Background: Dimerization of G protein-coupled receptors has an impact on their signaling properties.
Results: Dimerization with its truncated splice variant blocks ghrelin receptor activation.
Conclusion: The dominant effect exerted by the splice variant of the ghrelin receptor on the full-length one is due to allosteric conformational events within dimeric assemblies.
Significance: Unraveling the molecular mechanisms responsible for heteromer-directed selectivity is crucial for understanding GPCR-mediated signaling.
Keywords: 7-Helix Receptor, Arrestin, Fluorescence, G Protein-coupled Receptors (GPCR), G Proteins, Membrane Proteins, Dimerization, Ghrelin
Abstract
Heterodimerization of G protein-coupled receptors has an impact on their signaling properties, but the molecular mechanisms underlying heteromer-directed selectivity remain elusive. Using purified monomers and dimers reconstituted into lipid discs, we explored how dimerization impacts the functional and structural behavior of the ghrelin receptor. In particular, we investigated how a naturally occurring truncated splice variant of the ghrelin receptor exerts a dominant negative effect on ghrelin signaling upon dimerization with the full-length receptor. We provide direct evidence that this dominant negative effect is due to the ability of the non-signaling truncated receptor to restrict the conformational landscape of the full-length protein. Indeed, associating both proteins within the same disc blocks all agonist- and signaling protein-induced changes in ghrelin receptor conformation, thus preventing it from activating its cognate G protein and triggering arrestin 2 recruitment. This is an unambiguous demonstration that allosteric conformational events within dimeric assemblies can be directly responsible for modulation of signaling mediated by G protein-coupled receptors.
Introduction
Although it has been shown that G protein-coupled receptor (GPCR)2 monomers can efficiently activate G proteins (1–3) and recruit arrestins (3–5), homo- and heterodimers have been described for many receptors (6). Association between different GPCRs can lead to the formation of complexes with distinct and unique signaling properties (7). Some of these complexes activate distinct signaling effectors upon activation by the same ligand, a process named heteromer-directed signaling. Heteromer-directed signaling has been shown to be involved in the biological role of receptors but also in disease-associated deregulations of GPCR signaling (7). Despite of its functional relevance, the molecular mechanisms underlying modulation of GPCR pharmacology by dimers/oligomers remain elusive.
An increased diversity in signaling is caused by the generation of GPCR splice variants (8). A particular case of heteromer-directed selectivity is that associated with dimerization between GPCR splice variants and full-length receptors. Indeed, many of the splice variants have been shown to dimerize with a full-length counterpart and, by doing so, affect the pharmacological profile of the receptor with which they associate (8). For instance, association of a splice variant of the ghrelin receptor with the neurotensin NTS1 receptor results in a species that functions as a neuromedin U receptor (9). In many cases, dimerization between truncated splice variants and full-length receptors results in a dominant-negative effect on signaling (10–12). This is the case for the ghrelin receptor (13).
Ghrelin is a neuroendocrine peptide hormone that acts through its cognate GHS-R1a receptor to control major processes such as growth hormone secretion, food intake, or reward-seeking behaviors (14). Alternative splicing of the GHS-R1a gene results in a non-signaling truncated GHS-R1b protein that includes the TM1 to TM5 domains of the full-length receptor (15). GHS-R1a and GHS-R1b have been reported to coexist, particularly in several cancer cell lines (16). Importantly, GHS-R1b has been shown to dimerize with the full-length receptor with a dominant negative effect on GHS-R1a-mediated signaling as a direct consequence (13). This could be of importance under pathological conditions where both receptors are coexpressed (13, 16).
There is increasing evidence that GPCRs explore complex conformational landscapes in response to the binding of ligands (17, 18). The conformational states stabilized by pharmacologically distinct compounds likely interact with various efficacies with different signaling proteins, thus explaining the observed differences in efficacy toward the multiple pathways associated with a single receptor. However, how specific protein-protein contacts within heterodimeric species can affect the conformational landscape a receptor can adopt in response to ligand binding is still an open question.
We showed recently that monomeric GHS-R1a inserted into lipid discs adopts a complex conformational landscape in response to ligand and signaling protein binding (18). To explore the influence of dimerization on the structural dynamics of the ghrelin receptor, here we reconstituted, within a single lipid nanodisc, GHS-R1a as a monomer, homodimer, or heterodimer with GHS-R1b. This was made possible by an original method we developed to selectively purify well oriented dimeric species in lipid discs. Getting these dimeric assemblies opened the way to a strict comparison between the functional and structural features of the ghrelin receptor monomer and those in homo- and heterodimers. By doing so, we show that, although homodimerization does not significantly impact the functional and structural properties of the ghrelin receptor, GHS-R1b prevents GHS-R1a from activating its cognate signaling partners by restricting its conformational repertoire. This is to account for the dominant negative GHS-R1b exerts on ghrelin-associated signaling and illustrates how dimerization can affect signaling by modulating changes in GPCR conformation.
EXPERIMENTAL PROCEDURES
BLT1 Production, Labeling, and Lipid Disc Assembly
BLT1 was expressed in Escherichia coli inclusion bodies as a fusion with a fragment of α5 integrin (2). The protein was refolded in detergent-lipid mixed micelles (19), and ligand-competent proteins were purified on a 5bα affinity column (2). BLT1 was labeled with either Alexa Fluor 488 or Alexa Fluor 568 (Invitrogen) at a unique reactive cysteine in position 47 or 176 of a cysteine-free receptor (20). To this end, the receptor was incubated for 16 h at 4 °C at a dye:protein molar ratio of 10:1 and subsequently desalted using a PD10 column (Bio-Rad). Under the conditions used, labeling yields of 80–85% were achieved, as assessed by the absorbance of the protein at 276 nm and that of the dye at its maximal absorbance. For dimer assembly, receptors labeled either with the fluorophore donor or with the acceptor were mixed in equimolar amounts. Receptor dimers were reconstituted in lipid nanodiscs with MSP1E3(-) as the scaffolding protein and 1-palmitoyl-2-oleoylphosphatidylcholine/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG)/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS) as the lipids and purified as described for rhodopsin dimers (21). Preparations obtained under such conditions essentially corresponded to receptor dimers within a single disc.
PGD-based Purification of Receptor Dimers in Lipid Discs
To purify parallel dimers, purified bovine calmodulin was added to the receptor-containing particles at a 1:5 receptor:CaM molar ratio (protein concentrations in the 50–100 μm range) in a 20 mm Tris-HCl, 100 mm NaCl, 1 mm CaCl2 (pH 8 buffer), incubated for 1 h at 4 °C, and submitted to SEC. SEC was carried out on a Superdex 200 column (16 × 700 mm, GE Healthcare). The column was first equilibrated with the corresponding buffer, the protein fractions were loaded on the column (loaded volume not exceeding 1% of the total column volume), and elution was carried out with the equilibration buffer at a 0.2 ml/min flow rate. 0.3-ml fractions were collected. For complex dissociation, the CaM-receptor complex was incubated for 1 h in a 20 mm Tris-HCl, 300 mm KCl, 10 mm EGTA (pH 8) buffer, and the protein mixture was again submitted to SEC.
GHS-R1a-GHS-R1a Dimer Preparation
The GHS-R1a receptor was expressed, purified, and reconstituted in 0.2% (w/v) n-dodecyl-β-d-maltoside:0.02% (w/v) cholesteryl hemisuccinate micelles (3). The homodimer was then assembled into lipid nanodiscs and purified as described above for BLT1. For the bimane emission experiments, the receptor containing the unique reactive cysteine at position 304 was mixed in equivalent amounts with a GHS-R1a cysmin mutant devoid of all its reactive cysteines (18) before assembly into lipid discs. The cysmin mutant included a C-terminal S-tag for heterodimers purification (22). The receptor dimer with a single protomer that could be labeled with bimane was purified using the two-step procedure we developed with the leukotriene B4 receptors (22, 23). Parallel dimers were finally purified using the PGD-based procedure. For the GHS-R1aE124Q:GHS-R1a dimer, the mutant receptor included a C-terminal S-tag for heterodimer purification (22). In this case also, either the wild type or the mutant receptor bore the reactive Cys-304 residue. Receptor dimers were reconstituted by mixing equivalent amounts of GHS-R1a and GHS-R1aE124Q in 0.2% (w/v) n-dodecyl-β-d-maltoside:0.02% (w/v) cholesteryl hemisuccinate micelles. The GHS-R1aE124Q-GHS-R1a “heterodimers” were assembled into lipid discs and purified as above. In all cases, labeling of Cys-304 with bimane was carried out as in Ref. 18. Similar labeling ratios were obtained in all cases.
GHS-R1a-GHS-R1b Dimer Purification
The ghrelin receptors were produced as described (3). GHS-R1b included a C-terminal S tag for heterodimer purification (22). After mixing equivalent amounts of GHS-R1a and GHS-R1b in 0.2% (w/v) n-dodecyl-β-d-maltoside:0.02% (w/v) cholesteryl hemisuccinate micelles, receptor heterodimers were assembled into a disc and purified as above.
FRET-monitored Ligand Binding and GTPγS Binding Assays
Ligand binding assays were carried out as described in Ref. 3. Briefly, direct ligand binding experiments were performed using fluorescence energy transfer with the purified receptor labeled with Alexa Fluor 350 at its N terminus and a ghrelin peptide labeled with FITC (JMV 4946). Titration experiments were carried out with protein concentrations in the 10 nm protein concentration range and increasing ligand concentrations. Competition experiments were carried out by adding increasing concentrations of the competing compound to a receptor-JMV 4946 mixture (100 nm concentration range). Fluorescence emission spectra were recorded at 20 °C between 400 and 600 nm on a Cary Eclipse spectrofluorimeter (Varian) with an excitation at 346 or 488 nm. Buffer contributions were systematically subtracted. The FRET ratio corresponds to the ratio of the acceptor-emitted fluorescence at 520 nm from excitation at two different wavelengths, 346 and 488 nm (24). All binding data were analyzed using GraphPad Prism software (version 4.0).
G Protein Activation Assays
Purified Gαqβ1γ2 trimer expressed in Sf9 cells (25) was used in all assays. GTPγS binding experiments were carried out for 5 min at 20 °C using the fluorescent BODIPY FL GTPγS analog (26). The reaction mixture consisted in 200 nm Gq, 200 nm BODIPY FL GTPγS and 20 nm ghrelin receptor-containing nanodiscs in a buffer 20 mm Tris-HCl pH 7.4, 150 mm NaCl, 100 μm EDTA, 3 mm MgCl2. Fluorescence emission was recorded at 511 nm with an excitation at 500 nm (Cary Eclipse spectrofluorimeter, Varian). All data were normalized to the fluorescence measured under the same conditions with empty discs.
Arrestin recruitment assay
The phosphorylation independent arrestin-2 mutant L68C-R169E was produced in E. coli (27) and labeled on Cys-68 with the thiol alkylating fluorescent compound monobromobimane (28). For the recruitment assay, arrestin was added at a 5:1 arrestin:ghrelin receptor-containing discs molar ratio. A 20 mm Tris-HCl, 250 mm NaCl, 0.5 mm EDTA (pH 7.4) buffer was used in all experiments. Incubation was carried out for 45 min at 4 °C. The different ligands were then added at a 10 μm concentration (or at increasing concentration for the dose-response experiment) and incubated for 45 additional min at 4 °C. Fluorescence emission was recorded at 20 °C between 400 and 600 nm on a Cary Eclipse spectrofluorimeter (Varian) with an excitation at 380 nm. All data were normalized to the fluorescence measured under the same conditions with empty discs.
Fluorescence Spectroscopy
For the bimane fluorescence measurements, all experiments were carried out at the same bimane concentration (5 μm range with similar labeling ratios in all cases). The ghrelin receptor-containing discs were incubated with either increasing or saturating concentrations (50 μm) of the different ligands for 30 min. In the experiments with the purified effector proteins, the latter were added to the ligand-free receptor (the receptor-to-G-protein ratio was 1:10 and the receptor-to-arrestin ratio was 1:5) and incubated for 30 min. The different ligands were then added, and incubation was pursued for another 30 min. Fluorescence emission spectra were recorded using a Cary Eclipse spectrofluorimeter (Varian) with an excitation wavelength at 380 nm. For Trp emission, the experimental setup was similar, with the exception that excitation was at 295 nm and emission between 300 and 500 nm.
RESULTS
An Original Peptide Tag Enables Purification of GPCR Parallel Dimers
Before delineating the functioning of ghrelin receptor dimers incorporated into lipid discs, it was crucial to devise a method to control the relative orientation of the receptors when assembled into the lipid discs. Indeed, two receptor molecules incorporated in individual nanodiscs adopt both the natural parallel and the non-natural antiparallel orientations (29). We designed here a method for purifying particles where the two protomers are in the parallel orientation. This method is depicted schematically in Fig. 1A. It relies on the use of the CaM binding domain from petunia glutamate decarboxylase (named here PGD tag). The CaM-binding domain of PGD is a 26-residue region that makes a unique and stable complex with CaM of well defined stoichiometry, i.e. a single calcium-loaded CaM with two peptides (30). Because the PGD peptide interacts with CaM only as a dimer, if it is fused to the N terminus of the receptor, only lipid discs where the peptides are sufficiently close in space, i.e. particles where the receptor N termini are on the same side of the lipid disc, should bind CaM.
FIGURE 1.
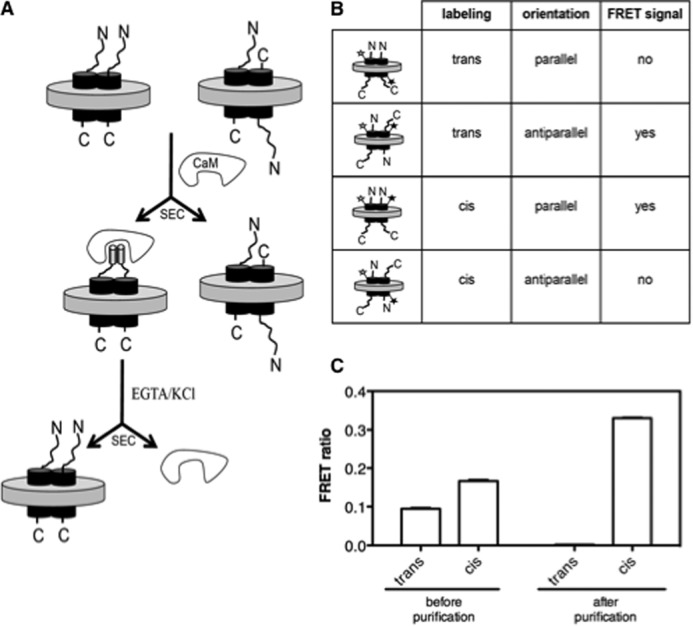
Parallel dimer purification. A, schematic representation of the PGD tag-based purification method. The tag is fused to the N terminus of the receptor and is represented schematically as a cylinder in the presence of CaM to account for its helical structure upon binding to CaM. Parallel dimers were separated from non-parallel ones using SEC. Another possibility would be to consider a CaM affinity-based method using calmodulin immobilized on a chromatographic matrix (CaM-Sepharose 4B). B, schematic representation and putative associated interprotomer FRET signal of the different species considered. The white star represents the fluorescence donor and the black one the acceptor. C, FRET ratio measured between Alexa Fluor 488 and Alexa Fluor 568-labeled BLT1 protomers reconstituted in lipid nanodiscs before and after purification using the PGD tag. Results are given as mean ± S.D. from three different experiments.
The leukotriene B4 BLT1 receptor was first used to validate the PGD tag-based purification procedure. We selected this receptor as a model because we have in hand a full series of unique cysteine-containing mutants that allowed a detailed exploration of the relative arrangement of receptor proteins within the dimer using interprotomer FRET (2). Receptor dimers in which one of the protomers bore the fluorescent donor and the other the acceptor were assembled into lipid discs, and the orientation of the two receptors within the dimeric assembly was investigated using fluorescence transfer between these two probes. Two different kinds of fluorescent labeling were considered for assessing BLT1 orientation in the lipid discs. In the first one, the donor was on the extracellular loop of one protomer and the acceptor attached to Cys-47 in the intracellular loop of the other (trans-labeling, Fig. 1B). In the second one, the donor and the acceptor were both attached to Cys-176 in the extracellular loop of each of the two protomers (cis-labeling, Fig. 1B). On the basis of the R0 value we measured for the two probes and the distance between the extracellular and intracellular loops of the receptor inferred from the three-dimensional model of BLT1, FRET is observed only when the fluorophores are located on the same side of the dimeric assembly (Fig. 1B). In agreement with this assertion, no FRET was observed when the fluorescence donor was positioned in the extracellular loop and the acceptor in the intracellular loop of the same receptor protein (supplemental Fig. S1A). As shown in Fig. 1C, a significant interprotomer FRET signal was obtained after dimer reconstitution into lipid discs when cis- or trans-labeling was considered. This signal was independent of particle concentration (supplemental Fig. S1B), indicating that it originates exclusively from two receptor molecules within the same lipid disc. A significant signal for trans-labeled dimer preparations attests to the presence of the antiparallel orientation in our preparation (Fig. 1B). We then purified parallel receptors using the PGD tag as described under “Experimental Procedures” (see SEC profile in supplemental Fig. S2) and again analyzed the geometrical features of the dimer with FRET. In this case, FRET was observed with cis-labeling exclusively (Fig. 1C). This is strong evidence that, in contrast to what was obtained just after disc reconstitution, the receptor dimers are all in the parallel orientation.
We then applied this purification method to ghrelin receptors to get both the purified GHS-R1a-GHS-R1a homodimer and the purified GHS-R1a-GHS-R1b heterodimer. In all cases, each of the protomer included a different purification tag so that complexes of specific protomer composition (see below) were isolated using the two-step procedure we developed previously (23). Of importance, this procedure not only allowed the selection of dimers of well defined composition but it also allowed specific removal of any residual monomeric species, giving rise to homogeneous dimer preparations. Parallel homo- and heterodimers were then efficiently purified using the PGD tag (supplemental Fig. S3).
GHS-R1a Dimerization and Ligand Binding
We then assessed whether homo- and heterodimerization of the ghrelin receptor affected its ligand binding. To this end, we first monitored the ghrelin-binding properties of the GHS-R1a-GHS-R1a homodimer using a FRET-based assay (3). As shown in Fig. 2, the receptor homodimer displays a Kd value for the fluorescein-labeled ghrelin-derived JMV 4946 (3) closely related to that of the receptor monomer (70 and 75 nm for the monomer and homodimer, respectively). Only the shape of the dose-response profile was slightly different, depending on whether the monomer and the homodimer were considered. Indeed, the shape of the dose-response curve obtained for the homodimer is suggestive of the occurrence of negative cooperativity effects. This would be in agreement with what had been reported for the ghrelin receptor upon binding its ghrelin agonist (31). We further assessed the pharmacological profile of the monomer and dimer in competition assays using JMV 4946 as the fluorescent tracer. As shown in Table 1, similar Ki values were obtained for all the ligands regardless of whether the monomer or the dimer was considered. These data indicate that homodimerization of GHS-R1a does not have a major impact on its ligand-binding properties.
FIGURE 2.
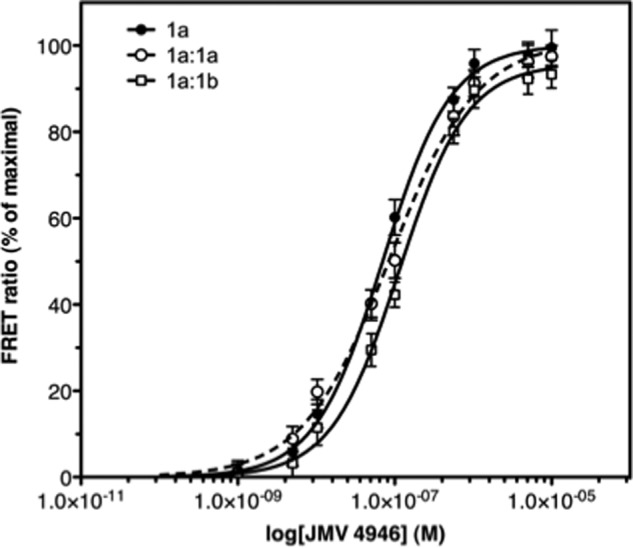
Influence of GHS-R1a dimerization on ligand binding. FRET-monitored binding of a FITC-labeled ghrelin peptide (JMV 4946) to Alexa Fluor 350-labeled GHS-R1a assembled in lipid discs. The binding data are presented as the variations in FRET ratio as a function of JMV 4946 concentration. 1a, monomer; 1a:1a, homodimer; 1a:1b, GHS-R1a-GHS-R1b heterodimer. The data represent the mean ± S.D. from three independent experiments.
TABLE 1.
Ki values (in nm) of compounds from different pharmacological classes determined from competition binding fluorescent assays between JMV 4946 and the different monomeric and dimeric purified receptors
| Ghrelin | MK 0677 | JMV 3002 | JMV 3011 | SPA | |
|---|---|---|---|---|---|
| GHS-R1a | 78.4 ± 8.2 | 65.8 ± 6.3 | 9.2 ± 2.7 | 8.3 ± 3.3 | 293.7 ± 10.2 |
| GHS-R1b | |||||
| GHS-R1aE124Q | 912 | 993 | 915 | 852 | |
| GHS-R1a:GHS-R1a | 75.6 ± 7.7 | 68.4 ± 7.2 | 9.9 ± 2.5 | 10.2 ± 3.9 | 286.3 ± 12.8 |
| GHS-R1a:GHS-R1b | 109.6 ± 6.2 | 90.6 ± 5.3 | 19.3 ± 3.3 | 9.9 ± 2.8 | 291.8 ± 9.2 |
| GHS-R1a:GHS-R1aE124Q | 79.4 ± 5.6 | 68.9 ± 2.3 | 8.7 ± 3.8 | 8.9 ± 4.2 | 299.6 ± 6.5/988 |
We then analyzed the influence of GHS-R1b on the ligand-binding properties of GHS-R1a. The purified truncated receptor did not bind the ghrelin-derived peptide JMV 4946, as assessed using monomeric GHS-R1b (Table 1). This indicates that the TM6 and TM7 domains of the ghrelin receptor are mandatory for ligand recognition either through direct interactions or for correct structuring of the receptor ligand-binding pocket. In contrast, specific JMV 4946 binding was observed within the GHS-R1a-GHS-R1b heterodimer (Fig. 2). Because GHS-R1b does not bind its ligands by itself, it is reasonable to assume that GHS-R1a is responsible for JMV 4946 binding in the heterodimer. Interestingly, although within the same range, the Kd value of the heterodimer for JMV 4946 is slightly reduced compared with that of the monomer (70 and 113 nm for the monomer and heterodimer, respectively). In the same way, the Ki values for full (ghrelin, MK0677) and partial (JMV3002) agonists were slightly reduced, whereas those for the neutral antagonist (JMV3011) and the inverse agonist (SPA) were essentially unaffected (Table 1). This indicates that the ghrelin receptor essentially maintains its ligand-binding ability within the GHS-R1a:GHS-R1b dimer.
GHS-R1a Dimerization and Arrestin 2 Recruitment
We then assessed the impact of dimerization on the ability of the ghrelin receptor to recruit arrestin 2 in response to agonist binding. As reported previously (3, 18), the GHS-R1a monomer recruits arrestin in response to the binding of the full agonist ghrelin, whereas no recruitment was observed with either the JMV 3002 Gq-biased agonist (32), the JMV 3011 neutral antagonist (32), or the inverse agonist SPA (Fig. 3). The ghrelin receptor homodimer displayed a similar behavior, i.e. it recruited arrestin 2 to a similar extent in response to ghrelin binding only (Fig. 3). Half-saturation of ghrelin-catalyzed arrestin recruitment was reached at comparable agonist concentrations with the monomer and the homodimer (Fig. 3A), indicating that homodimerization does not significantly affect the way the ghrelin receptor interacts with arrestin 2.
FIGURE 3.
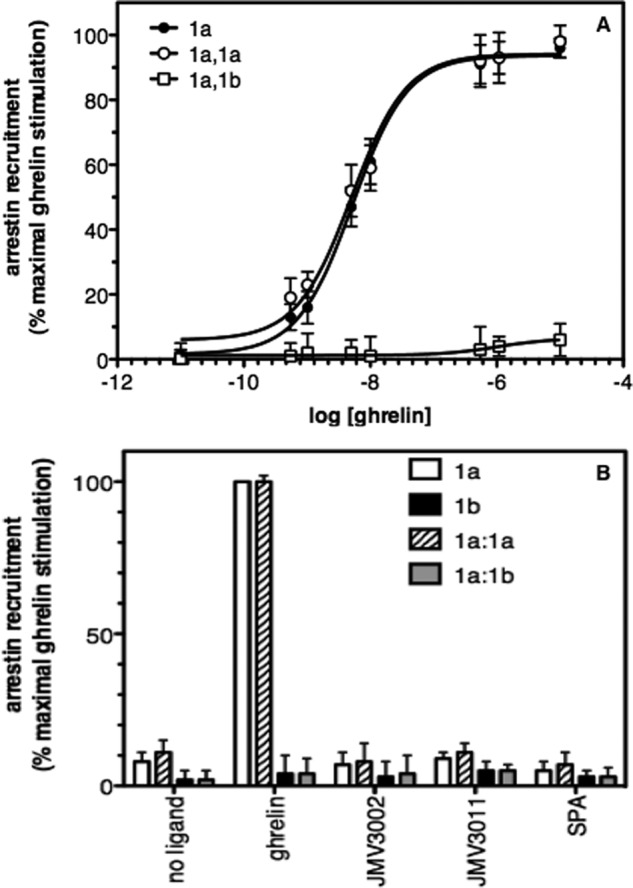
Influence of GHS-R1a dimerization on arrestin recruitment. A, changes in bimane emission intensity of labeled arrestin 2 induced by GHS-R1a in the presence of increasing concentrations in ghrelin. Data are presented as the percentage of maximum bimane fluorescence change measured in the presence of ghrelin. B, changes in emission intensity of bimane-labeled arrestin 2 induced by the purified receptors in the absence and presence of ligands (full agonist, ghrelin; partial agonist, JMV3002; neutral antagonist, JMV3011; inverse agonist, SPA; ligand concentration, 10 μm). 1a, GHS-R1a monomer; 1b, GHS-R1b monomer; 1a:1a, GHS-R1a-GHS-R1a homodimer; 1a:1b, GHS-R1a-GHS-R1b dimer. In all cases, data are presented as the mean ± S.D. from three independent experiments.
However, in contrast to what occurred with the monomer or with the homodimer, no measurable arrestin 2 recruitment was observed with the GHS-R1a:GHS-R1b dimer, even in the presence of saturating concentrations in ghrelin (Fig. 3). This indicates that GHS-R1a does not recruit arrestin in response to full agonist binding when associated with GHS-R1b in the same disc (Fig. 3). This is not the mere consequence of reconstituting two receptors within a unique disc because neither associating two GHS-R1a receptors (Fig. 3) nor GHS-R1a with the unrelated LTB4 receptor BLT1 (supplemental Fig. S4) affected ghrelin-induced recruitment of arrestin by GHS-R1a.
GHS-R1a Dimerization and G Protein Activation
We next investigated the ability of the ghrelin receptor in the homo- and heterodimer to activate its cognate Gq protein. As shown in Fig. 4, the ghrelin receptor homodimer displayed a constitutive activity within the same range than that of the monomer, indicating that homodimerization does not significantly impact the high basal activity of the ghrelin receptor. In contrast, we could not measure any basal Gq activation after reconstitution of GHS-R1a with GHS-R1b within the same lipid disc (Fig. 4). Logically, with this absence of constitutive activity was associated an absence of any significant effect of the inverse agonist SPA (Fig. 4). Again, this effect was not due to the simple association of two receptors within the same disc because reconstitution of the homodimer (Fig. 4) or of GHS-R1a with BLT1 (supplemental Fig. S5) did not alter the constitutive activity of the ghrelin receptor.
FIGURE 4.
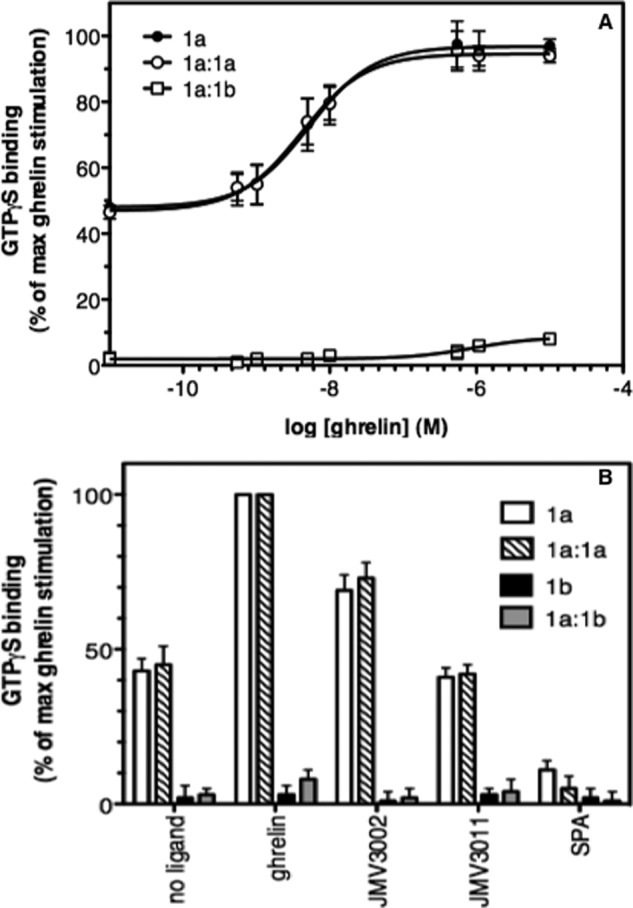
Influence of GHS-R1a dimerization on G protein activation. A, BODIPY FL GTPγS binding to Gαq (Gαqβ1γ2) induced by GHS-R1a-containing discs in the presence of an increasing concentration in ghrelin. Data are presented as the percentage of maximum BODIPY FL fluorescence change measured in the presence of ghrelin. B, BODIPY FL GTPγS binding to Gαq triggered by the purified receptors in the absence and in the presence of ligands (full agonist, ghrelin; partial agonist, JMV3002; neutral antagonist, JMV3011; inverse agonist, SPA; ligand concentration, 10 μm). 1a, GHS-R1a monomer; 1b, GHS-R1b monomer; 1a:1a, GHS-R1a-GHS-R1a homodimer; 1a:1b, GHS-R1a-GHS-R1b dimer. In all cases, the data represent the mean ± S.D. from three independent experiments.
Monomeric GHS-R1a in lipid nanodiscs further activated Gq in an agonist-dependent manner (Fig. 4). This was the case also for the purified homodimer that was able to trigger Gq activation in a ghrelin dependent manner (Fig. 4). Half-saturation of ghrelin-catalyzed GTPγS binding was reached at comparable agonist concentrations with the monomer and the homodimer (Fig. 4A). As is the case for the basal activity, however, no significant ghrelin-induced Gq activation was observed when GHS-R1a was associated with GHS-R1b (Fig. 4). The lack of ghrelin-induced G protein activation is a specific effect resulting from the association of GHS-R1a with GHS-R1b because it occurs neither when GHS-R1a is associated with BLT1 nor when GHS-R1b is assembled with BLT1 (supplemental Fig. S5). Overall, our observations imply that assembling GHS-R1a with its truncated counterpart abolishes both its constitutive activity and its ability to trigger Gq protein activation in an agonist-dependent manner.
GHS-R1a Dimerization and Receptor Activation
We then investigated the structural determinants underlying the dominant negative activity of GHS-R1b with respect to arrestin recruitment and G protein coupling. A crowding effect resulting from the assembly of two receptor proteins within the same disc cannot be invoked because homodimerization has no effect on these processes. Another possibility would be that dimerization with GHS-R1b affects the conformational landscape of GHS-R1a preventing it from reaching the active conformation responsible for recruitment and/or activation of signaling partners. To assess this point on an experimental basis, we used bimane attached to Cys-304 in the extracellular tip of TM7 as a fluorescent reporter to explore changes in GHS-R1a conformation (18). Variations in bimane emission are very likely to account for global changes in receptor conformation, as evidenced by the strict concordance between the changes in bimane emission and those in receptor tryptophan fluorescence observed upon agonist binding (supplemental Fig. S6).
We thus compared the emission properties of bimane bound to GHS-R1a in the monomer, the homodimer, and the GHS-R1a-GHS-R1b heterodimer. For the homodimer, only one of the two protomers bore the reactive Cys-304 residue that can be labeled with bimane. As reported previously with the leukotriene B4 receptor (19, 23), labeling only one protomer within the dimer allows to specifically monitor the changes in the conformation of the labeled subunit without being blurred by the mixed contribution of the two receptors. This is also the case for the heterodimer, where only the full-length receptor was labeled with the fluorescent probe.
In the absence of any ligand, the emission properties of bimane were similar whether GHS-R1a was monomeric, homodimeric, or heterodimeric (Fig. 5A). This suggests that the conformational features of the ligand-free ghrelin receptor are not primarily dependent on its oligomerization state, at least as far as the environment of the bimane moiety attached to the extracellular tip of TM7 is concerned. This suggests that dimerization may not primarily affect the basal conformational state of the ghrelin receptor.
FIGURE 5.
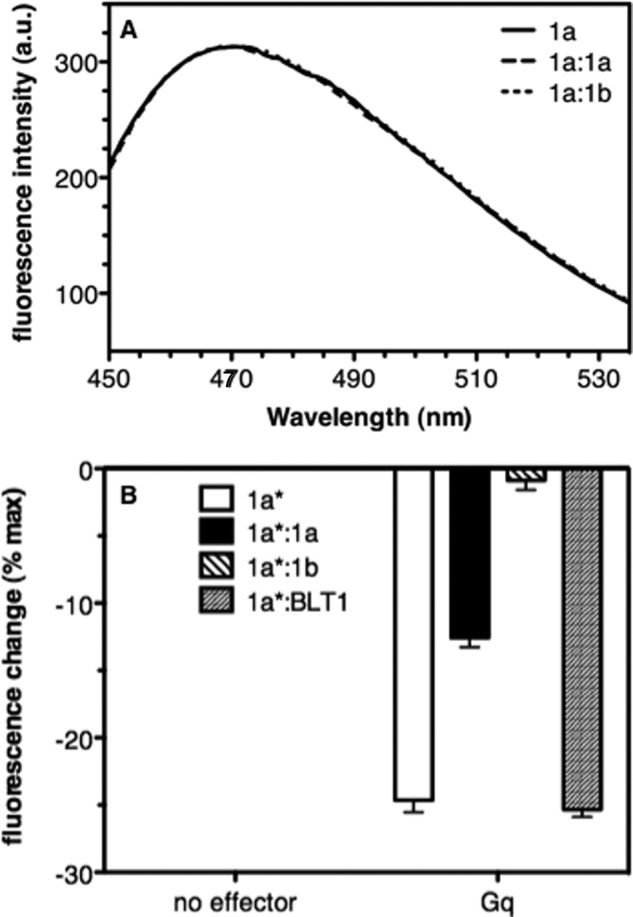
Influence of GHS-R1a dimerization on Gq-induced GHS-R1a conformational changes. A, fluorescence emission spectra of bimane attached to Cys-304 in GHS-R1a in the absence of ligand. B, changes in maximum emission intensity of bimane attached to GHS-R1a in the absence and presence of Gαqβ1γ2 (receptor-to-G protein ratio, 1:10). 1a, GHS-R1a monomer; 1a:1a, GHS-R1a-GHS-R1a homodimer; 1a:1b, GHS-R1a-GHS-R1b dimer; 1a:BLT1, GHS-R1a:BLT1 “dimer.” The asterisk indicates which protomer is labeled with bimane. Data represent the mean ± S.D. from three independent experiments.
Because the high constitutive activity is an important characteristic of the ghrelin receptor, we subsequently investigated the influence of Gq on the ligand-free receptor conformation. Adding the G protein trimer to the ligand-free receptor monomer is associated with a significant change in bimane emission intensity (Fig. 5B). This change has been attributed to the stabilization of the active conformation of the ghrelin receptor by the G protein in the absence of any agonist and is likely responsible for the high constitutive activity of the ghrelin receptor (18). In the case of the homodimer, the change in bimane emission was of a strict intermediate value compared with that of the monomer (Fig. 5B). Such an intermediate change in the spectroscopic signature of a GPCR in a dimer had already been reported in the case of the leukotriene B4 receptors and was attributed to an asymmetry in the dimeric assembly, with only one of the two protomers in the fully active state (19). Keeping in mind that only one of the two equivalent protomers in the ghrelin receptor homodimer is labeled with bimane, our data are, therefore, indicative of a model where the two protomers in the dimer differ in their activation state with only one in the active conformation. However, we cannot totally exclude, at this stage of our analysis, an alternative model where the intermediate change in bimane emission intensity would result from a different active conformation compared with that reached in the monomer.
In contrast to what was obtained with the ghrelin receptor monomer and homodimer, where Gq addition to the ligand-free receptor triggered a significant change in bimane emission, essentially no change in the emission properties of the ligand-free GHS-R1a labeled with bimane was observed upon addition of Gq to the GHS-R1a-GHS-R1b complex (Fig. 5B). This is a specific effect of the reconstitution with GHS-R1b because the variations in bimane emission upon addition of Gq were the same whether GHS-R1a was monomeric or associated to BLT1 (Fig. 5B). These data suggest that the truncated receptor prevents the full-length receptor from undergoing the Gq-triggered change in conformation that occurs with the monomer and the homodimer. This is likely to be responsible for the absence of constitutive activity of GHS-R1a in the dimer with GHS-R1b.
We then compared the ligand-induced changes in the emission of bimane attached to GHS-R1a in the monomer to those measured when homodimeric or associated to GHS-R1b. As is the case for Gq-induced changes, the variations in bimane emission intensity of one of the protomers within the homodimer were systematically of intermediate amplitude compared with those observed with the monomeric species (Fig. 6A). As stated above, this is likely due to an asymmetric dimeric assembly where only one of the protomer reaches its fully active conformation.
FIGURE 6.
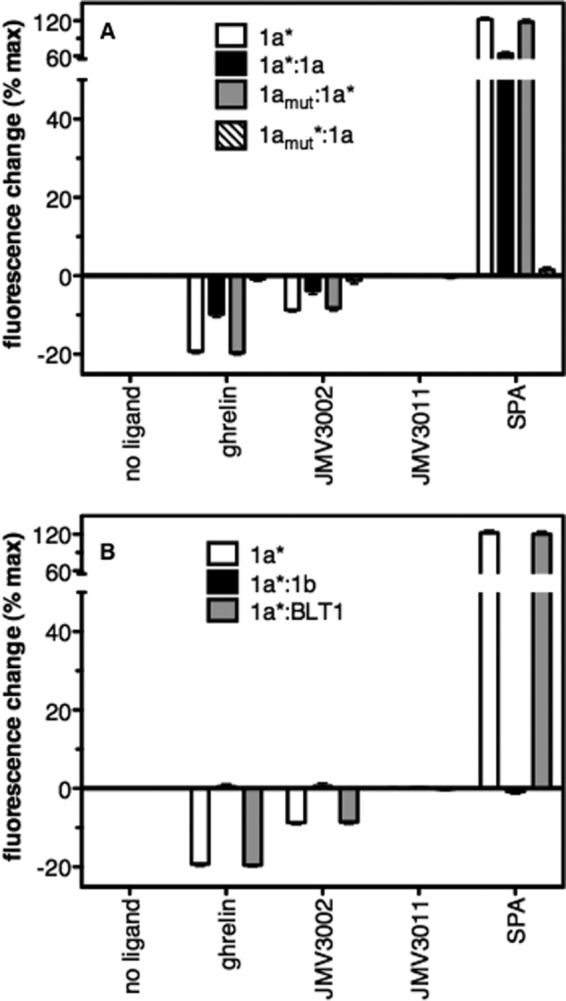
Influence of dimerization on ligand-induced GHS-R1a conformational changes. Shown are the changes in maximum emission intensity of bimane attached to GHS-R1a in the absence and presence of ligands (full agonist, ghrelin; partial agonist, JMV3002; neutral antagonist, JMV3011; inverse agonist, SPA; ligand concentration, 10 μm). Data are presented as percent changes in maximum emission intensity compared with that of the monomeric receptor in the absence of ligand. 1a, GHS-R1a monomer; 1a:1a, GHS-R1a-GHS-R1a homodimer; 1amut:1a, GHS-R1aE124Q-GHS-R1a dimer; 1a:1b, GHS-R1a-GHS-R1b dimer; 1a:BLT1, GHS-R1a-BLT1 dimer. The asterisk indicates the protomer labeled with bimane. In all cases, data represent the mean ± S.D. from three independent experiments.
To further explore this asymmetry model, we produced a GHS-R1a dimer where only one protomer can bind its ligand following the strategy we developed with BLT1 (22). To this end, we introduced in the GHS-R1a receptor the E124Q mutation in TM3 that had been shown to abolish the ability of the ghrelin receptor to bind its ligands without significantly affecting its constitutive activity (33). As is the case in more complex cellular systems, this mutation abolished agonist binding when introduced in the purified receptor (Table 1). As a consequence, the purified mutant receptor did not display agonist-induced Gq activation and arrestin recruitment, whereas its basal activity at Gq was preserved (supplemental Fig. S7). Importantly, the E124Q mutation is not likely to affect the global conformation of the receptor because the Trp emission profiles of the wild-type and mutant receptors are very similar (supplemental Fig. S8). Its effects on agonist binding are therefore probably because of a modification of local receptor-ligand contacts, as proposed initially (33). The E124Q mutant receptor was then assembled with a wild-type protomer within the same disc. As for the homodimer, either the wild-type or the mutant receptor bore the unique reactive cysteine for bimane labeling. We then monitored the changes in bimane emission of this dimeric species upon binding the MK0677 agonist. The latter was selected rather than ghrelin because it displays essentially no residual binding to the E124Q mutant (Table 1). We reported previously that ghrelin and MK0677, both full agonists of the ghrelin receptor, strictly trigger the same changes in GHS-R1a conformation (18). As shown in Fig. 6A, the variation in bimane emission intensity observed when the labeled protomer was the ligand-competent one was very similar to that observed with the monomer. In contrast, no change was observed in bimane emission when the probe was attached to the mutant receptor (Fig. 6A). Importantly, the receptor assembly with only one of the two protomers bound with the agonist and, in the active conformation, activated the G protein and recruited arrestin to a similar extent as the wild-type, unmodified homodimer (supplemental Fig. S9). In the same way, similar effects were observed with the partial agonist, the inverse agonist, and the neutral antagonist when comparing the monomer and the homodimer with only one ligand-competent protomer (Fig. 6A). Overall, these data indicate that, when assembled in a homodimer, the ghrelin receptor can reach an active state upon binding its full agonist similar to that observed with the monomer.
In contrast to what was observed with the homodimer, assembling GHS-R1a and GHS-R1b within the same disc essentially abolished all the ligand-induced conformational changes observed with monomeric GHS-R1a whether the ligand was a full, partial, or inverse agonist (Fig. 6B). As is the case for Gq-induced changes, this was not due to the mere association with a second receptor because assembling GHS-R1a with itself (Fig. 6A) or with BLT1 (B) did not alter the agonist-induced changes. Likewise, the effects observed are to be specific because GHS-R1b did not affect LTB4-induced changes in BLT1 conformation either (supplemental Fig. S10). This indicates that GHS-R1b restricts the conformational dynamics of the full-length ghrelin receptor, preventing it from undergoing any of the conformational changes that occur in the monomeric receptor in response to ligand binding and G protein coupling.
DISCUSSION
Biophysical methods applied to purified receptors and receptor complexes can provide dynamic information that, at the moment, cannot be totally supplied by crystallography. In this context, model membrane systems such as lipid discs provide invaluable tools to characterize GPCR dimers through biophysical methods applied to individual particles containing two proteins. However, before delineating the functioning of receptor dimers incorporated into lipid discs, it was crucial to devise a method to control the relative orientation of the receptors incorporated into individual discs. Indeed, it had been shown that two receptor molecules incorporated into the same lipid disc adopt both the natural parallel and the non-natural antiparallel orientations (29). We have designed a totally original method aimed at purifying model membrane particles where the two protomers are in the parallel orientation. Of importance, in contrast to other dimerization motives (34), the PGD peptide is totally unstructured and unable to dimerize per se as long as CaM is not added (30) so that fusing it to the N terminus of the receptor shall not affect its expression, purification, folding, or dimeric arrangement. The efficient PGD tag-based purification of the ghrelin and LTB4 receptor parallel dimers suggests that the method may be of general use for functional reconstitution of GPCR dimers into lipid discs.
This dimer purification procedure provided us with an unprecedented possibility to compare the pharmacological properties of ghrelin receptor monomers, homodimers, and heterodimers. By doing so, we clearly establish that homodimerization of the ghrelin receptor does not significantly affect its ligand binding, arrestin recruitment, and G protein activation properties.
Our data suggest that the ghrelin receptor homodimer is an asymmetric assembly upon agonist activation, with only one of the two protomers in the active conformation, indicating that this may be a general property of GPCR dimers (19, 35–37) or even of higher-order assemblies (38). Interestingly, our observations imply that activating only one protomer in the homodimer is sufficient not only for Gq activation, as reported previously for the leukotriene B4 receptors (19, 23), but also for arrestin recruitment. The latter observation may explain the cross-internalization effects observed in more complex cellular systems for different receptors (39–41). Furthermore, our data with the ligand-free receptor homodimer in the presence of Gq also suggest that asymmetry is not restricted to the activation process with agonists but may also apply in the context of constitutive activity. Indeed, our results suggest that the transition from the active to the inactive state that occurs upon precoupling of the ligand-free receptor with the G protein is different for each of the protomers within the dimer. The systematic occurrence of asymmetry in the dimer upon activation by either ligands or signaling proteins could mean, as proposed recently (42), that the structural architecture of a GPCR dimeric assembly is hardly compatible with the two protomers being in the active conformation at the same time.
Besides, our data with the GHS-R1a-GHS-R1b dimer indicate that the full-length ghrelin receptor within this heterodimeric complex essentially maintains its ligand-binding ability. In contrast to homodimerization, however, heterodimerization with the non-signaling splice variant abolishes GHS-R1a-mediated activation of associated signaling proteins. Indeed, neither arrestin recruitment nor Gq protein activation was detected when the ghrelin receptor GHS-R1a was associated to GHS-R1b within the same disc. Strikingly, in the case of G proteins, not only agonist-mediated Gq activation but also constitutive activity was abolished. These observations establish that the dominant negative effect observed in vivo (13) is the direct consequence of the physical association between both receptors within the same membrane space. In this context, it is to be noted that our purified system easily allowed the unambiguous identification of the heteromer signature (43) with dimer-directed selectivity effects on functional outputs (G protein activation, arrestin recruitment), something that is actually difficult to distinguish from functional cross-talk effects when using more complex cellular model systems (44).
In contrast to what happens in monomers and homodimers, none of the ligand- and Gq-induced changes in GHS-R1a conformation were observed in the GHS-R1a-GHS-R1b dimer. These observations plead for a mechanism where GHS-R1b exerts its dominant negative effect on GHS-R1a-mediated activation of G proteins and recruitment of arrestin 2 by restricting the conformational dynamics of the full-length ghrelin receptor and freezing it in a basal, non-active conformation (Fig. 7). Such a restriction in receptor conformational changes that prevents GHS-R1a from reaching its active structure is likely due to specific protein-protein contacts within the dimeric assembly. An alternative possibility would be that dimerization of GHS-R1a with GHS-R1b prevents signaling proteins to interact with the full-length receptor because of steric and not conformational effects, as reported in the MT1-GPR50 dimer (45). However, an effect originating from crowding on the surface of the nanodisc cannot be invoked because assembling GHS-R1a within a homodimer or with the unrelated BLT1 receptor in the same disc does not prevent the ghrelin receptor from interacting with arrestin 2 and G proteins. Conversely, assembling BLT1 with GHS-R1b within the same nanodisc does not prevent the LTB4 receptor from activating Gi.
FIGURE 7.
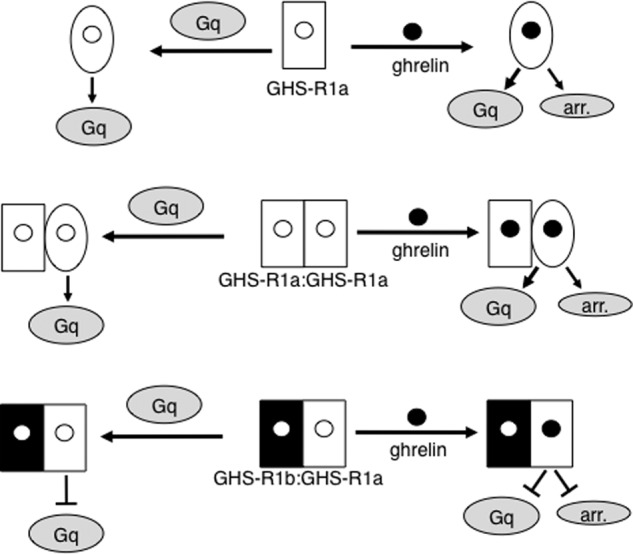
Agonist-induced activation of GHS-R1a in monomers and dimers. Shown is a schematic representation of the proposed mechanism for ghrelin receptor activation in the different assemblies (monomer, homodimer, and heterodimer) considered in this work and its consequence of Gq activation and arrestin (arr.) recruitment. The different shapes represent different conformations of the ghrelin receptor. The assumption that GHS-R1b in the heterodimer does not bind its ligand is made on the basis that the monomeric truncated receptor is totally ligand-incompetent.
Interestingly, the ligand-free GHS-R1a appears to display similar conformational features whether monomeric, homodimeric, or associated with GHS-R1b. This suggests that dimerization with GHS-R1b does not significantly affect the basal conformational state of the ghrelin receptor, at least as far as the environment of the bimane probe is considered. This similarity in conformation is puzzling because the ligand-free GHS-R1a differs in its functional properties in the monomer/homodimer and in the heterodimer with GHS-R1b. Indeed, as stated above, GHS-R1a completely loses its constitutive activity when associated to its splice variant. We proposed previously that the constitutive activity of the monomeric ghrelin receptor could be due to an inherent flexibility of the basal conformational state that would allow it to undergo a transition toward an active state upon precoupling with the G protein in the absence of any agonist (18). In agreement with this model, the absence of constitutive activity of GHS-R1a within the dimer with GHS-R1b was associated with an inability of Gq to trigger any significant change in the conformation of ligand-free GHS-R1a in contrast to what is observed with the ghrelin receptor monomer and homodimer. Of importance, these data also imply that the basal conformation we observed in the absence of any ligand or effector protein (18) is a non-signaling conformational state because the ghrelin receptor is not able to activate Gq when frozen in this conformation upon interaction with GHS-R1b.
In closing, the occurrence of truncated receptors resulting from alternative splicing has been reported for several GPCRs besides the ghrelin receptor (8). The exact function of these alternative splice forms is still to be assessed, but in many cases they have been shown to dimerize with a full-length counterpart and, as such, affect the pharmacological profile of the receptor with which they associate. We provide here direct evidence that, through dimerization, a naturally occurring, non-signaling splice variant of the ghrelin receptor profoundly restrains GHS-R1a conformational dynamics and freezes it in a non-signaling conformation. Such an impairment in receptor conformational dynamics upon dimerization results in a loss of the ability of the ghrelin receptor to activate its cognate signaling partners, and this likely affects ghrelin-mediated signaling in pathological situations where the full-length receptor and its splice variants are coexpressed.
Acknowledgment
We thank Jean-Philippe Pin (IGF, Montpellier) for helpful discussions.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), by Montpellier I University, and by Agence Nationale pour la Recherche Contracts PCV08_323163 and ANR-10-BLAN-1208.

This article contains supplemental Figs. S1–S10 and references.
- GPCR
- G protein-coupled receptor
- TM
- transmembrane
- SEC
- size exclusion chromatography
- CaM
- calmodulin
- PGD
- petunia glutamate decarboxylase
- SPA
- substance P analog.
REFERENCES
- 1. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arcemisbéhère L., Sen T., Boudier L., Balestre M. N., Gaibelet G., Detouillon E., Orcel H., Mendre C., Rahmeh R., Granier S., Vivès C., Fieschi F., Damian M., Durroux T., Banères J. L., Mouillac B. (2010) Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J. Biol. Chem. 285, 6337–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damian M., Marie J., Leyris J. P., Fehrentz J. A., Verdié P., Martinez J., Banères J. L., Mary S. (2012) High constitutive activity is an intrinsic feature of ghrelin receptor protein. A study with a functional monomeric GHS-R1a receptor reconstituted in lipid discs. J. Biol. Chem. 287, 3630–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayburt T. H., Vishnivetskiy S. A., McLean M. A., Morizumi T., Huang C. C., Tesmer J. J., Ernst O. P., Sligar S. G., Gurevich V. V. (2011) Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J. Biol. Chem. 286, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahmeh R., Damian M., Cottet M., Orcel H., Mendre C., Durroux T., Sharma K. S., Durand G., Pucci B., Trinquet E., Zwier J. M., Deupi X., Bron P., Banères J. L., Mouillac B., Granier S. (2012) Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 109, 6733–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lohse M. J. (2010) Dimerization in GPCR mobility and signaling. Curr. Opin. Pharmacol. 10, 53–58 [DOI] [PubMed] [Google Scholar]
- 7. Rozenfeld R., Devi L. A. (2011) Exploring a role for heteromerization in GPCR signalling specificity. Biochem. J. 433, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wise H. (2012) The roles played by highly truncated splice variants of G protein-coupled receptors. J. Mol. Signal. 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi K., Furukawa C., Takano A., Ishikawa N., Kato T., Hayama S., Suzuki C., Yasui W., Inai K., Sone S., Ito T., Nishimura H., Tsuchiya E., Nakamura Y., Daigo Y. (2006) The neuromedin U-growth hormone secretagogue receptor 1b/neurotensin receptor 1 oncogenic signaling pathway as a therapeutic target for lung cancer. Cancer Res. 66, 9408–9419 [DOI] [PubMed] [Google Scholar]
- 10. Grosse R., Schöneberg T., Schultz G., Gudermann T. (1997) Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol. Endocrinol. 11, 1305–1318 [DOI] [PubMed] [Google Scholar]
- 11. Nag K., Sultana N., Kato A., Hirose S. (2007) Headless splice variant acting as dominant negative calcitonin receptor. Biochem. Biophys. Res. Commun. 362, 1037–1043 [DOI] [PubMed] [Google Scholar]
- 12. Córdoba-Chacón J., Gahete M. D., Durán-Prado M., Luque R. M., Castaño J. P. (2011) Truncated somatostatin receptors as new players in somatostatin-cortistatin pathophysiology. Ann. N.Y. Acad. Sci. 1220, 6–15 [DOI] [PubMed] [Google Scholar]
- 13. Leung P. K., Chow K. B., Lau P. N., Chu K. M., Chan C. B., Cheng C. H., Wise H. (2007) The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin receptor. Cell. Signal. 19, 1011–1022 [DOI] [PubMed] [Google Scholar]
- 14. Kojima M., Kangawa K. (2010) Ghrelin. More than endogenous growth hormone secretagogue. Ann. N.Y. Acad. Sci. 1200, 140–148 [DOI] [PubMed] [Google Scholar]
- 15. Howard A. D., Feighner S. D., Cully D. F., Arena J. P., Liberator P. A., Rosenblum C. I., Hamelin M., Hreniuk D. L., Palyha O. C., Anderson J., Paress P. S., Diaz C., Chou M., Liu K. K., McKee K. K., Pong S. S., Chaung L. Y., Elbrecht A., Dashkevicz M., Heavens R., Rigby M., Sirinathsinghji D. J., Dean D. C., Melillo D. G., Patchett A. A., Nargund R., Griffin P. R., DeMartino J. A., Gupta S. K., Schaeffer J. M., Smith R. G., Van der Ploeg L. H. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273, 974–977 [DOI] [PubMed] [Google Scholar]
- 16. Cassoni P., Ghé C., Marrocco T., Tarabra E., Allia E., Catapano F., Deghenghi R., Ghigo E., Papotti M., Muccioli G. (2004) Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur. J. Endocrinol. 150, 173–184 [DOI] [PubMed] [Google Scholar]
- 17. Kahsai A. W., Xiao K., Rajagopal S., Ahn S., Shukla A. K., Sun J., Oas T. G., Lefkowitz R. J. (2011) Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 7, 692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mary S., Damian M., Louet M., Floquet N., Fehrentz J. A., Marie J., Martinez J., Banères J. L. (2012) Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc. Natl. Acad. Sci. U.S.A. 109, 8304–8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Damian M., Martin A., Mesnier D., Pin J. P., Banères J. L. (2006) Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 25, 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baneres J. L., Martin A., Hullot P., Girard J. P., Rossi J. C., Parello J. (2003) Structure-based analysis of GPCR function: conformational adaptation of both agonist and receptor upon leukotriene B4 binding to recombinant BLT1. J. Mol. Biol. 329, 801–814 [DOI] [PubMed] [Google Scholar]
- 21. Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., Sligar S. G. (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 22. Mesnier D., Banères J. L. (2004) Cooperative conformational changes in a G-protein-coupled receptor dimer, the leukotriene B(4) receptor BLT1. J. Biol. Chem. 279, 49664–49670 [DOI] [PubMed] [Google Scholar]
- 23. Damian M., Mary S., Martin A., Pin J. P., Banères J. L. (2008) G protein activation by the leukotriene B4 receptor dimer. Evidence for an absence of trans-activation. J. Biol. Chem. 283, 21084–21092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hickerson R., Majumdar Z. K., Baucom A., Clegg R. M., Noller H. F. (2005) Measurement of internal movements within the 30 S ribosomal subunit using Forster resonance energy transfer. J. Mol. Biol. 354, 459–472 [DOI] [PubMed] [Google Scholar]
- 25. Kozasa T. (2004) Purification of G protein subunits from Sf9 insect cells using hexahistidine-tagged α and β γ subunits. Methods Mol. Biol. 237, 21–38 [DOI] [PubMed] [Google Scholar]
- 26. McEwen D. P., Gee K. R., Kang H. C., Neubig R. R. (2001) Fluorescent BODIPY-GTP analogs. Real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 291, 109–117 [DOI] [PubMed] [Google Scholar]
- 27. Gurevich V. V., Benovic J. L. (2000) Arrestin. Mutagenesis, expression, purification, and functional characterization. Methods Enzymol. 315, 422–437 [DOI] [PubMed] [Google Scholar]
- 28. Sommer M. E., Smith W. C., Farrens D. L. (2005) Dynamics of arrestin-rhodopsin interactions. Arrestin and retinal release are directly linked events. J. Biol. Chem. 280, 6861–6871 [DOI] [PubMed] [Google Scholar]
- 29. Banerjee S., Huber T., Sakmar T. P. (2008) Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J. Mol. Biol. 377, 1067–1081 [DOI] [PubMed] [Google Scholar]
- 30. Yuan T., Vogel H. J. (1998) Calcium-calmodulin-induced dimerization of the carboxyl-terminal domain from petunia glutamate decarboxylase. A novel calmodulin-peptide interaction motif. J. Biol. Chem. 273, 30328–30335 [DOI] [PubMed] [Google Scholar]
- 31. Holst B., Brandt E., Bach A., Heding A., Schwartz T. W. (2005) Nonpeptide and peptide growth hormone secretagogues act both as ghrelin receptor agonist and as positive or negative allosteric modulators of ghrelin signaling. Mol. Endocrinol. 19, 2400–2411 [DOI] [PubMed] [Google Scholar]
- 32. Moulin A., Demange L., Ryan J., Mousseaux D., Sanchez P., Bergé G., Gagne D., Perrissoud D., Locatelli V., Torsello A., Galleyrand J. C., Fehrentz J. A., Martinez J. (2008) New trisubstituted 1,2,4-triazole derivatives as potent ghrelin receptor antagonists. 3. Synthesis and pharmacological in vitro and in vivo evaluations. J. Med. Chem. 51, 689–693 [DOI] [PubMed] [Google Scholar]
- 33. Holst B., Frimurer T. M., Mokrosinski J., Halkjaer T., Cullberg K. B., Underwood C. R., Schwartz T. W. (2009) Overlapping binding site for the endogenous agonist, small-molecule agonists, and ago-allosteric modulators on the ghrelin receptor. Mol. Pharmacol. 75, 44–59 [DOI] [PubMed] [Google Scholar]
- 34. Sano T., Vajda S., Smith C. L., Cantor C. R. (1997) Engineering subunit association of multisubunit proteins. A dimeric streptavidin. Proc. Natl. Acad. Sci. U.S.A. 94, 6153–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jastrzebska B., Ringler P., Palczewski K., Engel A. (2013) The rhodopsin-transducin complex houses two distinct rhodopsin molecules. J. Struct. Biol. 182, 164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hlavackova V., Goudet C., Kniazeff J., Zikova A., Maurel D., Vol C., Trojanova J., Prézeau L., Pin J. P., Blahos J. (2005) Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 24, 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Comps-Agrar L., Kniazeff J., Nørskov-Lauritsen L., Maurel D., Gassmann M., Gregor N., Prézeau L., Bettler B., Durroux T., Trinquet E., Pin J. P. (2011) The oligomeric state sets GABAB receptor signalling efficacy. EMBO J. 30, 2336–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terrillon S., Barberis C., Bouvier M. (2004) Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with β-arrestin and their trafficking patterns. Proc. Natl. Acad. Sci. U.S.A. 101, 1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sartania N., Appelbe S., Pediani J. D., Milligan G. (2007) Agonist occupancy of a single monomeric element is sufficient to cause internalization of the dimeric β2-adrenoceptor. Cell. Signal. 19, 1928–1938 [DOI] [PubMed] [Google Scholar]
- 41. Canto I., Trejo J. (2013) Transactivation of the PAR1-PAR2 heterodimer by thrombin elicits β-arrestin-mediated endosomal signaling. J. Biol. Chem. 288, 15900–15912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manglik A., Kruse A. C., Kobilka T. S., Thian F. S., Mathiesen J. M., Sunahara R. K., Pardo L., Weis W. I., Kobilka B. K., Granier S. (2012) Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferré S., Baler R., Bouvier M., Caron M. G., Devi L. A., Durroux T., Fuxe K., George S. R., Javitch J. A., Lohse M. J., Mackie K., Milligan G., Pfleger K. D., Pin J. P., Volkow N. D., Waldhoer M., Woods A. S., Franco R. (2009) Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 5, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prezeau L., Rives M. L., Comps-Agrar L., Maurel D., Kniazeff J., Pin J. P. (2010) Functional crosstalk between GPCRs. With or without oligomerization. Curr. Opinion Pharmacol. 10, 6–13 [DOI] [PubMed] [Google Scholar]
- 45. Levoye A., Dam J., Ayoub M. A., Guillaume J. L., Couturier C., Delagrange P., Jockers R. (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 25, 3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]


