Abstract
It has been suggested that anergic T cells may not be only inert cells but may rather play an active role, for example by regulating immune responses. We have previously reported the existence of “anergic” IL-10-producing CD4+ T cells generated in vivo by continuous antigenic stimulation. Using a gene transfer system where the antigen recognized by such T cells is expressed in skeletal muscle by two different DNA viral vectors, we show that these cells not only remain tolerant toward their cognate antigen but also can suppress the immune response of naïve T cells against the immunogenic adenoviral proteins. Furthermore, they can completely inhibit tissue destruction that takes place as a result of an immune response. The system presented here is unique in that the T cells have been anergized in vivo, their antigen specificity and functional status are known, and the amount, form, and timing of antigen expression can be manipulated. This model will therefore permit us to carefully dissect the mechanisms by which these anergic T cells regulate the priming and/or effector function of naïve T cells.
Central deletion plays an essential role in tolerance induction toward thymus-expressed and circulating proteins (1, 2) and even toward some antigens that were thought to be tissue-restricted (3). However, it is not the only mechanism of immune tolerance. Mature T cells need to be tolerized toward tissue-restricted antigens that are not present in sufficient quantity in the thymus. An increasing number of experiments have demonstrated that, in vivo, tissue-specific proteins can be crosspresented by neighboring antigen-presenting cells (APCs) that can then, depending on largely undefined parameters, prime (4–7) or tolerize (8–12) MHC class I and II restricted antigen-specific T cells. In some in vivo models of peripheral tolerance induction, it has been found that anergic T cells secrete significant levels of IL-10 (13, 14), a cytokine known for its immunoregulatory function (15–18). Nevertheless, it was not clear whether such in vivo anergized cells could exert an immunoregulatory function on naïve T cells. CD4+CD25+ (19, 20), CD4+CD62Lhigh (21), and CD4+CD45RBlow cells (22), generated in mice under physiological conditions, have been shown to regulate certain autoimmune responses and, in some cases, appear to do so in an IL-10-dependent manner (23). However, such populations consist of heterogenous cells, and it is difficult to pinpoint their antigen specificity. In the present study, we have used T cells that express a MHC class II-restricted T cell antigen receptor (TCR) specific for the influenza hemagglutinin (HA) that were generated in mice where the antigen is present on hemopoietic cells (24). The CD4+ cells expressing high levels of the transgenic TCR (detected by the 6.5 mAb) are “anergic” in terms of in vitro-induced proliferation but exhibit activation markers and secrete significant levels of IL-10 (14). To study a regulatory function of these cells, we have exploited a gene transfer system in which HA is targeted to the skeletal muscle. This was done by using two different viral vectors: a recombinant adeno-associated virus (rAAV) exclusively expressing the HA-transgene (25, 26) and eliciting an HA-specific immune response through uptake of the protein by surrounding APCs (27–30); the second vector consisted of a recombinant E1-deleted adenovirus (rAd) that expresses, besides the HA transgene, several highly immunogenic viral proteins and that elicits immune responses directed against the HA as well as adenoviral epitopes. Both vectors can induce immune responses that led to destruction of transduced muscle fibers (31–33). This system allowed us to study the effect of the “anergic” HA-specific CD4+ T cells on the immune response toward the HA protein alone (rAAV-HA vectors) or toward the HA protein and the adenoviral proteins (rAd-HA vectors).
Materials and Methods
Mice.
TCR-HA mice, IG-HA mice, and TCR-HA × IG-HA mice, previously described (24, 34), are on the BALB/c background and were bred in our animal facility at Institut Necker. BALB/c and BALB/c nude mice were purchased from Iffa/Credo.
Recombinant Virus Vectors.
The rAd-HA and rAAV-HA vectors were prepared as described (30) and injected i.m. in a volume of 25 μl into the tibialis anterior of anesthetized mice. rAdLacZ described in Jooss et al. (33) was injected i.v. into BALB/c or TCR-HA × IG-HA mice.
Histology.
Frozen muscle sections were fixed in acetone and incubated with biotinylated H37/38 mAb, directed against a conformational epitope of HA (35) (kindly provided by Walther Gerhard, Wistar Institute, Philadelphia) in PBS/2% goat serum, washed, and incubated in avidin-biotin-chromagen solution (Vector Laboratories). The slides were revealed in diaminobinzidine (DAB fast, Sigma) and counterstained with methyl green or hematoxylin.
Antiadenoviral Cytotoxic Assay.
The cytotoxic T lymphocyte assay was done as previously described (33).
Adoptive Transfers.
Total lymph node cells from a TCR-HA RAG−/− mouse were used as source of naïve HA-specific T cells. Staining of these cells with CD4-PE and 6.5-Fluos (mAb specific for the transgenic TCR) revealed that 80% of total lymph node cells were CD4+6.5+. These cells (2 × 105) were injected into BALB/c nude recipients. Some of these recipients received also “anergic” HA-specific T cells. For this, splenocytes from 3- to 4-month-old TCR-HA × IG-HA mice were partially depleted of sIg+ cells by using Dynabeads (Dynal, Oslo). Splenocytes (25 × 106) (of which 4% were CD4+6.5+) were coinjected to achieve a 5:1 ratio of “anergic”/naïve T cells. Five days after the transfer, recipients were injected i.m. with rAd-HA or rAAV-HA vectors. Muscles were harvested 27 days after vector administration and tested for persistence of HA expression.
Results
HA-Specific Tolerance Results in Stable Gene Expression of rAAV-HA but Not rAd-HA Vectors.
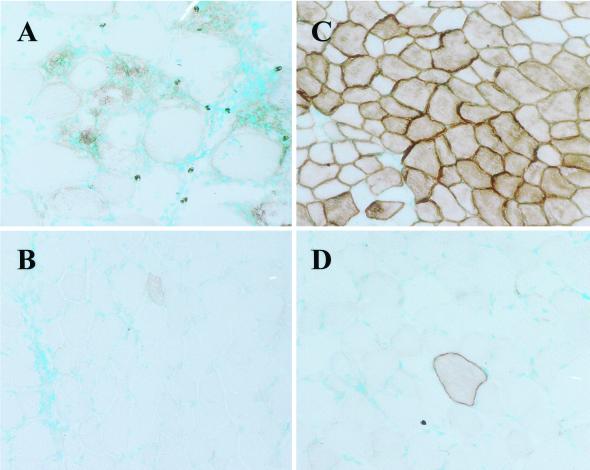
Vector-mediated gene transfer to skeletal muscle leading to the expression of membrane-bound HA in the muscle fibers of BALB/c mice results in the induction of an immune response directed toward the HA protein and the viral backbone in the case of adenoviral vectors (rAd-HA) and exclusively toward the HA protein in the case of rAAV-HA vectors (30). In both cases, the immune response leads to muscle fiber destruction. Using TCR transgenic mice, we could determine that T cell priming was a very local event that occurred in the lymph nodes draining the injected leg and was because of crosspresentation of antigenic material by surrounding APCs. To test whether T cell tolerance resulting from ubiquitous expression of HA would prevent muscle fiber destruction after rAd-HA- or rAAV-HA-mediated gene transfer, we tested the immune response to both viral vectors in IG-HA transgenic mice. These mice express the HA antigen under the Igκ promoter and enhancer, leading to HA expression on circulating hemopoietic cells (34). As shown in Fig. 1, BALB/c control mice injected with rAAV-HA vector showed muscle fiber destruction (Fig. 1A), whereas IG-HA mice showed stable gene expression 27 days after vector administration (Fig. 1C). In contrast, both BALB/c and IG-HA mice showed muscle fiber destruction when injected with rAd-HA vectors, as early as 7 days after vector administration (Fig. 1 B and D). These results show that IG-HA mice, although tolerant to HA, still respond to the adenoviral proteins, resulting in tissue destruction. Tolerance to HA does not seem to be broken in IG-HA mice on rAd-HA administration: in one experiment in which IG-HA mice received rAd-HA in the left leg and rAAV-HA in the right leg, tissue destruction was observed only in the left leg (data not shown). These results indicate that expression of a self protein by viral vectors that is also expressed elsewhere in the body will not result in an immune response unless the vector itself is immunogenic.
Figure 1.
Persistence of gene expression after rAAV-HA- but not rAd-HA-mediated gene transfer in IG-HA transgenic mice. The tibialis anterior muscle of BALB/c (A and B) or IG-HA (C and D) mice was injected with rAAV-HA (1 × 1011 particles) (A and C) or rAd-HA (2.5 × 1010 particles) (B and D). Muscles were isolated 27 days after vector administration and frozen sections tested for HA expression. Sections were counterstained with methyl green. (×200.)
Regulation of Immune Responses in TCR-HA Transgenic Mice.
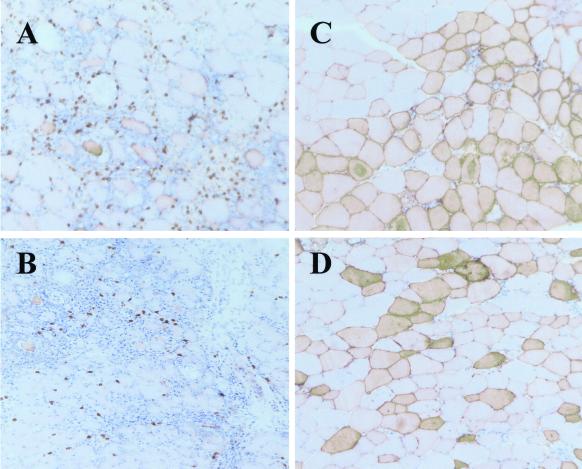
TCR-HA × IG-HA double transgenic mice express HA on the hemopoietic cells and a class II-restricted HA-specific TCR. In these mice, despite efficient thymic deletion of T cells expressing high levels of the HA-specific TCR, a significant number of cells with the transgenic TCR can be found in the spleen and lymph nodes, and their percentage among CD4 T cells, determined by flow cytometry with CD4 and 6.5 antibodies, increases with the age of the mouse up to 15% of all CD4+ T cells. These cells are “anergic” in terms of proliferation (24) but exhibit an activated phenotype in vivo and secrete significant amounts of IL-10 (14), suggesting that these “anergic” cells could in fact act as regulatory T cells. These cells are heterogenous in that up to 30% in lymph nodes and up to 20% in spleen express CD25. All of these cells have down-regulated CD45RB when compared with naïve cells with the same specificity. Intramuscular gene transfer in these double transgenic TCR-HA × IG-HA mice was performed to determine the effect of high numbers of “anergic” HA-specific T cells on the immune response directed to the adenoviral proteins as well as to the HA protein. As can be seen in Fig. 2, TCR-HA × IG-HA mice that received rAAV-HA showed stable expression of HA 27 days after gene transfer (Fig. 2C), similar to what was observed in IG-HA animals (Fig. 1C). Surprisingly, muscles of TCR-HA × IG-HA mice having received rAd-HA also showed stable gene expression and no infiltration at both early (not shown) and late timepoints (Fig. 2D). As expected, TCR-HA single transgenic control mice showed a strong response with both viral vectors (Fig. 2 A and B). The histology results shown in Figs. 1 and 2 are summarized in Table 1.
Figure 2.
Persistence of gene expression with both rAAV-HA and rAd-HA vectors in TCR-HA × IG-HA transgenic mice. TCR-HA (A and B) or TCR-HA × IG-HA (C and D) mice were injected with rAAV-HA (1 × 1011 particles) (A and C) or rAd-HA (2.5 × 1010 particles) (B and D). Muscles were isolated 5 (A) or 17 days (B) after vector administration and frozen sections tested for HA expression. Sections were counterstained with hematoxylin/eosin. The differences in HA staining observed between rAAV and rAd viral vectors result from the different efficiency of these vectors in transducing muscle fibers: rAAV vectors are highly tropic for muscle and, with the amounts injected in this study, generally result in the homogenous transduction of 60–75% of muscle fibers, whereas rAd-HA vectors are less efficient in transducing muscle fibers of animals that age and result in a more “patchwork-like” staining profile. (×200.)
Table 1.
Immune response to rAAV-HA or rAd-HA vectors in different mice
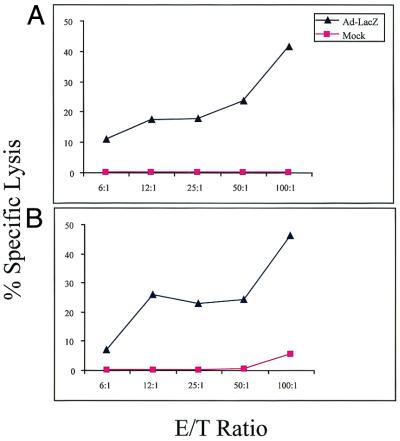
That rAd-HA gene transfer is stable in TCR-HA × IG-HA mice but not in IG-HA mice could have different explanations: (i) the absence of an antiadenoviral T cell repertoire because of the introduction of the transgenic TCR in the TCR-HA × IG-HA mice; (ii) a deficiency in the priming capacity of the APCs of TCR-HA × IG-HA mice; or (iii) suppression of the adenoviral-specific T cell response by high numbers of “anergic” HA-specific T cells because of the high frequency of anergic T cells with the transgenic TCR. A similarly high frequency of anergic T cells would not be expected in IG-HA transgenic mice because of the low frequency of HA-specific T cells in such mice. To address the first two possibilities, we tested the capacity of TCR-HA × IG-HA mice to mount a cytotoxic response against adenoviral proteins. As can be seen in Fig. 3, the cytotoxic response against the adenoviral backbone was equally strong in control BALB/c (Fig. 3 Upper) and TCR-HA × IG-HA (Fig. 3 Lower) mice that were injected with a recombinant adenoviral vector expressing the Escherichia coli β-galactosidase protein 8 days before the assay. This assay measures a class I-restricted response, but it has been shown that antiadenoviral responses need class II help (36), therefore it is likely that the class II-restricted antiviral repertoire is not significantly impaired in the transgenic mice. These results show that TCR-HA × IG-HA mice contain APCs that can efficiently prime T cells toward adenoviral proteins, and that they possess a significant antiadenoviral T cell repertoire. Thus, the absence of an immune response toward the adenoviral backbone in TCR-HA × IG-HA mice is likely to depend on a regulatory function of the large numbers of “anergic” HA-specific T cells.
Figure 3.
Induction of an adenoviral-specific cytotoxic T cell response in TCR-HA × IG-HA and BALB/c mice. BALB/c (A) and TCR-HA × IG-HA (B) mice were injected i.v. with rAd-LacZ (1 × 1011 particles) and splenocytes harvested 8 days after vector administration. The cells were restimulated in vitro for 5 days with rAd-LacZ and tested for specific lysis on syngeneic target cells (KD2SV). Target cells were uninfected (Mock, squares) or infected with Ad-LacZ (Ad-LacZ, triangles).
Cotransfer of “Anergic” T Cells Inhibits Tissue Destruction by Naïve T Cells.
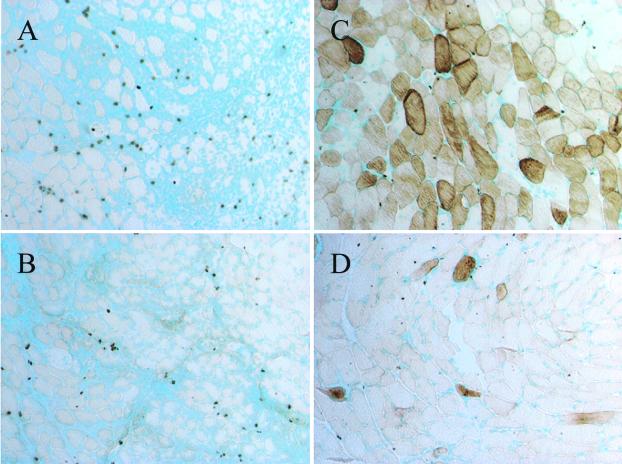
To confirm that the “anergic” T cells in the TCR-HA × IG-HA mice were acting as regulatory T cells, we tested their ability to abrogate the immune response of naïve TCR-HA cells toward muscle fibers expressing recombinant vector-encoded HA. For this, nu/nu BALB/c mice (deficient in T cells) were transferred with 2 × 105 naïve monospecific TCR-HA cells from a TCR-HA RAG−/− mouse and with T-cell-enriched splenocytes from TCR-HA × IG-HA mice containing 106 CD4+TCR-HAhigh cells. Control mice received naïve TCR-HA cells only. Four days after adoptive transfer, recipients received either of the recombinant viral vectors (rAd-HA or rAAV-HA) into the tibialis anterior muscle, and 28 days later, muscles were prepared, and frozen sections were tested for HA expression and T cell infiltration. As can be seen in Fig. 4, the immune response toward HA-expressing muscle fibers observed in recipient mice transferred with naïve T cells only (Fig. 4 A and B) was completely inhibited by cotransfer of the “anergic” TCR-HA T cells. This immune-suppressive effect was observed with both rAd-HA (Fig. 4C) and rAAV-HA (Fig. 4D) viral vectors. No suppressive effect was observed on cotransfer of naïve TCR-HA cells with T cell-enriched splenocytes from an IG-HA mouse (data not shown). Thus, these results indicate that the “anergic” T cells that develop and accumulate in TCR-HA × IG-HA mice can indeed act as regulatory T cells and inhibit immune responses toward recombinant vector-encoded HA as well as toward adenoviral proteins.
Figure 4.
Regulation of the HA-specific immune response by cotransfer of “anergic” and naïve HA-specific T cells. Naïve TCR-HA cells (2 × 105) obtained from the lymph nodes of RAG−/− TCR-HA mice were injected i.v. into nu/nu BALB/c recipients. Some of the recipients also received a 5-fold amount (106) of “anergic” TCR-HA cells obtained from the spleen of 3- to 4-month-old TCR-HA × IG-HA mice, as described in Materials and Methods. Five days after the adoptive transfer, recipients that received naïve cells alone (A and B) or naïve plus anergic cells (C and D) were injected i.m. with rAAV-HA (A and C) or rAd-HA (B and D) vectors. Muscles were isolated 27 days after vector administration, and frozen sections were tested for HA expression. (×200.)
Discussion
The possibility has been suggested that “anergic” CD4+ T cells, which accumulate in vivo when antigen is expressed in hemopoietic tissue, may have an active immunoregulatory role in vivo rather than being inert cells, but this possibility has been difficult to prove. In the present study, we have analyzed the immune response toward a viral vector-encoded protein, HA, in mice containing HA-specific MHC class II-restricted T cells that have been anergized in vivo by hemopoietic cells expressing the HA protein. We found that expression of HA by a viral vector in the muscle leads to stable gene expression, also when the number of antigen-specific T cells with HA-specific TCRs is artificially high, as is the case of TCR-HA × IG-HA mice. That there was no overt immune response against viral vector-encoded HA in the TCR-HA × IG-HA mice is interesting, because it was previously shown that these double transgenic mice contain in the periphery T cells that express two αβTCRs at the cell surface, enabling them to escape thymic and peripheral deletion, and cause diabetes when isolated and transferred into immunodeficient mice that express HA on pancreatic β cells (37). The reason why the same cells did not cause destruction of HA-expressing muscle fibers in TCR-HA × IG-HA double transgenic mice could be that they are held in check by the “anergic” immunoregulatory cells with high levels of the transgenic TCR. This hypothesis is supported by the finding that there is a striking difference between IG-HA and TCR-HA × IG-HA mice with regard to the immune response that is induced on rAd-HA administration. Although the former mice mounted an immune response toward the adenoviral proteins resulting in tissue destruction, the latter showed stable gene expression and absence of lymphocyte infiltration even at late timepoints. The latter mice contain high numbers of T cells expressing high levels of the HA-specific TCR that are “anergic” in terms of proliferation but produce IL-10 (14), which has been reported to represent an immunoregulatory cytokine and has been found associated with in vivo tolerance induction in other models (13, 20, 38, 39). These results suggested, therefore, that these “anergic” T cells, which, because of the transgenic TCR-HA, are present in high numbers in TCR-HA × IG-HA but not in IG-HA mice, had suppressed the adenoviral-specific T cells. Whether the regulatory HA-specific T cells were recruited to the same site as the adenovirus-specific T cells and thus suppressed their response remains to be formally proven but is a likely possibility, as TCR-HA × IG-HA mice injected with a rAd vector expressing β-gal exhibited tissue destruction comparable to that observed in TCR-HA mice (data not shown). Because the T cell priming to the virally encoded antigen occurs in the draining lymph nodes by APCs that present the antigenic material (30), it is possible that the HA-specific T cells regulate the adenovirus-specific T cells because of close proximity when recognizing antigen on the same APC. The possibility that the “anergic” T cells are acting indirectly by reducing the expression of costimulatory molecules and cytokines of local APCs (15, 40) cannot be ruled out. The cotransfer experiments of “anergic” and naïve T cells into nude recipients confirm that the former can act as regulatory cells and inhibit the destruction of HA-expressing muscle fibers by the naïve T cells. The regulatory capacity of these anergic T cells is long-lived because even in mice cotransferred 2 months before vector administration, no immune response toward rAd-HA was observed (data not shown). Even though we enriched for T cells, in the cotransfer experiments, some HA-expressing hematopoietic cells could have been transferred and could be contributing to the maintenance of the regulatory capacity of the “anergic” T cells, because in other systems it has been reported that antigen needs to be present to maintain anergy (41, 42). As one would expect, a certain ratio of “anergic”/naive cells is required because transfer of the former into immunocompetent BALB/c mice was not sufficient to suppress an immune response toward rAd-HA (not shown).
The mechanisms and molecules involved in such immune regulation remain to be determined. In our system, despite the fact that the “anergic” HA-specific T cells secrete IL-10, their regulatory role does not seem to depend exclusively on such cytokine: continuous administration of high quantities of the anti-IL-10 receptor neutralizing antibody, 1B1.3a (43), into TCR-HA × IG-HA mice injected with rAd-HA or rAAV-HA (0.5 mg i.p. every other day from day −1 to day 15 after viral vector administration) did not result in breakdown of tolerance and tissue destruction (data not shown). Transforming growth factor-β (TGF-β) could also be involved in such immunoregulation (22), although the HA-specific “anergic” cells were not found to express significantly more TGF-β mRNA than naïve cells (unpublished results). However, TGF-β mRNA levels do not always correlate with TGF-β activity. The immunoregulatory molecules CTLA4 and PD1 are expressed at very high levels in the cytoplasm and cell membrane, respectively, of these “anergic” T cells (44) and could play an important role in the function of these cells. In fact, it has recently been reported that blocking of CTLA4 inhibits the regulatory function of CD4+CD25+ T cells in inflammatory bowel disease (45, 46). PD1, a receptor that shares 23% identity with CTLA4 (47), has been shown to down-regulate immune responses, its loss leading to breakdown of peripheral tolerance (48). The adoptive transfer system we describe in the present study will permit determination of the role of these and other molecules in the immunoregulation exerted by “anergic” T cells with known specificity for antigen.
Acknowledgments
We thank Benedita Rocha for helpful discussions, Sebastien Lavie for excellent animal care, and Sabine Camugli and Estelle Morillon for technical assistance. This work was supported by the Association Française contre les Myopathies, the European Community Biotech (contract CT98–0503), and the Institut National de la Santé et Recherche Médicale. H.v.B. is supported by the Körber Foundation.
Abbreviations
- TCR
T cell antigen receptor
- HA
hemagluttinin
- APC
antigen-presenting cell
- rAd
recombinant adenovirus
- rAAV
recombinant adeno-associated virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.von Boehmer H, Kisielow P. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- 2.Kappler J W, Roehm N, Marrack P. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D. Curr Opin Immunol. 1998;10:656–662. doi: 10.1016/s0952-7915(98)80085-x. [DOI] [PubMed] [Google Scholar]
- 4.Bevan M. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo D, Reilly C R, Scott B, Liblau R, McDevitt H O, Burkly L C. Eur J Immunol. 1993;23:1693–1698. doi: 10.1002/eji.1830230744. [DOI] [PubMed] [Google Scholar]
- 6.Sigal L J, Crotty S, Andino R, Rock K L. Nature (London) 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 7.Sarukhan A, Lechner O, von Boehmer H. Eur J Immunol. 1999;29:3410–3416. doi: 10.1002/(SICI)1521-4141(199910)29:10<3410::AID-IMMU3410>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.von Boehmer H, Hafen K. Nature (London) 1986;320:626–628. doi: 10.1038/320626a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhong G, Sousa C, Germain R. J Exp Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman R M. J Exp Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler A J, Marsh D W, Yochum G S, Guzzo J L, Nigam A, Nelson W G, Pardoll D M. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarukhan A, Trautmann A, Rocha B. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- 14.Buer J, Lanoue A, Franzke A, Garcia C, von Boehmer H, Sarukhan A. J Exp Med. 1998;187:177–183. doi: 10.1084/jem.187.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 16.Macatonia S E, Doherty T M, Knight S C, O'Garra A. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 17.de Waal Malefyt R, Yssel H, de Vries J E. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 18.Groux H, Bigler M, de Vries J E, Roncarolo M G. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 20.Papiernik M, do Carmo Leite-de-Moraes M, Pontoux C, Joret A-M, Rocha B, Pénit C, Dy M. J Immunol. 1997;158:4642–4653. [PubMed] [Google Scholar]
- 21.Lepault F, Gagnerault M-C. J Immunol. 2000;164:240–247. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 22.Powrie F, Carlino J, Leach M W, Mauze S, Coffman R L. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asseman C, Mauze S, Leach M W, Coffman R L, Powrie F. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanoue A, Bona C, von Boehmer H, Sarukhan A. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski R J. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockstedt D G, Podsakoff G M, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engelman E G. Clin Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 28.Liu D-W, Tsao Y-P, Kung J T, Ding Y-A, Sytwu H-K, Xiao X, Chen S-L. J Virol. 2000;74:2888–2894. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning W C, Zhou S, Bland M P, Escobedo J A, Dwarki V. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 30.Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O, Jooss K. J Virol. 2001;75:269–277. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Su Q, Wilson J M. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathy S, Black H, Goldwasser E, Leiden J. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 33.Jooss K, Ertl H C J, Wilson J M. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalberer C, Reininger L, Melchers F, Rolink A. Eur J Immunol. 1997;27:2400–2407. doi: 10.1002/eji.1830270939. [DOI] [PubMed] [Google Scholar]
- 35.Staudt L M, Gerhard W. J Exp Med. 1983;157:687–704. doi: 10.1084/jem.157.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang K, Xiang Z, Ertl H C, Wilson J M. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarukhan A, Garcia C, Lanoue A, von Boehmer H. Immunity. 1998;8:563–570. doi: 10.1016/s1074-7613(00)80561-0. [DOI] [PubMed] [Google Scholar]
- 38.Sundstedt A, Hoiden I, Rosendahl A, Kalland T, van Rooijen N, Dohlsten M. J Immunol. 1997;158:180–186. [PubMed] [Google Scholar]
- 39.Bacchetta R, Bigler M, Touraine J-L, Parkman R, Tovo P A, Abrams J, de Waal Malefyt R, de Vries J E, Roncarolo M G. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 41.Rocha B, Tanchot C, von Boehmer H. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsdell F, Fowlkes B J. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 43.O'Farrell A M, Liu Y, Moorre K W, Miu A L F. EMBO J. 1998;17:1006–1018. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechner O, Lauber J, Franzke A, Sarukhan A, von Boehmer H, Buer J. Curr Biol. 2001;11:1–20. [Google Scholar]
- 45.Read S, Malmstrom V, Powrie F. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak T W, Sakaguchi S. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman G J, Long A J, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz L J, Malenkovich N, Okazaki T, Byrne M C, et al. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Immunity. 1999;2:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]