Abstract
Background
In 2011, we reported occurrence of natural human infections with Brugia pahangi, a filarial worm of dogs and cats, in a surburb of Kuala Lumpur, the capital city of Malaysia. Our preliminary entomological survey at that time suggested the mosquito species Armigeres subalbatus as the vector of the zoonotic infections. In this present report, we provide biological evidence to confirm our preliminary finding.
Findings
A total of 1798 adult female Ar. subalbatus mosquitoes was caught in the vicinity of the suburb, and 1599 were dissected for the presence of filarial larvae. Sixty-two mosquitoes were positive, and 27 of these were infected with L3 larvae. The L3 were inoculated into male gerbils. Microfilariae could be detected in the gerbils 92 days post-infection. Post-mortem on the gerbils recovered adult worms in the peritoneal cavity, heart, lungs, tail and testis. Male adult worms were confirmed to be B. pahangi by the ratio length of their spicules (left spicule: right spicule). Female adult worms were confirmed by the absence of minute cuticular bosses in the tail region. The worms were further confirmed to be B. pahangi by PCR.
Conclusions
Our results showed that Ar. subalbatus was the vector for the zoonotic Brugia pahangi infections. This mosquito species should now be categorised as a medically important mosquito species in Malaysia. Its role in the transmission of zoonotic B. pahangi must therefore be considered in future studies on filarial infections.
Keywords: Armigeres subalbatus, Vector, Zoonotic, Brugia pahangi, Filariasis
Findings
Background
Brugia pahangi, a closely related species of B. malayi, is a lymphatic filarial worm of mammals, but essentially of domestic cats and dogs [1]. This worm is not known to cause human disease in the natural environment. Although there was a report on the presence of its microfilariae (mf) in human blood in South Kalimantan, the finding was not conclusive as the method used to identify the mf relied on measuring phosphatase distribution in the mf [2]. Experimental B. pahangi infection on human volunteers produced signs and symptoms of lymphatic filariasis, but hardly produced microfilaraemia [3].
Recently, we reported several cases of natural human infection with B. pahangi in a suburb of Kuala Lumpur, the capital city of Malaysia [4]. The life cycle of B. pahangi involves an intermediate mosquito vector and primary mammalian hosts. Mosquito species Mansonia annulata and Ma. dives are known to be natural vectors of B. pahangi[5]. However, these two species live in a forest environment, and they were not found in our preliminary entomological survey of the suburb. Instead, the most prevalent mosquito species in the suburb was Armigeres subalbatus and some were found to be infected with filarial larvae.
Ar. subalbatus is commonly found close to human dwellings especially in sub-urban areas with poor sanitation that contain polluted water such as septic tanks [6]. This species has been known to be a vector of Japanese encephalitis virus [7]. It has also been reported to be a vector of filarial worm Wuchereria bancrofti in India [8] and the dog heartworm Dirofilaria immitis in Peninsular Malaysia [9]. In the early 1960s, it was found that Ar. subalbatus could serve as an efficient vector in experimental infections involving B. pahangi. Since then, numerous B. pahangi-Ar. subalbatus research work has been conducted. However, despite being widely used as a laboratory vector for several decades, hitherto there is no detailed description of Ar. subalbatus as a vector for B. pahangi in nature. In this report, we present biological findings to confirm Ar. subalbatus as a natural vector, particularly for the transmission of zoonotic B. pahangi filariasis.
Methods
Location of entomological survey
Entomological survey was carried out in the vicinity of Bukit Gasing–Kampung Kerinchi (3°5′47″N, 101°39′25″ E – 3°6′51″N, 101°39′47″E), where zoonotic B. pahangi infections were previously reported [4]. This suburb consisted of houses, apartments, several construction projects and slum villages. A recreational secondary forest straddles the center of the suburb. Originally, the secondary forest was a plantation site and had been left idle for 50 years.
Mosquito collection method
Mosquito collection was carried out from 0600–0900 hours and from 1800–2030 hours using the human landing catches method. Approval for the collection was obtained from the University of Malaya Ethical and Research Review Committee [Ref. No. PAR/19/02/2013/AA (R)]. The mosquitoes were brought alive to the laboratory in the Department of Parasitology, Faculty of Medicine, University of Malaya to be counted and identified.
Dissection of mosquitoes
Dissection of mosquitoes was performed at the earliest possible time to check for the presence of filarial larvae. Only female mosquitoes were dissected. The mosquito was placed on a slide under a binocular dissecting microscope. The wings and legs were removed and the body separated into three major parts: head with proboscis, thorax and abdomen. Each part was placed in a separate drop of saline. Live larvae could be seen wriggling out into the normal saline. The number of infected mosquitoes, and the number and the stage of the larvae obtained were recorded.
Inoculation of stage 3 larvae (L3) into gerbils (Meriones unguiculatus)
The L3 larvae were pooled and inoculated immediately into male gerbils by intra-peritoneal and subcutaneous injections for development into adult worms. Sluggish and inactive larvae were not used. After 3 months, thick blood smears of the gerbils were made and stained with Giemsa for mf detection [10]. Approval for using gerbils in our study was granted by the University of Malaya Animal Care and Use Committee [Ref. No. PAR/29/06/2012/RM(R)].
Post mortem and recovery of adult worms
The gerbils were sacrificed for recovery of adult worms 95 days post-infection. A midline incision was made with iris scissors. After skinning and opening up the abdominal cavity, the testis, lungs, heart, lymph nodes and tail tissues were removed and placed in separate small petri dishes containing normal saline. The adult worms were then isolated from the tissues using a dissecting needle and transferred to a cavity block containing normal saline [11].
Identification of adult worm species by morphometric method
The adult worm was mounted in glycerin in a permanent hanging drop preparation on a slide. It was then examined under the microscope which was attached with an Image Analyzer. The length of the left spicule (LS) and right spicule (RS) of male worms was measured and the LS:RS ratio was calculated. The ratio range for B. pahangi is 1.80-2.50:1, while for B. malayi is 2.90-3.80:1 [12]. The tail region of female worms was also examined to determine the presence of minute cuticular bosses, a characteristic of adult female worm of B. malayi, but absent in B. pahangi.
Identification of adult worm species by PCR
DNA was extracted from adult worms using DNeasy Blood and Tissue Kit (Spin-Column protocol, QIAGEN, Germany). The extracted DNA was amplified using primer pairs specific for the cytochrome oxidase I (COXI) gene of B. pahangi (forward primer 5′ TATTGCCTGTTATGC 3′, reverse primer 5′ TGTATATGTGATGAC 3′). PCR was carried out in a 25 ml reaction mixture containing 10 mM Tris–HCl (pH 8·3), 2 mM MgCl2, 50 mM KCl, 0·01% gelatin, 200 mM of each deoxynucleoside triphosphate, 20 pmol of each primer, 1 U of Taq polymerase (Fermentas Life Sciences, Canada). The PCR mixture was pre-heated at 95°C for 10 min for initial denaturation before 30 cycles of amplification, which consisted of denaturation at 94°C for 1 min, annealing at 54°C for 1 min, and elongation at 72°C for 2 min. Final extension of the reaction was carried out at 72°C for 10 min. PCR product was analysed by agarose gel electrophoresis. The expected size of the PCR product was 633 bp.
Results
Mosquito collection
In the entomological survey, a total of 1878 mosquitoes was collected (Table 1). Four species of mosquitoes from two genera were found during the collection period. The predominant mosquitoes caught were Ar. subalbatus (95.7%). Another Armigeres species, Ar. kesseli, was also caught, albeit in small number. Other mosquitoes collected were Aedes albopictus and Ae. aegypti.
Table 1.
Mosquito species and number collected in the entomological survey
| Mosquito species | Number of mosquitoes collected (%) |
|---|---|
|
Armigeres subalbatus |
1798 (95.7) |
|
Armigeres kesseli |
16 (0.9) |
|
Aedes albopictus |
55 (2.9) |
|
Aedes aegypti |
9 (0.5) |
| Total | 1878 |
Mosquito dissection and larvae recovery
Of the 1798 Ar. subalbatus mosquitoes collected, 1599 were dissected for the presence of filarial larvae. Sixty-two mosquitoes were positive, and 27 of these were infected with L3. Majority of the infected mosquitoes harboured between 7 and 25 L3, although there was one which had 45 L3. Most of the L3 were found in the proboscis. However, in some mosquitoes which had a high number of larvae, L3 could be found in the entire body. Developing larvae (L1 and L2) were found mostly in the thorax. The total larvae recovered were 346 L3, 259 L2 and 16 L1. Table 2 summarises the number of positive mosquitoes, infection and infective rates, and number of larvae recovered. None of the Ar. kesseli, Ae. albopictus and Ae. aegypti mosquitoes were positive for larvae.
Table 2.
Number of positive mosquitoes, infection and infective rates, and number of larvae recovered
| Infection/infective rate | Number of infected mosquitoes (%cih) |
|---|---|
| Total positive (infection rate) |
62 (3.9) |
| with L3 (infective rate) |
27 (1.7) |
| with L2 |
32 (2.0) |
| with L1 |
3 (0.2) |
|
Larva stage |
Total larvae recovered in mosquitoes |
| L3 |
346 |
| L2 |
259 |
| L1 | 16 |
Pre-patent period, recovery of adult worms and species identification
Only L3 larvae were injected into two gerbils. Thirty-four were inoculated intra-peritoneally into the first gerbil, and 5 subcutaneously into the second gerbil. Blood from the infected gerbils was taken at 1000 hours and examined for the presence of mf for six days, from day 90–95 post infection. The blood smear was stained with Giemsa. Mf could be detected on days 92–95, indicating pre-patent period in gerbil of 92 days.
Post mortem on the gerbils was carried out 95 days post-infection. Eighteen adult worms were recovered, in which 16 were recovered in the peritoneal cavity, heart, lungs and tail of the first gerbil. Two were recovered from the testis of the second gerbil. Five of the 7 adult male worms were cleared and mounted for identification. They were confirmed to be B. pahangi by determining the ratio length of their spicules (LS:RS). The ratios of the adult male worms obtained were 2.47:1, 2.36:1, 2.38:1, 2.06:1 and 2.00:1 (Figure 1A), respectively. These ratios were within the ratio range for B. pahangi (1.80-2.50:1) which is much smaller than the ratio of B. malayi (2.90-3.80:1) [12]. The 9 adult female worms were confirmed to be B. pahangi by examining their body cuticle in the tail region. The body cuticle of B. pahangi adult female worm is devoid of the minute cuticular bosses (Figure 1B), which are only seen in female B. malayi adult worm [12].
Figure 1.
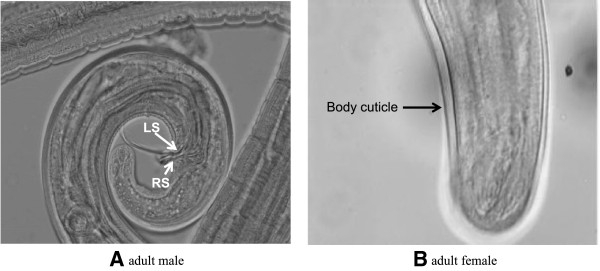
Identification of species based on: (A) the ratio length of spicules (left spicule:right spicule, LS:RS) in adult male, the worm was confirmed to be B. pahangi with LS:RS = 2.00:1; and (B) tail region of adult female of B. pahangi, with body cuticle devoid of minute cuticular bosses (x40).
Species identification of adult worm by PCR
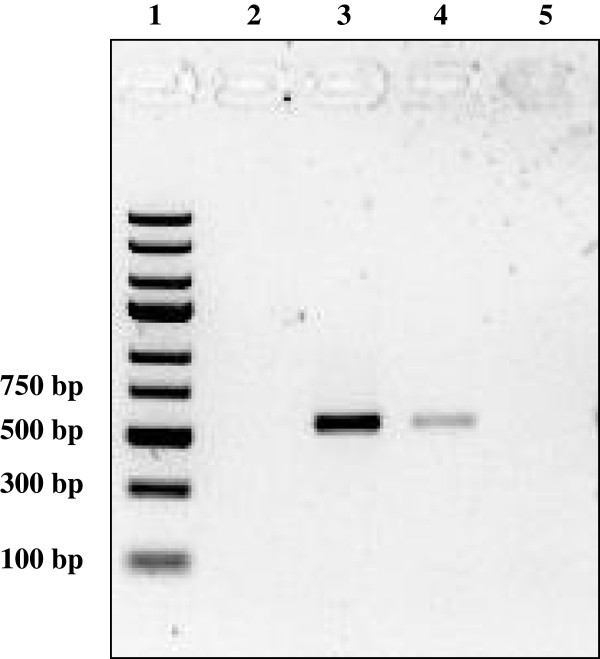
The adult worms were further confirmed to be B. pahangi by PCR using primers specific for the COXI gene. The 633 bp region of the B. pahangi COXI gene was successfully amplified (Figure 2).
Figure 2.
Agarose gel electrophoresis of amplicons from PCR using primers specific for Brugia pahangi COXI gene. Lane 1, DNA molecular mass standards (Fermentas, Lithuania); lane 3, amplicon (633 bp) from PCR on DNA of adult male worm; lane 4, amplicon (633 bp) from PCR on DNA of adult female worm; no amplicon was detected from PCR on DNA of B. malayi adult male worm (lane 2) and negative control (water only) (lane 5).
Discussion
Armigeres subalbatus is widely distributed in Malaysia. Previously, this species has never been considered a medically important mosquito in Malaysia as compared to other mosquitoes such as Culex, Aedes, Mansonia and Anopheles spp. Our present study highlights the role of Ar. subalbatus as a vector of zoonotic filariasis in Peninsular Malaysia.
Pioneering work on the vectors of B. pahangi in Malaysia was carried out mainly in the 1960s. Field and laboratory studies established Ma. annulata and Ma. dives as vectors of B. pahangi in nature [5]. The typical breeding place for Ma. annulata is the forest verge where open swamp and forest meet. Swamp forest having rootlets of trees, rattans and palms is the usual breeding ground for Ma. dives. Our study site, on the other hand, was a suburb with residential houses and flats, villages and a recreation hill park. This therefore explains the absence of Mansonia species in the site. The surroundings of the site were favourable for Ar. subalbatus, which breeds well around human dwellings with poor sanitation that contain polluted water [6]. This explains the abundance and predominance of Ar. subalbatus in the site as compared to other mosquito species, such as Ar. kesseli, Ae. albopictus and Ae. aegypti. The human landing catch method was used to collect the mosquitoes, and the high number of Ar. subalbatus obtained was surprising since it was previously reported that this species did not feed readily on humans [13]. Infection and infective rates obtained in our study were 3.9% and 1.7%, respectively. These rates are higher than those observed in previous entomological studies in endemic areas in Malaysia, which ranged from 0.1% to 3% [14-17].
A unique biological feature of Ar. subalbatus is its high susceptibility to B. pahangi but refractoriness to B. malayi mf infection. The mf of these filarial species are morphologically similar, although genetically they can be differentiated by various molecular methods. Whereas B. pahangi mf can easily develop to the infective L3 stage, B. malayi mf are rapidly destroyed in the haemocoel by melanotic encapsulation [18,19]. Before the advent of biochemical and molecular methods, distinguishing of B. malayi and B. pahangi mf could only be done by letting Ar. subalbatus feed on the infected animal or human, and detect the development of larvae in the mosquito [20]. Hence, the developing and infective larvae recovered in the Ar. subalbatus in our study were undoubtedly B. pahangi.
In laboratory experimental settings, Ar. subalbatus has been demonstrated to be an extremely efficient host, producing high number of L3 larvae [5]. In an experiment in which Ar. subalbatus was allowed to feed on a B. pahangi animal carrier with more than 1 mf/μl, all become infected and the larvae reached maturity as early as the 7th day after feeding. Almost all (99%) larvae were L3 by the 11th day. When fed on an animal carrier with 6.2 mf/μl, an average of 38.7 larvae per mosquito was obtained. Wharton [13] reported experimental infection index of 7.1 ± 1.1, for mf counts of 1 mf/μl. In other words, of an average of 7 L3 per mosquito could be produced. In our study, a relatively high number of L3 larvae was seen in the naturally-infected mosquitoes, ranging between 7 and 25, with one reaching up to 45. This high infective rate is likely due to the size of the female Ar. subalbatus, which is comparatively larger than any other mosquito species. In a single blood meal, a female Ar. subalbatus can take an average of 4.5 μl of blood [13].
Upon ingestion of an infective blood meal by the female Ar. subalbatus, the mf penetrate the midgut epithelium and migrate to the thoracic musculature of the mosquito. Here, the mf moult several times to develop into infective L3. The L3 migrate to the head region and finally to the proboscis of the mosquito. The L3 are then transmitted to a vertebrate host when the infected mosquito takes a blood meal. In our study, most of the larvae were seen in the usual sites in the mosquitoes: L1 and L2 in the thorax, and L3 in the proboscis. Oddly, in some highly infected mosquitoes, L3 could be found in the entire body. Zahedi et al. [21], who found developing larvae L2 in the head, commented that erratic migration of larvae to unusual sites might be the consequence of heavy infection.
In our survey, Ar. kesseli was found but in smaller number. It shares similar morphology with Ar. subalbatus. The latter, however, can be identified by the presence of white scaling in the hind femur which tapers and terminates before the knee, and the broad black band on sternite 3–6. The white scaling in the hind femur of Ar. kesseli is broad, and the black band on its sternite is narrower [22]. Despite having morphological similarities and occupying similar habitats, none of the Ar. kesseli in our study was found to be infected with larvae.
Conclusions
Our study was conducted in a suburb where several cases of zoonotic B. pahangi infection were recently reported [4]. The findings of our study show that Ar. subalbatus was indeed the vector for the zoonotic infections. With a capacity to naturally harbour a high number of B. pahangi larvae and being a human biter which thrives in areas of human habitations, Ar. subalbatus should be considered a medically important mosquito species in Malaysia along with other mosquitoes such as Aedes, Anopheles, Culex and Mansonia spp. Its role in the transmission of zoonotic B. pahangi must be considered in future studies on filarial infections.
Abbreviations
L: Larva; LS: Left spicule; RS: Right spicule.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM, MYF, and RM designed the study. AZ and SS performed field collection, mosquito identification and gerbil infection. AZ and YLL performed molecular identification of the adult worms. AZ and MYF drafted the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Azdayanti Muslim, Email: azdayanti@salam.uitm.edu.my.
Mun-Yik Fong, Email: fongmy@um.edu.my.
Rohela Mahmud, Email: rohela@ummc.edu.my.
Yee-Ling Lau, Email: lauyeeling@um.edu.my.
Sinnadurai Sivanandam, Email: sivanandamsinna@hotmail.com.
Acknowledgements
This research was supported by the University of Malaya High Impact Research Grant UM-MOHE UM.C/625/1/HIR/MOHE/MED/18 from the Ministry of Higher Education Malaysia and UMRG (RG333/11HTM). The authors are grateful to Hidaitul Masalaina, Mohd Azli Kamaruzzaman, Sukri Jaafar, Aidil, Akbar Ishak, and Kashfi for their assistance in field collection of mosquito samples.
References
- Denham DA, McGreevy PB. Brugian filariasis: epidemiological and experimental studies. Adv Parasitol. 1977;15:243–309. doi: 10.1016/s0065-308x(08)60530-8. [DOI] [PubMed] [Google Scholar]
- Palmieri JR, Ratiwayanto S, Masbar S, Tirtokusumo S, Rusch J, Marwoto HA. Evidence of possible natural infections of man with Brugia pahangi in South Kalimantan (Borneo), Indonesia. Trop Geogr Med. 1985;37:239–244. [PubMed] [Google Scholar]
- Edeson JF, Wilson T, Wharton RH, Laing AB. Experimental transmission of Brugia malayi and B. pahangi to man. Trans R Soc Trop Med Hyg. 1960;54:229–234. doi: 10.1016/0035-9203(60)90066-3. [DOI] [PubMed] [Google Scholar]
- Tan LH, Fong MY, Mahmud R, Muslim A, Lau YL, Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol Int. 2011;60:111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Edeson JF, Wharton RH, Laing AB. A preliminary account of the transmission, maintenance and laboratory vectors of Brugia pahangi. Trans R Soc Trop Med Hyg. 1960;54:439–449. doi: 10.1016/0035-9203(60)90089-4. [DOI] [PubMed] [Google Scholar]
- Rajavel AR. Larval habitat of Armigeres subalbatus (COQ) and its characteristics in Pondicherry. Southeast Asian J Trop Med Public Health. 1992;23:470–473. [PubMed] [Google Scholar]
- Liu H, Lu HJ, Liu ZJ, Jing J, Ren JQ, Liu YY, Lu F, Jin NY. Japanese encephalitis virus in mosquitoes and swine in Yunnan province, China 2009–2010. Vector Borne Zoonotic Dis. 2013;13:41–49. doi: 10.1089/vbz.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Bhattacharya S, Palit CA, Das S, Ghosh KK, Hati AK. Diurnal man-biting activity of Armigeres subalbatus (Coquillet, 1898) in a village in West Bengal. Indian J Med Res. 1983;78:794–798. [PubMed] [Google Scholar]
- Cheong WH, Mak JW, Naidu S, Mahadevan S. Armigeres subalbatus incriminated as an important vector of the dog heartworm Dirofilaria immitis and the bird Cardiofilaria in urban Kuala Lumpur. Southeast Asian J Trop Med Public Health. 1981;12:611–612. [PubMed] [Google Scholar]
- Sivanandam S, Fredericks HJ. The “Innenkorper” in differentiation between the microfilanriae of Brugia pahangi and B. malayi (sub-periodic form) Med J Malaya. 1966;20:337–338. [PubMed] [Google Scholar]
- Lim PKC, Sim BKL. Laboratory techniques in filariasis. Bull Inst Med Res Malaysia. 1983;19:95–104. [Google Scholar]
- Buckley JJC, Edeson JFB. On the adult morphology of Wuchereria sp. (malayi?) from a monkey (Macaca irus) and from cats in Malaya, and on Wuchereria pahangi sp. from a dog and a cat. J Helminthol. 1956;30:1–20. doi: 10.1017/S0022149X00032922. [DOI] [PubMed] [Google Scholar]
- Wharton RH. The biology of Mansonia mosquitoes in relation to the transmission of filariasis in Malaya. Bull Inst Med Res Malaysia. 1978;11:85–89. [PubMed] [Google Scholar]
- Ramachandran CP, Cheong WH, Sivanandam S, Omar AH, Mahadevan S. Filariasis in Ulu Trengganu, West Malaysia: parasitological and entomological observations. Southeast Asian J Trop Med Public Health. 1970;1:505–515. [Google Scholar]
- Cheah WC, Cheong WH, Mahadevan S, Lai KPF, Sivanandam S. Filariasis in Negri Sembilan - a follow-up study. Med J Malaysia. 1977;32:103–110. [PubMed] [Google Scholar]
- Mak JW, Cheong WH, Omar AH, Sivanandam S, Mahadevan S. Filariasis in Perlis, Peninsular Malaysia. Med J Malaysia. 1977;31:198–203. [PubMed] [Google Scholar]
- Mak JW, Lye MS, Sim BKL, Cheong WH, Lee CP. Studies on malaria and filariasis in Hilir Perak, Peninsular Malaysia. Trop Biomed. 1985;2:40–46. [Google Scholar]
- Yamamoto H, Kobayashi M, Ogura N, Tsuruoka H, Chigusa Y. Studies on filariasis. VI. The encapsulation of Brugia malayi and B. pahangi larva in the mosquito, Armigeres subalbatus. Japanese J Sanitary Zool. 1985;36:1–6. [Google Scholar]
- Beerntsen BT, Luckhart S, Christensen BM. Brugia malayi and Brugia pahangi: Inherent difference in immune activation in the mosquitoes Armigeres subalbatus and Aedes aegypti. J Parasitol. 1989;75:76–81. doi: 10.2307/3282940. [DOI] [PubMed] [Google Scholar]
- Cheong WH, Omar AH, Sivanandam S. An attempt to separate a mixed Brugia infection by biological means. Singapore Med J. 1965;5:43. [PubMed] [Google Scholar]
- Zahedi M, Denham DA, White GB. An unusual site of development of Brugia pahangi in the mosquito, Armigeres subalbatus. Trop Biomed. 1990;7:203–205. [Google Scholar]
- Ramalingam S. On the restriction of Armigeres durhami Edwards and the description of Armigeres kesseli species (Diptera: Culicidae) Trop Biomed. 1987;4:55–65. [Google Scholar]