Abstract
In T cell-deficient conditions, naïve T cells undergo spontaneous “homeostatic” proliferation in response to contact with self-MHC/peptide ligands. With the aid of an in vitro system, we show here that homeostatic proliferation is also cytokine-dependent. The cytokines IL-4, IL-7, and IL-15 enhanced homeostatic proliferation of naïve T cells in vitro. Of these cytokines, only IL-7 was found to be critical; thus, naïve T cells underwent homeostatic proliferation in IL-4− and IL-15− hosts but proliferated minimally in IL-7− hosts. In addition to homeostatic proliferation, the prolonged survival of naïve T cells requires IL-7. Thus, naïve T cells disappeared gradually over a 1-month period upon adoptive transfer into IL-7− hosts. These findings indicate that naïve T cells depend on IL-7 for survival and homeostatic proliferation.
In peripheral lymphoid tissues, the overall number of mature T cells is tightly regulated through cell survival, division, and death (1–4). The specific signals that regulate T cell homeostasis are largely unknown but are thought to differ depending on the state of T cell activation/differentiation (5). Under normal physiological conditions, the longevity of naïve CD8+ T cells in a resting state depends on continuous contact with self-MHC/peptide ligands (6–8). As for CD8+ cells, the survival of naïve CD4+ cells is reported to require contact with self-MHC ligands (9–12), although the validity of this idea recently has been questioned (13, 14). In contrast to naïve T cells, survival of most memory T cells is MHC independent and is associated with periodic cell division. For CD8+ memory cells, cell division and survival are largely controlled by IL-15 (15–18). The signals required for proliferation and survival of memory CD4+ cells are still unknown.
In lymphopenic conditions, both naïve and memory T cells have the capacity to undergo spontaneous “homeostatic” proliferation in an attempt to restore the size of the depleted T cell populations (recently reviewed in refs. 3 and 4). As with survival, naïve and memory T cells exhibit different characteristics for homeostatic proliferation. Thus, in T-deficient hosts, proliferation of memory T cells is rapid and MHC independent, whereas proliferation of naïve T cells is relatively slow and depends on contact with self-MHC/peptide ligands (8, 13, 19–27). For naïve T cells, the implication therefore is that reducing T cell numbers somehow augments the signals received through the TCR from contact with self-MHC/peptide ligands; TCR signaling changes from being covert to overt, thus pushing T cells into cell division.
One possible mechanism by which T cell depletion causes overt activation of residual T cells is that TCR signaling is enhanced by increased exposure to certain cytokines. Thus, through lack of competition, T cell depletion may increase the level of certain growth-promoting cytokines such as IL-2, IL-4, IL-7, and IL-15. Of these cytokines, IL-4 and IL-7 are known to prolong the survival of naïve T cells in vitro (28–30). Moreover, IL-7 has recently been implicated in regulating naïve T cell homeostasis (31, 32).
To investigate the role of cytokines, we have devised an approach for studying homeostatic proliferation in excised lymphoid organs (LOs) cultured in vitro. Of the various cytokines tested, only IL-4, IL-7, and IL-15 had the capacity to enhance homeostatic proliferation of naïve CD8+ cells in vitro. Under in vivo conditions, only IL-7 was found to be essential for homeostatic proliferation of naïve T cells. IL-7 was also found to be crucial for survival as naïve T cells failed to persist in IL-7− hosts.
Materials and Methods
Mice.
C57BL/6, B6.PL, and B6.Ly5.1 congenic mice were purchased from the breeding colony at The Scripps Research Institute. B6.OT-I RAG-1− (33) and B6.2C (34) transgenic mice were obtained from S. Hedrick (University of California at San Diego, La Jolla) and D. Loh (Nippon Roche Research Center), respectively. B6.RAG-1−, B6.bcl-2 transgenic line 36 (35), and B6.IL-4− (36) were purchased from The Jackson Laboratory. B6.IL-7− (37) and B6.IL-15− (16) mice were kind gifts from DNAX and Immunex, respectively; β2m−K−D− mice (8, 38), generated by F. Lemonnier (Institut Pasteur), were provided by R. Ahmed (Emory University).
Adoptive Transfer of T Cells.
Lymph node (LN) cells were obtained from donor mice and 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) labeled as described (23, 39). Aliquots of 2–3 × 106 cells were injected i.v. into unmanipulated mice or mice exposed to 600 cGy whole body irradiation 1 day before cell transfer. At the indicated days after transfer, LN and spleen cells were stained with the appropriate antibodies and analyzed by flow cytometry (23). Purified CD8+ cells were obtained by treating LN cells with a mixture of anti-HSA, anti-H-2Ab, and anti-CD4 plus complement and then panning on anti-CD8 coated plates (23). For studies on T cell survival, 15 × 106 purified CD4 and CD8 (purity 99%) LN T cells from B6.Ly5.1+ donors were CFSE-labeled and transferred into unirradiated B6 or B6.IL-7− mice. LN and spleen cells were harvested at the indicated time points and stained for Ly5.1 and CD8 and analyzed by flow cytometry.
In Vitro Analysis of T Cell Homeostasis.
CFSE-labeled donor LN cells (2–5 × 106) were injected into unirradiated B6, irradiated B6, or unirradiated RAG-1− mice. Several (3–15) hours later, LNs and spleens were harvested from host mice; spleens were cut into small 1-mm cubes. The organs were cultured in DMEM supplemented with 10% γ-globulin-free horse serum, glutamine, penicillin/streptomycin, and neomycin for 7 days at 37°C in a 93% O2/7% CO2 atmosphere; the culture medium was changed every other day (40). Single cell suspensions were made from the LNs and spleen fragments and stained with the appropriate antibodies followed by flow cytometry analysis. The following cytokines were used: recombinant murine (rm)IL-2, rmIL-4, rmIL-7, and recombinant human (rh)IL-15 (R & D Systems). For blocking studies, anti-IL-2Rβ (clone Tmβ1), anti-IL-4 (clone 11B11), and anti-common γ chain (γc) (a mixture of clones TUGM2, 3E12, and 4G3) were obtained from PharMingen, and anti-IL-7 polyclonal antibody was obtained from R & D Systems.
FACS Analysis.
LN or spleen cells were stained for donor cells as previously described (23). To detect OT-I specific cells, phosphatidylethanolamine (PE)-conjugated anti-Vβ2 (PharMingen) and Cy5-conjugated anti-CD8 (clone YTS-169) were used. To detect 2C-specific cells, PE-conjugated anti-CD8 (eBioscience, San Diego) and Cy5-conjugated anti-clonotypic mAb1B2 were used. To detect Thy-1.1+ cells from B6.PL donor mice, cells were stained with biotinylated anti-Thy-1.1 (PharMingen) followed by Cy5-conjugated streptavidin (Jackson ImmunoResearch) and PE-conjugated anti-CD8 (eBiosicence). In studies examining T cell survival in IL-7− mice, Ly5.1 donor cells were stained with PE-conjugated anti-CD8 (clone YTS-169), and Cy5-conjugated Ly5.1. To determine expression of cytokine receptors on T cells, LN cells from RAG-1− OT-I, B6, and 2C mice were stained with PE-conjugated anti-CD25 (PharMingen), PE-conjugated anti-IL-2Rβ (PharMingen), biotinyated anti-IL-7Rα (clone A7R34) (41) followed by PE-conjugated streptavidin (Jackson ImmunoResearch), or anti-γc (clone 4G3) (PharMingen) followed by biotinylated anti-rat IgG (Jackson ImmunoResearch) followed by PE-conjugated streptavidin; the cells were then stained for CD4 and CD8 or CD8 and 1B2 (for 2C cells). PE, biotin, or unconjugated rat IgG antibody (PharMingen) was used for background staining.
Results
An in Vitro System for Studying Homeostatic Proliferation.
Current studies on homeostatic T cell proliferation are strictly limited to in vivo experiments on live animals. Thus, examining homeostatic proliferation under conventional culture conditions in vitro is impractical because single cell suspensions of T cells die within a few days. Moreover, even if T cell death is reduced, e.g., by using bcl-2 transgenic T cells, spontaneous proliferation of T cells cultured with APC is very limited; this applies even with a high APC to T cell ratio, i.e., the conditions that induce strong homeostatic proliferation in vivo (data not shown). The failure to detect homeostatic proliferation of T cells in vitro could reflect that naïve T cells need to interact with APC within the three-dimensional architecture of the lymphoid tissues and/or that the stromal cells of the lymphoid tissues play a crucial role in supporting homeostatic proliferation, perhaps by secreting essential cytokines. If this reasoning is correct, one would expect naïve T cells to undergo homeostatic proliferation in vitro in whole lymphoid tissues, i.e., in tissues that were not physically disrupted.
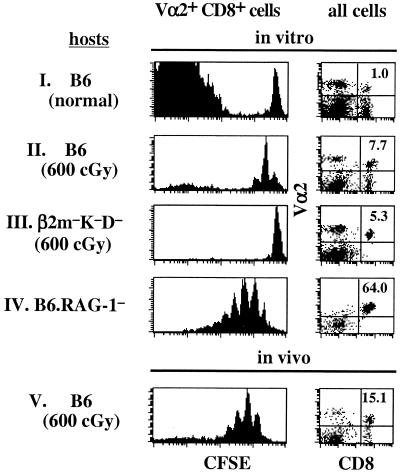
To test this idea, CD8+ T cells from OT-I TCR transgenic mice on a RAG-1− background were injected into T-depleted (irradiated) syngeneic B6 mice; to measure proliferation, the injected T cells were labeled with the intracellular dye, CFSE (39). LNs and spleens were removed 4–15 h later and cultured in vitro; LNs were cultured as whole organs, whereas the spleens were minced into 1-mm fragments. The LNs were either cultured at the air/liquid interphase on top of media-saturated sponge foams or submerged in DMEM supplemented with 10% serum in a 93% O2/7% CO2 atmosphere for 7 days; spleen fragments were cultured submerged in media (40). Although the total recoveries of viable cells from these LO cultures were 5-fold lower than from freshly harvested tissues (2.4 ± 0.6 × 106 in vivo vs. 0.5 ± 0.2 × 106 in vitro, after injection of 2 × 106 whole LN cells), the donor OT-I cells underwent significant homeostatic proliferation (as defined by dilution of CFSE in Vα2+ CD8+ cells) (Fig. 1). Thus, the donor cells proliferated in LO cultures prepared from T cell-depleted syngeneic hosts, i.e., irradiated (Fig. 1, group II) or RAG-1− (Fig. 1, group IV) hosts, but not in cultures from T cell-sufficient unirradiated B6 hosts (Fig. 1, group I); note that the large CFSE− peak for unirradiated B6 host denotes unlabeled host Vα2+ cells (Fig. 1, group I). Consistent with the notion that homeostatic proliferation is directed to MHC molecules (MHC class I for CD8+ cells), homeostatic proliferation of OT-I CD8+ cells was undetectable in MHC class I− hosts, i.e., in irradiated β2m−K−D− (H2b) mice (Fig. 1, group III). The recovery of donor OT-I cells from irradiated MHC class I− hosts (0.5 ± 0.1 × 104) was significantly lower than that from B6 hosts (1.6 ± 0.5 × 104), presumably reflecting the difference in the levels of proliferation and survival.
Figure 1.
Homeostatic proliferation of RAG-1− OT-I TCR transgenic T cells in vitro. Small doses (2–5 × 106 cell/mouse) of CFSE-labeled LN cells from OT-I mice in a RAG-1-deficient background were injected into various irradiated (600 cGy,1 day before) or unirradiated hosts. For in vitro analysis, LNs from host mice were removed 15 h after injection of donor OT-I cells. Whole LNs and spleen fragments were cultured for 7 days as described in Materials and Methods. For in vivo analysis, LNs from host mice were harvested 8 days after donor cell injection. Shown are CFSE profiles of gated donor OT-I cells (CD8+ Vα2+) or CD8 and Vα2 staining on all cells. Similar results were obtained with spleens.
Cytokines Enhance Homeostatic Proliferation of CD8+ Cells in Vitro.
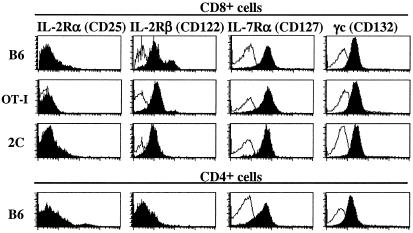
Although homeostatic proliferation of OT-I cells was clearly detectable in LO cultures prepared from irradiated hosts, for B6 mice the number of cell divisions observed in vitro was consistently less than that observed under in vivo conditions (Fig. 1, compare group II with group V). This difference could reflect greater death of T cells in LO cultures than in vivo. To examine this possibility, LO cultures were incubated in medium supplemented with cytokines known to prevent death of naïve T cells in vitro, i.e., IL-4 and IL-7 (28–30). Both for normal (B6) and TCR transgenic (OT-I, 2C) T cells, most naïve T cells express receptors for IL-4 (42, 43) and IL-7 (Fig. 2) and also express the common cytokine γ chain (γc or cd132), which is required for signaling via IL-4 and IL-7 (and also IL-2, IL-9, and IL-15) (Fig. 2 and ref. 44). IL-2Rβ (CD122), a receptor for IL-2 and IL-15, is expressed on naïve CD8+ cells, although not on CD4+ cells, whereas IL-2Rα (CD25), a high affinity receptor for IL-2, is not expressed on naïve T cells (Fig. 2).
Figure 2.
Expression of receptors for IL-2, IL-7, and IL-15 and the common cytokine γ chain (γc) on LN T cells from TCR transgenic and normal B6 mice. LN cells from B6, RAG-1− OT-I, and 2C mice were triple-stained for expression of the indicated cytokine receptors and CD4 and CD8. Shown are gated CD4+ or CD8+ cells that were stained for the indicated receptors (filled) or background staining with rat IgG (unfilled).
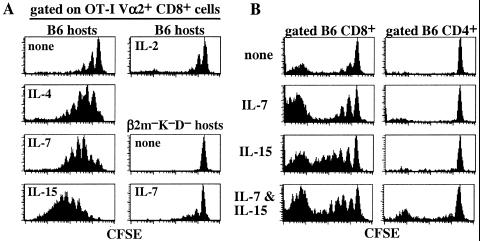
In LO cultures prepared from irradiated B6 hosts injected with RAG-1− OT-I CD8+ cells, the striking finding was that supplementing the media with low concentrations (1–10 ng/ml) of IL-4, IL-7, or IL-15 significantly increased overall T cell recoveries (2- to 4-fold) and, more importantly, enhanced homeostatic proliferation of donor T cells (Fig. 3A). Thus, with addition of these cytokines, homeostatic proliferation of OT-I cells in LO cultures was as high or higher than in vivo. Similar results were observed when the injected RAG-1− OT-I cells were depleted of contaminating CD44hi cells (data not shown), which makes it unlikely that the enhanced proliferation by cytokines reflected selective expansion of memory/activated T cells. In contrast to IL-4, IL-7, and IL-15, supplementing the media with similar concentrations of IL-2, IL-6, or IL-9 failed to enhance homeostatic proliferation of OT-I cells in LO cultures (Fig. 3 and data not shown).
Figure 3.
IL-4, IL-7, and IL-15 promote proliferation of TCR transgenic and normal CD8+ cells in LO cultures. (A) LNs from irradiated (600 cGy) B6 or β2m−Kb−Db− mice previously injected with CFSE-labeled RAG-1− OT-I cells were cultured in vitro in media only or in media supplemented with recombinant cytokines (10 ng/ml) for 7 days. Shown are CFSE profiles of gated donor OT-I cells. (B) LNs from irradiated (600 cGy) B6 mice previously injected with CFSE-labeled normal polyclonal LN cells from Thy-1-congenic B6.PL LN cells were incubated with or without the indicated cytokines (10 ng/ml) for 7 days. Shown are gated donor CD4+ (Thy-1.1+ CD8−) and CD8+ (Thy-1.1+ CD8+) cells.
Significantly, addition of IL-4, IL-7, or IL-15 had little effect on the inability of OT-I cells to undergo homeostatic proliferation in LO cultures prepared from irradiated MHC class I-deficient β2m−K−D− hosts (Fig. 3A and data not shown); this also applied when a mixture of all three cytokines was added (not shown). Hence, the enhancing effect of the cytokines depended on TCR–MHC interaction.
As with the transgenic CD8+ OT-I cells, polyclonal Thy-1-congenic (B6.PL) CD8+ cells also underwent significant homeostatic proliferation in LO cultures prepared from irradiated B6 hosts, although proliferation in the absence of cytokines was less marked than with OT-I cells (Fig. 3B). However, proliferation increased considerably upon addition of IL-7 or IL-15. Interestingly, in contrast to CD8+ cells, homeostatic proliferation of B6 CD4+ cells in LO cultures was very low and was not enhanced by addition of IL-4, IL-7, or IL-15, even in combinations (Fig. 3B and data not shown).
Requirement for Signaling Through γc for Homeostatic Proliferation and Survival.
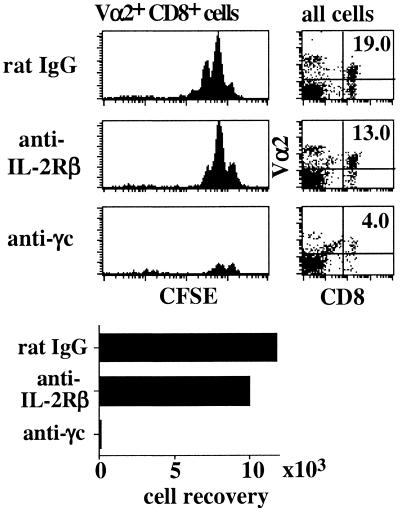
For CD8+ cells, the above data suggest that homeostatic proliferation could depend on steady-state “background” production of IL-4, IL-7, or IL-15. In favor of this possibility, supplementing LO cultures (prepared from irradiated B6 hosts) with a mixture of three anti-γc mAbs substantially inhibited homeostatic proliferation of OT-I CD8+ cells (Fig. 4). Surprisingly, anti-γc antibodies also reduced the overall recovery of OT-I cells by >10-fold (Fig. 4). This effect did not appear to reflect opsonization of T cells because addition of an isotype-matched antibody to IL-2Rβ (a receptor for IL-2 and IL-15) only slightly decreased OT-I cell proliferation and recovery (Fig. 4).
Figure 4.
Blocking signaling through the γc prevents survival and homeostatic proliferation of naïve T cells. LNs from irradiated B6 mice previously injected with CFSE-labeled Rag-1− OT-I LN cells were cultured in vitro in media supplemented with normal rat IgG (50 μg/ml), mAb to IL-2Rβ (clone TMβ-1, 50 μg/ml), or a mixture of mAbs to γc (clones 4G3, 3E12, and TUGm2 at 20 μg/ml each) for 7 days. Shown are Vα2 and CD8 staining on all viable cells, CFSE levels on gated donor OT-I cells, and the total numbers of cells recovered from the three cultures.
IL-7 Is Essential for Homeostatic Proliferation in Vivo.
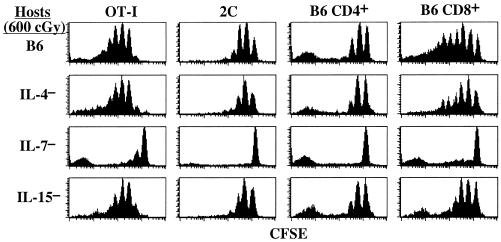
The above finding that homeostatic proliferation is abrogated by blocking the γc but not by blocking the IL-2Rβ chain implies that homeostatic proliferation is controlled by background production of either IL-4 or IL-7 (although not IL-15). However, attempts to prove this idea by blocking proliferation with commercially available anti-IL-4 and anti-IL-7 neutralizing antibodies in LO cultures were unsuccessful (data not shown). Because it was unclear whether these antibodies were functionally efficient in LO cultures, we examined homeostatic proliferation in vivo in cytokine-gene knockout mice. In these hosts, the notable finding was that homeostatic proliferation of OT-I CD 8+ cells was the same or only slightly reduced in irradiated IL-4− and IL-15− hosts but was greatly reduced in irradiated IL-7− hosts (Fig. 5). Similar findings applied to CD8+ cells from another TCR transgenic line (2C) and also to normal B6.PL CD8+ and CD4+ cells (Fig. 5). These findings indicate that homeostatic proliferation of naïve T cells in vivo depended crucially on background production of IL-7 with little contribution from IL-4 or IL-15. Interestingly, donor cells giving rise to CFSE− peaks, indicative of multiple rounds of cell division, were observed in IL-7− hosts; these cells were probably contaminating memory T cells, which undergo very rapid homeostatic proliferation.
Figure 5.
Homeostatic proliferation of naïve T cells in vivo crucially depends on IL-7. Groups of irradiated (600 cGy) normal B6 mice and B6 mice deficient in IL-4, IL-7, or IL-15 were injected with 2C, RAG-1− OT-I, or B6.PL LN cells. LNs from host mice were harvested 7 days later and stained for donor markers. Shown are CFSE levels of gated donor T cells.
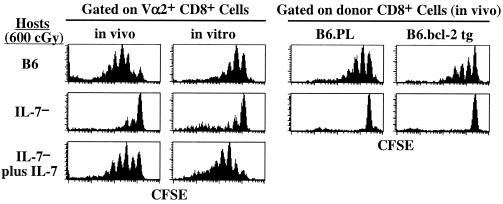
Because IL-7 is essential for early development of T and B lymphocytes, the secondary lymphoid tissues in IL-7− mice are extremely small and hypocellular (37). It is thus possible that T cells fail to undergo homeostatic proliferation in IL-7− hosts because the lymphoid tissues in these mice are aberrant. To address this concern, we tested whether infusion of exogenous IL-7 would restore homeostatic proliferation of donor T cells in IL-7− mice. This was indeed found to be the case, both under in vivo and in vitro conditions (Fig. 6 Left). Thus, injection of IL-7 (500 ng/mouse every 12 h for 7 days) restored the ability of irradiated IL-7− hosts to support homeostatic proliferation of OT-I cells. Similarly, addition of exogenous IL-7 (40 ng/ml) in the media restored the capacity of donor OT-I cells to proliferate in LO cultures prepared from irradiated IL-7− hosts.
Figure 6.
Restoration of homeostatic proliferation of OT-I cells in IL-7− mice by addition of exogenous IL-7 but not by up-regulation of bcl-2. (Left) Groups of irradiated B6.IL-7− mice were injected with RAG-1− OT-I LN cells. For in vivo analysis, mice were either injected with PBS or 500 ng of recombinant IL-7 every 12 h for 7 days. For in vitro analysis, spleen fragments from the hosts were treated with media only or media containing 40 ng/ml IL-7 for 7 days. Irradiated B6 hosts injected with OT-I cells and analyzed either in vivo or in vitro conditions served as controls. Shown are gated donor cells. (Right) A group of irradiated B6 mice was injected with a mixture of wild-type B6.PL and B6.Ly5.1 bcl-2 transgenic cells. Mice were analyzed 7 days later by staining LN cells for Thy-1.1, Ly5.1, and CD8; shown are gated CD8+ cells.
Bcl-2 Cannot Replace the Requirement for IL-7 in Homeostatic Proliferation.
One of the actions of IL-7 is to up-regulate bcl-2 (45), and overexpression of bcl-2 has been shown to partially overcome the requirement for IL-7 for T cell lymphopoiesis in the thymus (46). In contrast to thymocyte development, however, overexpression of bcl-2 failed to replace the requirement for IL-7 in homeostatic proliferation. Thus, bcl-2 transgenic T cells failed to undergo proliferation in irradiated IL-7− hosts, although these cells proliferated in irradiated B6 hosts (Fig. 6 Right).
Survival of Naïve CD4+ and CD8+ T Cells Depends on IL-7.
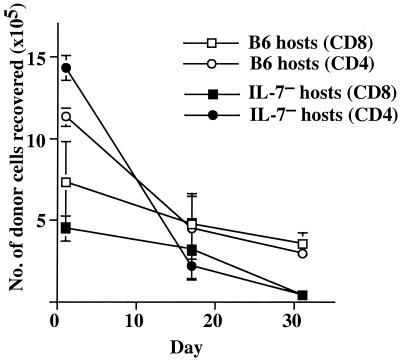
To determine whether IL-7 is essential for naïve T cells to survive in interphase, large numbers (15 × 106) of purified CD4 and CD8 cells from young B6.Ly5.1 mice were CFSE-labeled and injected into unirradiated, i.e., non-T cell-depleted, IL-7−, or control B6 mice and analyzed on days 1, 17, and 30. As shown in Fig. 7, most of the donor Ly5.1+ CFSEhi (i.e., undivided) T cells survived in IL-7− hosts at the same frequency as in control B6 hosts for a 2-week period. However, the donor CFSEhi cell numbers decreased dramatically in IL-7− hosts after this period and were almost undetectable at 30 days after cell transfer. Thus, IL-7 is also important for prolonged survival of naïve T cells.
Figure 7.
Survival of naïve T cells requires IL-7. CFSE labeled purified LN CD4 and CD8 T cells (15 × 106 cells/mouse) from B6. Ly5.1 mice were labeled with CFSE and transferred into unirradiated B6 (unfilled) or IL-7− (filled) hosts. One, 17, and 30 days after transfer, spleen cells were harvested, and donor CFSEhi CD8+ T cells (squares) were detected by staining for Ly5.1 and CD8. Donor CD4+ T cells (circles) are Ly5.1+, CFSEhi, and CD8− T cells. Total numbers of donor CFSEhi cells recovered from host LN and spleens (2–3 hosts analyzed individually per time point) are shown.
Discussion
The findings in this article confirm and extend the recent observation of Schluns et al. (47) that IL-7 plays a crucial role in controlling survival and homeostatic proliferation of naïve T cells. In the absence of IL-7, homeostatic proliferation of naïve T cells is almost completely abolished, and the lifespan of naïve T cells is greatly reduced. Therefore, in addition to its known role in controlling early T cell production, IL-7 plays a key role in regulating T cell homeostasis in peripheral lymphoid tissues. The following working model is proposed for how IL-7 regulates homeostasis. Acting in conjunction with low-level TCR signals from contact with self-MHC/peptide ligands, IL-7 serves to keep naïve T cells alive at a resting state. The overall size of the naïve T cell pool is thus a reflection of the basal level of free IL-7 in the body, the production of IL-7 presumably being tightly regulated. When the total number of naïve T cells drops below a certain level, consumption of IL-7 is reduced; residual T cells then encounter increased basal concentration of IL-7, which augments TCR signaling and thereby drives naïve T cells to undergo homeostatic proliferation in response to self-MHC/peptide ligands.
One minor difference between Schluns et al. (47) and our data is the estimated lifespan of naïve T cells in the absence of IL-7, i.e., in the order of days in the former study compared with several weeks in our study. This difference may reflect that we examined the survival of normal T cells in IL-7− hosts, whereas Schluns et al. transferred IL-7Rα− T cells to normal hosts. Because IL-7 is crucial for thymopoiesis, the few mature T cells produced in IL-7Rα− mice may not necessarily be representative of normal T cells. Thus, perhaps reflecting increased sensitivity to activation-induced cell death (48), these cells may have a very abbreviated lifespan and thus disappear rapidly after adoptive transfer. A more interesting possibility is that IL-7Rα is not a unique receptor for IL-7 but can also bind other cytokines. In favor of this possibility, a new IL-7-like cytokine, termed thymic stromal lymphopoietic (TSLP), binds to a receptor on T cells composed of IL-7Rα and TSLP receptor (49–51). Whether TSLP plays a role in T cell homeostasis/survival is still unclear. Interestingly, unlike IL-7, signaling via TSLP does not involve γc.
Considering that several different cytokines, including IL-4, IL-6, and IL-7, are known to prevent apoptosis of T cells in vitro (28–30, 52), it is striking that absence of only IL-7 was detrimental to cell survival. It is, however, possible that the survival of T cells in vivo is under the control of multiple cytokines, with IL-7 assuming the major role (32, 53). Thus, in the absence of both IL-4 and IL-7, the lifespan of T cells may be even shorter. In favor of this idea, we found that the survival of naïve T cells in LO cultures was greatly reduced after mAb blockade of γc. It is also notable that residual T cells in γc− mice have a short lifespan (54).
In addition to cell survival, it is also possible that the role of IL-7 in homeostatic proliferation is not unique but simply reflects that IL-7 is expressed at higher levels in vivo than other cytokines. Here, a key question, which we have yet to address, is whether the failure of T cells to undergo homeostatic proliferation in IL-7− mice can be overcome by adding high concentrations of other cytokines, e.g., IL-4 or IL-15. This question is important because, in addition to IL-7, these two cytokines considerably enhanced homeostatic proliferation in LO cultures. It also should be mentioned that there may be distinct differences between CD4+ and CD8+ cells in terms of the efficacy of various cytokines to drive cell survival and homeostatic proliferation. Thus, a receptor for IL-15, IL-2Rβ, is expressed at a much higher level on naïve CD8+ than CD4+ cells. In addition, in LO cultures, CD4+ cells were much less sensitive to IL-4, IL-7, and IL-15 than CD8+ cells.
With regard to the sites of IL-7 production in vivo, we have found that homeostatic proliferation of T cells requires that the cells directly enter the T cell zones of the LN and spleen (55). This point is of interest as IL-7 can be synthesized by dendritic cells (56), i.e., by cells that are a dominant population in the T cell zones and that presumably play a crucial role as APC for homeostatic proliferation, in addition to stromal cells in the thymus and bone marrow.
As a final point, it should be emphasized that the specific roles played by IL-7 and other cytokines in homeostatic T cell proliferation and survival remain to be clarified. These cytokines may act by providing costimulation for TCR signaling and/or a passive survival signal. Perhaps the simplest idea is that cytokines induce T cells to up-regulate anti-apoptotic molecules. If so, it is striking that we found no evidence that constitutive up-regulation of Bcl-2, i.e., in Bcl-2 transgenic T cells, failed to overcome the inability of T cells to undergo homeostatic proliferation in IL-7− hosts. The role of other anti-apoptotic molecules, e.g., Bcl-XL, has yet to be studied. Alternatively, a more complex scenario is possible. For example, survival of naïve T cells may be associated with occasional compulsory cell division through contact with the IL-7. Cell death could thus arise from the pressure to undergo cell division in the absence of IL-7.
In summary, our data indicate that IL-7 plays a key role in T cell homeostasis, both for homeostatic proliferation and survival. This finding raises the possibility that the overproduction of T (and B) cells in IL-7 transgenic mice (57, 58) reflects the action of IL-7 on mature T cells. The data also suggest that the paucity of naïve T cells in aging may reflect a decline in IL-7 production, in which case infusion of exogenous IL-7 could serve to replenish the T cell pool.
Acknowledgments
We thank M. Von Herrath for providing IL-4− mice and P. Marrack for sending antibodies to IL-7Rα. We thank Dr. N. Klinman for technical advice on LO culture system. We also thank B. Bondi-Boyd, J. Kuhns, and B. Marchand for various support. This work was supported by U.S. Public Health Service Grants AI21487, CA38355, and AI46710 (to J.S.); HL54729 (to K.I.W.), and AI41079 and AI45809 (to C.D.S.). J.T.T. is supported by U.S. Public Health Service Institutional National Research Service Award HL07195. C.D.S. is a Scholar of the Leukemia and Lymphoma Society.
Abbreviations
- γc
common γ chain
- LN
lymph node
- LO
lymphoid organ
- TSLP
thymic stromal lymphopoietic
- CFSE
5,6-carboxyfluorescein diacetate succinimidyl ester
- PE
phosphatidylethanolamine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Freitas A A, Rocha B B. Immunol Today. 1993;14:25–29. doi: 10.1016/0167-5699(93)90320-K. [DOI] [PubMed] [Google Scholar]
- 2.Bell E B, Sparshott S M. Semin Immunol. 1997;9:347–353. doi: 10.1006/smim.1997.0092. [DOI] [PubMed] [Google Scholar]
- 3.Goldrath A W, Bevan M J. Nature (London) 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 4.Surh C D, Sprent J. J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanchot C, Rosado M M, Agenes F, Freitas A A, Rocha B. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 6.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 7.Nesic D, Vukmanovic S. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 8.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Rodewald H-R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 10.Brocker T. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rooke R, Waltzinger C, Benoist C, Mathis D. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 13.Clarke S R, Rudensky A Y. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman J R, Stefanova I, Yasutomo K, Germain R N. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 15.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 18.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 19.Beutner U, MacDonald H R. Int Immunol. 1998;10:305–310. doi: 10.1093/intimm/10.3.305. [DOI] [PubMed] [Google Scholar]
- 20.Oehen S, Brduscha-Riem K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 21.Viret C, Wong F S, Janeway C A., Jr Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 22.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 24.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muranski P, Chmielowski B, Ignatowicz L. J Immunol. 2000;164:3087–3094. doi: 10.4049/jimmunol.164.6.3087. [DOI] [PubMed] [Google Scholar]
- 27.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boise L H, Minn A J, June C H, Lindsten T, Thompson C B. Proc Natl Acad Sci USA. 1995;92:5491–5495. doi: 10.1073/pnas.92.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vella A, Teague T K, Ihle J, Kappler J, Marrack P. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishimoto H, Sprent J. J Immunol. 1999;163:1817–1826. [PubMed] [Google Scholar]
- 31.Webb L M, Foxwell B M, Feldmann M. Immunology. 1999;98:400–405. doi: 10.1046/j.1365-2567.1999.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer B C, Swanson B, Kappler J. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H, Loh D Y. Nature (London) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 35.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn R, Rajewsky K, Muller W. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 37.von Freeden-Jeffry U, Vieira P, Lucian L A, McNeil T, Burdach S E G, Murray R. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perarnau B, Saron M F, San Martin B R, Bervas N, Ong H, Soloski M J, Smith A G, Ure J M, Gairin J E, Lemonnier F A. Eur J Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 40.Froscher B G, Klinman N R. Methods Enzymol. 1987;150:196–208. doi: 10.1016/0076-6879(87)50077-5. [DOI] [PubMed] [Google Scholar]
- 41.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H. Proc Natl Acad Sci USA. 1993;90:9125–9129. doi: 10.1073/pnas.90.19.9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohara J, Paul W E. Nature (London) 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Huang H, Guo L, Stonehouse T, Watson C J, Hu-Li J, Paul W E. J Exp Med. 2000;192:1125–1134. doi: 10.1084/jem.192.8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 45.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 46.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 47.Schluns K S, Kieper W C, Jameson S C, Lefrancois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 48.Maraskovsky E, Teepe M, Morrissey P J, Braddy S, Miller R E, Lynch D H, Peschon J J. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 49.Levin S D, Koelling R M, Friend S L, Isaksen D E, Ziegler S F, Perlmutter R M, Farr A G. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 50.Park L S, Martin U, Garka K, Gliniak B, Di Santo J P, Muller W, Largaespada D A, Copeland N G, Jenkins N A, Farr A G, et al. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sims J E, Williams D E, Morrissey P J, Garka K, Foxworthe D, Price V, Friend S L, Farr A, Bedell M A, Jenkins N A, et al. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marrack P, Mitchell T, Bender J, Hildeman D, Kedl R, Teague K, Kappler J. Immunol Rev. 1998;165:279–285. doi: 10.1111/j.1600-065x.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
- 53.Boursalian T E, Bottomly K. J Immunol. 1999;162:3795–3801. [PubMed] [Google Scholar]
- 54.Lantz O, Grandjean I, Matzinger P, Di Santo J P. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 55.Dummer W, Ernst B, LeRoy E, Lee D-S, Surh C D. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 56.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu Y J, Lebecque S, Caux C. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- 57.Samaridis J, Casorati G, Traunecker A, Iglesias A, Gutierrez J C, Muller U, Palacios R. Eur J Immunol. 1991;21:453–460. doi: 10.1002/eji.1830210230. [DOI] [PubMed] [Google Scholar]
- 58.Mertsching E, Burdet C, Ceredig R. Int Immunol. 1995;7:401–414. doi: 10.1093/intimm/7.3.401. [DOI] [PubMed] [Google Scholar]