Abstract
Objective
To investigate the independent effects of antihypertensive treatment and blood pressure (BP) levels on physical and mental health status in patients with arterial disease.
Design
Cross-sectional analyses within the Secondary Manifestations of ARTerial disease (SMART) study, a single centre cohort study.
Setting
Hospitalized care.
Subjects
5,877 patients (mean age 57) with symptomatic and asymptomatic arterial disease who underwent a standardized vascular screening.
Main outcome measure
Self-rated physical and mental health assessed with the Short Form (SF)-36.
Results
In the total population, antihypertensive drug use and increased intensity of antihypertensive treatment was associated with poorer health status independent of important confounders including BP levels; adjusted mean differences (95%CI) in physical and mental health between 0 and ≥3 antihypertensives were -1.2 (-2.1, -0.3) and -3.5 (-4.4; -2.6). Furthermore, lower systolic and lower diastolic BP levels were related to poorer physical and mental health status independently of antihypertensive treatment. Mean differences (95%CI) in physical and mental health status per SD decrease in systolic BP were -0.56 (-0.84; -0.27) and -0.32 (-0.61; -0.03), and per SD decrease in diastolic BP -0.50 (-0.78; -0.23) and -0.08 (-0.36; 0.20). The association between low BP and poor health status was particularly present in patients with coronary artery disease.
Conclusions
In a population of patients with asymptomatic and symptomatic arterial disease, antihypertensive treatment and lower BP levels are independently associated with poorer self-rated physical and mental health. These results might indicate that there are different underlying mechanisms explaining these independent associations.
Keywords: antihypertensive treatment, blood pressure, physical health, mental health, cardiovascular disease
Introduction
Cardiovascular disease is a major health problem worldwide, with ischemic heart disease and stroke as leading causes of disability and mortality[1]. Improved possibilities to treat cardiovascular diseases have led to increased survival rates of patients with cardiovascular disease and have augmented the interest in measuring patient-rated health status including physical and mental health.
Compared to the general population, self-rated health and quality of life is substantially lower in patients with symptomatic arterial disease [2-4]. Reduced physical and mental health not only interferes with daily living, but is among the best predictors of the use of general medical and mental health services [5], and a strong predictor of new vascular events and mortality [6-8]. It is therefore important to identify risk factors for reduced physical and mental health, particularly in high-risk populations.
Hypertension is an established and strong risk factor for cardiovascular disease and has been associated with poorer health status [9]. Although cardiovascular morbidity and mortality associated with hypertension has been considerably reduced by antihypertensive drug therapy, many people experience adverse effects subsequently compromising health status [10], irrespective of their BP level [11]. With respect to the relation between BP and health status, both high and low BP have been linked to subjective complaints and reduced health status [12, 13]. The Hypertension Optimal Treatment (HOT) study showed that lowering of BP resulted in more subjective complaints but an overall better quality of life [14]; however, no earlier study has investigated the independent effects of antihypertensive treatment and both higher and lower BP levels on health status. Also, since patients with vascular disease are at increased risk for high BP as well as poor functioning, and will also be more intensively treated for high BP, it is important to study the contributions of BP and treatment to health status in these patients.
In this study we determined the independent cross-sectional associations of antihypertensive treatment and BP levels with self-rated physical and mental health, in a large cohort of patients with symptomatic and asymptomatic arterial disease.
Methods
The SMART study
The Secondary Manifestations of ARTerial disease (SMART) study is an ongoing single-centre cohort study, started in 1996 in patients who were newly referred to the University Medical Center Utrecht for the treatment of clinical manifestations of arterial disease or for the treatment of vascular risk factors. The rationale and design of the SMART study have been described in detail elsewhere [15]. In short, patients underwent a standardized vascular screening program and were screened for additional risk factors and severity of atherosclerosis. Patients were classified into symptomatic or asymptomatic patients based on referral diagnosis and vascular history. Symptomatic patients were those with manifest coronary artery disease (CAD), cerebrovascular disease (CVD), or peripheral arterial disease (PAD). Patients could have more than one diagnosis. CAD was defined as myocardial infarction, angina pectoris, coronary artery bypass graft surgery or coronary angioplasty in the past or at inclusion. CVD was defined as transient ischemic attack or stroke at inclusion and patients who reported stroke in the past. PAD was defined as surgery or angioplasty of the arteries supplying the lower extremities, a history of intermittent claudication or rest pain at inclusion confirmed by a resting ankle-brachial index <0.9 in at least one leg, a history of aneurysm of the abdominal aorta (AAA; distal aortic diameter≥3cm) or previous AAA surgery. Asymptomatic patients were those without symptoms of clinically manifest arterial disease but with vascular risk factors, such as hypertension or diabetes. The Ethics Committee of our institution approved the study and all patients gave their written informed consent.
Blood pressure
BP was measured twice in sitting position with a sphygmomanometer and the average of the two measures was calculated. Systolic and diastolic BP measures were categorized into 4 categories; SBP in ≤120, 121-140, 141-160, and >160mmHg and DBP in ≤70, 71-80, 81-90, and >90mmHg. Pulse pressure (PP) was calculated as the difference between SBP and DBP. Antihypertensive drug use was assessed with questionnaires. Antihypertensive treatment was categorized in using 0, 1, 2, or ≥3 antihypertensive drugs. In addition, antihypertensive drugs were categorized in use of beta-blockers, diuretics, calcium-channel blockers (CCB), ACE-inhibitors (ACEi), angiotensin receptor blockers (ARBs), or other antihypertensives.
Self-rated physical and mental health
Patients completed the SF-36, which is a widely used and well-validated questionnaire for assessing multidimensional aspects of self-rated health status[16]. The SF-36 is organized into eight multi-item health domains: general health perception, bodily pain, physical functioning, role limitations because of physical health problems, mental health, vitality, social functioning, and role limitations because of emotional problems. Raw scale scores are transformed into a variable (range 0–100), with lower scores indicating lower levels of well-being. As a measure of physical health status, the first four domains were combined into a physical component summary scale. The last four domains were combined into a mental component summary scale as a measure of mental health status. Component scales were positively scored and normalized to a general population mean of 50. Since the domain scales were not normally distributed, scores were additionally dichotomized in low (lowest quartiles) and high (highest three quartiles).
Covariates
At inclusion, subjects completed a questionnaire covering medical history, symptoms of and risk factors for cardiovascular disease, family history, current medication use, smoking habits, and alcohol intake. A standardized diagnostic protocol was performed, including physical examination and laboratory tests. Body mass index (BMI) was calculated as kg/m2. Total cholesterol, HDL, and triglycerides were measured with commercial enzymatic kits. LDL was calculated with Friedewald’s formula. Hyperlipidemia was defined as a referral diagnosis of hyperlipidemia, use of lipid-lowering drugs, a known history of hyperlipidemia, or high cholesterol levels (total cholesterol ≥5.0mmol/L, LDL cholesterol ≥3.0mmol/L). Diabetes mellitus was defined as a referral diagnosis of diabetes, self-reported use of glucose-lowering agents, a known history of diabetes or a fasting plasma glucose level ≥7.0mmol/L at baseline. Ultrasonography was performed with a 10-MHz linear-array transducer (ATL Ultramark 9) by certified ultrasound technicians at the Department of Radiology. Mean carotid intima-media thickness (IMT)(mm) was calculated for each patient based on 6 far-wall measurements of the left and right common carotid arteries as previously described[17].
Analytical sample
The SF-36 questionnaire was added to the assessment in October 2001. Between October 2001 and March 2010 5,877 patients were included in the SMART study.
Data-analysis
We used multiple imputation (10 datasets) to address the missing baseline values in the study sample of 5,877 patients using the statistical program R (AregImpute)[18]. Data were analyzed with SPSS version 19.0 (Chicago, IL, USA) by pooling the 10 imputed data sets. Percentages of missing values before imputation are given in the footnote of table 1. Patients characteristics were calculated according to categories of SBP (≤120, 121-140, 141-160, >160mmHg) for patients with and without antihypertensive treatment.
Table 1.
Patient characteristics according to combined categories of antihypertensive treatment and systolic blood pressure.
| No antihypertensive treatment (N=1,903) | Antihypertensive treatment (N=3,974) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Systolic Blood Pressure (mmHg) | ≤120 | 121-140 | 141-160 | >160 | ≤120 | 121-140 | 141-160 | >160 |
| N=329 | N=828 | N=505 | N=241 | N=512 | N=1,437 | N=1,226 | N=799 | |
| Demographics | ||||||||
| Male sex, % men | 54% | 64% | 68% | 56% | 72% | 71% | 70% | 63% |
| Age, years* | 45 (13) | 49 (13) | 53 (13) | 59 (11) | 57 (11) | 57 (11) | 60 (11) | 62 (10) |
| Arterial disease category | ||||||||
| Asymptomatic arterial disease, % | 55% | 53% | 52% | 46% | 10% | 19% | 28% | 34% |
| Coronary heart disease, % | 11% | 12% | 15% | 12% | 75% | 64% | 54% | 43% |
| Cerebrovascular disease, % | 23% | 23% | 19% | 20% | 14% | 16% | 17% | 18% |
| Peripheral arterial disease, % | 16% | 15% | 20% | 30% | 11% | 13% | 13% | 16% |
| Blood pressure measures | ||||||||
| Systolic blood pressure, mmHg* | 114 (6) | 131 (5) | 149 (6) | 174 (13) | 114 (7) | 131 (5) | 150 (6) | 177 (14) |
| Diastolic blood pressure, mmHg* | 72 (7) | 81 (7) | 88 (9) | 97 (11) | 71 (8) | 80 (8) | 87 (9) | 98 (12) |
| Pulse pressure, mmHg* | 42 (7) | 50 (8) | 61 (9) | 78 (14) | 42 (7) | 51 (8) | 62 (10) | 79 (15) |
| Antihypertensive medication use | ||||||||
| Beta-blockers, % | - | - | - | - | 76% | 67% | 62% | 58% |
| Diuretics, % | - | - | - | - | 28% | 32% | 34% | 37% |
| ACE-inhibitors, % | - | - | - | - | 42% | 40% | 41% | 48% |
| Angiotensin receptor blockers, % | - | - | - | - | 11% | 16% | 21% | 25% |
| Calcium channel blockers, % | - | - | - | - | 19% | 24% | 25% | 24% |
| Other, %‡ | - | - | - | - | 1% | 1% | 2% | 3% |
| Other risk factors | ||||||||
| BMI, kg/m2* | 24 (6) | 26 (5) | 27 (6) | 27 (6) | 27 (4) | 28 (5) | 28 (4) | 28 (5) |
| Smoking, packyrs† | 9 (0-25) | 7 (0-23) | 10 (0-27) | 14 (2-32) | 12 (1-29) | 12 (1-29) | 11 (0-29) | 11 (0-27) |
| Alcohol, % current | 84% | 84% | 85% | 81% | 80% | 82% | 80% | 78% |
| Diabetes, % | 13% | 14% | 18% | 13% | 13% | 21% | 25% | 23% |
| Hyperlipidemia, % | 81% | 84% | 84% | 86% | 94% | 92% | 92% | 91% |
| Carotid intima media thickness, mm* | 0.70 (0.18) | 0.76 (0.23) | 0.83 (0.23) | 0.94 (0.26) | 0.83 (0.21) | 0.87 (0.25) | 0.91 (0.26) | 0.98 (0.27) |
| Other medication use | ||||||||
| Platelet inhibitor medication, % | 32% | 34% | 35% | 35% | 80% | 72% | 64% | 59% |
| Lipid-lowering medication, % | 33% | 41% | 40% | 37% | 79% | 75% | 70% | 61% |
| Oral glucose-lowering medication, % | 4% | 5% | 9% | 7% | 8% | 14% | 17% | 16% |
| Insulin, % | 6% | 6% | 5% | 1% | 4% | 5% | 7% | 5% |
| Antidepressants, % | 8% | 5% | 6% | 4% | 8% | 6% | 5% | 4% |
| Total number of medications, % ≥5 | 19% | 16% | 13% | 16% | 66% | 61% | 58% | 55% |
Mean (SD),
Median (IQR)
alpha blockers, combination antihypertensive drugs, centrally acting agents
Percentage of missing values before imputation: blood pressure, 0.2%; smoking, 0.5%; alcohol use, 0.7%; carotid IMT, 1.8%; mental and physical health
Antihypertensive treatment
Linear regression models were used to estimate the cross-sectional association of antihypertensive treatment (yes/no) with the composite scores of physical and mental health status. Adjustments were made for demographics (age, sex) and BP levels (model 1) and additionally for cardiovascular risk factors (BMI, smoking, alcohol, DM, hyperlipidemia, carotid IMT) and other medication use (platelet inhibitor, lipid-lowering, glucose-lowering, or antidepressant medication) (model 2). To take into account the effect of BP levels in the relation between antihypertensive treatment and health status, these analyses were stratified for SBP categories (≤120, 121-140, 141-160, >160mmHg). Also, analysis of covariance (ANCOVA) was used to estimate the mean adjusted physical and mental health composite scores for the number of antihypertensive drugs used (0, 1, 2, ≥3). In addition, within patients using antihypertensive medication (N=3,974), types of antihypertensive drugs (beta-blockers, diuretics, CCB, ACEi, ARBS) were associated with health status. These analyses were adjusted according to model 2 and were further adjusted for use of other antihypertensive drugs. To further take into account the confounding effect of use of other antihypertensive drugs, additional analyses were performed restricting the sample size to patients using antihypertensive monotherapy (N=1,714).
Blood pressure
Regression analyses were used to estimate the cross-sectional association of BP levels (SBP, DBP, and PP; continuous) with self-rated physical and mental health status (composite scores). Analyses were adjusted for demographics and antihypertensive treatment (model 1) and additionally for cardiovascular risk factors and other medication use (model 2). To explore whether there was a non-linear relation between BP and health status, quadratic terms of SBP, DBP, or PP were incorporated in the regression models. Also, ANCOVA was used to calculate mean (SE) adjusted mental and physical composite scores for categories of SBP (≤120, 121-140, 141-160, >160mmHg) and DBP in (≤70, 71-80, 81-90, >90mmHg). To take into account the effect of antihypertensive treatment in the relation between BP and health status, these analyses were stratified for antihypertensive treatment (yes/no).
To investigate whether the underlying arterial disease influences the association of antihypertensive treatment and BP measures with health status, through for example confounding by indication, additional analyses were performed within the separate arterial disease categories (asymptomatic, CAD, CVD, PAD) using the models described above and interaction terms between BP or antihypertensive treatment with arterial disease categories were added to the models. Also, log-binomial and Poisson regression models with robust standard errors were used to estimate relative risks of poor physical and mental health status (domain scores: lowest quartile vs. highest three quartiles) associated with antihypertensive treatment and BP levels.
Results
In the study sample of 5,877 patients, mean (range) age was 56 years (17-82) and the majority was men (67%). Of the sample, 1,933 (33%) had asymptomatic arterial disease, 2,547 (43%) had CAD, 1,061 (18%) had CVD, and 869 (15%) had PAD. The mean (range) of self-rated physical and mental health scores were 43 (7-68) and 49 (9-75), respectively. In addition, 82% had hypertension and 3,971 (68%) used antihypertensive medication.
Patients using antihypertensive medication were older, more often men, had more symptomatic arterial disease, diabetes, and hyperlipidemia, had thicker carotid IMT, and used more other medications such as platelet inhibitor, lipid-lowering, and glucose-lowering medication (Table 1). Both in patients with and without antihypertensive treatment, those with higher SBP levels were older, had higher DBP and PP levels, more often had DM, and had thicker carotid IMT (Table 1). Patients with lower SBP used more medication including antidepressants. Within patients on antihypertensive treatment, those with lower SBP levels more often had CAD and used more beta-blockers (Table 1).
Antihypertensive treatment
Patients on antihypertensive treatment had poorer self-rated health status than patients not on treatment, particularly physical health status (Table 2). These findings were independent of SBP and DBP levels (model 1) and the effect estimates only slightly changed after further adjustments for cardiovascular risk factors and other medication use (model 2).
Table 2.
Association of antihypertensive treatment and measures of blood pressure with self-rated physical and mental health status (SF36).
| Model | Physical health (composite score)
|
Mental health (composite score)
|
|||
|---|---|---|---|---|---|
| B | (95% CI) | B | (95% CI) | ||
|
|
|
||||
| Antihypertensive treatment (yes/no) † | 1 | -3.03 | (-3.66; -2.40)** | -0.83 | (-1.46; -0.19)* |
| 2 | -2.26 | (-2.88; -1.63)** | -0.80 | (-1.43; -0.16)* | |
| Systolic blood pressure (per SD) ‡ | 1 | 0.38 | (0.09; 0.66)* | 0.40 | (0.12; 0.69)** |
| 2 | 0.56 | (0.27; 0.84)** | 0.32 | (0.03; 0.61)* | |
| Diastolic blood pressure (per SD) ‡ | 1 | 0.42 | (0.15; 0.70)** | 0.20 | (-0.08; 0.48) |
| 2 | 0.50 | (0.23; 0.78)** | 0.08 | (-0.20; 0.36) | |
| Pulse pressure (per SD) ‡ | 1 | 0.18 | (-0.12; 0.48) | 0.43 | (0.13; 0.73)** |
| 2 | 0.38 | (0.09; 0.68)* | 0.42 | (0.11; 0.72)** | |
SD: SBP=21mmHg, DBP=12mmHg, PP=15mmHg.
P-linear trend <0.05;
P-linear trend<0.01.
Model 1:adjusted for age, sex, SBP, DBP;
Model 1: adjusted for age, sex, antihypertensive treatment;
Model 2: additionally adjusted for BMI, smoking, alcohol, diabetes, hyperlipidemia, carotid IMT, other medication use
Linear regression analysis estimating the association between antihypertensive treatment and self-rated health status within strata of SBP categories showed that this relation was less pronounced for the highest SBP category; B’s (95%CI) for the relation treatment and physical health across the SBP categories (≤120, 121-140, 141-160, >160mmHg) were -2.92 (-4.57,-1.28), -3.65 (-4.63, -2.67), -2.85 (-4.00, -1.69), -1.53 (-3.15, 0.09), and for relation treatment and mental health B’s (95%CI) were-0.72 (-2.43, 1.00), -1.22 (-2.22, -0.22), -0.85 (-2.00, 0.30),-0.16 (-1.42, 1.73).
In addition, increasing number of antihypertensive drugs was related to poorer mental and physical health status (Figure 1); adjusted mean differences (95%CI) in mental and physical health between use of 0 and ≥3 antihypertensive drugs according to model 2 were -1.11 (-2.05, -0.24) and -3.66 (-4.57; -2.79), respectively.
Figure 1.
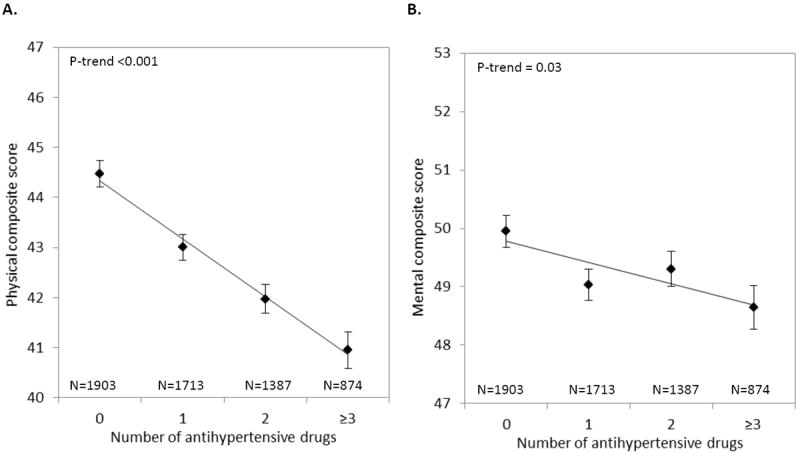
Mean (SE) adjusted scores of physical (A) and mental health status (B) across categories of number of antihypertensive drugs in the total sample (N=5,877).
Adjustments were made for age, sex, BMI, smoking, alcohol, diabetes, hyperlipidemia, carotid IMT, other medication use, and systolic and diastolic blood pressure.
Next, we related the different types of antihypertensive medication to health status within patients using antihypertensive medication (N=3,974). ANCOVA showed that those using diuretics or CCB had significantly poorer physical health status compared to those who used other antihypertensive drugs; adjusted mean differences (95%CI) were -1.20 (-1.93; -0.47) and -2.37 (-3.15; -1.58), respectively. These differences were most pronounced in patients with CAD and CVD (Supplemental Figure 1). Also, asymptomatic patients or patients with CVD or PAD who used beta-blockers had significantly lower scores on physical health compared to those who used other antihypertensive drugs.
To further investigate the effect of the different types of antihypertensive medication on health status, the analyses were repeated in patients on antihypertensive monotherapy. In this subsample of 1,714 patients, those on beta-blockers (N=911), diuretics (N=124), CCB (N=135), or ACE inhibitors (N=385) had similar physical health scores. However, patients on ARBs monotherapy (N=159) had significantly higher physical health scores compared to those using other antihypertensive drugs; adjusted mean difference (95%CI) was 2.34 (0.64; 3.99).
Type of antihypertensive medication was not associated with mental health status (data not shown).
Blood pressure
Linear regression analysis estimating the association between BP and self-rated health status showed that lower BP levels (SBP, DBP, and PP) were linearly associated with poorer physical health and that lower SBP and lower PP were linearly associated with poorer mental health (Table 2). These findings were independent of age, sex, cardiovascular risk factors, other medication use, and use of antihypertensives.
Figure 3 shows the results of ANCOVA estimating the association of BP with self-rated health status within strata of antihypertensive treatment (yes/no). Both in patients with and without antihypertensive treatment, lower SBP and DBP were associated with poorer physical health (Figures 3A and 3B). However, in patients without antihypertensive treatment these associations were less pronounced and not statistical significant. Also, lower SBP tended to be related to poor mental health, independent of antihypertensive treatment yes/no (Figure 3C). In addition, a non-linear relation was found between DBP and mental health status in patients without antihypertensive treatment (P-quadratic=0.03); suggesting that both low and high DBP resulted in poorer mental health (Figure 3D).
Additional analyses estimating the association of antihypertensive treatment and BP with health status for the different arterial disease categories showed that antihypertensive treatment was associated with risk of poor physical health in all disease groups except for patients with CAD (Supplemental Table 1); however, p-values for interaction between antihypertensive treatment and arterial disease categories were all >0.10. In contrast, the association of low SBP and DBP with poor physical and mental health status was mainly present in patients with CAD (Supplemental Table 1). For physical health status, p-values for interaction for SBP*CAD and DBP*CAD were 0.12 and 0.07; for mental health status these p-values were 0.29 and 0.55. In addition, the estimated association of antihypertensive treatment with risk of poor mental health was primarily present in patients with CVD.
Additional analyses estimating the association of antihypertensive treatment and BP with the domain scales of the SF36 showed that antihypertensive treatment was particularly associated with risk of lower scores on “general health”, “physical function”, “role limitations because of physical health problems”, “vitality”, and “social function”. In addition, the estimated association of low BP with poorer health status in CAD patients was mainly driven by the “general health” domain scale (Supplemental Table 2). In sensitivity analyses we found no change in the associations after excluding those with very low SBP (<100mmHg, N=42) or DBP (<60mmHg, N=89). Moreover, the presented associations were similar for younger (<65years) and older (≥65 years) patients. Finally, adjusting for total number of drugs (as proxy of extent of comorbidity) did not change the found relation between antihypertensive treatment and self-rated health status (data not shown).
Discussion
Within this large-scale cohort study in patients with symptomatic and asymptomatic arterial disease the two main observations were (1) that antihypertensive drug use and increased intensity of antihypertensive treatment were associated with poorer self-rated physical and mental health, independent of BP levels; and (2) that lower BP levels were associated with poorer physical and mental health status, independently of antihypertensive treatment.
To our knowledge, this is the first study investigating the independent association of antihypertensive treatment and BP levels with health status. Also, it is important to study this relation in a population with high vascular risk, since we know that in this population both lower BP levels and reduced health status have been related to an increased risk of events and mortality [6, 19]. Also, patients with poorer self-rated health status might have lower medication adherence, which can lead to an even greater risk of events and mortality [20].
Our findings that antihypertensive drug use and increased intensity of antihypertensive treatment contributed to poorer health status, independently of BP, is in line with a previous study showing that those using antihypertensive medication had more physically unhealthy days [21]. However, other studies relating antihypertensive treatment to health status show inconsistent findings with either no relation [22] or even better self-rated health status for those on antihypertensive treatment [23].
The relation between antihypertensive treatment and poorer health status could be explained by medication related adverse effects [10]. A previous study has identified a considerable incidence of adverse events related to antihypertensive drugs use [24], which could consequently result in a reduction in self-rated health. Since most side effects are type and dose dependent, it has been suggested that a strategy using a combination of low dose drugs is probably the best approach to BP control without interfering with mental or physical functioning [10]. Our study indicates that CCB and diuretics most strongly affects health status, possibly due to side effects as postural hypotension, dizziness, constipation, and increased urination. However, when we restricted our analyses to patients using monotherapy the effect of CCB and diuretics on self-rated health status were not present. This might suggest that these drugs only affect health status when they were used as a second, third, or fourth option. Therefore, another explanation for the relation between antihypertensive treatment and poor health status could be that patients using antihypertensive treatment, particularly those using more than one drug, represent a group with more comorbidity and thus lower health status. This is confirmed by our findings that patients using antihypertensive medication also used more other medications. Yet, multivariable adjustment and additional adjustment for total number of drugs, as proxy of severity of comorbidity, did not change the results.
Our findings that lower BP levels were associated with poorer self-rated health status, independently of life-style factors, cardiovascular risk factors, and presence of atherosclerosis, are of interest and in line with one earlier cross-sectional population-based study in middle-aged men [12]. These results and our findings are in line with the growing body of evidence that not only high BP, but also lower BP, is associated with adverse health outcomes, such as an increased risk of cardiovascular events and mortality [19, 25, 26], and alterations in brain function and structure [27-32].
Several possible mechanisms for the relation between low BP and poor health status have been hypothesized. First, low BP could be the result of use of antihypertensive treatment, which in turn could cause reduced self-rated health due to side effects. Even though the relation between lower BP and poorer health status was somewhat stronger in those with antihypertensive treatment, it was also present in those without treatment. Second, patients with reduced self-rated health may suffer from more advanced atherosclerosis resulting in increased arterial stiffness or reduced cardiac output, which may both reflect in lower BP levels. Increased PP, an indicator of arterial stiffness, was not related to poorer health status. However, we did find that in patients with CAD, low BP was more strongly related with poorer health status. This might suggest that reduced cardiac output resulted in low BP and poorer functioning, although the vascular screening program of this study did not include measures of cardiac function. Also, patients with lower BP levels used more other medications, which could suggest that these patients have more co-morbidity. However, adjusting for other medications did not change the results. Another explanation for our findings could be that low BP leads to poor health perception through depressive symptoms or mood problems [28-31]. However, no association was found between low BP and the mental health domain scale, which has been used to assess mood problems; and although patients with lower BP used more antidepressants, adjusting for antidepressants did not change our results. Finally, a causal mechanism explaining low BP related mental and physical dysfunctioning should be considered. Chronic low BP could cause decreased tissue perfusion, for example cerebral hypoperfusion, that could lead to brain pathology [27], psychological and cognitive disturbances[28-32], and chronic somatic symptoms like dizziness and fatigue [12], subsequently leading to poorer health status.
Major strengths of our study include the large sample size, which made it possible to not only investigate the association of antihypertensive treatment and BP with health status, but also investigate this relation within the separate arterial disease categories. Also, self-rated health status was assessed using a validated questionnaire and the large sample size allowed analyzing the underlying domains of health status. In addition, the extensive information on cardiovascular risk factors and the extent of clinical and subclinical arterial disease measures made it possible to relate BP to health status independent of these important confounders.
A limitation of our study is that our results are based on cross-sectional data, which precluded affirmation of the direction of causality. Future longitudinal studies should investigate whether lower BP levels are an actual risk factor for poor health status or whether low BP is merely a risk-indicator or marker of underlying comorbidity. Second, since our population consists of patients with arterial disease our results might not be applicable to patients without arterial disease and to the general population. Also, concurrent diseases might bias our results. However, extensive adjustment for other risk factors and markers of disease severity did not diminish our findings.
In summary, in patients with arterial disease antihypertensive treatment and low BP are independently related to poorer self-rated mental and physical health status. These independent effects could be the result of different underlying mechanisms associated with poor health status, such as side effects of antihypertensive treatment and low BP related comorbidities.
Supplementary Material
Figure 2.
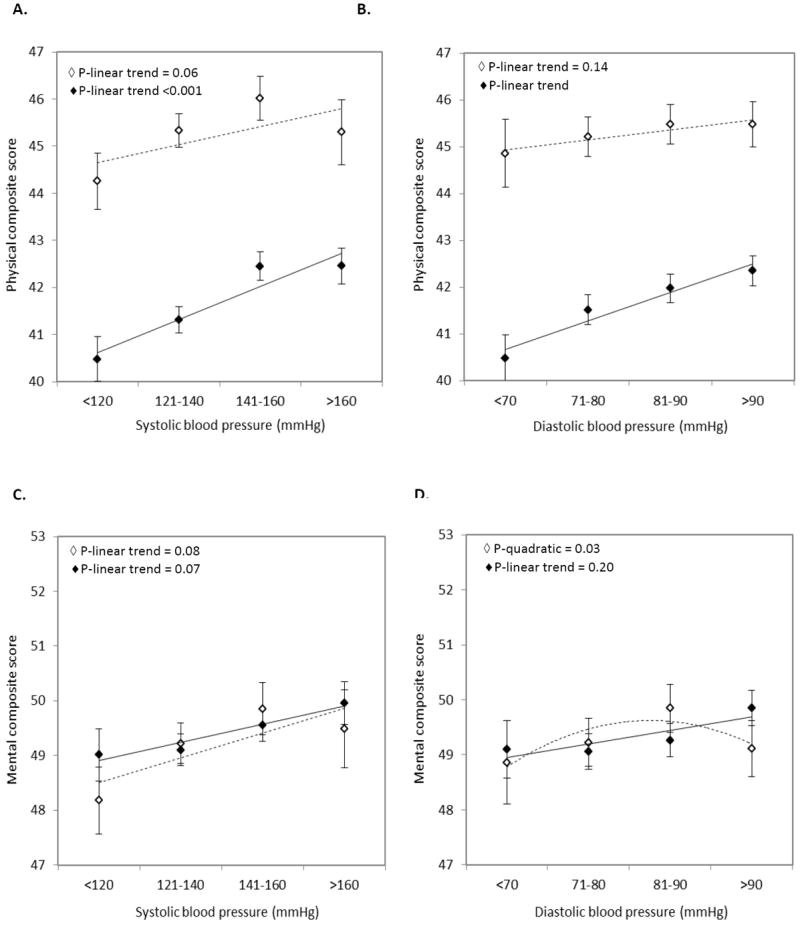
Mean (SE) adjusted scores of physical and mental health status across categories of systolic blood pressure (A & C) or diastolic blood pressure (B & D) for patients without antihypertensive treatment (marker ◇; trend line ----), and patients with antihypertensive treatment (marker ◆; trend line ——).
Adjustments were made for age, sex, BMI, smoking, alcohol, diabetes, hyperlipidemia, carotid IMT, other medication use, and antihypertensive drug use.
Acknowledgments
We gratefully acknowledge the members of the SMART Study Group of University Medical Center Utrecht: A. Algra, MD, PhD, Julius Center for Health Sciences and Primary Care and Rudolf Magnus Institute for Neurosciences, Department of Neurology; P.A. Doevendans, MD, PhD, Department of Cardiology; Y. van der Graaf, MD, PhD, D.E. Grobbee, MD, PhD, and G.E.H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary Care; L.J. Kappelle, MD, PhD, Department of Neurology; W.P.Th.M. Mali, MD, PhD, Department of Radiology; F.L. Moll, MD, PhD, Department of Vascular Surgery; and F.L.J. Visseren, MD, PhD, Department of Vascular Medicine.
This study was supported by a program grant from the Netherlands Organization for Scientific Research (NWO-MW: project-no. 904-65-095) and research grants from the ‘Internationale Stichting Alzheimer Onderzoek’ (ISAO: project No. 09502) and ‘Alzheimer Nederland’ (project No. WE.15-2011-02)
Footnotes
Conflicts of interest
Nothing to declare
References
- 1.Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31:642–8. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Wu EQ, Zheng ZJ, Sullivan PW, Zhan L, Labarthe DR. Patient-reported health status in coronary heart disease in the United States: age, sex, racial, and ethnic differences. Circulation. 2008;118:491–7. doi: 10.1161/CIRCULATIONAHA.107.752006. [DOI] [PubMed] [Google Scholar]
- 3.Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke; a journal of cerebral circulation. 2006;37:2567–72. doi: 10.1161/01.STR.0000240506.34616.10. [DOI] [PubMed] [Google Scholar]
- 4.Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health-related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. Br J Gen Pract. 2004;54:826–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Jama. 1995;273:59–65. [PubMed] [Google Scholar]
- 6.Grool AM, van der Graaf Y, Visseren FL, de Borst GJ, Algra A, Geerlings MI. Self-rated health status as a risk factor for future vascular events and mortality in patients with symptomatic and asymptomatic atherosclerotic disease: the SMART study. J Intern Med. 2012 doi: 10.1111/j.1365-2796.2012.02521.x. [DOI] [PubMed] [Google Scholar]
- 7.Myint PK, Surtees PG, Wainwright NW, et al. Physical health-related quality of life predicts stroke in the EPIC-Norfolk. Neurology. 2007;69:2243–8. doi: 10.1212/01.wnl.0000296010.21252.78. [DOI] [PubMed] [Google Scholar]
- 8.Myint PK, Luben RN, Surtees PG, et al. Self-reported mental health-related quality of life and mortality in men and women in the European Prospective Investigation into Cancer (EPIC-Norfolk): a prospective population study. Psychosom Med. 2007;69:410–4. doi: 10.1097/psy.0b013e318068fcd4. [DOI] [PubMed] [Google Scholar]
- 9.Trevisol DJ, Moreira LB, Kerkhoff A, Fuchs SC, Fuchs FD. Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2011;29:179–88. doi: 10.1097/HJH.0b013e328340d76f. [DOI] [PubMed] [Google Scholar]
- 10.Handler J. Quality of life and antihypertensive drug therapy. J Clin Hypertens (Greenwich) 2005;7:274–85. doi: 10.1111/j.1524-6175.2005.04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trevisol DJ, Moreira LB, Fuchs FD, Fuchs SC. Health-related quality of life is worse in individuals with hypertension under drug treatment: results of population-based study. J Hum Hypertens. 2012;26:374–80. doi: 10.1038/jhh.2011.48. [DOI] [PubMed] [Google Scholar]
- 12.Rosengren A, Tibblin G, Wilhelmsen L. Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ. 1993;306:243–6. doi: 10.1136/bmj.306.6872.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss NS. Relation of high blood pressure to headache, epistaxis, and selected other symptoms. The United States Health Examination Survey of Adults. N Engl J Med. 1972;287:631–3. doi: 10.1056/NEJM197209282871303. [DOI] [PubMed] [Google Scholar]
- 14.Wiklund I, Halling K, Ryden-Bergsten T, Fletcher A. Does lowering the blood pressure improve the mood? Quality-of-life results from the Hypertension Optimal Treatment (HOT) study. Blood Press. 1997;6:357–64. doi: 10.3109/08037059709062095. [DOI] [PubMed] [Google Scholar]
- 15.Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15:773–81. doi: 10.1023/a:1007621514757. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Kosinki M, Keller SD. SF-36 Physical and Mental health Summary Scales: A User’s Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 17.Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART study. Circulation. 1999;100:951–7. doi: 10.1161/01.cir.100.9.951. [DOI] [PubMed] [Google Scholar]
- 18.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Dorresteijn JA, van der Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL. Relation Between Blood Pressure and Vascular Events and Mortality in Patients With Manifest Vascular Disease: J-Curve Revisited. Hypertension. 2012;59:14–21. doi: 10.1161/HYPERTENSIONAHA.111.179143. [DOI] [PubMed] [Google Scholar]
- 20.Holt EW, Muntner P, Joyce CJ, Webber L, Krousel-Wood MA. Health-related quality of life and antihypertensive medication adherence among older adults. Age Ageing. 2010;39:481–7. doi: 10.1093/ageing/afq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes DK, Denny CH, Keenan NL, Croft JB, Greenlund KJ. Health-related quality of life and hypertension status, awareness, treatment, and control: National Health and Nutrition Examination Survey, 2001--2004. J Hypertens. 2008;26:641–7. doi: 10.1097/HJH.0b013e3282f3eb50. [DOI] [PubMed] [Google Scholar]
- 22.Maatouk I, Wild B, Herzog W, et al. Longitudinal predictors of health-related quality of life in middle-aged and older adults with hypertension: results of a population-based study. J Hypertens. 2012 doi: 10.1097/HJH.0b013e328353d81b. [DOI] [PubMed] [Google Scholar]
- 23.Grimm RH, Jr, Grandits GA, Cutler JA, et al. Relationships of quality-of-life measures to long-term lifestyle and drug treatment in the Treatment of Mild Hypertension Study. Archives of internal medicine. 1997;157:638–48. [PubMed] [Google Scholar]
- 24.Goncalves CB, Moreira LB, Gus M, Fuchs FD. Adverse events of blood-pressure-lowering drugs: evidence of high incidence in a clinical setting. Eur J Clin Pharmacol. 2007;63:973–8. doi: 10.1007/s00228-007-0352-y. [DOI] [PubMed] [Google Scholar]
- 25.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? AnnInternMed. 2006;144:884–93. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan NM. The diastolic J curve: alive and threatening. Hypertension. 2011;58:751–3. doi: 10.1161/HYPERTENSIONAHA.111.177741. [DOI] [PubMed] [Google Scholar]
- 27.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes. The SMART-MR study. J Hypertens. 2010;28:1498–505. doi: 10.1097/HJH.0b013e32833951ef. [DOI] [PubMed] [Google Scholar]
- 28.Barrett-Connor E, Palinkas LA. Low blood pressure and depression in older men: a population based study. BMJ. 1994;308:446–9. doi: 10.1136/bmj.308.6926.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenoir H, Lacombe JM, Dufouil C, et al. Relationship between blood pressure and depression in the elderly. The Three-City Study. J Hypertens. 2008;26:1765–72. doi: 10.1097/HJH.0b013e3283088d1f. [DOI] [PubMed] [Google Scholar]
- 30.Pilgrim JA, Stansfeld S, Marmot M. Low blood pressure, low mood? BMJ. 1992;304:75–8. doi: 10.1136/bmj.304.6819.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Licht CM, de Geus EJ, Seldenrijk A, van Hout HP, Zitman FG, van Dyck R, Penninx BW. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–8. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- 32.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


