Abstract
Introduction
One of the bacterial agents that has been found to be associated with colorectal cancer is Streptococcus bovis, with 13% of infective endocarditis cases caused by this pathogenic species.
Case presentation
We describe the case of a 57-year-old Caucasian man with infiltrating and ulcerating metastatic adenocarcinoma of the sigmoid colon. The patient was receiving second-line chemotherapy treatment and, on the eighth day of the second cycle, he developed a grade IV pancytopenia. We diagnosed a severe sepsis with positive blood cultures for Streptococcus bovis/gallolyticus with a secondary endocarditis.
Conclusions
A recent study suggests that the majority of patients affected by colonic cancer have a Streptococcus bovis/gallolyticus colonization that becomes apparent as an overt infection only when immunosystem disorders or cardiac valve lesions occur. This correlation is important for involving more specialists in a correct and early diagnosis of this rare, but potentially fatal, complication.
Keywords: Streptococcus bovis/gallolyiticus, Endocarditis, Colon cancer
Introduction
The human gastrointestinal tract is colonized by different commensal bacterial species. This bacterial population prevents the invasion of pathogenic bacteria [1].
Among the commensal bacteria, Streptococcus bovis (S. bovis) is found in 2.5 to 15% of the population. Several studies have shown a correlation between the presence of S. bovis biotype I bacteremia and colon cancer.
In addition, 13% of infective endocarditis cases are caused by this pathogenic species [2,3].
The aim of this report is to present the clinical, diagnostic, and therapeutic aspects of a patient with advanced colon cancer who developed secondary neutropenia after antineoplastic treatment with subsequent onset of meningoencephalitis and endocarditis related to S. bovis/gallolyticus infection.
The clinical, diagnostic and therapeutic aspects will be discussed.
Case presentation
A 57-year-old Caucasian man with no family history of neoplastic diseases and no comorbidities, after severe weight loss and abdominal pain, was examined by his general practitioner for tumor markers (carcinoembryonic antigen (CEA)=4448 and cancer antigen (CA)19.9=4728). His fecal occult blood test was positive and the biopsies obtained during a colonoscopy were positive for infiltrating and ulcerated poorly differentiated adenocarcinoma (G3) of the sigmoid colon.
An immunohistochemistry analysis revealed epidermal growth factor receptor (EGFR) positivity and molecular analysis showed that both KRAS and BRAF were not mutated.
A staging computed tomography (CT) scan showed liver, lung, and spleen metastases and the presence of peritoneal carcinomatosis. Because of the advanced stage of disease, no indication was given for surgical treatment. The patient underwent 10 cycles of chemotherapy according to the FOLFOX6 regimen (oxaliplatin 100mg/mq; leucovorin 400mg/mq; 5-fluorouracil 400mg/mq bolus followed by 5-fluorouracil 2400mg/mq by continuous infusion over 46 hours every two weeks) with markers reduction (CEA=681 and CA19.9=330), improved performance status and weight gain. His chemotherapy treatment was continued with capecitabine, 1000mg/mq twice a day, as a maintenance therapy for two additional months. After this treatment, a CT scan showed progression of the disease in the lungs and liver, and laboratory examinations showed an increase in the tumor markers (CEA=820 and CA19.9=507).
The patient started a second-line chemotherapy with cetuximab, 400mg/mq loading dose, and irinotecan 200mg/mq. On the eighth day of the second cycle the patient developed fever (39°C), diarrhea, and confusion and the neurological examination by the emergency service revealed severe opisthotonus and retroversion of the eyes. A brain CT scan was negative for ischemic events or secondary lesions and his electroencephalogram (EEG) was normal.
In the afternoon, the patient was admitted to the medical oncology unit, where he was diagnosed with severe sepsis with clinical involvement of the meningoencephalic system and grade IV pancyopenia (hemoglobin (Hb)=8.5g/dL, red blood cells (RBC)=2.8×10^6/mL, platelets (PLT)=31000mL, white blood cells (WBC)=300mL, neutrophils=100mL).
While waiting for blood culture results, he started empiric antibiotic therapy with 500mg levofloxacin administered intravenously every 12 hours and 200mg intravenous fluconazole once a day in association with granulocyte colony-stimulating factor (G-CSF). After five days, the pancytopenia resolved but the neurological disorder persisted.
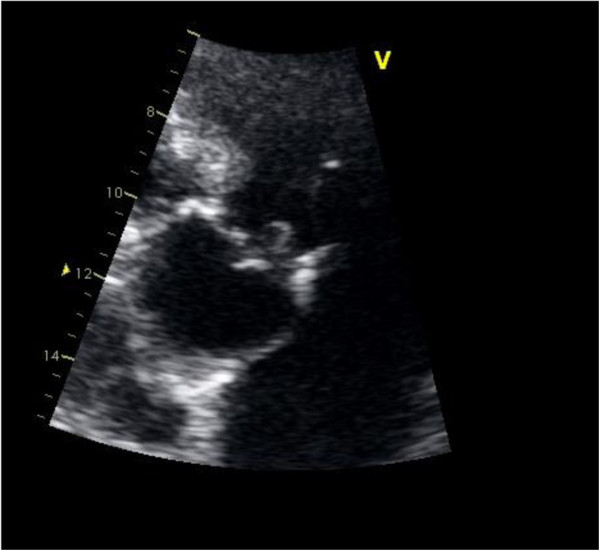
In the meantime, Streptococcus gallolyticus subsp gallolyticus was isolated in the blood cultures. Echocardiography showed a moderate aortic valve insufficiency due to an endocardial vegetation stack on the right coronary cusp (about 10mm in diameter) protruding in the outflow tract of the left ventricle (Figure 1). A cerebrospinal fluid sample was not taken due to the very low platelet count (5000/mL on the second day after admission).
Figure 1.
Endocardial vegetation stack on the right coronary cusp.
Ampicillin and sulbactam (3g intravenous every six hours) were added to the antibiotic treatment.
On the fourteenth day, the patient’s neurological status improved and he exhibited only residual mild opisthotonus with no cognitive deficit.
A total body CT scan was performed on the third week after admission, which showed progressive disease, thus antineoplastic therapy was ceased.
Discussion
The incidence of colon cancer with S. bovis endocarditis has been shown to be between 18 and 62% [4-10].
The co-occurrence of a bacterial endocarditis and colon carcinoma was first reported in 1951 [11] but the association of S. bovis and colorectal neoplasia was not recognized until 1974 [12]. S bovis was documented by Klein et al. as the pathogenic agent specifically related to the presence of a colon cancer in 1977 [13].
Bacteria are linked to cancer by two mechanisms: chronic inflammation and the production of carcinogenic metabolites [14].
S. bovis/gallolyticus has been reported to increase the production of inflammatory cytokines in the colonic mucosa of rats, suggesting a direct interaction between S. bovis and colonic mucosal cells, which is thought to lead to the development of colorectal cancer. It should be noted that in humans most colonic neoplasms associated with S. bovis bacteremia are ulcerated adenomas, a well-known precursor of invasive cancer [15-19].
In our clinical case, the neoplastic disease was already present when the patient developed bacteremia and endocarditis secondary to the infection with S. bovis. The infection occurred in the context of severe neutropenia. We assume that due to the critical condition of the patient, he developed endocarditis and possibly a meningoencephalitis as a result of the transition of the S. bovis/gallolyticus through the ulcerated tumoral lesion of the colon into the bloodstream.
A recent study suggested that the majority of patients affected by colonic cancer develop a silent infection, but it becomes apparent when immunosystem disorders or cardiac valve lesions occur [20].
Considering the correlation between S. bovis/gallolyticus, endocarditis and tumors, colorectal endoscopic evaluation would be useful in patients who have developed bacterial endocarditis caused by S. bovis/gallolyticus in order to identify silent colorectal cancer as early as possible.
Conclusions
In these cases, the teamwork of several specialists (cardiologist, infectivologist, endoscopist, oncologist) remains the gold standard for an accurate diagnostic workup and prompt treatment decisions.
Therefore, understanding whether other cases like ours have occurred and what to do in such situations would be helpful.
In addition, the peculiar aspect of this case is the presence of a neurological involvement, which could be due to both the septic status and to a specific sensibility of the brain membrane to Streptococcus bovis/gallolyticus, albeit a cerebrospinal fluid (CSF) analysis was not done.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AC, RL, BP, OC, BCP and ZA diagnosed and followed the patient. BCP performed and interpreted the echocardiography. AC, RL and OC prepared the manuscript and ZA evaluated the draft and suggested revisions. All authors read and approved the final manuscript.
Contributor Information
Chiara Abeni, Email: chiara.abeni@poliambulanza.it.
Luigina Rota, Email: luigina.rota@poliambulanza.it.
Chiara Ogliosi, Email: chiara.ogliosi@poliambulanza.it.
Paola Bertocchi, Email: paola.bertocchi@poliambulanza.it.
Pietro Berra Centurini, Email: pietro.berracenturini@poliambulanza.it.
Alberto Zaniboni, Email: alberto.zaniboni@poliambulanza.it.
References
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2011;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Waisberg J, Matheus CO, Pimenta J. Infectious endocarditis from streptococcus bovis associated with colonic carcinoma: case report and literature review. Arq Gastroenterol. 2002;39:177–180. doi: 10.1590/S0004-28032002000300008. [DOI] [PubMed] [Google Scholar]
- Sharara AL, Abou Hamdan T, Malli A, El-Halabi MM, Hashash JG, Ghaith OA, Kanj SS. Association of Streptococcus bovis endocarditis and advanced colorectal neoplasia: a case–control study. J Dig Dis. 2013;14:382–387. doi: 10.1111/1751-2980.12059. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Thompson RL, Wilkowske CJ, Washington JA, Giuliani ER, Geraci JE. Short-term therapy for streptococcal infective endocarditis. Combined intramuscular administration of penicillin and streptomycin. JAMA. 1981;245:360–363. doi: 10.1001/jama.1981.03310290028017. [DOI] [PubMed] [Google Scholar]
- Reynolds JG, Silva E, McCormack WM. Association of Streptococcus bovis bacteremia with bowel disease. J Clin Microbiol. 1983;17:696–697. doi: 10.1128/jcm.17.4.696-697.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leport C, Bure A, Leport J, Vilde JL. Incidence of colonic lesions in Streptococcus bovis and enterococcal endocarditis. Lancet. 1987;1:748. doi: 10.1016/s0140-6736(87)90391-6. [DOI] [PubMed] [Google Scholar]
- Zarkin BA, Lillemoe KD, Cameron JL, Effron PN, Magnuson TH, Pitt HA. The triad of Streptococcus bovis bacteremia, colonic pathology, and liver disease. Ann Surg. 1990;211:786–791. doi: 10.1097/00000658-199006000-00019. discussion 791–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok H, Jureen R, Soon CY, Tey BH. Colon cancer presenting as Streptococcus gallolyticus infective endocarditis. Singapore Med J. 2007;48:e43–e45. [PubMed] [Google Scholar]
- Malkin J, Kimmitt PT, Ou HY, Bhasker PS, Khare M, Deng Z, Stephenson I, Sosnowski AW, Perera N, Rajakumar K. Identification of Streptococcus gallolyticus subsp. macedonicus as the etiological agent in a case of culture-negative multivalve infective endocarditis by 16S rDNA PCR analysis of resected valvular tissue. J Heart Valve Dis. 2008;17:589–592. [PubMed] [Google Scholar]
- Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis; a silent sign for colonic tumour. Colorectal Dis. 2010;12:164–171. doi: 10.1111/j.1463-1318.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- McCoy W, Mason JM. Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala. 1951;21:162–166. [PubMed] [Google Scholar]
- Keusch GT. Opportunistic infections in colon carcinoma. Am J Clin Nutr. 1974;27:1481–1485. doi: 10.1093/ajcn/27.12.1481. [DOI] [PubMed] [Google Scholar]
- Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of streptococcus bovis and carcinoma of the colon. N Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- Parsonnet J. Bacterial infection as a cause of cancer. Environ Health Perspect. 1995;Suppl 8:263–268. doi: 10.1289/ehp.95103s8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmerich S, Scholler M, Duranton B, Gosse F, Galluser M, Klein JP, Raul F. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis. 2000;21:753–756. doi: 10.1093/carcin/21.4.753. [DOI] [PubMed] [Google Scholar]
- Biarc J, Nguyen IS, Pini A, Gossé F, Richert S, Thiersé D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP, Schöller-Guinard M. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis) Carcinogenesis. 2004;25:1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- Abdulamir AS, Hafidh RR, Abu Bakar F. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249. doi: 10.1186/1476-4598-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulamir AS, Hafidh RR, Mahdi LK, Al-jeboori T, Abubaker F. Investigation into the controversial association of Streptococcus gallolyticus with colorectal cancer and adenoma. BMC Cancer. 2009;9:403. doi: 10.1186/1471-2407-9-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkin BA, Lillemoe KD, Cameron JL, Effron PN, Magnuson TH, Pitt HA. The triad of Streptococcus bovis bacteriemia, colonic pathology and liver disease. Ann Surg. 1990;211:791–792. doi: 10.1097/00000658-199006000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Schöller-Guinard M, Lasonder E, Ruers TJ, Willems HL, Swinkels DW. Profiling the humoral immune response in colon cancer patients: diagnostic antigens from Streptococcus Bovis. Int J Cancer. 2006;119:2127–2135. doi: 10.1002/ijc.22116. [DOI] [PubMed] [Google Scholar]