Abstract
Introduction
Cardiovascular diseases (CVDs) are a leading cause of morbidity and mortality in low-income countries including India. There is a need for effective, low-cost methods to prevent CVDs in rural India. One strategy is to identify and implement interventions at high-risk individuals using community health workers (CHWs). There is a paucity of CHW-based CVD intervention trials from low-income countries.
Methods
We designed a multicenter, household-level, cluster-randomized trial with 1:1 allocation to intervention and control arms. The CHWs undertook a door-to-door survey and screened 5,699 households in 28 villages from 3 rural regions in India to identify at-risk households. The households were defined as those with ≥1 individual aged ≥35 years and at moderate or high risk for CVD based on the non–laboratory-based National Health and Nutrition Examination Survey score. All at-risk individuals were invited to attend a physician-led village clinic that provided a CVD risk reduction prescription and education about target risk factor levels for CVD control. All households in which at least 1 member at moderate to high risk for CVD had received a risk reduction prescription were eligible for randomization. Households randomized to the CHW-based intervention will receive 1 household visit by a CHW every 2 months, for 12 months. During these visits, CHWs will measure blood pressure, ascertain and reinforce adherence to prescribed therapies, and modify therapy to meet targets. Households randomized to the control arm do not receive CHW visits. At 12 months after randomization, we will evaluate 2 primary outcomes of systolic blood pressure and adherence to antihypertensive drugs and secondary outcomes of INTERHEART risk score, body mass index, and waist-to-hip ratios. At 18 to 24 months after randomization and 6 to 12 months after the last intervention, we will record these outcomes to evaluate sustainability of intervention.
Results
Community health workers screened a total of 5,033 households that included 9,248 individuals and identified 2,571 households with 3,784 at-risk individuals. We randomized 2,438 households (1,219 to intervention and 1,219 to control groups).
Conclusion
Our large trial of CHWs in rural India will provide important information regarding a promising approach to primary prevention of CVDs.
Cardiovascular diseases (CVDs) due to coronary artery disease are a leading cause of mortality both in urban as well as rural areas in India.1,2 Various studies have estimated the prevalence of CVD to be between 7% and 11% in urban and 1% and 6% in rural India.3 The INTERHEART Study, a large global coronary heart disease case-control study, identified 9 potentially modifiable risk factors (ie, abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, low consumption of fruit and vegetables, alcohol use, and low levels of physical activity) that accounted for 90% of the risk for a myocardial infarction.4 These findings were consistent across the globe, among men and women and in all ages. In low- and middle-income countries (LMICs), risk factors rates are higher and manifest approximately 8 to 10 years earlier.4 Cardiovascular disease primary prevention trials showed variable success in high-income countries and provide useful insights for designing effective strategies to reduce the burden of CVDs in LMICs. 5-8
A community health worker (CHW) is defined as a member of the community who has received training to promote health or to carry out specific health care services but is not a health care professional.9 Community health workers are central to primary health care delivery in India, and using CHWs has been successful in various infectious disease and maternal-child health programs.10,11 Currently, CHWs are not involved in chronic disease management in India but are potentially useful, as demonstrated in studies done in high-income12 and, more recently, in other LMICs (Table).
Table.
Interventions aimed at primary prevention of cardiovascular diseases in low and middle income countries
| First author | Year of publication |
Country/ region |
Study type | Follow-up duration |
Participants | Interventions | Outcome |
|---|---|---|---|---|---|---|---|
| Randomized control trials involving community health workers | |||||||
| Jafar et al22 | 2009 | Pakistan | Community based 2 × 2 factorial cluster randomized trial |
24 m | 12 communities (1341 individuals) from rural Pakistan |
Home-based health education and a providerlevel intervention in 2 × 2 factorial design |
10.8 mm Hg BP reduction in combined homebased health education and provider-level intervention |
| Joshi et al24 | 2012 | India | Community- based double cluster randomized trial |
Between 12 and 24 m |
44 villages (3712 individuals) from rural India |
Health promotion and algorithmbased care by nonphysician health workers |
No difference in gain in knowledge or identification of high-risk individuals using an algorithm |
| Community based primary care programs involving community health workers | |||||||
| Balcazar et al25 | 2009 | US-Mexico Border |
Program with intervention arm alone |
3 m | High-risk Hispanic subgroups (256 participants, outcomes analyzed in 85) |
Health education campaign through promotoras |
Before and after comparison showed reduction in DBP, LDL cholesterol, and HbA1c levels |
| Mohammadifard et al27 | 2009 | Iran | Isfahan healthy heart program survey-resurvey technique |
6 y | 12514 individuals in baseline and 5000 in follow-up survey |
Health education campaigns though community-based and legislative actions |
Reduction in fat consumption index and meat consumption index and improvement in global dietary index |
| Facility-based programs/interventions involving care coordinators or health educators | |||||||
| Prabhakaran et al28 | 2009 | India | Industrial sites who agreed for worksite interventions vs those who did not |
4 y | 6 intervention and 1 control site (5828 participants) |
Multimodal health educational interventions delivered by worksite health officers |
Reduction in weight, waist circumference, blood sugar, and lipid levels in intervention worksites |
| Shah et al29 | 2010 | India | School based program preand postanalysis |
6 m | 40000 children, 25000 parents, and 1500 teachers |
Nutritional education through lectures, discussions, and small group activities |
Improved knowledge and behavior among children aged 8-11 y |
| Mendis et al31 | 2011 | Nigeria and China |
Primary health care facility based cluster randomized trial |
12 m | 40 primary health facilities, 2397 patients |
Facility-level patient education and standardized treatment for hypertension |
SBP reduction 13.28 vs 9.41 (China)11.01 vs 6.62 (Nigeria) |
| Ongoing studies | |||||||
| Lijing Y (NCT01259700) |
Ongoing | China | Community- based cluster randomized trial |
2 y | 10000 individuals | CVD risk reduction and salt reduction educational interventions |
Primary outcome is BP reduction |
| Prabhakaran D (NCT01212328) |
Ongoing | India | Individual patient randomized control trial |
42 m | 1120 patients with diabetes mellitus |
Clinical care coordinator and decision support system |
Multiple CVD risk control targets |
| Anchala et al32 (CTRI/2012/03/002476) |
Ongoing | India | Facility-based cluster- randomized trial |
12 m | 16 primary health center clusters (8 in each arm) |
Physicians using a decision support system vs no such system |
4 mm Hg reduction in SBP |
NCT refers to National Institutes of Health clinical trials registry number (clinicaltrials.gov); CTRI refers to clinical trial registry of India number (www.ctri.nic.in). Abbreviation: DBP, Diastolic BP.
Low-income countries need cost-efficient solutions for prevention of chronic vascular diseases. This calls for screening for high-risk individuals and approaching them with effective low-cost interventions to promote adherence to preventive therapies. Improving adherence in the intermediate-term is essential to achieve a long-term reduction in cardiovascular morbidity. Approximately two-thirds of Indians reside in villages, where key barriers to CVD prevention include lack of awareness about its risk factors, cost of preventive therapies, cultural practices related to tobacco use, and poor access to health systems. We believe that trained CHWs can address some of these barriers and reduce the burden of CVDs in rural India. The hypothesis for the current trial is that households with individuals at risk for a cardiovascular event counseled and monitored by CHWs will have improved risk factor levels, compared with households not receiving such visits.
In 2008, the UnitedHealth Group and the National Heart Lung Blood Institute (NHLBI) collaborated to support a global network of centers of excellence to help reduce the burden of chronic diseases in middle- and low-income countries.13 St John’s Research Institute in Bangalore, India, is one of the 11 centers of excellence.14 PrePAre trial is one of the studies being conducted by this center of excellence. In this article, we describe the design and methods of this trial and discuss similar initiatives in LMICs.
Methods
Design
PrePAre is a multicenter, household-level, cluster-randomized trial with 1:1 allocation to intervention and control arms.
Ethics statement
We obtained approval from institutional ethics committees of the participating institutes in India (Rajah Muthaiah Medical College Annamalainagar, St John’s Medical College Bengaluru, Mahatma Gandhi Institute of Medical Sciences, Sevagram); Population Health Research Institute, Hamilton, Canada; NHLBI Bethesda, USA; and Health Ministry Screening Committee, Government of India. The study protocol is registered with Clinical Trial Registry of India (CTRI/2012/09/002981). Study investigators conducted community meetings with key stake-holders in each village to explain the study and to seek their cooperation. Community health workers prepared hand-drawn maps to locate households within a village, and a study investigator at each participating site approached all the eligible individuals and sought a written informed consent in one of the local languages (Kannada, Marathi, and Tamil).
Setting
The PrePAre trial is being conducted in selected rural communities from health subcenters in proximity of participating institutes in India. Three villages in Thunisaramedu and Veerasolagan subcenters (Cuddalore district) near Annamalainagar in Tamil Nadu (population ~4000); 15 villages in Ettakodi, Puraand, Chikkatirupathi subcenters (Kolar district) near Bengaluru in Karnataka (population ~10,000); 10 villages in Goji and Hamdapur subcenters (Wardha district) near Sevagram, Maharashtra (population ~10,000), were selected as trial sites. Altogether, these 28 villages have 5,699 households (Figure 1).
Figure 1.
Map of India depicting the participating sites.
Study personnel
The study team at each site consists of a principal investigator, coinvestigators, a study physician, a supervisor, and 4 to 6 CHWs. We identified CHWs from the local communities. A CHW was required to have completed high school, to be living in one of the villages included in the study, and to be approved by members of village administrative body. At each site, a supervisor coordinated the CHWs, and a study physician conducted field level clinics. This organizational structure was designed to demonstrate the feasibility of integration of a CVD prevention model in existing public health delivery system. Currently, public health workers at village, subcenter, and primary health center level in India are not assigned tasks that would prevent CVD (online Appendix Supplementary Table).
Study participants
We defined a household as a group of individuals living together and sharing a common kitchen. The unit of study randomization was a household at risk for CVD. To be enrolled in the study, a household had to have (a) at least 1 member ≥35 years old, at 10% or higher risk of developing an adverse cardiovascular outcome in the next 5 years (as determined by the non–laboratory-based National Health and Nutrition Examination Survey score [NHANES score]),15 and (b) its members living in the village at the time of the baseline screening survey. We excluded households from the trial if the individual members either refused consent or did not participate in the pre-randomization clinic visit.
Study procedures
Training of CHW
We trained CHWs in communication skills, qualitative techniques such as social mapping, conducting a baseline survey, and delivering key health messages to the intervention group. We also trained them to measure blood pressure (BP) with a digital BP–measuring device, weight by a digital weighing scale, height by a stadiometer, and waist circumference by a nonstretchable measuring tape. We developed plain language training modules on health and disease, communication and organizational skills, the cardiovascular system, measurement of cardiovascular risk factors and their interpretation, levels of prevention with a special focus on primary prevention, and linkages with existing public health systems. Every module explains basic concepts for a classroom teaching, followed by either a short story, a role-play exercise, or an example related to the topic. In addition, training material also included charts, models, and instruments CHWs that they would use in their actual work. We translated and adapted the entire training material in the local languages (Kannada, Marathi, and Tamil). Initially, the study physician and a supervisor from each site were trained as trainers, at a 7-day course. These trainers then returned to their sites to identify and train CHWs.
Baseline survey for risk stratification and screening
Community health workers visited and allocated an identification number to each house and listed its members. To ensure complete inclusion of households, CHWs visited locked houses at least thrice on different days of the week and at different times. We excluded houses whose residents were either temporary agricultural workers or stayed outside the village. We excluded individuals known to have cardiovascular, cerebrovascular, or peripheral vascular diseases (acute myocardial infarction, stroke, documented unstable angina, or peripheral vascular disease).
Community health workers administered a structured questionnaire to collect the following data: age, sex, socioeconomic status (standard of living index),16 tobacco (smoking, smokeless, and passive smoking), alcohol consumption, history of hypertension, diabetes mellitus, physical activity levels (work time and leisure time), dietary intake of various foods (salty, fried foods, fruit, and vegetables), and psychosocial factors (depression and stress). In addition, CHWs measured height and weight (height measured by a stadiometer, accuracy 0.1 cm, and weight measured by a digital scale, accuracy 0.1 kg), waist and hip circumference (measured by a nonstretchable tape, accuracy 0.1 cm), and BP (3 measurements, 5 minutes apart, using a Omron digital BP equipment). We calculated body mass index (BMI), waist-to-hip ratio (WHR), and average systolic BP (SBP) and diastolic BP using these measures. Community health workers and study physicians used baseline survey data to perform risk stratification using NHANES non–laboratory-based risk score15 and to estimate the baseline non–laboratory-based INTERHEART risk score of individual participants.17 Because the prevalence of smokeless tobacco in the study villages is high and based on the INTERHEART data, we decided a priori to replace smoking with tobacco consumption in the NHANES. We categorized the 5-year risk of first CVD event as low (<10%), moderate (10%-20%), and high (>20%). We plan to recapture all the variables captured during baseline after 12 and 24 months.
Prerandomization clinic visit
Study physicians conducted clinics in each village. The CVD risk reduction advice and prescriptions used in the study were based on the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure18 and the National Cholesterol Education Program (NCEP).19
Cardiovascular disease risk reduction prescriptions are in the form of a booklet with pictorial depiction of CVD risk factors. Three lifestyle interventions included in the prescription are tobacco cessation (prescribed to individuals who smoke or chew tobacco), healthy diet (to consume fewer salt containing and fried foods and more fruits and vegetables), and physical activity (brisk walking or cycling at least 120 minutes a week). Target for tobacco cessation and quit date were set after discussing with the participants. To assess adherence to the prescribed drugs, the CHWs asked subjects who were prescribed CVD medications to preserve the empty drug blisters or bills of the prescribed drugs. Subsequent to the clinic visit, we provided all individuals with appointments to attend follow-up clinics. Individuals were free to continue follow-up with their other physicians and to continue any previous drug therapies.
Randomization and blinding
We used a central computer-generated randomization process, with 1: 1 allocation of eligible households to intervention and control arms. The study physicians providing care in the clinics, but not the CHWs, were blind to the allocation of household to the 2 arms of the study. Given the nature of the intervention, we did not blind the CHWs to whether allocation of households was allocated to the intervention and nonintervention arm. We did, however, blind the study physician providing medical care in the clinics.
Intervention
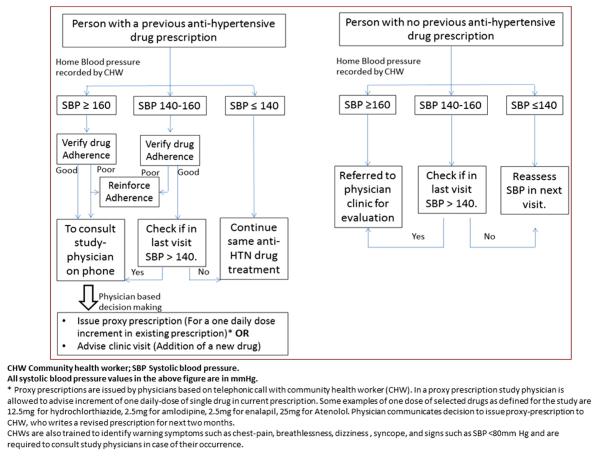
Community health worker visit all households in the intervention arm once every 2 months (6 visits in the first year). During each visit, CHWs perform 4 tasks. First, they ascertain adherence to prescribed pharmacological and nonpharmacological therapies by administering a questionnaire and inspecting the empty blister packets or proof of drug purchase if available. Second, they measure BP and ascertain if BP values meet the preset targets. If BP values do not meet targets, CHWs telephonically seek advice by asking the study physicians who use a simplified target-oriented algorithm to titrate dose of antihypertensive drugs (Figure 2). Third, CHWs ensure that individuals adhere to the prescribed treatment. They use educational messages to target tobacco use, adherence to medication, and promote lifestyle changes. Lifestyle changes include dietary modification (reduction in salt consumption, fried foods, salty foods, and increased consumption of fruits and vegetables) and physical activity (promotion of walking and cycling). The CHWs place clear and short goal-directed slogans printed on common household objects in the household to promote integration of preventive therapies with activities of daily living. Fourth, CHWs set goals for BP and weight and reduction in tobacco use and weight reduction goals for the next visit. These goals are individualized and mutually agreed upon. The CHWs also discuss simple, practical, and socially acceptable means for achieving these targets and lifestyle changes.
Figure 2.
A simple algorithm for community health workers to act upon blood pressure values.
Community health workers spend approximately 30 to 45 minutes for a household visit; approximately half the time is spent on measuring BP and the rest on reinforcing adherence to prescriptions on life style and drugs. To address the problem of contamination, the CHWs are instructed to visit only the intervention households. Whenever individuals from control households approach the CHWs and request house visits, the CHWs refer them to the clinic.
Follow-up clinics
Every month, throughout the duration of the trial, a study physician and a supervisor will organize clinics at the health subcenter. All eligible members residing in the villages around the subcenter, regardless of their enrollment or allocation to either arm of the study, can access clinic services, at no cost. In villages, it is difficult to access antihypertensive drugs because they are not available, public health supplies are erratic, or private pharmacies stock only high-priced brand-name drugs. To address these issues, participating institutes are allowed to dispense low-cost generic medications (amlodipine, hydrochlorothiazide, atenolol, ramipril, and atorvastatin) the day they run the clinic.
Outcome evaluation
To measure the early and sustained impact of intervention, we will evaluate the study outcomes at 12 and 24 months from randomization. The first follow-up will assess the early impact, whereas the second will determine the sustained impact of the interventions. The primary outcome measures are SBP and medication adherence. We will average SBP measurements of at-risk individuals and obtain mean SBP for a household. The study has 85% power to detect difference of 3 mm Hg in mean SBP between the intervention and control households. We hypothesize this reduction in SBP to be across all intermediate- and high-risk individuals in intervention households, large in those with stage 2 hypertension, and modest in those with stage 1 hypertension or prehypertension. We anticipate that about half of all individuals at high and moderate risk will have hypertension and a third of them will need to be on medication.
During 2-monthly visits to the households, CHWs inquire about medication adherence. Study supervisors collect the same measure every 6 months in control households. Every at-risk individual self-reports the number of antihypertensive pills that they were asked to consume, and in the preceding 2 months, the number of pills that they actually consume. The pill count is used to calculate mean daily number of prescribed medications consumed for the household. For example, if 1 of the 2 at-risk individuals in a household consumes a pill a day, the mean for the household is 0.5. If this individual consumes 12 of 30 prescribed pills in a month, mean for the household is 0.2. We define improvement in adherence to pharmacotherapy if individuals randomized to the intervention arm consumed ≥30% antihypertensive pills daily compared with those in the control arms (eg, 0.35 in control arm vs 0.5 in the intervention arm). We ask for medication adherence for at-risk individuals in control households at a lower frequency, as merely such an inquiry may be deemed as an intervention.
Secondary outcome measures are the INTERHEART risk score, WHR, and BMI. The study also has 85% power to detect an absolute difference of 2 points in the average INTERHEART risk score and reduction in the WHR and BMI of 0.01 and 0.75 kg/m2, respectively.
Sample size and statistical analyses
We calculated the sample size for the trial, using data from the PURE-India study. We calculated intraclass correlation (ICC) for different risk factor estimates. The ICC estimates ranged from 0.09 (WHR) to 0.38 (BMI). Using this range of ICC, average cluster size of 1.2 individuals, 85% power, and 2-sided α = .05, we estimated that we needed to enroll 900 households in each group to detect our primary outcome of at least a 3-mm difference in SBP and secondary outcomes of 2-point difference in INTERHEART risk score, 0.75 kg/m2 difference in BMI, and 0.01-cm difference in WHR.
Data will be presented as means and SDs for normally distributed continuous variables, median and interquartile ranges for continuous variables not normally distributed, and counts and percentages for dichotomous data. We will compare continuous variables (SBP, medication adherence, BMI, and WHR) in the intervention and control groups through a linear mixed model and a binary or ordinal mixed model for categorical variables; we shall treat, in each case, household as the random effect. As the individuals will be clustered within the household, we will use multilevel modeling to evaluate the relationship between individual-level exposures and outcomes of interest. We will also compare the score before and after the intervention using paired t test and develop a model for the differences in scores adjusting for baseline estimates and intervention as a fixed factor.
Quality control and trial monitoring
We are ensuring quality of CHW-based interventions, by reinforcing key concepts taught to them during initial training. In each household visit, the CHWs carry with them printed pocket cards—a set of 24-picture and text-based cards that serve as checklists for standard procedures, key health messages, and protocols to be followed. Furthermore, study supervisors and study physicians monitor the CHWs and help them hone their skills. A 3-tier monitoring process has also been set up to ensure quality of interventions. First, study supervisors verify the data of 10% of all participants in baseline surveys and household visits. Critical errors, which result in misclassification of participants or change in treatment decisions, are acted upon by the supervisor, who takes corrective (supervisor level checks in database) and educational (reinforcing key concepts with health workers) measures. Second, study physicians monitor and verify all risk stratification classifications. Third, the central coordination team periodically visit each study site, to evaluate CHW skills and accuracy of information.
The current trial is funded by NHLBI (HHSN2682200900025C; 2009–2014) as part of UnitedHealth Group-NHLBI initiative to counter chronic diseases in developing countries.
Trial progress
Training of the trainers, study physicians, and supervisors was completed in November 2010. After a week-long training course, we assessed trainers by using Objective Structured Practical Examination. The results indicated that all the trainers were well equipped to train CHWs at their respective sites.
We identified 14 CHWs (11 women) from within the communities they had been working for. Each CHW was allocated a population between 1,500 and 2,000 individuals, who either lived in the same or nearby villages. Between March 2011 and June 2011, trial physicians and supervisors trained CHWs at each site. We evaluated CHW training using pretest and posttest evaluations and completion of workbook and practicum-based assignments. All selected CHWs successfully completed their trainings, and all have continued in their role until September 2012.
Community health workers completed baseline surveys in 2 steps. In the first step, in August 2011, CHWs listed 24,661 individuals residing in 5,699 households in 28 villages. Of these, 10,310 individuals were aged ≥35 years old and 9,248 (89.6%) agreed for a baseline screening. In the second step, CHWs completed baseline surveys (administration of questionnaire and obtaining anthropometric and BP measurements) and performed risk stratification based on these measurements between September 2011 and February 2012 (Figure 3).
Figure 3.
Study flow.
Of 9,248 individuals who agreed to participate in baseline screening, 5,374 (58.2%) were at low risk and 3,874 (41.8%) were at moderate or high risk for future cardiovascular events. Subsequently, we conducted 82 village-level clinics between November 2011 and March 2012, to provide risk reduction prescription to moderate- and high-risk individuals. Of the 2,571 households identified as at risk, 3,285 individuals from 2,438 households (94.8%) participated in the village-level clinics and were eligible for randomization. Those who attended the clinic tended to be older as compared with those who did not (mean age [SD] 61.2 [10.5] vs 58.1 [9.36] years, P < .001). Gender and socioeconomic scores were similar across 2 groups. Of the individuals who attended the clinic visit, 207 (6.6%) were already on antihypertensive drugs, and 585 additional individuals (18.6%) were initiated on drug therapy.
We randomized 2,438 households and allocated 1,219 households with 1,661 at-risk individuals to the intervention group and 1,219 households with 1,624 at-risk individuals to the control group. Randomization was completed between February and May 2012. Community health worker–based intervention began in March 2012, and is currently ongoing. The 12- and 24-month follow-up visits will take place in March 2013 and 2014, respectively.
Community-based interventions in chronic diseases
Although many CHW based interventions have been shown to be beneficial in promoting childhood immunization uptake, initiation of breastfeeding, and improving pulmonary tuberculosis cure rates,9 there is a paucity of literature on their role in CVD risk reduction, especially in LMICs. In a meta-analysis of CVD risk reduction programs until 2008, all the 36 identified programs were from high-income settings. 20 Overall, there was a 0.65% absolute reduction in 10-year CVD risk. Another systematic review of CHW-based intervention trials21 identified 10 trials, which targeted hypertension, diabetes mellitus, or CVDs. Nine of these were from high-income countries. These trials showed that CHW interventions can increase knowledge levels, improve follow-up rates, and achieve better glycemic or BP control.
We reviewed published and ongoing studies aimed at CVD risk reduction from LMICs. A cluster randomized trial from Pakistan22 showed that a multilevel intervention involving home-based patient education delivered by CHWs and general practitioner education achieved a significant reduction in SBP, as compared with no such intervention. This combined strategy was also more cost-effective.23 Another cluster randomized trial from rural India24 evaluated CHW-based health promotion intervention (using population-level strategies such as rallies, street theater, posters, and community meetings) and did not find any difference in knowledge levels of participants in intervention and nonintervention arms. Two other cardiovascular risk reduction programs (promotores de salud from US-Mexico border25 and Isfahan healthy heart program from Iran26,27) report significant reductions in CVD risk factors and consumption of a more healthy diet, respectively. A few other educational interventions at worksites,28 schools,29,30 and health care facilities,31 which do not involve CHWs in the true sense, have also reported an improvement in risk factor levels and knowledge (Table).
Our study has several strengths. Our sample is large and covers 3 diverse linguistic and culturally diverse locations in India. The intervention is a multiple risk reduction strategy at a community level. The study uses a robust cluster randomized design to evaluate the impact of these interventions. Interventions are intensive and are executed through repetitive household-level interpersonal contact between CHWs and those at risk. Community health workers are not only involved in health promotion but also maintain continuity of clinical care. Our study will generate evidence to determine if CHWs can effectively reduce the burden of CVDs in rural communities in India.
Supplementary Material
Appendix Supplementary Table. Organization of public health workforce in India
Acknowledgements
We acknowledge all community health workers, study supervisors, study physicians, database team members, and division of clinical trials staff who are currently helping with the conduct of this study. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
References
- 1.Gaziano T, Reddy KS, Paccaud F. Cardiovascular disease. In: Jamison DT, editor. Disease control priorities in developing world. Oxford University Press; Oxford: 2006. pp. 645–62. [Google Scholar]
- 2.Joshi R, Cardona M, Iyengar S. Chronic diseases now a leading cause of death in rural India: mortality data from the Andhra Pradesh Rural Health Initiative. Int J Epidemiol. 2006;35:1522–9. doi: 10.1093/ije/dyl168. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Joshi P, Mohan V, et al. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008 doi: 10.1136/hrt.2007.132951. http://dx.doi.org/10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 4.Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA. 2007;297(3):286–94. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar JW, Fortman SP, Flora JA, et al. Effects of community-wide education on cardiovascular disease risk factors. The Stanford Five-City-Project. JAMA. 1990;264:359–65. [PubMed] [Google Scholar]
- 6.Luepker RV, Murray DM, Jacobs DR, et al. Community education for cardiovascular disease prevention: risk factor changes in the Minnesota Heart Health Program. Am J Public Health. 1994;84:1383–93. doi: 10.2105/ajph.84.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nissinen A, Berrios X, Puska P. Community-based noncommunicable disease interventions: lessons from developed countries for developing ones. Bull World Health Organ. 2001;79:963–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Puska P, Salonen JT, Nissinen A, et al. Change in risk factors for coronary heart disease during 10 years of a community intervention programme (North Karelia Project) BMJ. 1983;7:1840–4. doi: 10.1136/bmj.287.6408.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewin S, Munabi-Babigumira S, Glenton C, et al. The effect of lay health workers on mother and child health and infectious diseases. 2010. [DOI] [PMC free article] [PubMed]
- 10.Bang AT, Bang RA, Tale O. Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet. 1990;336:201–6. doi: 10.1016/0140-6736(90)91733-q. [DOI] [PubMed] [Google Scholar]
- 11.Baqui AH, El-Arifeen S, Darmstadt GL, et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 12.Ferdinand KC, Patterson KP, Taylor C, et al. Community-based approaches to prevention and management of hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 14(5):336–43. doi: 10.1111/j.1751-7176.2012.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabel EG, Stevens S, Smith R. Combating chronic disease in developing countries. Lancet. 2009;373(9680):2004–6. doi: 10.1016/S0140-6736(09)61074-6. [DOI] [PubMed] [Google Scholar]
- 14.National Heart Lung and Blood Institute . Bangalore India Center of Excellence. National Institute of Health; Bethesda: 2012. [Google Scholar]
- 15.Gaziano TA, Young CR, Fitzmaurice G, et al. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008;371:923–31. doi: 10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Institute for Population Sciences (IIPS) and ORC Macro . National Family Health Survey (NFHS-2), 1998–99. IIPS; Mumbai, India: 2000. [Google Scholar]
- 17.McGorrian C, Yusuf S, Islam S, et al. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–90. doi: 10.1093/eurheartj/ehq448. [DOI] [PubMed] [Google Scholar]
- 18.National Heart Lung Blood Institute . Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) NHLBI; Bethesda: 2003. Report No.: 03–5233. [DOI] [PubMed] [Google Scholar]
- 19.National Heart Lung Blood Institute . Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) NHLBI; Bethesda: 2002. Report No.: 02–5215. [Google Scholar]
- 20.Pennant M, Davenport C, Bayliss S, et al. Community programs for the prevention of cardiovascular disease: a systematic review. Am J Epidemiol. 172(5):501–16. doi: 10.1093/aje/kwq171. [DOI] [PubMed] [Google Scholar]
- 21.Global Health Workforce Alliance . Global experience of community health workers for delivery of health related millennium development goals: a systematic review, country case studies, and recommendations for integration into National Health Systems. World Health Organization; Geneva: 2010. [Google Scholar]
- 22.Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med. 2009;151(9):593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Jafar TH, Islam M, Bux R, et al. Cost-effectiveness of community-based strategies for blood pressure control in a low-income developing country: findings from a cluster-randomized, factorial-controlled trial. Circulation. 124(15):1615–25. doi: 10.1161/CIRCULATIONAHA.111.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi R, Chow CK, Raju PK, et al. The Rural Andhra Pradesh Cardiovascular Prevention Study (RAPCAPS) J Am Coll Cardiol. 2012;59(13):1188–96. doi: 10.1016/j.jacc.2011.10.901. [DOI] [PubMed] [Google Scholar]
- 25.Balcazar H, Alvarado M, Cantu F, et al. A promotora de salud model for addressing cardiovascular disease risk factors in the US-Mexico border region. Prev Chronic Dis. 2009;6(1):A02. [PMC free article] [PubMed] [Google Scholar]
- 26.Sarrafzadegan N, Kelishadi R, Esmaillzadeh A, et al. Do lifestyle interventions work in developing countries? Findings from the Isfahan Healthy Heart Program in the Islamic Republic of Iran. Bull World Health Organ. 2009;87:39–50. doi: 10.2471/BLT.07.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadifard N, Kelishadi R, Safavi M, et al. Effect of a community-based intervention on nutritional behaviour in a developing country setting: the Isfahan Healthy Heart Programme. Public Health Nutr. 2009;12:1422–30. doi: 10.1017/S1368980008004230. [DOI] [PubMed] [Google Scholar]
- 28.Prabhakaran D, Jeemon P, Goenka S, et al. Impact of a worksite intervention program on cardiovascular risk factors: a demonstration project in an Indian industrial population. J Am Coll Cardio. 2009;53:1718–28. doi: 10.1016/j.jacc.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 29.Shah P, Misra A, Gupta N, et al. Improvement in nutrition related knowledge and behaviour of urban Asian Indian school children: findings from the “medical education for children/adolescents for realistic prevention of obesity and diabetes and for healthy aging” (MARG) intervention study. Br J Nutrition. 2010;104:427–36. doi: 10.1017/S0007114510000681. [DOI] [PubMed] [Google Scholar]
- 30.Singhal N, Misra A, Shah P, et al. Effects of controlled school-based multi-component model of nutrition and lifestyle interventions on behaviour modification, anthropometery and metabolic risk profile of urban Asian Indian adolescents in North India. Eur J Clin Nutr. 2010;64:364–73. doi: 10.1038/ejcn.2009.150. [DOI] [PubMed] [Google Scholar]
- 31.Mendis S, Johnston SC, Fan W, et al. Cardiovascular risk management and its impact on hypertension control in primary care in low-resource settings: a cluster-randomized trial. Bull World Health Organ. 88(6):412–9. doi: 10.2471/BLT.08.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anchala R, Pant H, Prabhakaran D, et al. Decision support system (DSS) for prevention of cardiovascular disease (CVD) among hypertensive (HTN) patients in Andhra Pradesh, India’ - a cluster randomised community intervention trial. BMC Public Health. 12(1):393. doi: 10.1186/1471-2458-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Supplementary Table. Organization of public health workforce in India