Abstract
Background
Hyponatremia is often observed in patients with Legionella pneumonia. However, other electrolyte abnormalities are uncommon and the mechanism remains to be clarified.
Case presentation
We experienced two male cases of acquired Fanconi syndrome associated with Legionella pneumonia. The laboratory findings at admission showed hypophosphatemia, hypokalemia, hypouricemia and/or hyponatremia. In addition, they had the generalized dysfunction of the renal proximal tubules presenting decreased tubular reabsorption of phosphate (%TRP), increased fractional excretion of potassium (FEK) and uric acid (FEUA), low-molecular-weight proteinuria, panaminoaciduria and glycosuria. Therefore, they were diagnosed as Fanconi syndrome. Treatment for Legionella pneumonia with antibiotics resulted in the improvement of all serum electrolyte abnormalities and normalization of the %TRP, FEK, FEUA, low-molecular-weight proteinuria, panaminoaciduria and glycosuria, suggesting that Legionella pneumophila infection contributed to the pathophysiology of Fanconi syndrome.
Conclusion
To the best of our knowledge, this is the first report demonstrating Fanconi syndrome associated with Legionella pneumonia.
Keywords: Fanconi syndrome, Legionella pneumonia, Electrolyte abnormality
Background
Fanconi syndrome is a generalized dysfunction of the renal proximal tubules without primary glomerular involvement. It is typically characterized by variable degrees of phosphate, amino acids or glucose wasting by the proximal tubules. In addition, hypokalemia, hypouricemia, metabolic acidosis and low-molecular-weight proteinuria can be part of the clinical spectrum [1]. Acquired forms of Fanconi syndrome are caused by paraproteinemia, Sjögren syndrome, primary biliary cirrhosis (PBC) and drugs such as cisplatin [2-4]. However, infectious diseases have not been reported as a cause of Fanconi syndrome.
In this report, we present extremely rare cases of Legionella pneumonia complicated by Fanconi syndrome.
Case presentation
Patient 1
A 75-year-old man was admitted to our hospital with fever and general malaise.
A physical examination at the time of admission showed a body temperature of 38.9°C, blood pressure of 120/70 mmHg and a pulse rate of 80 beats/min. Laboratory findings showed a white blood cell count of 13,400/mm3 and C-reactive protein (CRP) of 23.7 mg/dL. A chest computed tomography (CT)-scan showed a ground-glass appearance in the right middle and lower lobes. Because the urinary antigen test for Legionella pneumophila serotype 1 (BinaxNOW® Legionella, Binax, Inc. ME, USA) was positive, he was diagnosed as Legionella pneumonia.
Several electrolyte abnormalities coexisted including hyponatremia, hypokalemia, hypophosphatemia and hypouricemia. His renal function was normal and the arterial blood gases analysis showed mild metabolic acidosis (Table 1). The tubular reabsorption of phosphate (%TRP) was decreased, although the fractional excretion of uric acid (FEUA) was increased, indicating disturbed proximal tubular reabsorption. Despite the hypokalemia, his urinary potassium excretion was not suppressed. Elevations in urinary levels of β2-microglobulin (β2-MG) and N-acetyl-β-D-glucosaminidase (NAG), glycosuria and panaminoaciduria were also observed (Tables 1 and 2). Therefore, he was diagnosed as Fanconi syndrome.
Table 1.
Laboratory data on admission
| Normal range | Patient 1 | Patient 2 | |
|---|---|---|---|
| BUN |
8–22 mg/dL |
17 |
41 |
| Serum creatinine |
0.71–1.20 mg/dL |
0.88 |
2.23 |
| Serum sodium |
136–147 mEq/L |
121 |
143 |
| Serum potassium |
3.6–5.0 mEq/L |
3.4 |
3.5 |
| Serum phosphorus |
2.5–4.3 mg/dL |
1.3 |
2.0 |
| Serum uric acid |
< 7.0 mg/dL |
2.4 |
2.7 |
| Serum chloride |
101–109 mEq/L |
83 |
107 |
| Serum CPK |
56–244 IU/L |
670 |
2,330 |
| Bicarbonate |
22.0–26.0 mEq/L |
20.8 |
15.7 |
| Anion gap |
12 ± 2 mEq/L |
15.2 |
20.3 |
| Serum osmolarity |
285–295 mOsm/kg |
255 |
N.D. |
| Urinary osmolarity |
mOsm/kg |
673 |
N.D. |
| ADH |
0.3–4.2 pg/mL |
1.7 |
N.D. |
| Proteinuria |
Negative; (−) |
(2+) |
(2+) |
| Leukocyturia |
Negative; (−) |
(−) |
(−) |
| Urinary pH |
4.8–7.5 |
6.5 |
5.5 |
| Urinary glucose |
Negative; (−) |
(3+) |
(2+) |
| Urinary RBC |
0–4/HPF |
10–19/HPF |
10–19/HPF |
| %TRP |
81–90% |
54.0 |
51.0 |
| FEK |
10.0–20.0% |
18.4 |
33.1 |
| FEUA |
5.5–11.0% |
18.7 |
42.8 |
| β2-MG |
< 250 μg/L |
80,397 |
110,556 |
| NAG | < 7.0 U/L | 18.0 | 19.7 |
Definition of the abbreviations: ADH antidiuretic hormone, BUN blood urea nitrogen, CPK creatine phosphokinase, FEK fractional excretion of potassium, FEUA fractional excretion of uric acid, HPF high power field, NAG N-acetyl-β-D-glucosaminidase, N.D. not determined, RBC red blood cell, %TRP tubular reabsorption of phosphate, WBC white blood cell, β2-MG β2-microglobulin. Conversion factors for units: BUN in mg/dL to mmol/L, × 0.357, creatinine in mg/dL to mmol/L, × 88.4, phosphorus in mg/dL to mmol/L, × 0.3229, uric acid in mg/dL to μmol/L, × 59.48, and ADH in pg/mL to pmol/L, × 0.923. No conversion is necessary for sodium, potassium, chloride, CPK, bicarbonate, anion gap, and osmolarity.
Table 2.
Data of the analysis of urinary amino acids on admission
| Normal range (mmol/day) | Patient 1 | Patient 2 | |
|---|---|---|---|
| Taurine |
322.2–5214.5 |
1318.6 |
272.5 |
| Phosphoethanolamine |
31.0–110.0 |
49 |
34.4 |
| Urea |
130.3–493.2 |
223.9 |
341.3 |
| Aspartic acid |
< 12.7 |
N.D. |
6.1 |
| Hydroxyproline |
N.D. |
N.D. |
N.D. |
| Threonine |
79.9–528.3 |
1530.2 |
380.7 |
| Serine |
208.8–1020.0 |
1529.6 |
478.3 |
| Asparagine |
60.7–372.3 |
747.4 |
393.6 |
| Glutamic acid |
11.3–42.7 |
30.1 |
15.5 |
| Glutamine |
207.0–1357.3 |
1071.2 |
170.3 |
| Sarcosine |
< 99.0 |
24.8 |
N.D. |
| α-Aminoadipic acid |
16.7–118.6 |
52.4 |
34.4 |
| Proline |
N.D. |
18.4 |
25.6 |
| Glycine |
652.1–3670.6 |
852.4 |
336.2 |
| Alanine |
141.2–833.9 |
839.3 |
538.6 |
| Citrulline |
13.5–55.6 |
63.4 |
N.D. |
| α-Aminobutylic acid |
< 27.1 |
N.D. |
N.D. |
| Valine |
24.8–82.2 |
97 |
104.2 |
| Cystine |
23.7–170.9 |
226.2 |
101.3 |
| Cystathionine |
< 44.7 |
21 |
334.6 |
| Methionine |
< 20.2 |
11.5 |
15.5 |
| Isoleucine |
7.5–23.5 |
26.5 |
29.2 |
| Leucine |
24.6–89.3 |
95.5 |
96.3 |
| Tyrosine |
50.6–308.4 |
327.4 |
111.8 |
| Phenylalanine |
27.2–110.2 |
150.7 |
199.8 |
| γ-Amino β-hydroxybutyric acid |
N.D. |
N.D. |
N.D. |
| β-Alanine |
< 153.0 |
58.4 |
9.4 |
| β-Amino-iso-butyric acid |
< 1623.9 |
151.8 |
288.5 |
| γ-Aminobutyric acid |
N.D. |
N.D. |
N.D. |
| Monoethanolamine |
195.3–606.2 |
292.8 |
356.8 |
| Homocystine |
N.D. |
N.D. |
N.D. |
| Histidine |
436.4–2786.5 |
1392 |
496.8 |
| 3-Methylhistidine |
113.4–480.9 |
202.9 |
396 |
| 1-Methylhistidine |
59.3–2816.2 |
821.2 |
16.7 |
| Carnosine |
< 87.6 |
N.D. |
21.2 |
| Anserine |
< 231.4 |
49.4 |
N.D. |
| Tryptophan |
20.7–150.7 |
156.7 |
59.2 |
| Hydroxylysine |
< 22.9 |
3.8 |
N.D. |
| Ornithine |
6.9–43.9 |
46.3 |
151.4 |
| Lysine |
51.6–1639.6 |
794.9 |
148.1 |
| Arginine | 11.6–54.8 | 51.5 | 31.3 |
Underlining indicates an abnormal value.
N.D. means not detected.
In addition, his urinary osmolarity was greater than his serum osmolarity and the level of antidiuretic hormone (ADH) was improperly high for the indicated serum osmolarity. There was no sign of extracellular fluid volume depletion. Therefore, a diagnosis of the syndrome of inappropriate secretion of ADH (SIADH) was also made.
He was treated for Legionella pneumonia with pazufloxacin mesilate (PZFX) at 1,000 mg per day. After treatment, his physical status recovered and his electrolyte abnormalities improved. Furthermore, his %TRP, FEUA, urinary β2-MG and NAG normalized, and the panaminoaciduria became undetectable (Figure 1A).
Figure 1.
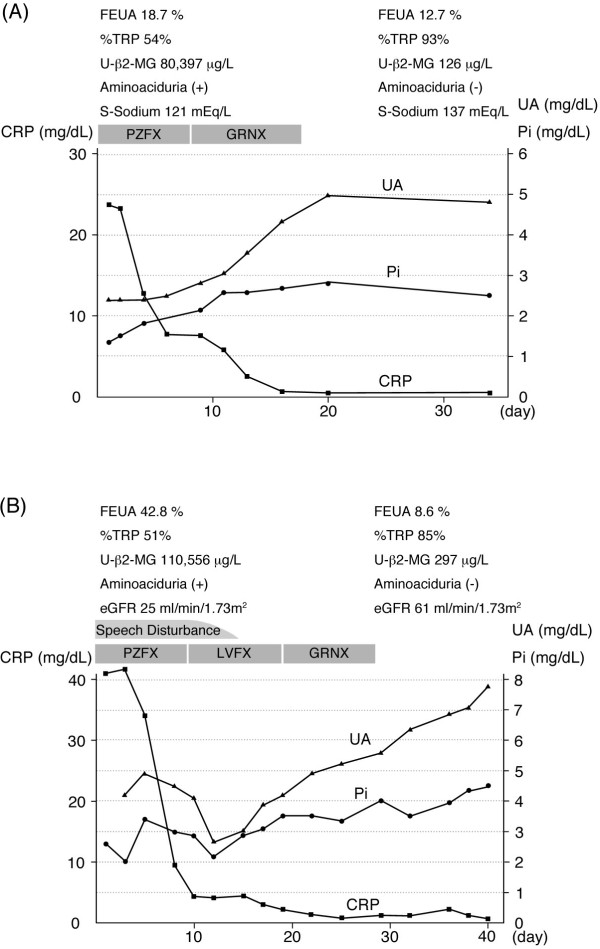
Clinical course in Patient 1 (A) and Patient 2 (B). Definition of the abbreviations: CRP = C-reactive protein, eGFR = estimated glomerular filtration rate, FEUA = fractional excretion of uric acid, GRNX = garenoxacin mesilate hydrate, LVFX = levofloxacin hydrate, Pi = phosphate (inorganic), PZFX = pazufloxacin mesilate, S-Sodium = serum sodium, UA = uric acid, U-β2-MG = urinary β2-microglobulin, %TRP = tubular reabsorption of phosphate.
Patient 2
A 57-year-old man was admitted to our hospital with fever and dysarthria.
A physical examination showed a body temperature of 39.1°C, a blood pressure of 112/48 mmHg and a pulse rate of 98 beats/min. Laboratory findings showed a white blood cell count of 6,100/mm3 and CRP of 46.7 mg/dL. A chest CT scan showed a ground-glass appearance in the left upper and lower lobes. Because of the positive urinary antigen test, he was diagnosed as Legionella pneumonia.
Several electrolyte abnormalities coexisted including hypophosphatemia, hypokalemia and mild hypouricemia. The arterial blood gas analysis showed metabolic acidosis (Table 1). The %TRP was decreased, although his fractional excretion of potassium (FEK) and FEUA were increased. Furthermore, his urinary β2-MG and NAG were remarkably increased (Table 1). Glycosuria and aminoaciduria were also observed (Table 2). Therefore, a diagnosis of Fanconi syndrome was made. Although his renal function was deteriorated, post-renal obstructive nephropathy and congenital anomalies were ruled out by the finding of abdominal CT scan.
Treatment for Legionella pneumonia was started with PZFX at 1,000 mg per day. After treatment, his symptoms disappeared and his electrolyte abnormalities improved. In addition, his %TRP, FEK, FEUA, urinary β2-MG and NAG normalized, and the aminoaciduria became undetectable (Figure 1B).
Conclusions
Because the electrolyte abnormalities disappeared after treatment for Legionella pneumonia and there was no evidence of other causes of Fanconi syndrome, it is most likely that Fanconi syndrome was associated with Legionella pneumonia in our cases. This is the first report of acquired Fanconi syndrome caused by infectious diseases.
Hyponatremia is known as a relatively common electrolyte abnormality in patients with Legionella pneumonia [5]. The responsible mechanism of hyponatremia is thought to be SIADH. In accordance with this mechanism, the hyponatremia in Patient 1 was accompanied by improperly high levels of ADH and improved following the recovery from Legionella pneumonia.
However, other electrolyte abnormalities are uncommon in patients with Legionella pneumonia, and the precise mechanism remains to be clarified [5]. In our cases, the laboratory findings at admission also showed hypophosphatemia, hypokalemia and hypouricemia. In addition, remarkably increased urinary excretion of low-molecular-weight proteins such as β2-MG, decreased %TRP, increased FEK and FEUA, panaminoaciduria and glycosuria were coexisted. Therefore, our cases suggest that the proximal tubular dysfunction caused these serum electrolyte abnormalities.
A relationship has recently been proposed between mitochondrial disorders and Fanconi syndrome [6,7]. For instance, it is known that patients with mitochondrial disorders, including mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), Kearns-Sayre syndrome, and anti-mitochondrial M2 antibody-positive primary biliary cirrhosis (PBC), often complicate with Fanconi syndrome [4,8,9]. Moreover, Fanconi syndrome related to antiviral agents, such as cidofovir, is thought to be caused by mitochondrial toxicity [10]. Because the mitochondria play a critical role in generating adenosine triphosphate (ATP) through oxidative phosphorylation and tubular epithelial cells, particularly in the renal proximal tubules, require a high energy supply from the mitochondria, it is reasonable that mitochondrial disorders can cause Fanconi syndrome. Interestingly, Evans et al. have reported that Legionella pneumophila, which is a facultative intracellular bacterium, was found inside renal tubular cells in autopsy cases of Legionella pneumonia [11]. In addition, it has been reported that Legionella pneumophila infection affects mitochondrial functions in mammalian cells [12,13]. Therefore, Legionella pneumophila infected the proximal tubular cells might interfere with their mitochondria and finally cause Fanconi syndrome in our cases.
However, we cannot explain the following issues: (i) from where Legionella pneumophila entered inside the renal proximal tubular cells (from basolateral or apical) and (ii) why the reabsorption defects developed only in the proximal tubules, not in the distal tubules. Furthermore, we cannot rule out the possibility that acute tubulointerstitial nephritis was occurred in the setting of Legionella pneumonia [14], although this possibility should be low because of the absence of typical symptoms such as rash and leukocyturia in our cases.
In summary, we experienced two rare cases of Legionella pneumonia complicated with Fanconi syndrome. Further studies are required to clarify the precise mechanisms responsible for the association between Legionella pneumophila infection and Fanconi syndrome.
Consent
Written informed consents were obtained from the patients for publication of this Case report and any accompanying images. Copies of the written consents are available for review by the Series Editor of this journal.
Abbreviations
ADH: Antidiuretic hormone; ATP: Adenosine triphosphate; BUN: Blood urea nitrogen; CRP: C-reactive protein; CT: Computed tomography; FEK: Fractional excretion of potassium; FEUA: Fractional excretion of uric acid; GRNX: Garenoxacin mesilate hydrate; LVFX: Levofloxacin hydrate; MELAS: Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; NAG: N-acetyl-β-D-glucosaminidase; PBC: Primary biliary cirrhosis; PZFX: Pazufloxacin mesilate; SIADH: Syndrome of inappropriate ADH secretion; S-Sodium: Serum sodium; %TRP: Tubular reabsorption of phosphate; UA: Uric acid; β2-MG: β2-microglobulin.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NK, SaI, ShinI and ShirI treated the patient. NK wrote the first draft, and also evaluated the data. HF, RF and YF wrote the final draft. All authors reviewed and approved the final version of this manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Naoko Kinoshita-Katahashi, Email: knstnk1984@yahoo.co.jp.
Hirotaka Fukasawa, Email: hfukasawaucsd@gmail.com.
Sayaka Ishigaki, Email: sayakahonda@hotmail.co.jp.
Shinsuke Isobe, Email: uptodate20xx@yahoo.co.jp.
Shiro Imokawa, Email: fmrcw925@ybb.ne.jp.
Yoshihide Fujigaki, Email: yf0516@hama-med.ac.jp.
Ryuichi Furuya, Email: r-furuya@isis.ocn.ne.jp.
References
- Gaboardi F, Case L, Edefonti A, De Vecchi A, Graziani G. [Fanconi syndrome] Minerva Med. 1979;70:3075–3083. [PubMed] [Google Scholar]
- Maldonado JE, Velosa JA, Kyle RA, Wagoner RD, Holley KE, Salassa RM. Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am J Med. 1975;58:354–364. doi: 10.1016/0002-9343(75)90601-4. [DOI] [PubMed] [Google Scholar]
- Cachat F, Nenadov-Beck M, Guignard JP. Occurrence of an acute Fanconi syndrome following cisplatin chemotherapy. Medical and Pediatric Oncology. 1998;31:40–41. doi: 10.1002/(SICI)1096-911X(199807)31:1<40::AID-MPO11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lino M, Binaut R, Noel LH, Patey N, Rustin P, Daniel L, Serpaggi J, Varaut A, Vanhille P, Knebelmann B. et al. Tubulointerstitial nephritis and Fanconi syndrome in primary biliary cirrhosis. Am J Kidney Dis. 2005;46:e41–46. doi: 10.1053/j.ajkd.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Yu VL, Kroboth FJ, Shonnard J, Brown A, McDearman S, Magnussen M. Legionnaires’ disease: new clinical perspective from a prospective pneumonia study. Am J Med. 1982;73:357–361. doi: 10.1016/0002-9343(82)90727-6. [DOI] [PubMed] [Google Scholar]
- Niaudet P. Mitochondrial disorders and the kidney. Arch Dis Child. 1998;78:387–390. doi: 10.1136/adc.78.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emma F, Bertini E, Salviati L, Montini G. Renal involvement in mitochondrial cytopathies. Pediatr Nephrol. 2012;27:539–550. doi: 10.1007/s00467-011-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet P, Rotig A. The kidney in mitochondrial cytopathies. Kidney Int. 1997;51:1000–1007. doi: 10.1038/ki.1997.140. [DOI] [PubMed] [Google Scholar]
- Campos Y, Garcia-Silva T, Barrionuevo CR, Cabello A, Muley R, Arenas J. Mitochondrial DNA deletion in a patient with mitochondrial myopathy, lactic acidosis, and stroke-like episodes (MELAS) and Fanconi’s syndrome. Pediatr Neurol. 1995;13:69–72. doi: 10.1016/0887-8994(95)00082-Q. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi’s syndrome. Am J Kidney Dis. 2003;41:292–309. doi: 10.1053/ajkd.2003.50037. [DOI] [PubMed] [Google Scholar]
- Evans CP, Winn WC Jr. Extrathoracic localization of Legionella pneumophila in Legionnaires’ pneumonia. Am J Clin Pathol. 1981;76:813–815. doi: 10.1093/ajcp/76.6.813. [DOI] [PubMed] [Google Scholar]
- Zhang C, Kuspa A. Transcriptional down-regulation and rRNA cleavage in Dictyostelium discoideum mitochondria during Legionella pneumophila infection. PLoS One. 2009;4:e5706. doi: 10.1371/journal.pone.0005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A, Lima CA, Allan DS, Nasrallah GK, Garduno RA. The purified and recombinant Legionella pneumophila chaperonin alters mitochondrial trafficking and microfilament organization. Infect Immun. 2009;77:4724–4739. doi: 10.1128/IAI.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitarumizu K, Tokuda Y, Uehara H, Taira M, Taira K. Tubulointerstitial nephritis associated with Legionnaires’ disease. Intern Med. 2000;39:150–153. doi: 10.2169/internalmedicine.39.150. [DOI] [PubMed] [Google Scholar]


