunoblot analysis with anti-FLAG in the presence of E2. E, ChIP analysis of the occupancy of ERα on the indicated estrogen-responsive promoters in MCF7 cells stably transfected with control siRNA or MEMO siRNA1 and treated with 10 nm E2 for 1 h. UP, upstream of the indicated promoters. IgG, normal serum; IP, immunoprecipitation.
To investigate whether MEMO affects promoter occupancy of ERα, we performed ChIP experiments for the estrogen-responsive pS2, c-Fos, and CCND1 promoters. As expected, ERα was recruited to the pS2, c-Fos, and CCND1 promoters but not to a region ∼2-kb upstream of the pS2, c-Fos, or CCND1 promoters (Fig. 3E). Importantly, MEMO knockdown decreased ERα recruitment to the c-Fos and CCND1 promoters, but not the pS2 promoter, indicating that MEMO selectively regulates ERα recruitment to estrogen-responsive promoters.
MEMO Increases ERα Target Gene Expression through Activation of MAPK and AKT and Subsequent ERα Phosphorylation
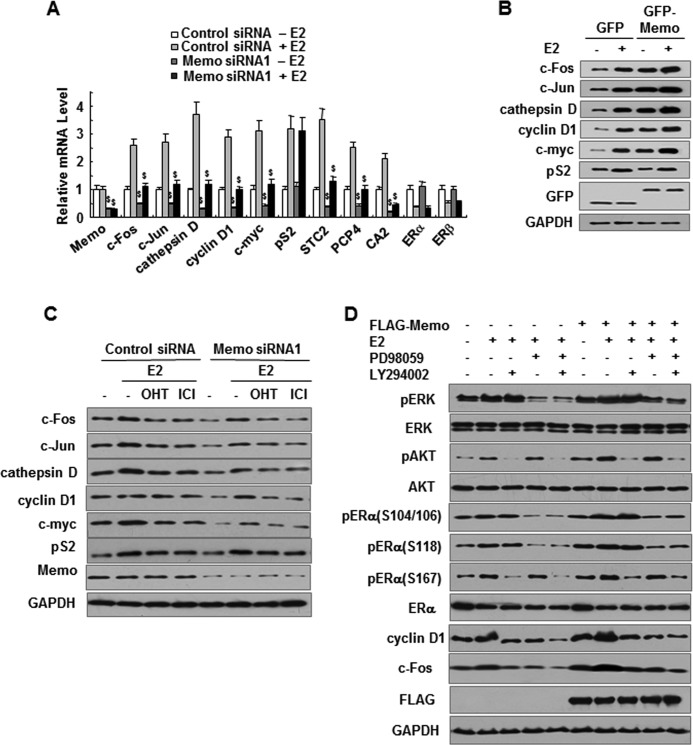
To validate the reporter assays in which MEMO increases ERα transcriptional activity, we performed real time RT-PCR analysis using MCF7 cells stably transfected with MEMO siRNA or control siRNA. The results showed that, in the presence or absence of E2, MEMO knockdown reduced the transcription of eight previously reported E2-regulated genes (Fig. 4A), including c-Fos (cellular FBJ osteosarcoma oncogene), c-Jun (cellular ju-nana), cathepsin D, CCND1/cyclin D1, c-myc (cellular myelocytomatosis virus oncogene), STC2 (stanniocalcin 2), PCP4 (Purkinje cell protein 4), and CA2 (carbonic anhydrase 2), many of which play key roles in cell proliferation regulation (27–30). Consistent with the results of ChIP experiments, MEMO did not alter pS2/TFF1 (trefoil factor 1) mRNA expression (Fig. 4A). In addition, MEMO did not regulate ER mRNA expression. Importantly, stable MEMO expression in MCF7 cells increased the protein levels of c-Fos, c-Jun, cathepsin D, cyclin D1, and c-Myc, but not pS2, in an E2-independent manner (Fig. 4B). In contrast, siRNA knockdown of endogenous MEMO in MCF7 cells reduced the protein expression of c-Fos, c-Jun, cathepsin D, cyclin D1, and c-Myc, but not pS2 (Fig. 4C), suggesting that MEMO selectively regulates estrogen-responsive gene expression. Similar results were obtained in ZR75-1 cells (supplemental Fig. 4, D and E). Moreover, 4-OHT and ICI182,780 antagonized the estrogen-mediated effect on the target gene expression in both control siRNA- and MEMO siRNA-transfected MCF7 cells (Fig. 4C).
FIGURE 4.
MEMO enhances ERα phosphorylation as well as the expression of estrogen-responsive genes through activation of ERK1/2 and AKT. A, real time RT-PCR analysis of estrogen-responsive genes in MEMO knockdown MCF7 cells treated with 10 nm E2 for 24 h. B and C, immunoblot analysis of estrogen-responsive gene expression in MCF7 cells stably transfected with GFP-tagged MEMO (B) or MEMO siRNA1 (C) in the presence of 10 nm E2, 10 nm E2 plus 100 nm 4-OHT, or 10 nm E2 plus 10 nm ICI182,780 for 24 h. D, MEMO stably expressed MCF7 cells were treated with E2 or E2 plus PD98059 or E2 plus LY294002 for 24 h. Cell lysates were blotted with the indicated antibodies.
To investigate whether activation of MAPK and AKT is responsible for MEMO modulation of ERα phosphorylation and target gene expression, PD98059 and LY294002, which are MAPK and PI3K/AKT inhibitors, respectively, were used to treat MCF7 cells stably transfected with either MEMO or empty vector. As expected, the MAPK and AKT inhibitors decreased phosphorylation of ERK1/2 and AKT as well as phosphorylation of ERα at serines 104, 106, 118, and 167 (Fig. 4D). Such inhibition impaired the effect of MEMO on the expression of the estrogen-responsive proteins cyclin D1 and c-Fos. These data suggest that MEMO increases ERα phosphorylation and estrogen-responsive gene expression at least in part through activated MAPK and AKT.
MEMO and ERα Interaction Is Required for Activation of MAPK and AKT as Well as Estrogen-responsive Gene Expression
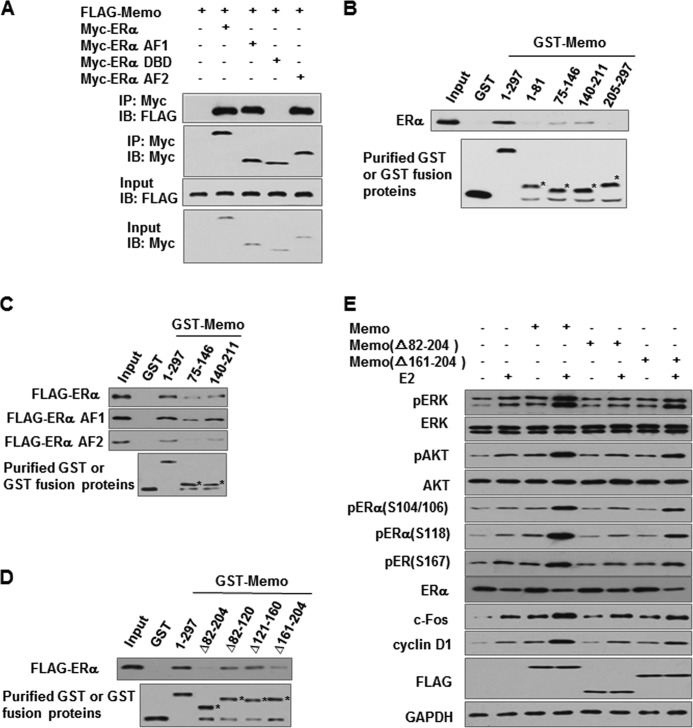
To further determine whether the interaction of MEMO and ERα is necessary for activation of MAPK and AKT as well as estrogen-responsive gene expression, we first used deletion analysis to map the interaction domains of ERα in coimmunoprecipitation assays. The AF1 and AF2 domains of ERα interacted with MEMO, whereas the ERα DNA binding domain did not (Fig. 5A). Next, we determined which MEMO protein region mediates interaction with ERα by GST pulldown assay. GST-MEMO(75–146) and GST-MEMO(140–211) bound ERα, whereas GST-MEMO(1–81), GST-MEMO(205–297), or GST alone did not (Fig. 5B). The MEMO(75–146) and the MEMO(140–211) interacted with ERα more weakly than full-length MEMO, suggesting that both regions of MEMO are required for maximal interaction with ERα. The AF1 and AF2 domains of ERα also interacted with MEMO(75–146) and MEMO(140–211), and MEMO(140–211) had higher binding affinity to the AF1 and AF2 of ERα than MEMO(75–146) (Fig. 5C). Further domain mapping showed that deletion of the amino acid region 161–204 of MEMO greatly attenuated the interaction of MEMO with ERα (Fig. 5D). Importantly, the MEMO(Δ82–204) deletion mutant, which lacks two ERα-binding sites, or the MEMO(Δ161–204) deletion mutant, which greatly reduces the interaction of MEMO with ERα, completely or almost abolished the ability of MEMO to activate MAPK and AKT, ERα phosphorylation, and estrogen-responsive gene expression (Fig. 5E). Combined with the previous results (Fig. 2C), these data suggest that interaction of MEMO with ERα is required for MEMO modulation of ERα function.
FIGURE 5.
Interaction of MEMO with ERα is required for MEMO modulation of phosphorylation of ERK1/2, AKT, and ERα as well as the expression of estrogen-responsive genes. A, mapping of the MEMO interaction regions in ERα. HEK293T cells transfected with FLAG-tagged MEMO and Myc-tagged ERα, ERα AF1, ERα DNA binding domain, or ERα AF2 were immunoprecipitated (IP) with anti-Myc followed by immunoblotting (IB) with the indicated antibodies. B–D, mapping of the regions of MEMO that interact with ERα. Purified GST or GST-MEMO deletion mutant proteins were incubated with lysates of HEK293T cells expressing FLAG-tagged ERα (B–D), ERα AF1, or ERα AF2 (C). Bound proteins were subjected to Western blot with anti-FLAG. Asterisks indicate the positions of the expected purified proteins. E, immunoblot analysis of activation of ERK1/2 and AKT, ERα phosphorylation, and estrogen-responsive gene expression in MCF7 cells transfected with FLAG-tagged MEMO, MEMO(Δ82–204), or MEMO(Δ161–204) and treated with 10 nm E2 for 24 h.
MEMO Activation of MAPK and AKT Requires IGF1R and ErbBs
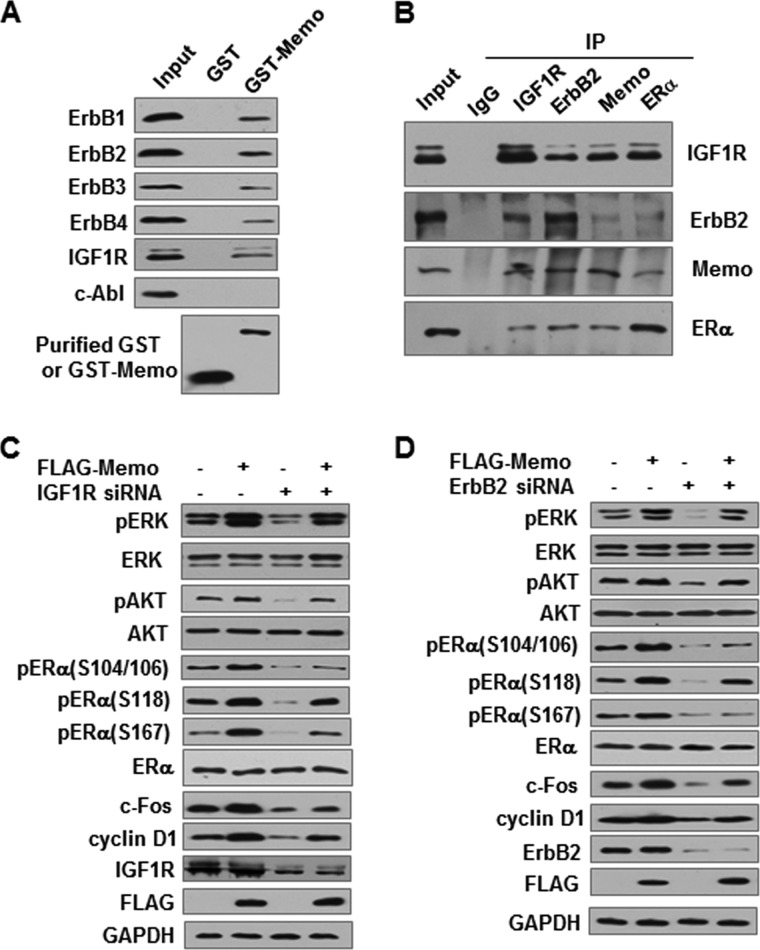
Phosphorylation of MAPK and AKT plays a critical role in transducing signals from membrane ER to nuclear ER (6). ER has no intrinsic kinase domain and thus is not capable of phosphorylating other proteins. Like ER, MEMO also did not have intrinsic kinase activity by an in vitro kinase assay (data not shown). It has been reported that ER initiates E2 rapid nongenomic signals by forming a protein complex with many signaling molecules, including IGF1R and EGFR/ErbB1, which have kinase activities (31). The formation of the protein complex leads to the activation of MAPK and AKT pathways. MEMO has been shown to interact with ERBB2, another EGFR family member (15). Thus, we hypothesized that MEMO may activate MAPK and AKT through its interaction with IGF1R and the members of the EGFR family. Indeed, GST pulldown experiments showed that MEMO interacted not only with IGF1R but also with the EGFR family members ErbB1–4 (Fig. 6A). As a control, MEMO did not interact with the nonreceptor tyrosine kinase c-Abl (cellular Abelson leukemia oncogene). These results suggested that MEMO, ERα, and IGF1R or ErbBs might coexist in the same functional complex. To verify this, we performed reciprocal coimmunoprecipitation assays with each of the antibodies against these proteins. MEMO, ERα, IGF1R, and ERBB2 could form a protein complex in MCF7 cells (Fig. 6B).
FIGURE 6.
MEMO activates ERK1/2 and AKT and increases ERα phosphorylation through its interaction with IGF1R and ERBB2. A, GST pulldown analysis of purified GST or GST-MEMO fusion protein incubated with MCF7 whole cell lysates. Bound proteins were subjected to Western blot with the indicated antibodies. B, identification of MEMO, ERα, ERBB2, and IGF1R within the same complex by reciprocal coimmunoprecipitation assays. MCF7 cell lysates were immunoprecipitated with each of the antibodies or normal IgG as the control. All the immunoprecipitates were detected by immunoblotting with the indicated antibodies. C and D, immunoblot analysis of phosphorylation of ERK1/2, AKT, and ERα as well as the expression of estrogen-responsive genes in MEMO expressed MCF7 cells infected with lentiviral particles carrying IGF1R siRNA (C) or ERBB2 siRNA (D).
Next, we investigated whether MEMO activates MAPK and AKT through IGF1R and ERBB2. Knockdown of endogenous IGF1R or ERBB2 in MCF7 cells greatly attenuated MEMO activation of ERK1/2 and AKT, leading to reduced phosphorylation of ERα at serines 104, 106, 118, and 167 as well as reduced expression of the estrogen-responsive proteins c-Fos and cyclin D1 (Fig. 6, C and D). These results suggest that MEMO activates MAPK and AKT and subsequent ER targets through IGF1R and ERBB2.
MEMO Is Required for Estrogen-mediated Breast Tumor Growth
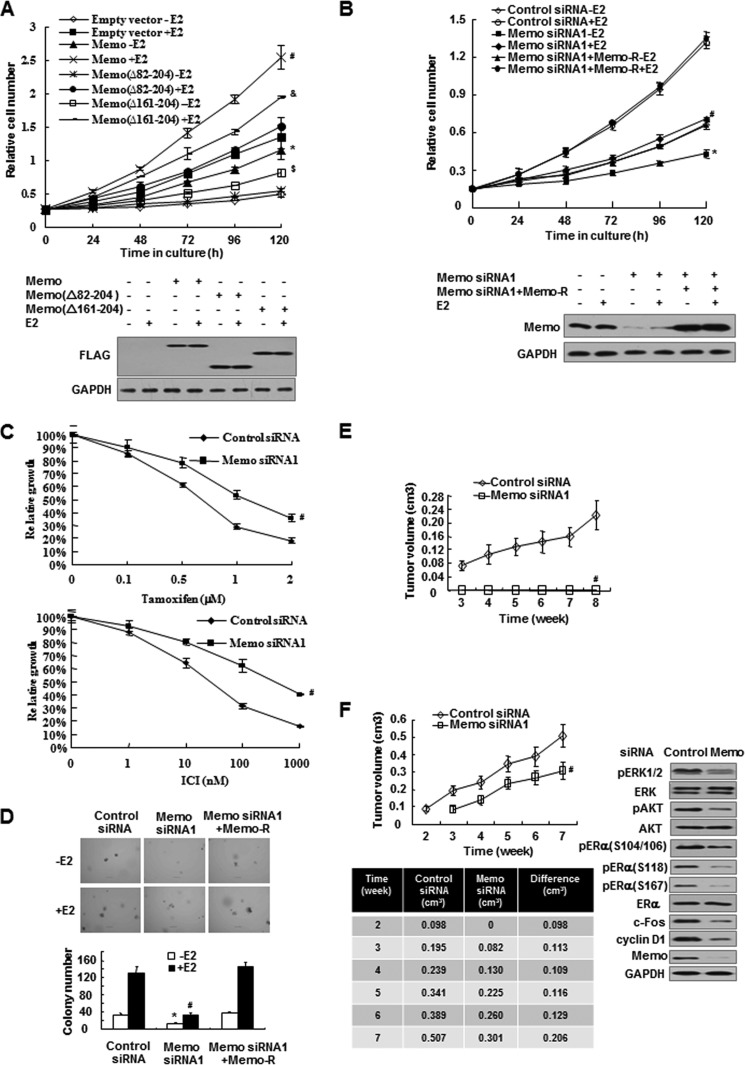
Next, we determined the effect of MEMO on breast cancer cell growth. In assays of anchorage-dependent growth, MCF7 cells stably transfected with MEMO grew faster than those transfected with empty vector both in the presence and in the absence of E2 (Fig. 7A). The above-mentioned deletion mutants, MEMO(Δ82–204) and MEMO(Δ161–204), completely or almost abolished the ability of MEMO to increase the growth of MCF7 cells. In contrast, MEMO knockdown greatly reduced E2-mediated growth stimulation of MCF7 cells (Fig. 7B), and this phenotype was rescued by MEMO re-expression. Similar results were observed in ZR75-1 cells (supplemental Fig. 5, A and B). As determined by anchorage-dependent growth assays, knockdown of MEMO reduced the sensitivity of MCF7 cells to the estrogen antagonists 4-OHT and ICI182,780 (Fig. 7C). MEMO knockdown also greatly inhibited anchorage-independent growth of MCF7 and ZR75-1 cells in the presence or absence of E2 (Fig. 7D and supplemental Fig. 5, C and D), and again, the observed effects were rescued by MEMO re-expression in MCF7 cells (Fig. 7D). Furthermore, all mice inoculated with MCF7 or ZR75-1 cells expressing control siRNA developed tumors in the presence of E2 but not in the absence of E2 (Fig. 7, E and F, and data not shown), suggesting that both MCF7 and ZR75-1 cell lines are estrogen-dependent. In contrast, none of the mice inoculated with MCF7 cells expressing MEMO siRNA developed tumors in the presence of E2 (Fig. 7E), and mice inoculated with ZR75-1 cells expressing MEMO siRNA showed late latency and a much smaller tumor size (Fig. 7F). The tumors in mice inoculated with ZR75-1 cells expressing MEMO siRNA had reduced phosphorylation of ERK1/2, AKT, ERα(Ser-104 and Ser-106), ERα(Ser-118), and ERα(Ser-167), as well as expression of c-Fos and cyclin D1 (Fig. 7F).
FIGURE 7.
MEMO is required for ERα-positive breast tumor growth. A, anchorage-dependent growth assays in MCF7 cells transfected with FLAG-tagged MEMO, MEMO(Δ82–204), or MEMO(Δ161–204) with or without 10 nm E2 treatment. Cell viability was assessed at the indicated times. Data are presented as means ± S.D. of three independent experiments. Bottom, immunoblot analysis with anti-FLAG. B, anchorage-dependent growth assays in MCF7 cells transfected with MEMO siRNA1 or MEMO siRNA1 plus siRNA-resistant MEMO. Cells were treated and analyzed as in A. Bottom, immunoblot analysis with anti-MEMO. C, MCF7 cells stably transfected with control siRNA or MEMO siRNA1 were treated with different doses of 4-OHT or ICI182,780. Cell viability was assessed 5 days after treatment. D, anchorage-independent growth assays in MCF7 cells transfected as in B. Scale bar, 50 μm. E and F, volume of xenograft tumors derived from MCF7 (E) or ZR75-1 (F) cells expressing control siRNA or MEMO siRNA1. Detailed tumor volumes were listed in the table (F). Data are presented as mean ± S.D. (n = 10). Representative tumor tissues were subjected to immunoblot analysis with the indicated antibodies (F, right panel). *, p < 0.01 versus empty vector or control siRNA without E2. #, p < 0.01 versus empty vector or control siRNA with E2. $, p < 0.05 versus empty vector or control siRNA without E2. &, p < 0.05 versus empty vector or control siRNA with E2.
DISCUSSION
In this study, we have identified MEMO as a new component of extranuclear ERα signaling. MEMO integrates extranuclear and nuclear ERα actions. Importantly, MEMO plays an essential role in ERα-positive breast cancer cell growth through regulation of many growth-related genes. Thus, the findings provide novel mechanistic insights into the growth of ERα-positive breast cancer cells, the number of which increases during breast cancer development.
Several membrane and cytoplasmic adaptor proteins, including caveolins (32, 33), striatin (34), p130Cas (35), the adaptor protein Shc (36), hematopoietic PBX-interacting protein (37, 38), and modulator of nongenomic action of estrogen receptor/proline-, glutamic acid-, and leucine-rich protein (MNAR/PELP1) (39), have been shown to interact with membrane/cytoplasmic ERα. E2 increases the interaction of ERα with these proteins. However, MEMO interacts with ERα and regulates ERα transcriptional activity in an E2-independent manner. It has been reported that stimulation of numerous growth factor receptors leads to a ligand-independent increase in ERα transactivation, presumably by ERα phosphorylation (10). Indeed, MEMO forms a complex with the growth factor receptors IGF1R and ERBB2, resulting in the phosphorylation of the ligand-independent AF1 domain of ERα. The fact that E2-independent activation of ERα by MEMO suggests that MEMO may play roles in the development and progression of both E2-independent and -dependent cancers. Because two to three proteins are usually studied for protein-protein interaction, whether there is more than one extranuclear ERα-containing complex remains to be investigated.
Extranuclear ERα initiates rapid action response by binding to estrogen. Although we grew cells with phenol red-free DMEM supplemented with 10% charcoal-stripped FBS, there may be residual estrogens in the culture media, which activate rapid estrogen response, followed by ERα phosphorylation and transcriptional activation. In addition, the charcoal-stripped FBS also contained many growth factors, which activate growth factor signaling and may cross-talk with estrogen signaling (40). The fact that knockdown of MEMO can inhibit basal ERα transcriptional activity and MEMO regulates ERα transcriptional activity in a ligand-independent manner suggests that a small amount of estrogen may be sufficient for MEMO modulation of ERα transcriptional activation.
It has been shown that the ligand-independent activity of ERα is a result of ERα phosphorylation at multiple sites, including serines 104, 106, 118, 167, and 305 (41). Phosphorylation of ERα at these sites increases ERα transcriptional activity. However, the clinical significance of ERα phosphorylation at these sites is complicated. It has been reported that phosphorylation at some sites in ERα is associated with a better clinical outcome, whereas phosphorylation at other sites is associated with a poorer clinical outcome most often in patients treated with tamoxifen (42). For instance, ERα phosphorylation at serine 118 or 167 is associated with a better clinical outcome in patients treated with tamoxifen, whereas elevated phosphorylation at serine 305 predicts tamoxifen resistance. The clinical significance of ERα phosphorylation at serines 104 and 106 is unknown. Our study showed that MEMO increased ERα phosphorylation at serines 104, 106, 118, and 167, and tamoxifen antagonized such effect. Moreover, knockdown of MEMO induced tamoxifen resistance in cultured breast cancer cells. It will be interesting to determine whether MEMO is a predictive marker in breast cancer patients treated with tamoxifen.
Overexpression of ERBB2 is associated with aggressive breast tumors that are more likely to metastasize (18). MEMO was shown to be required for ERBB2-driven breast cancer cell migration (15). Whether MEMO has other biological functions remains unknown. We demonstrate that MEMO plays an essential role in ERα-positive breast cancer cell growth. MEMO not only activates MAPK and AKT, two important growth regulatory protein kinases (43), but also enhances the expression of many growth-related estrogen-responsive proteins, including c-Fos (44), c-Jun (45), cyclin D1 (46), and c-myc (47). c-Fos and c-Jun belong to the activating protein-1 (AP-1) transcription factor that plays a critical role in tumorigenesis and progression. They regulate the expression of AP-1 target genes by forming heterodimer (c-Jun/c-Fos) or homodimer (c-Jun/c-Jun) and by binding to the AP-1 site within gene promoters. Both c-Fos and c-Jun are overexpressed in human breast cancer. c-Fos knockdown with antisense cDNA suppresses breast cancer cell growth. c-Jun overexpression in MCF7 cells produces a tumorigenesis-, invasive-, and hormone-resistant phenotype. Overexpression of cyclin D1 is common in human cancers of epithelial cell origin. Cyclin D1 is required for mammary tumorigenesis induced by ERBB2. Like cyclin D1, c-Myc is also overexpressed in breast tumors. Overexpression of c-Myc contributes to breast cancer development and progression and is associated with poor clinical outcome. The fact that MEMO can regulate many key oncogenes suggests the importance of MEMO in breast cancer development and progression and as a therapeutic target.
MCF7 and ZR75-1 are two well characterized ERα-positive breast cancer cell lines. In our study, ZR75-1 cells are easier to form tumors in the presence of estrogen in nude mice than MCF7 cells. This may be due to differential gene expression profiling between the two cell lines. It has been reported that ZR75-1 cells selectively use genes for energy, protein synthesis, and sugar metabolism and other pathways differently from MCF7 cells (48). Such characteristics may impart more aggressiveness to ZR75-1 than MCF7. The difference in gene expression between MCF7 and ZR75-1 cells may also affect the ability of MEMO to regulate breast cancer cell growth in nude mice. None of the mice inoculated with MEMO siRNA-expressing MCF7 cells developed tumors in the presence of E2 and mice inoculated with MEMO siRNA-expressing ZR75-1 cells revealed late latency and a much smaller tumor size. Although MEMO siRNA-expressing ZR75-1 tumors grew with smilar kinetics to control tumors 2–6 weeks after inoculation, significant different kinetics seems to be observed 7 weeks after inoculation. This trend might be more obvious if tumors had grown for more than 7 weeks.
Overexpression of ERBB2 occurs in ∼30% of breast cancer and is associated with poor clinical outcome such as shorter survival and shorter time to relapse (18). ERBB2 is therefore an attractive target for drug development. Accordingly, humanized monoclonal antibodies against ERBB2 (Herceptin®, trastuzumab) have been developed and used in the treatment of patients with breast cancer overexpressing ERBB2. However, not all patients whose tumors overexpress ERBB2 respond to trastuzumab treatment (49). The mechanisms underlying trastuzumab resistance include the inability or reduced capacity of trastuzumab binding to ERBB2 and activation of downstream signaling pathways such as PI3K/AKT signaling. In some breast cancer patients, trastuzumab is unable to interfere with the ERBB2 heterodimers by EGFR or ErbB3. Interaction of ERBB2 with other proteins such as mucin-4 sterically hinders ERBB2 from binding to trastuzumab (49). Activation of PI3K/AKT signaling has been considered as the major determinant of trastuzumab resistance. Trastuzumab-mediated growth inhibition was lost in breast cancer cells that overexpressed both IGF1R and ERBB2. This may be due to cross-talk between IGF1R and ERBB2 signaling (50). IGF1R and ERBB2 interact with each other and synergistically stimulate PI3K/AKT signaling. The findings that MEMO, ERα, IGF1R, and ERBB2 form a complex and MEMO activates the AKT pathway raise the possibility that MEMO may be involved in trastuzumab resistance. Whether MEMO prevents ERBB2 from binding to trastuzumab remains to be elucidated. The essential role of MEMO in ERα-positive breast tumor growth might make it an interesting target for breast cancer therapy.
This work was supported by Major State Basic Research Development Program Grants 2011CB504202 and 2012CB945100, National Natural Science Foundation Grants 81072173, 81272913, and 30625035, and National Key Technologies R&D Program for New Drugs Grant 2009ZX09301-002.

This article contains supplemental Figs. 1–5 and Tables 1a and 1b.
- ER
- estrogen receptor
- MEMO
- mediator of ERBB2-driven cell motility
- ERE
- estrogen-response element
- E2
- 17β-estradiol
- IGF1R
- insulin-like growth factor 1 receptor
- EGFR
- EGF receptor.
REFERENCES
- 1. Deroo B. J., Korach K. S. (2006) Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yager J. D., Davidson N. E. (2006) Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 354, 270–282 [DOI] [PubMed] [Google Scholar]
- 3. Levin E. R., Pietras R. J. (2008) Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res. Treat. 108, 351–361 [DOI] [PubMed] [Google Scholar]
- 4. Fu X. D., Simoncini T. (2008) Extranuclear signaling of estrogen receptors. IUBMB Life 60, 502–510 [DOI] [PubMed] [Google Scholar]
- 5. Boonyaratanakornkit V. (2011) Scaffolding proteins mediating membrane-initiated extranuclear actions of estrogen receptor. Steroids 76, 877–884 [DOI] [PubMed] [Google Scholar]
- 6. Pietras R. J., Márquez-Garbán D. C. (2007) Membrane-associated estrogen receptor signaling pathways in human cancers. Clin. Cancer Res. 13, 4672–4676 [DOI] [PubMed] [Google Scholar]
- 7. Song R. X., Santen R. J. (2006) Membrane initiated estrogen signaling in breast cancer. Biol. Reprod. 75, 9–16 [DOI] [PubMed] [Google Scholar]
- 8. Song R. X., Chen Y., Zhang Z., Bao Y., Yue W., Wang J. P., Fan P., Santen R. J. (2010) Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J. Steroid Biochem. Mol. Biol. 118, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas R. S., Sarwar N., Phoenix F., Coombes R. C., Ali S. (2008) Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-α activity. J. Mol. Endocrinol. 40, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., Metzger D., Chambon P. (1995) Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494 [DOI] [PubMed] [Google Scholar]
- 11. Joel P. B., Traish A. M., Lannigan D. A. (1995) Estradiol and phorbol ester cause phosphorylation of serine 118 in the human estrogen receptor. Mol. Endocrinol. 9, 1041–1052 [DOI] [PubMed] [Google Scholar]
- 12. Bunone G., Briand P. A., Miksicek R. J., Picard D. (1996) Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15, 2174–2183 [PMC free article] [PubMed] [Google Scholar]
- 13. Simoncini T., Hafezi-Moghadam A., Brazil D. P., Ley K., Chin W. W., Liao J. K. (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin M. B., Franke T. F., Stoica G. E., Chambon P., Katzenellenbogen B. S., Stoica B. A., McLemore M. S., Olivo S. E., Stoica A. (2000) A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141, 4503–4511 [DOI] [PubMed] [Google Scholar]
- 15. Marone R., Hess D., Dankort D., Muller W. J., Hynes N. E., Badache A. (2004) Memo mediates ErbB2-driven cell motility. Nat. Cell Biol. 6, 515–522 [DOI] [PubMed] [Google Scholar]
- 16. Qiu C., Lienhard S., Hynes N. E., Badache A., Leahy D. J. (2008) Memo is homologous to nonheme iron dioxygenases and binds an ErbB2-derived phosphopeptide in its vestigial active site. J. Biol. Chem. 283, 2734–2740 [DOI] [PubMed] [Google Scholar]
- 17. Meira M., Masson R., Stagljar I., Lienhard S., Maurer F., Boulay A., Hynes N. E. (2009) Memo is a cofilin-interacting protein that influences PLCγ1 and cofilin activities, and is essential for maintaining directionality during ErbB2-induced tumor-cell migration. J. Cell Sci. 122, 787–797 [DOI] [PubMed] [Google Scholar]
- 18. Bazley L. A., Gullick W. J. (2005) The epidermal growth factor receptor family. Endocr. Relat. Cancer 12, S17–27 [DOI] [PubMed] [Google Scholar]
- 19. Zaoui K., Honoré S., Isnardon D., Braguer D., Badache A. (2008) Memo-RhoA-mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J. Cell Biol. 183, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H., Xie X., Zhu X., Zhu J., Hao C., Lu Q., Ding L., Liu Y., Zhou L., Liu Y., Huang C., Wen C., Ye Q. (2005) Stimulatory cross-talk between NFAT3 and estrogen receptor in breast cancer cells. J. Biol. Chem. 280, 43188–43197 [DOI] [PubMed] [Google Scholar]
- 21. Burns K. A., Li Y., Arao Y., Petrovich R. M., Korach K. S. (2011) Selective mutations in estrogen receptor α D-domain alters nuclear translocation and non-estrogen response element gene regulatory mechanisms. J. Biol. Chem. 286, 12640–12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding L., Wang Z., Yan J., Yang X., Liu A., Qiu W., Zhu J., Han J., Zhang H., Lin J., Cheng L., Qin X., Niu C., Yuan B., Wang X., Zhu C., Zhou Y., Li J., Song H., Huang C., Ye Q. (2009) Human four-and-a-half LIM family members suppress tumor cell growth through a TGF-β-like signaling pathway. J. Clin. Invest. 119, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Y., Ding L., Zhang H., Han J., Yang X., Yan J., Zhu Y., Li J., Song H., Ye Q. (2006) Potentiation of Smad-mediated transcriptional activation by the RNA-binding protein RBPMS. Nucleic Acids Res. 34, 6314–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ding L., Yan J., Zhu J., Zhong H., Lu Q., Wang Z., Huang C., Ye Q. (2003) Ligand-independent activation of estrogen receptor α by XBP-1. Nucleic Acids Res. 31, 5266–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng L., Li J., Han Y., Lin J., Niu C., Zhou Z., Yuan B., Huang K., Li J., Jiang K., Zhang H., Ding L., Xu X., Ye Q. (2012) PES1 promotes breast cancer by differentially regulating ERα and ERβ. J. Clin. Invest. 122, 2857–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levin E. R. (2005) Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 19, 1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stossi F., Barnett D. H., Frasor J., Komm B., Lyttle C. R., Katzenellenbogen B. S. (2004) Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145, 3473–3486 [DOI] [PubMed] [Google Scholar]
- 28. Creighton C. J., Cordero K. E., Larios J. M., Miller R. S., Johnson M. D., Chinnaiyan A. M., Lippman M. E., Rae J. M. (2006) Genes regulated by estrogen in breast tumor cells in vitro are similarly regulated in vivo in tumor xenografts and human breast tumors. Genome Biol. 7, R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frasor J., Danes J. M., Komm B., Chang K. C., Lyttle C. R., Katzenellenbogen B. S. (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144, 4562–4574 [DOI] [PubMed] [Google Scholar]
- 30. Carroll J. S., Brown M. (2006) Estrogen receptor target gene: an evolving concept. Mol. Endocrinol. 20, 1707–1714 [DOI] [PubMed] [Google Scholar]
- 31. Song R. X., Zhang Z., Chen Y., Bao Y., Santen R. J. (2007) Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF7 breast cancer cells. Endocrinology 148, 4091–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boulware M. I., Kordasiewicz H., Mermelstein P. G. (2007) Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 27, 9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schlegel A., Wang C., Katzenellenbogen B. S., Pestell R. G., Lisanti M. P. (1999) Caveolin-1 potentiates estrogen receptor α (ERα) signaling. Caveolin-1 drives ligand-independent nuclear translocation and activation of ERα. J. Biol. Chem. 274, 33551–33556 [DOI] [PubMed] [Google Scholar]
- 34. Lu Q., Pallas D. C., Surks H. K., Baur W. E., Mendelsohn M. E., Karas R. H. (2004) Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc. Natl. Acad. Sci. U.S.A. 101, 17126–17131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cabodi S., Moro L., Baj G., Smeriglio M., Di Stefano P., Gippone S., Surico N., Silengo L., Turco E., Tarone G., Defilippi P. (2004) p130Cas interacts with estrogen receptor α and modulates non-genomic estrogen signaling in breast cancer cells. J. Cell Sci. 117, 1603–1611 [DOI] [PubMed] [Google Scholar]
- 36. Song R. X., Barnes C. J., Zhang Z., Bao Y., Kumar R., Santen R. J. (2004) The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 101, 2076–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manavathi B., Acconcia F., Rayala S. K., Kumar R. (2006) An inherent role of microtubule network in the action of nuclear receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 15981–15986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X., Yang Z., Zhang H., Ding L., Li X., Zhu C., Zheng Y., Ye Q. (2008) The estrogen receptor-interacting protein HPIP increases estrogen-responsive gene expression through activation of MAPK and AKT. Biochim. Biophys. Acta 1783, 1220–1228 [DOI] [PubMed] [Google Scholar]
- 39. Fox E. M., Andrade J., Shupnik M. A. (2009) Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids 74, 622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osborne C. K., Shou J., Massarweh S., Schiff R. (2005) Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin. Cancer Res. 11, 865s–870s [PubMed] [Google Scholar]
- 41. Murphy L. C., Seekallu S. V., Watson P. H. (2011) Clinical significance of estrogen receptor phosphorylation. Endocr. Relat. Cancer. 18, R1–R14 [DOI] [PubMed] [Google Scholar]
- 42. de Leeuw R., Neefjes J., Michalides R. (2011) A role for estrogen receptor phosphorylation in the resistance to tamoxifen. Int. J. Breast Cancer 232435:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De, Luca A., Maiello M. R., D'Alessio A., Pergameno M., Normanno N. (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 16, S17–S27 [DOI] [PubMed] [Google Scholar]
- 44. Milde-Langosch K. (2005) The Fos family of transcription factors and their role in tumourigenesis. Eur. J. Cancer 41, 2449–2461 [DOI] [PubMed] [Google Scholar]
- 45. Lopez-Bergami P., Lau E., Ronai Z. (2010) Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat. Rev. Cancer 10, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Musgrove E. A., Caldon C. E., Barraclough J., Stone A., Sutherland R. L. (2011) Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 11, 558–572 [DOI] [PubMed] [Google Scholar]
- 47. Meyer N., Penn L. Z. (2008) Reflecting on 25 years with MYC. Nat. Rev. Cancer 8, 976–990 [DOI] [PubMed] [Google Scholar]
- 48. Mandal S., Davie J. R. (2007) An integrated analysis of genes and pathways exhibiting metabolic differences between estrogen receptor positive breast cancer cells. BMC Cancer 7, 1471–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang X., Gao L., Wang S., McManaman J. L., Thor A. D., Yang X., Esteva F. J., Liu B. (2010) Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to herceptin. Cancer Res. 70, 1204–1214 [DOI] [PubMed] [Google Scholar]
- 50. Vogel C., Chan A., Gril B., Kim S. B., Kurebayashi J., Liu L., Lu Y. S., Moon H. (2010) Management of ErbB2-positive breast cancer: insights from preclinical and clinical studies with lapatinib. Jpn. J. Clin. Oncol. 40, 999–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]